Abstract
High extracellular NaCl is known to change expression of numerous genes, many of which are regulated by the osmoprotective transcription factor nuclear factor of activated T cells-5 (NFAT5). In the present study we employed RNA-Seq to provide a comprehensive, unbiased account of genes regulated by high NaCl in mouse embryonic fibroblast cells (MEFs). To identify genes regulated by NFAT5 we compared wild-type MEFs (WT-MEFs) to MEFs in which mutation of the NFAT5 gene inhibits its transcriptional activity (Null-MEFs). In WT-MEFs adding NaCl to raise osmolality from 300 to 500 mosmol/kg for 24 h increases expression of 167 genes and reduces expression of 412. Raising osmolality through multiple passages (adapted cells) increases expression of 196 genes and reduces expression of 528. In Null-MEFs, after 24 h of high NaCl, expression of 217 genes increase and 428 decrease, while in adapted Null-MEFs 143 increase and 622 decrease. Fewer than 10% of genes are regulated in common between WT- and null-MEFs, indicating a profound difference in regulation of high-NaCl induced genes induced by NFAT5 compared with those induced in the absence of NFAT5. Based on our findings we suggest a mechanism for this phenomenon, which had previously been unexplained. The NFAT5 DNA-binding motif (osmotic response element) is overrepresented in the vicinity of genes that NFAT5 upregulates, but not genes that it downregulates. We used Gene Ontology and manual curation to determine the function of the genes targeted by NFAT5, revealing many novel consequences of NFAT5 transcriptional activity.
Keywords: NFAT5, hypertonicity, GO analysis, osmotic, RNA
hypertonicity, which is induced by high extracellular concentrations of solutes such as NaCl, can damage and even kill cells, but cells generally protect themselves by responses including accumulation of compatible organic osmolytes (reviewed in Ref. 2). The highest interstitial concentrations of NaCl in mammals occur in their renal medullas, consequent to the operation of the urinary concentrating mechanism, but interstitial NaCl can be elevated in other tissues as well, although not nearly to as great an extent in the renal medulla.
Hypertonicity decreases gene expression in general (30) but also increases expression of many genes (8). Nuclear factor of activated T cells-5 (NFAT5) (16, 22) is a transcription factor that increases expression of genes clearly involved in osmoprotection, but also of other genes in which the connection is not obvious (9). Previous screens identified many hypertonicity-induced genes, including those regulated by NFAT5. Most of those screens employed DNA microarrays, for example (17, 20, 24, 31). Recently, the development of RNA sequencing (RNA-Seq) has provided a means for a more comprehensive profiling of gene expression than previous methods, including DNA microarrays (3).
In the present study we employed RNA-Seq to provide a comprehensive, unbiased account of genes regulated by high NaCl in mouse embryonic fibroblast cells (MEFs). To distinguish which of the genes are regulated by NFAT5 we compared mRNA expression in wild-type MEFs (WT-MEFs) to MEFs in which mutation of the NFAT5 gene inhibits its transcriptional activity (Null-MEFs).
MATERIALS AND METHODS
Cell Culture
WT-MEFs and Null-MEFs (7) were a gift from Dr. Go (University of Washington, St. Louis, MO). Cells were fed with DMEM supplemented with 10% FBS. To create cells adapted to high NaCl, cells were passaged more than three times in medium at 500 mosmol/kg (NaCl added). For more acute stress, cells were grown at 300 mosmol/kg through the same passages as cells adapted to 500 mosmol/kg and then incubated at 500 mosmol/kg for 24 h or kept at 300 mosmol/kg as a control.
PolyA RNA Purification
Total RNA was extracted from cell lysates using the RNeasy mini kit (Qiagen, Valencia, CA). DNA was removed by DNase digestion on the included purification column. PolyA RNA was purified using the Dynabeads mRNA purification kit (Invitrogen, Carlsbad, CA) according to a modified manufacturer protocol, as previously described (25). Briefly, 150 μg total RNA was incubated with Dynabeads Oligo(dT)25 for 5 min to bind polyA RNA. After being washed, beads were heated at 75°C to elute polyA RNA. Binding and elution were repeated two times to obtain high purity polyA RNA.
RNA-Seq
PolyA RNA was chemically fragmented with fragmentation buffer (40 mM Tris acetate, pH 8.2, 100 mM potassium acetate, and 30 mM magnesium acetate) at 94°C for 3 min. Fragmented polyA RNA was reverse-transcribed to cDNA with random hexamers. cDNA fragments were enzymatically blunted, ligated to sequencing adaptors, and migrated on a gel. We extracted 250–350 bp cDNAs and amplified them by PCR. For deep sequencing, multiplexed sequencing for six DNA libraries from RNA-Seq was performed on a High-Seq 2000 Illumina genome analyzer.
RNA-Seq Data Analysis
We included 12 datasets in the RNA-Seq data analysis: two biological replicates each for WT-MEFs and Null-MEFs in medium at 300 mosmol/kg, WT-MEFs and Null-MEFs in medium at 500 mosmol/kg for 24 h, and WT-MEFs and Null-MEFs that were adapted to medium at 500 mosmol/kg. BWA software (18) was used to map RNA-Seq reads to the mm9 mouse reference genome (http://genome.ucsc.edu), allowing for two mismatches. Only uniquely mapped reads were retained for the following downstream analysis. Transcript expression levels were quantified by using rpkmForGene program (28). EdgeR (29) was used to identify differentially expressed transcripts. Fold-changes were calculated using ratios of the arithmetic mean of the normalized read counts for each pair of replicates. Statistical significance of differential gene expression in RNA-Seq data was determined with EdgeR. Differentially expressed genes were selected by requiring both a fold-change ≥ 2 and an false discovery rate (FDR) ≤ 0.05. The Gene Ontology (GO) analysis of the differentially expressed genes was carried out using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (12).
Supplemental Table S1 contains all of the genes identified by RNA-Seq, including evaluation of the confidence with which they are identified.1 There are four lists of significantly differentially expressed genes with exposure to elevated NaCl: upregulated and downregulated genes in WT-MEFs at high NaCl for 24 h compared with WT-MEFs in normal medium; upregulated and downregulated genes in WT-MEFs adapted to high-NaCl compared with WT-MEFs in normal medium. Such differential expression is specific to WT-MEFs; if the same gene also was differentially expressed at high NaCl in Null-MEFs, it was excluded from Supplemental Table S1. In each list, the first column contains the gene symbol; the second column lists the fold-change calculated by EdgeR software, which uses ratios of the arithmetic mean of the normalized read counts for each pair of replicates. The last column is the FDR output from EdgeR indicating the statistical significance of the expression change. Supplemental Table S1 also contains Gene Ontology (GO) (12) analysis of protein products of the identified genes. To simplify the GO functions table, we removed annotation clusters that have an enrichment score <1.5. In addition, only molecular functions (“GOTERM_MF_FAT”) and biological processes (“GOTERM_BP_FAT”) are retained in the table.
NFAT5 Activity
Adapted cells were reverse-transfected for 24 h with ORE-X-Luciferase (13) and phRL-CMV-Luciferase (Promega, Madison, WI) vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Then, media were either increased from 300 to 500 mosmol/kg or replaced with the same medium at 300 or 500 mosmol/kg. Twenty-four hours later cells were harvested with passive lysis buffer (Dual Luciferase Reporter Assay system, Promega). Absolute values per μg protein of ORE-X and phRL-CMV activities were measured and normalized to each activity in WT-MEFs at 300 mosmol/kg. The NFAT5 activity in Fig. 1A is presented as a ratio of ORE-X/phRL-CMV.
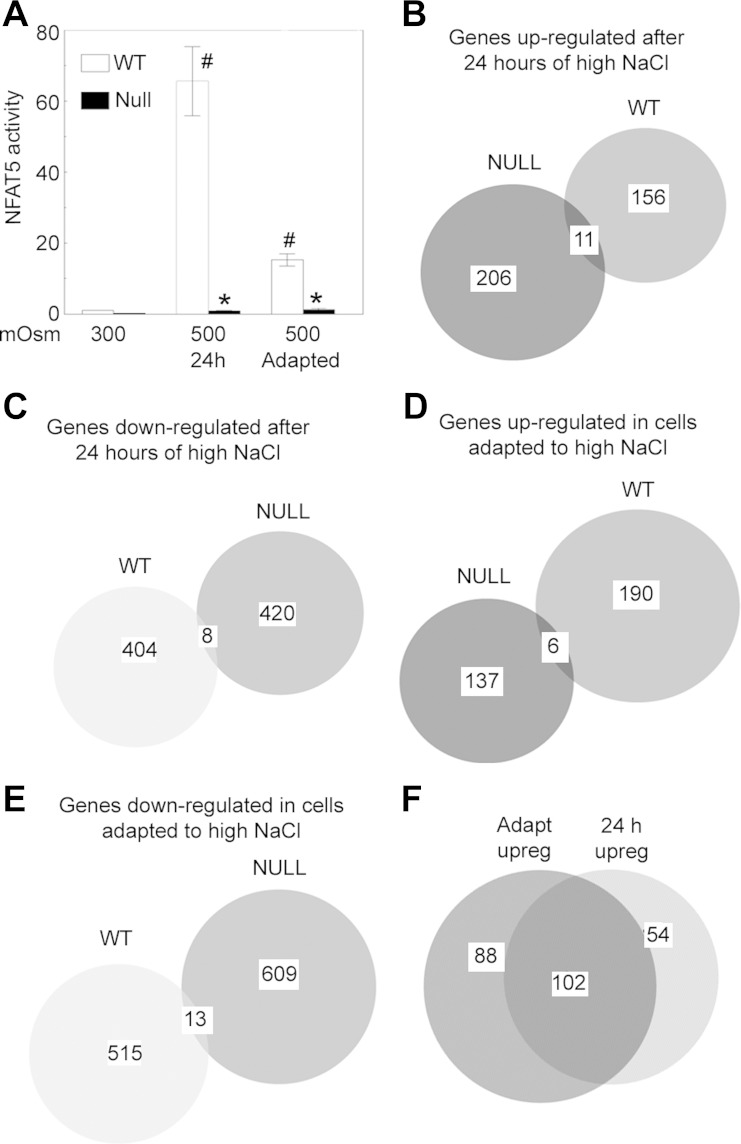
Fig. 1.
A: mouse embryonic fibroblast cells in which mutation of the NFAT5 gene inhibits its transcriptional activity (Null-MEFs) lack specific nuclear factor of activated T cells-5 (NFAT5) transcriptional activity. The MEFs were transfected with a luciferase reporter of NFAT5 transcriptional activity. Osmolality was increased from 300 to 500 mosmol/kg for 24 h in media bathing wild-type (WT-) or Null-MEFs or maintained at 300 or 500 mosmol/kg in media bathing adapted MEFs (#P < 0.05 vs. 300 mosmol/kg, *P < 0.05 vs. WT-MEFs). B–E: high NaCl regulates very few genes in common between WT- and Null-MEFs. B: number of genes upregulated after 24 h of high NaCl. C: number of genes downregulated after 24 h of high NaCl. D: number of genes that are upregulated in adapted MEFs. E: number of genes that are downregulated in adapted MEFs. F: number of genes upregulated in WT-MEFs and/or Null-MEFs.
RESULTS
Null-MEFs Express Mutant NFAT5 That Lacks Transcriptional Activity
The Null-MEFs were generated from mice in which exons 5 and 6 of NFAT5, which are critical for NFAT5 activity, were deleted (7). This region mediates critical sequence-specific contacts with DNA and also forms one of the two interfaces for dimerization within the DNA binding domain. High NaCl increases NFAT5 activity (measured with luciferase reporters) in WT-MEFs but not in Null-MEFs, confirming that the Null-MEFs lack NFAT5 transcriptional activity (Fig. 1A and Ref. 7). Also, WT-MEFs adapted to 500 mosmol/kg have higher NFAT5 transcriptional activity than those adapted to 300 mosmol/kg, but the difference is less than that of acute elevation of NaCl for only 24 h (Fig. 1A).
The Presence of NFAT5 Binding Sites Is Important for High NaCl-induced Upregulation of Genes in WT-MEFs, but Not for Their Downregulation
In WT-MEFs exposed to high NaCl for 24 h the NFAT5 DNA binding motif is enriched by 1.86-fold among upregulated genes, and in adapted cells it is enriched by 2.48-fold among upregulated genes (Table 1). In contrast, the result is markedly different for downregulated genes. Among downregulated genes, the motif is depleted to 0.89-fold in cells exposed to high NaCl for 24 h and to 0.68-fold in adapted cells. We conclude that NFAT5 binding to its specific DNA element is important for NFAT5-regulated increases in gene expression, but not for NFAT5-regulated decreases in gene expression.
Table 1.
IRF1 and NFAT5 binding sites are enriched at the promoter region (−10 to 2 kb around the transcription start site) of many up- and downregulated genes in WT- and Null-MEFs
| NFAT5 |
IRF1 |
|||
|---|---|---|---|---|
| WT-MEFs | Null-MEFs | WT-MEFs | Null-MEFs | |
| 24 h | ||||
| Upregulated | 1.86 (P = 0.059) | 0.66 | 2.09 (P = 0.09) | 0 |
| Downregulated | 0.89 | 1.28 | 2.90 (P = 0.006) | 0.49 |
| Adapted | ||||
| Upregulated | 2.48 (P = 0.003) | 1.40 | 3.10 (P = 0.03) | 2.00 |
| Downregulated | 0.68 | 0.80 | 3.24 (P = 0.002) | 0.62 |
IRF1, interferon regulatory factor 1; NFAT5, nuclear factor of activated T cells-5; MEF, mouse embryonic fibroblast cells; WT-MEF, wild-type MEF; Null-MEF, MEFs in which mutation of the NFAT5 gene inhibits its transcriptional activity.
High NaCl Alters Expression of Very Few Genes in Common between WT- and Null-MEFs
A previous microarray screen of MEF cells (17) demonstrated that the response to high NaCl is dominated by NFAT5 in that expression of a totally different set of genes is increased by high NaCl in WT-MEFs vs. Null-MEFs in which the NFAT5 gene is inactive. The observation is confirmed in the present study by RNA-Seq. Overall, high NaCl increases the expression of 156 genes in WT-MEFs and of 206 genes in Null-MEFS after 24 h, but only 11 of those genes are regulated in common (Fig. 1B). High NaCl decreases expression of 404 genes in WT-MEFs and of 420 genes in Null-MEFS after 24 h, but only eight of those genes are regulated in common (Fig. 1C). Similarly, adaptation of WT-MEFs to 500 mosmol/kg increases expression of 190 genes and adaptation of Null-MEFs increases expression of 137 genes, but only six increase in common (Fig. 1D), and adaptation decreases expression of 515 genes in WT-MEFs and of 609 genes in Null-MEFs, but expression of only 13 genes decrease in common (Fig. 1E). Thus, we confirm that, in response to high NaCl, expression of transcriptionally active NFAT5 alters the expression of a large set of genes and that expression of transcriptionally inactive NFAT5 also alters expression of a large set of genes, but that the genes in the sets differ markedly. It seems obvious that high NaCl-induced activity of wild-type NFAT5 should affect the expression of its target genes. However, it is not obvious how expression of transcriptionally inactive NFAT5 can affect expression of numerous genes. Lee et al. (17) did not offer an explanation for the finding.
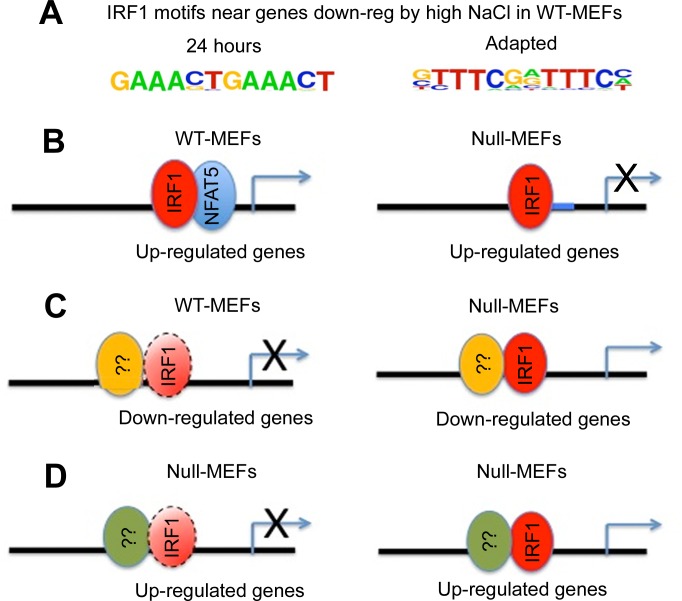
Motif analyses were thus performed to identify transcription factors potentially involved in the regulation. As expected, the NFAT5 motif, TGGAAAATTN (Matrix V$NFAT5.02 from Genomatix with references) (5, 14, 15), is enriched in genes upregulated under the high-salt treatment, in both 24 h (1.86-fold enrichment, P = 0.059) and adapted (2.48-fold enrichment, P = 0.003) conditions (Table 1). In addition, de novo motif search was used to identify motif(s) enriched in genes downregulated by high tonicity in wild-type cells. We found that interferon regulatory factor 1 (IRF1) binding site is the top motif overrepresented in the 24 h (P = 1e-18) and adapted (P = 1e-19) conditions, respectively (Fig. 2A). The results are counterintuitive because NFAT and IRF have been shown to synergize with each other in regulating in gene expression, and the two binding sites are partially overlapped at the nucleotide level (6, 10). This prompted us to determine the enrichment of the IRF1 binding site at the promoter region (−10 to 2 kb around the transcription start site) of up- and downregulated genes in WT and null cells. For genes repressed by high salt, the enrichment of the IRF1 motif is specific for WT cells (2.9-fold and 3.24-fold for 24 h and adapted conditions, respectively) but not null cells (Table 1, P = 0.006 and P = 0.002 for 24 h and adapted conditions, respectively). Interestingly, the IRF1 motif is also enriched in the promoter of the upregulated genes in WT cells (2.09-fold for 24 h and 3.1-fold for adapted, Table 1, P = 0.09 and P = 0.03 for 24 h and adapted conditions, respectively), and modestly in null cells under adapted condition (2.0-fold). Taking these results together, we propose (see discussion for details) a cooperative (or synergistic) model between NFAT5 and IRF1 (Fig. 2). In addition, we do not find any change in the mRNA expression of IRF1 or of NFAT5, itself, after NaCl has been increased for 24 h (Supplemental Table S1). Lack of change of NFAT5 mRNA is the expected result since we previously found that NFAT5 mRNA in mIMCD3 cells showed a transient increase, owing to stabilization of the mRNA, followed by a return to baseline within 8 h.
Fig. 2.
Proposed model to explain the profound differences in NFAT5 target gene expression. between WT-MEFs and Null-MEFs. See text for explanation.
Categories of NFAT5 Target Genes (i.e., Genes Expressed in WT-MEFs, but Not in Null-MEFs) Upregulated after 24 h or More of High NaCl
Many functions were previously identified of the genes upregulated by hypertonicity-induced increase of NFAT5 activity, including protective accumulation of compatible organic osmolytes (1). In the present study we used GO analysis (12) to characterize overrepresented NFAT5-regulated genes that we identify by RNA-Seq in MEF cells (Tables 2 and 3), and, since some interesting target genes might not be sufficiently overrepresented to be included in the GO results, we also manually curated the target genes shown in Table 4. We used GeneCards (http://www.genecards.org) and RefSeq (http://www.ncbi.nlm.nih.gov/refseq/) to identify functions of protein products of the genes identified in the analysis. In the following, * indicates targets that we identify after 24 h of high NaCl, but not after adaptation to high NaCl.
Table 2.
GO analysis of genes whose expression is upregulated in WT-MEFs after 24 h of high NaCl
| Gene | Reference | |
|---|---|---|
| Sodium symporter activity | ||
| Solute carrier family 1 (glial high affinity glutamate transporter), member 3 | Slc1a3 | |
| Solute carrier family 6 (neurotransmitter l-prolinetransporter), member 7 | Slc6a7 | |
| Solute carrier family 6 (neurotransmitter betaine/gaba transporter), member 12 | Slc6a12 | AJP 274:F753 |
| Solute carrier family 38, member 4 (Neutral amino acids) | Slc38a4 | |
| Protein tyrosine phosphatase activity | ||
| Protein tyrosine phosphatase, receptor type, B | Ptprb | |
| Protein tyrosine phosphatase, nonreceptor type 7 | Ptpn7 | |
| Protein tyrosine phosphatase, nonreceptor type 5 (striatum-enriched) | Ptpn5 | |
| Protein tyrosine phosphatase, receptor type, R | Ptprr | |
| Regulation of cell growth | ||
| Insulin-like growth factor binding protein 7 | IGFBP7 | AJP 300:F707 |
| HtrA serine peptidase 3 | HTRA3 | |
| Insulin-like growth factor binding protein 5 | IGFBP5 | AJP 300:F707 |
| Neurotrophic tyrosine kinase, receptor, type 3 | NTRK3 | |
| Phosphorylation/Protein tyrosine kinase | ||
| Guanylate cyclase 2G, pseudogene | GUCY2G | |
| Neurotrophic tyrosine kinase, receptor, type 3 | NTRK3 | |
| Platelet-derived growth factor receptor, beta polypeptide | PDGFRB | |
| TXK tyrosine kinase | TXK | |
| Colony stimulating factor 1 receptor | CSF1R | |
| SH2 domain containing 1B1 | SH2D1B1 | |
| Male germ cell-associated kinase | MAK | |
| RAR-related orphan receptor gamma | RORC | |
| Reelin | RELN | |
| Heparin binding | ||
| SPARC related modular calcium binding 2 | SMOC2 | |
| Cysteine-rich secretory protein LCCL domain containing 2 | CRISPLD2 | |
| Fibroblast growth factor 10 | FGF10 | |
| Chemokine (C-C motif) ligand 8 | CCL8 | |
| Chemokine (C-C motif) ligand 7 | CCL7 | |
| Response to abiotic stimulus | ||
| Solute carrier family 1 (glial high affinity glutamate transporter),member 3 | SLC1A3 | |
| Crystallin, alpha B | CRYAB | |
| Xylosyltransferase 1 | XYLT1 | |
| Heat shock 27 kDa protein | HSPB2 | |
| Heat shock protein 1A | HSPA1A | |
| Heat shock protein 1B | HSPA1B | |
| Immunoglobulin subtype | ||
| Neurotrophic tyrosine kinase, receptor, type 3 | NTRK3 | |
| Immunoglobulin superfamily, member 5 | IGSF5 | |
| Myomesin family, member 3 | MYOM3 | |
| Insulin-like growth factor binding protein 7 | IGFBP7 | |
| Platelet derived growth factor receptor, beta polypeptide | PDGFRB | |
| CD300 antigen like family member B | CD300LB | |
| CD28 antigen | CD28 | |
| Colony stimulating factor 1 receptor | CSF1R | |
| Immune system development | ||
| RAR-related orphan receptor gamma | RORC | |
| Spectrin alpha, erythrocytic 1 | SPNA1 | |
| CD28 antigen | CD28 | |
| Embryonic development | ||
| Mesenchyme homeobox 2 | MEOX2 | |
| Protein tyrosine phosphatase, receptor type, R | PTPRR | |
| Platelet derived growth factor receptor, beta polypeptide | PDGFRB | |
| PR domain containing 1, with ZNF domain | PRDM1 | |
| Epidermis development/keratinization | ||
| Forkhead box Q1 | FOXQ1 | |
| Small proline-rich protein 1A | SPRR1A | |
| Small proline-rich protein 2E | SPRR2E | |
| Small proline-rich protein 2K | SPRR2K | |
| Peptidase activity/Transition metal ion binding | ||
| ADAMTS-like 2 | ADAMTSL2 | |
| Phosphate regulating endopeptidase homolog | PHEX | |
| Matrix metallopeptidase 3 | MMP3 | |
| Matrix metallopeptidase 1b | MMP1B | |
| Complement component 1, r subcomponent B | C1RB | |
| Reelin | RELN | |
| HtrA serine peptidase 3 | HTRA3 | |
| Protease, serine 35 | PRSS35 | |
| Arachidonate lipoxygenase, epidermal | ALOX12E | |
| UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 15 | GALNTL2 | |
| RAR-related orphan receptor gamma | RORC | |
| LIM domain only 7 | LMO7 | |
| Ring finger protein 183 | RNF183 | |
| Superoxide dismutase 3, extracellular | SOD3 | |
| NLR family, apoptosis inhibitory protein 1 | NAIP1 | |
| PR domain containing 1, with ZNF domain | PRDM1 | |
| Metallothionein 4 | MT4 | |
| Muscle components | ||
| Troponin T2, cardiac | TNNT2 | |
| crystallin, alpha B | CRYAB | |
| Solute carrier family 4 (anion exchanger), member 1 | SLC4A1 | |
| Cytoskeleton components | ||
| Tubulin, alpha 8 | TUBA8 | |
| Keratin 76 | KRT76 | |
| Spectrin alpha, erythrocytic 1 | SPNA1 | |
| Small proline-rich protein 2E | SPRR2E | |
| Solute carrier family 4 (anion exchanger), member 1 | SLC4A1 | |
| Small proline-rich protein 2K | SPRR2K | |
| Keratin 23 | KRT23 | |
| Ion transport | ||
| FXYD domain-containing ion transport regulator 2 | FXYD2 | |
| Solute carrier family 38, member 4 | SLC38A4 | |
| Sodium channel, voltage-gated, type XI, alpha | SCN11A | |
| Predicted gene 5868 | GM5868 | |
| Chloride channel calcium activated 2 | CLCA5 | |
| Anoctamin 7 | ANO7 | |
| Nucleotide biosynthetic process | ||
| Adenosine monophosphate deaminase 3 | AMPD3 | |
| ATPase, class I, type 8B, member 3 | ATP8B3 | |
| Male germ cell-associated kinase | MAK | |
| GTP binding protein (gene overexpressed in skeletal muscle) | GEM | |
| Heat shock protein 1A | HSPA1A | |
| Heat shock protein 1B | HSPA1B | |
| Neurotrophic tyrosine kinase, receptor, type 3 | NTRK3 | |
| Tubulin, alpha 8 | TUBA8 | |
| Platelet derived growth factor receptor, beta polypeptide | PDGFRB | |
| TXK tyrosine kinase | TXK | |
| NLR family, apoptosis inhibitory protein 1 | NAIP1 | |
| Inositol hexaphosphate kinase 3 | IP6K3 | |
| ATPase, class I, type 8B, member 3 | ATP8B3 | |
| ATP-binding cassette, sub-family A (ABC1), member 12 | ABCA12 | |
| Colony stimulating factor 1 receptor | CSF1R | |
| Cellular component morphogenesis | ||
| Troponin T2, cardiac | TNNT2 | |
| Cholecystokinin | CCK | |
| Solute carrier family 1 (glial high affinity glutamate transporter),member 3 | SLC1A3 | |
| Netrin G1 | NTNG1 | |
| Reelin | RELN | |
| EF-hand/calcium binding | ||
| S100 calcium binding protein A4 | S100A4 | |
| SPARC related modular calcium binding 2 | SMOC2 | |
| Spectrin alpha, erythrocytic 1 | SPNA1 | |
| Endoplasmic reticulum lumen | ||
| Endoplasmic reticulum protein 27 | ERP27 | |
| Olfactomedin 1 | OLFM1 | |
| Calsequestrin 2 | CASQ2 | |
| Regulation of transcription | ||
| Sex comb on midleg-like 4 (Drosophila) | SCML4 | |
| Avian musculoaponeurotic fibrosarcoma (v-maf) AS42 oncogene homolog | MAF | |
| Forkhead box Q1 | FOXQ1 | |
| Regulatory factor X, 4 (influences HLA class II expression) | RFX4 | |
| Mesenchyme homeobox 2 | MEOX2 | |
| SRY (sex determining region Y)-box 5 | SOX5 | |
| RAR-related orphan receptor gamma | RORC | |
| PR domain containing 1, with ZNF domain | PRDM1 | |
| G protein-coupled receptor | ||
| Purinergic receptor P2Y, G protein-coupled 12 | P2RY12 | |
| Somatostatin receptor 2 | SSTR2 | |
| RIKEN cDNA A630033H20 gene | A630033H20RIK | |
| Thyrotropin releasing hormone receptor 2 | TRHR2 | |
| G protein-coupled receptor 149 | GPR149 | |
| MAS-related GPR, member F | MRGPRF | |
| Leucine-rich repeat-containing G protein-coupled receptor 6 | LGR6 | |
GO, Gene Ontology; AJP, American Journal of Physiology.
Table 3.
GO analysis of genes whose expression is upregulated by high NaCl in WT-MEFs after adaption, but not after 24 h
| Peptidase activity | |
| ADAM metallopeptidase with thrombospondin type 1 motif, 17 | ADAMTS17 |
| ADAMTS-like 1 | ADAMTSL1 |
| Protease, serine, 2 (Trypsin 2) | PRSS2 |
| Peptidase domain containing associated with muscle regeneration 1 | PAMR1 |
| OTU deubiquitinase 7A | OTUD7A |
| Tubulointerstitial nephritis antigen | TINAG |
| Thrombospondin, type 1 repeat | |
| Sema domain, seven thrombospondin repeats (type 1 and type 1-like), transmembrane domain (TM) and short cytoplasmic domain, (semaphorin) 5B | SEMA5B |
| ADAMTS-like 1 | ADAMTSL1 |
| Brain-specific angiogenesis inhibitor 1 | BAI1 |
| Embryonic morphogenesis/Tube development | |
| Cytochrome P450, family 26, subfamily b, polypeptide 1 | CYP26B1 |
| Fibroblast growth factor 10 | FGF10 |
| Paired box 2 | PAX2 |
| Cysteine-rich secretory protein LCCL domain containing 2 | CRISPLD2 |
| V-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian) | MYCN |
| Polysaccharide binding | |
| Cysteine-rich secretory protein LCCL domain containing 2 | CRISPLD2 |
| Fibroblast growth factor 10 | FGF10 |
| Chemokine (C-C motif) ligand 7 | CCL7 |
| Response to organic substance | |
| Cytochrome P450, family 11, subfamily a, polypeptide 1 | CYP11A1 |
| Fatty acid binding protein 4, adipocyte | FABP4 |
| Fibroblast growth factor 10 | FGF10 |
| Response to wounding | |
| Coagulation factor II (thrombin) receptor-like 3 | F2RL3 |
| Integrin beta 2-like | ITGB2L |
| Chemokine (C-C motif) ligand 7 | CCL7 |
| CD163 antigen | CD163 |
| Metal ion binding | |
| ADAM metallopeptidase with thrombospondin type 1 motif, 17 | ADAMTS17 |
| Additional sex combs like 3 (Drosophila) | ASXL3 |
| ADAMTS-like 1 | ADAMTSL1 |
| Cytochrome P450, family 11, subfamily a, polypeptide 1 | CYP11A1 |
| RAR-related orphan receptor alpha | RORA |
| Monooxygenase, DBH-like 1 | MOXD1 |
| Protein kinase C, beta | PRKCB |
| Polo-like kinase 5 | PLK5 |
| Ras association (RalGDS/AF-6) domain family member 5 | RASSF5 |
| Eyes absent 2 homolog (Drosophila) | EYA2 |
| Deoxynucleotidyltransferase, terminal | DNTT |
| Protease, serine 2 | PRSS2 |
| Early B cell factor 1 | EBF1 |
| Cytochrome P450, family 26, subfamily b, polypeptide 1 | CYP26B1 |
| Basonuclin 1 | BNC1 |
| OTU domain containing 7A | OTUD7A |
| Potassium voltage-gated channel, subfamily H (eag-related), member 5 | KCNH5 |
| Cadherin 11 | CDH11 |
| Cell junction | |
| Claudin 19 | CLDN19 |
| Cholinergic receptor, nicotinic, alpha polypeptide 1 | CHRNA1 |
| Homer homolog 2 (Drosophila) | HOMER2 |
| Lin-7 homolog A (C. elegans) | LIN7A |
| Extracellular matrix | |
| ADAMTS-like 1 | ADAMTSL1 |
| Cysteine-rich secretory protein LCCL domain containing 2 | CRISPLD2 |
| Tenascin R | TNR |
| Leucine-rich repeat | |
| Additional sex combs like 3 (Drosophila) | ASXL3 |
| Leucine-rich repeat transmembrane neuronal 1 | LRRTM1 |
| Leucine-rich repeat containing 15 | LRRC15 |
| Chemical homeostasis | |
| Fatty acid binding protein 4, adipocyte | FABP4 |
| Cholinergic receptor, nicotinic, alpha polypeptide 1 | CHRNA1 |
| Rhesus blood group-associated A glycoprotein | RHAG |
| Protein kinase C, beta | PRKCB |
| Regulation of phosphorylation | |
| Fatty acid binding protein 4, adipocyte | FABP4 |
| Nik related kinase | NRK |
| Bradykinin receptor, beta 2 | BDKRB2 |
| Cation channel activity | |
| Cholinergic receptor, nicotinic, alpha polypeptide 1 | CHRNA1 |
| Potassium voltage-gated channel, subfamily H (eag-related), member 5 | KCNH5 |
| Potassium channel, subfamily K, member 10 | KCNK10 |
| G protein-coupled receptor | |
| Corticotropin releasing hormone receptor 1 | CRHR1 |
| Coagulation factor II (thrombin) receptor-like 3 | F2RL3 |
| 5-hydroxytryptamine (serotonin) receptor 1B | HTR1B |
| Chemokine-like receptor 1 | CMKLR1 |
| Brain-specific angiogenesis inhibitor 1 | BAI1 |
| Bradykinin receptor, beta 2 | BDKRB2 |
| Prostaglandin F receptor | PTGFR |
| Neuropeptide Y receptor Y5 | NPY5R |
| 5-hydroxytryptamine (serotonin) receptor 1F | HTR1F |
| Negative regulation of nitrogen compound metabolic process | |
| 5-hydroxytryptamine (serotonin) receptor 1B | HTR1B |
| Fatty acid binding protein 4, adipocyte | FABP4 |
| Paired box 2 | PAX2 |
| Transcription regulation | |
| Avian erythroblastosis virus E-26 (v-ets) oncogene related | ERG |
| Early B cell factor 1 | EBF1 |
| RAR-related orphan receptor alpha | RORA |
| Paired box 2 | PAX2 |
| V-myc myelocytomatosis viral related oncogene, neuroblastoma derived (avian) | MYCN |
| Potassium voltage-gated channel, subfamily H (eag-related), member 5 | KCNH5 |
| Additional sex combs like 3 (Drosophila) | ASXL3 |
| Eyes absent 2 homolog (Drosophila) | EYA2 |
| Arrestin, beta 1 | ARRB1 |
| Basonuclin 1 | BNC1 |
Table 4.
| 24 h and Adapted | 24 h Only | Adapted Only |
|---|---|---|
| 1700016c15rik | 1500015o10rik | 1700026d08rik |
| 1700016m24rik | 2310030 g06rik | 4930547c10rik |
| 1700018a04rik | A4 gnt | Abi3 |
| 4930511m06rik | Ano7 | Adamts17 |
| 5430421f17rik | Atp8b3 | Adamtsl1 |
| A430107o13rik | C1rb | Akr1b3+Gm6644 |
| A630033 h20rik | Calr4 | Ankrd35 |
| Abca12 | Cd28 | Arrb1 |
| Accsl | Cd300lh | Asxl3 |
| Adamtsl2 | Ces1b | Bai1 |
| Alox12e | Cplx2 | Bdkrb2 |
| Ampd3 | D630039a03rik | Bmper |
| Ankrd34c | E330012b07rik | Bnc1 |
| Cables1 | Fam180a | Ccl7 |
| Casq2 | Fbxo2 | Cd163 |
| Cbln4 | Galntl2 | Cdh11 |
| Cck | Gem | Chrna1 |
| Ccl8 | Gm16445 | Cldn19 |
| Cd200 | Gm5082 | Cmklr1 |
| Cd300lb | Gm5868 | Crhr1 |
| Cdh5 | Gm5886 | Crispld2 |
| Ces1a | Gm9758 | Crtam |
| Chdh | Gucy2 g | Ctxn3 |
| Clca5 | Hhla1 | Cyp11a1 |
| Cnnm1 | Hspb2 | Cyp26b1 |
| Col23a1 | Igfbp5 | D430036j16rik |
| Cryab | Il33 | Dntt |
| Csf1r | Krt76 | Ebf1 |
| Cytip | Lad1 | Erg |
| Dll4 | Maf | Eya2 |
| Dpt | Mmp3 | F2rl3 |
| E430016f16rik | Myom3 | Fabp4 |
| Erp27 | Paqr5 | Fabp5 |
| Fat2 | Phex | Fam20a |
| Foxq1 | Prl7d1 | Fgf10 |
| Fxyd2 | Prr15 | Fgl2 |
| Gal3 st1 | Ptgds | Gm13889 |
| Gm14207 | Ptpn5 | Gm5126 |
| Gm17019 | Ptpn7 | Gm53 |
| Gpr149 | Ptprb | Gm885 |
| Gpr30 | Ptprr | Gngt2 |
| Hspa1a | Renbp | Homer2 |
| Hspa1b | Rfx4 | Hpse2 |
| Htra3 | Rnf183 | Hs3 st5 |
| Ifitm6 | Scn11a | Hs3 st6 |
| Igfbp7 | Slc4a1 | Htr1b |
| Igsf5 | Speer8-Ps1 | Htr1f |
| Il17rb | Srgn | Itgb2l |
| Ip6k3 | Tfpi2 | Kcnh5 |
| Itga10 | Tnfrsf23 | Kcnk10 |
| Kbtbd11 | Tnfrsf26 | Kprp |
| Krt23 | Tnnt2 | Lin7a |
| Lgr6 | Wfdc3 | Lrrtm1 |
| Lmo7 | Xylt1 | Lypd6b |
| Mak | Moxd1 | |
| Meox2 | Muc1 | |
| Mmp1b | Mug-Ps1 | |
| Mrgprf | Mug2 | |
| Mt4 | Mycn | |
| Naip1 | Myct1 | |
| Ntng1 | Nap1l2 | |
| Ntrk3 | Notum | |
| Olfm1 | Npy5r | |
| P2ry12 | Nrk | |
| Pdgfrb | Otud7a | |
| Pdzd2 | Pamr1 | |
| Plekhb1 | Pax2 | |
| Prdm1 | Pde1a | |
| Prss35 | Plk5 | |
| Ranbp3l | Pnmal2 | |
| Reln | Prkcb | |
| Rftn2 | Prl2c2 | |
| Rorc | Prss2 | |
| S100a4 | Ptgfr | |
| Scml4 | Rassf5 | |
| Sec1 | Resp18 | |
| Serinc2 | Rgs4 | |
| Sh2d1b1 | Rhag | |
| Sla | Rora | |
| Slc15a2 | Rps4y2 | |
| Slc15a3 | Sema5b | |
| Slc1a3 | Serpina11 | |
| Slc38a4 | Slc6a4 | |
| Slc6a12 | Tchh | |
| Slc6a7 | Tinag | |
| Slco4a1 | Tmem108 | |
| Smoc2 | Tnr | |
| Sod3 | Vip | |
| Sox5 | ||
| Speer4d | ||
| Spn-Ps | ||
| Spna1 | ||
| Sprr1a | ||
| Sprr2e | ||
| Sprr2k | ||
| Srpx2 | ||
| Sstr2 | ||
| Trhr2 | ||
| Tuba8 | ||
| Txk | ||
| Wdr95 | ||
| Zar1 |
Angiogenesis/rheumatoid arthritis.
Our RNA-Seq analysis identifies the NFAT5 targets Srpx2, Dll4, and Cdh5, previously noted to be involved in the angiogenesis associated with rheumatoid arthritis (9). We also identify Ptprb* and Pdgfrb, which play essential roles in blood vessel development by promoting proliferation, migration, and recruitment of pericytes and smooth muscle cells to endothelial cells, and Smoc2, which can stimulate endothelial cell proliferation and migration, as well as angiogenic activity.
Apoptosis.
Tnfrsf23* is a TNF receptor that recruits apoptotic suppressors; Naip1 is an antiapoptotic protein that inhibits activities of Casp3, Casp7, and Casp9.
Bone and cartilage.
NFAT5 is a regulator of nucleus pulposus cell function and survival in the intervertebral disc (33). The NFAT5 target genes involved are (9) Ifitm6, Sox5, Maf, and As42. We find (Table 4) that Ifitm6, Sox5, Maf, and As42 are also NFAT5 targets in WT-MEFs. B3gat1 was identified as an NFAT5 target in nucleus pulposus cells (9), but we do not identify it in WT-MEFs (Table 4). Additional NFAT5 targets involved in bone and cartilage that we identify by RNA-Seq in WT-MEFs exposed to high NaCl for 24 h or more are Phex*, which is involved in bone and dentin mineralization and Itga10, which combines with the integrin beta 1 chain to form a novel collagen type II-binding integrin expressed in cartilage tissue.
Cancer.
Olfm1, Cbln4, Fat, Sstr2, Htra3, Mmp1b, Ptprr, S100a4, foxq1, Gal3st1, and Lgr6 were previously identified (9) as NFAT5 targets involved in cancer. We find (Table 4) that all of these genes, except Fat, are NFAT5 targets in WT-MEFs. Additional NFAT5 targets involved in cancer that we identify by RNA-Seq in WT-MEFs exposed to high NaCl for 24 h or more (Table 4) are Mmp3, which is involved in the breakdown of extracellular matrix in metastasis; Clca5, which is involved in cancers of lung and breast; Ano7*, Pdzd2, and Mak, which are involved in prostate cancer; Ntrk3, which is involved in fibrosarcoma; Fat2, which is involved in skin squamous cell carcinoma; and Prdm1, which is involved in plasmacytoma and central nervous system lymphoma.
Cell growth.
Igfbp7 inhibits cell proliferation acting through autocrine/paracrine pathways; Prl7d1* encodes the anterior pituitary hormone prolactin, which is a growth regulator for many tissues, including cells of the immune system; Mak is a serine/threonine protein kinase related to kinases involved in cell cycle regulation; and Cables1 is involved in regulation of the cell cycle.
Cell junction.
Igsf5 provides, together with MagI1, an adhesion machinery at tight junctions, which may regulate the permeability of the kidney glomerulus and small intestinal epithelium.
Clotting.
P2ry12 is a G protein-coupled receptor involved in platelet aggregation.
Cytoskeleton.
The cytoskeletal tubulin Tuba8 was previously identified as an NFAT5 target (9), as we now find in WT-MEFs (Table 4). In addition, Lad1* is an anchoring filament that is a component of basement membranes.
Development.
We find NFAT5-dependent expression in WT-MEFs of Meox2 (Table 4), previously noted as an NFAT5 target involved in development (9). Scm14 was also previously identified as an NFAT5-dependent target involved in development (9), but we do not find its expression in WT-MEFs (Table 4). Additional NFAT5 targets involved in development that we identify by RNA-Seq in WT-MEFs exposed to high NaCl for 24 h or more (Table 4) are Pdgfrb, which plays an essential role in cardiovascular and renal development, both of which are deficient in NFAT5 null mice (21); Mmp1b, a matrix metalloproteinase involved in the breakdown of extracellular matrix in embryonic development; Smoc2, which is highly expressed during embryogenesis; Scml4, which is a Polycomb group (PcG) protein required to maintain the transcriptionally repressive state of homeotic genes throughout development; Maf, a transcription factor involved in embryonic lens fiber cell development; Foxq1, which is involved in embryonic development, Ntng1, which is involved in axon guidance; Reln, which is involved in neuronal migration during brain development; Sstr2, which may participate in neuron development and maturation during brain development; Fat2, which controls cell proliferation during development; Mt4, which plays a special role in regulating zinc metabolism during the differentiation of stratified epithelia; Cables1, which is critical for neuronal development; Alox12e, which is involved in terminal differentiation of keratinocytes; and Zar1, an oocyte-specific protein that is thought to function in the initiation of embryogenesis.
Enzymes.
Wfdc3* is a serine-type endopeptidase inhibitor; Renbp* catalyzes the interconversion of N-acetylglucosamine to N-acetylmannosamine and binds to renin, inhibiting its activity; Xylt1* catalyzes the first step in biosynthesis of glycosaminoglycan; Ptgds* catalyzes the conversion of PGH2 to PGD2, a prostaglandin involved in smooth muscle contraction/relaxation and a potent inhibitor of platelet aggregation; A4gnt* catalyzes the transfer of N-acetylglucosamine (GlcNAc) to core 2 branched O-glycans; Galntl2* catalyzes the initial reaction in O-linked oligosaccharide biosynthesis; Fbxo2* is the substrate recognition component of a ubiquitin-protein ligase complex that mediates the ubiquitination and subsequent proteasomal degradation of target proteins; Tfpi2* can inhibit a variety of serine proteases including factor VIIa/tissue factor, factor Xa, plasmin, trypsin, chymotryspin, and plasma kallikrein; and Lmo7 has ubiquitin-protein ligase activity.
Feedback regulation.
The targets of NFAT5 include many genes in signaling pathways that regulate NFAT5, itself, which implies feedback regulation. Thus, “Protein tyrosine phosphatase activity” (Table 2) includes NFAT5-regulated genes that code for phosphatases that dephosphorylate and thus affect the activity of signaling molecules that regulate the activity of NFAT5 itself. We had not previously screened for phosphatases regulated by NFAT5, but we had screened for phosphatases that affect NFAT5 activity (40, 41). Interestingly, Ptprb*, which codes for a phosphatase that supports high NaCl-induced NFAT5 activity in HEK293 cells (40), is also upregulated by NFAT5 activity, raising the possibility of a positive feedback loop that supports NFAT5 activity. Mitogen activated protein kinases (MAPKs, Table 5) regulate NFAT5 activity. Ptpn7* (26), Ptpn5* (27), and Ptprr* (32) dephosphorylate and, thus, reduce activity of MAPKs, again possibly feeding back on NFAT5 activity. Two of the “Regulation of cell growth” NFAT5 target genes (Igfbp7 and Igfbp5, Table 2) were previously identified in a microarray screen (17). Similar to the phosphatases just considered, Igfbp5 can activate MAPK signaling (37). Among NFAT5 target genes classified to have “Protein tyrosine kinase activity” (Table 2), Ntrk3 can increase activity of the MAPK/Erk pathway (19); Pdgfrb can increase c-fos (23); Gpr30 stimulates cAMP production and signals through the PI3K/ERK pathway; Il33* can phosphorylate MAPK3/ERK1 and/or MAPK1/ERK2, MAPK14, and MAPK8; and Csf1r can activate Erk (34), all of which can affect NFAT5 activity (Table 5). We conclude that many NFAT5 targets can activate pathways that regulate NFAT5 itself, suggesting feedback regulation of NFAT5.
Table 5.
Signaling molecules that affect NFAT5 activity
| Gene Symbol | Other Name |
|---|---|
| Akt1 | Akt1 |
| Cdk5 | Cdk5 |
| Csnk1a1 | Ck1 |
| Dusp14 | Mkp-1 |
| Fos | c-Fos |
| Fyn | Fyn |
| Gsk3b | Gsk-3β |
| Jun | c-Jun |
| Map2k6 | Mkk6 |
| Mapk13 | p38δ |
| Mapk14 | p38α |
| Mapk3k4 | Erk |
| Osm | Osm |
| Pik3cd | Pi3k |
| Plcg1 | Plc-γ1 |
| Pp1r3a | Pp1γ |
| Ppp1r3c | Ptg |
| Prkd1 | Pkcμ |
| Ptpn6 | Shp-1 |
| Rac1 | Rac1 |
| Rkaca | Pka |
From the diagram in Ref. 41.
Gastrointestinal.
Cck induces the release of pancreatic enzymes and the contraction of the gallbladder.
Heat shock proteins.
The stimulus in “Response to abiotic stimulus” (Table 2) presumably is high NaCl. Several heat shock proteins were previously found to be NFAT5 targets, namely Cryab, Hspb2*, and Hspa1b (9). We find that those genes are NFAT5 targets in WT-MEFs (Table 4). An additional heat shock protein that we find is a target of NFAT5 in WT-MEFs is Hspa1a. Heat shock proteins protect cells against hypertonicity. Hspa2 (HSP70-2) was previously found to be a target of NFAT5 in MDCK cells (35). However, we do not identify it as a target of NFAT5 in WT-MEFs.
Heparin binding.
NFAT5 target genes in this category can contribute to some of the known functions of NFAT5 (9). Thus, Smoc2 is involved in embryogenesis and angiogenic activity, Ccl8 in immunoregulation, and CCL7* in metastasis.
Immune function.
Some NFAT5 targets that we identify in WT-MEFs in the categories “Immunoglobulin subtype” and “Immune system development,” namely Rorc, Csf1r, Cd300lb, and Cd28* (Table 2), were previously noted to be NFAT5 targets that contribute to immunity (9). Additional immunity-related targets that we find by RNA-Seq in WT-MEFs exposed to high NaCl for 24 h or more (Table 4) are Prdm1, which contributes to maturation of B-lymphocytes, and Txk, a tyrosine kinase that regulates the development, function, and differentiation of T-cells. After adaptation to high NaCl (Table 3), but not after 24 h of high NaCl (Table 4), we identify Ccl7, a chemokine that attracts immune cells; Cd163, a sensor for bacteria and inducer of local inflammation; Rassf5, which is involved in lymphocyte adhesion; Prkcb, which is involved in B cell activation; Dntt, which is expressed in pre-B and pre-T lymphocytes during early differentiation; Cmklr1, which regulates cytokine production in macrophages by reducing the activation of MAPK1/3 (ERK1/2) and NF-κB; Sla, which negatively regulates T-cell receptor (TCR) signaling; Il17rb, which is a receptor for the proinflammatory cytokines Il17b; and Il17e, which may play a role in controlling the growth and/or differentiation of hematopoietic cells. Also, Srgn* plays a role in formation of mast cell secretory granules; Rfx4* is a transcription factor that influences HLA class II expression; Il33* is a cytokine that acts as a chemoattractant tor Th2 cells; Cd200 costimulates T-cell proliferation; Sh2d1b1 regulates signal transduction through receptors expressed on the surface of antigen-presenting cells; Arrb1 contributes to regulation of receptor-mediated immune functions; and C1rb* is a serine protease that combines with C1q and C1s to form C1, the first component of the classical pathway of the complement system.
Ion transport.
We find NFAT5-dependent expression in WT-MEFs of Fxyd2, Clca5, Slc6a7, and Slc1a3 (Table 4), previously identified as NFAT5 targets involved in ion transport (9). Slco4a1 was also previously identified as an NFAT5-dependent target involved in ion transport (9), but we do not find its expression in WT-MEFs (Table 4). Also, Scn11a* mediates the voltage-dependent sodium ion permeability of excitable membranes, and Slc4a1* (Band 3) is expressed in the erythrocyte plasma membrane, where it functions as a chloride/bicarbonate exchanger.
Keratinization of epidermis.
We find NFAT5-dependent expression in WT-MEFs of Krt76*, Sprr1a, Sprr2e, and Sprr2k, whose protein products are keratinocyte proteins that are cross-linked to membrane proteins resulting in the formation of an insoluble envelope beneath the plasma membrane. Sprr2e and Sprr2k were not previously identified as NFAT5 targets, but Sprr1a was identified (8) as a high NaCl-induced gene, dependent on mTOR. That these structural components in keratinocytes are NFAT5 targets is a novel finding that bears further investigation.
Metabolism.
Ampd3 is an AMP deaminase that plays a critical role in energy metabolism.
Muscle.
NFAT5 has an essential role in cardiac development, and cardiac failure is most likely responsible for the peripheral edema and death of NFAT5(−/−) embryos at embryonic day 14.5 (21). Accordingly, we find numerous NFAT5 targets in muscle, after 24 h or more of high NaCl. Thus, Casq2 is a calcium-binding protein that stores calcium for muscle function in the sarcoplasmic reticulum of cardiac and slow skeletal muscle cells; Tnnt2* is the tropomyosin-binding subunit of troponin, the thin filament regulatory complex that confers calcium-sensitivity to striated muscle actomyosin ATPase activity; Myom3* links the intermediate filament cytoskeleton to the M-disk of the myofibrils in striated muscle. Although Acta2 is an NFAT5 target in arterial smooth muscle cell (9), we do not identify it in WT-MEFs (Table 4).
Nerve.
Cplx2* negatively regulates the formation of synaptic vesicle clustering at active zone to the presynaptic membrane in postmitotic neurons.
Oxidative stress.
High NaCl causes mitochondrial-derived oxidative stress both in cell culture and in the renal inner medulla in vivo (38, 39). The NFAT5 targets Chdh and Sod3 are associated with oxidative stress (9). We do not identify NFAT5-dependent expression of Chdh in WT-MEFs (Table 4), but we do identify NFAT5-dependent expression of Sod3.
Receptors.
Paqr5* is a steroid membrane receptor that binds progesterone.
Sodium symporter activity.
Table 2 includes genes that code for sodium-coupled transporters. Slc1a3 (glutamate) and Slc6a12 (glycine betaine) were previously identified as hypertonicity-induced genes (8). We confirm that they are NFAT5 targets in WT-MEFs and identify the additional NFAT5 targets, Slc6a7 (L-proline) and Slc38a4 (neutral amino acids).
Transporters.
Atp8b3* is an aminophospholipid translocase that transports phosphatidylserine and phosphatidylethanolamine from one side of a bilayer to the other; Slc38a4 transports both cationic and neutral amino acids; Abca12 is an ATP-binding cassette transporter involved in lipid homeostasis; Slc15a2 is a renal proton-coupled peptide transporter that is responsible for the absorption of small peptides.
Categories of NFAT5 Target Genes Upregulated after Adaptation to High NaCl, but Not after as Little as 24 h of High NaCl.
The effect of high NaCl has often been studied in cell culture by elevating NaCl for minutes to hours, which is a convenient and instructive protocol. However, in some tissues in vivo, most notably the renal medulla, interstitial NaCl normally remains chronically elevated. That led us to investigate whether NFAT5 targets special categories of genes in cells adapted to high NaCl that are not activated after only 24 h of exposure to high NaCl. Numerous NFAT5 targets are upregulated by high NaCl in WT-MEFs after adaptation, but not after 24 h (Fig. 1D and Table 4). Interestingly, among those genes we do not find any in several categories whose expression increases after as little as 24 h of high NaCl, namely sodium symporter activity, heat shock proteins, immune function, keratinization of epidermis, and cytoskeleton. However, we do identify genes in the following categories.
Angiogenesis.
Bai1 inhibits angiogenesis; Cmklr1 enhances angiogenesis; Erg regulates angiogenesis.
Apoptosis.
Prkcb can contribute to its induction, Rassf5 is involved in regulation of Ras apoptotic function, and Erg is a transcription factor involved in regulation of apoptosis.
Bone and cartilage.
Cdh11 is implicated in bone development and maintenance; Bmper inhibits bone morphogenetic protein function.
Cancer.
Bai1, glioblastoma; Fgf10, tumor growth and invasion; Mycn, neuroblastoma; Rassf5, a tumor suppressor inactivated in a variety of cancers; Ptgfr, endometrial adenocarcinoma; Erg, Ewing's sarcoma and acute myeloid leukemia; ABI3 inhibits ectopic metastasis of tumor cells; and Muc1 promotes tumor progression.
Cell growth.
Fgf10 has mitogenic activity; Prkcb is involved in endothelial cell proliferation; Bnc1 is involved in keratinocyte proliferation; and Erg is involved in cell proliferation.
Cell junction.
Cldn19 is a claudin that is a component of tight junctions.
Cell polarity.
Lin7a establishes and maintains the asymmetric distribution of channels and receptors.
Circadian rhythm.
Rora is a nuclear hormone receptor that aids in the transcriptional regulation of some genes involved with circadian rhythm.
Clotting.
F2rl3 is a receptor for activated thrombin or trypsin involved in activation of platelets.
Cytoskeleton.
Abi3 regulates actin polymerization; Tchh confers mechanical strength to the hair follicle inner root sheath and to other toughened epithelial tissues.
Development.
Tinag is involved in nephrogenesis, which is defective in NFAT5-null mice; Sema5b, axon growth; Cyp26b1, skeleton; Fgf10, embryonic development; Pax2, urogenital tract, eyes, and CNS; Asxl3 maintains the transcriptionally repressive state of homeotic genes; Rora, a nuclear hormone receptor involved in organogenesis and differentiation; Eya2, a histone phosphatase that may contribute to organogenesis and muscle development; Nrk induces actin polymerization in late embryogenesis; Crhr1, involved in embryonic development of the adrenal gland; and Erg, a transcription factor regulating embryonic development.
DNA repair.
High NaCl causes DNA breaks and inhibits their repair (4). Eya2 is a protein phosphatase that promotes efficient DNA repair via the recruitment of DNA repair complexes containing MDC1.
Enzymes.
Hs3st5 catalyzes the rate-limiting step in the biosynthesis of heparin sulfate; Hs3st6 is a sulfotransferase that utilizes 3′-phospho-5′-adenylyl sulfate to catalyze the transfer of a sulfo group to heparin sulfate.
Epithelia.
Muc1 may provide a protective layer of mucous on epithelial cells that protects against bacterial and enzyme attack.
Extracellular matrix.
Adamtsl1 may have important functions in the extracellular matrix; Adamtsl2 is a secreted glycoprotein that binds the cell surface and extracellular matrix and interacts with latent transforming growth factor beta binding protein 1.
Eye.
Gngt2 plays a crucial role in cone phototransduction.
Feedback regulation.
Prkcb signals through the RAF1-MAPK/ERK pathway to promote activating cooperation between ATF2 and JUN; Nrk is a protein kinase that is required for activation of JNK; Crhr1 is a G protein-coupled receptor that activates adenylate cyclase; Htr1b and Htr1f are G protein-coupled receptors that inhibit adenylate cyclase; Cmklr1 is a receptor that signals through MAPK1/3 (ERK1/2), MAPK14/P38MAPK, and PI3K; Npy5r a receptor that inhibits adenylate cyclase activity.
Gastrointestinal.
Prss2 is a pancreatic trypsinogen; Prkcb is involved in intestinal sugar absorption.
Integrin.
Itgb2l is involved in cell adhesion and cell surface-mediated signaling.
Immunity.
Fam20a may function in hematopoiesis; Fgl2 may play a role in physiologic lymphocyte functions at mucosal sites.
Ion transport.
Kcnh5 is a voltage-gated potassium channel with diverse functions; Kcnk10 is a potassium channel.
Lipid metabolism.
Fabp4 is involved in fatty acid uptake, transport, and metabolism; Rora is a nuclear hormone receptor that regulates a number of genes involved in lipid metabolism; Cmklr1 enhances adipogenesis; Fabp5 plays a role in fatty acid uptake, transport, and metabolism.
Muscle.
Pamr1 is involved in regeneration of skeletal muscle; Vip stimulates myocardial contractility, causes vasodilation, increases glycogenolysis, lowers arterial blood pressure, and relaxes the smooth muscle of trachea, stomach, and gall bladder.
Nephritis.
Tinag encodes a glycoprotein in kidney basement membranes, autoantibodies against which are found in nephritis.
Nerve.
Homer2 is a dendritic protein that regulates glutamate receptor function; LIn7a localizes synaptic vesicles at synapses; Tnr is involved in neurite outgrowth, neural cell adhesion, and modulation of sodium channel function; Lrrtm1 is involved in synaptogenic activity.
Oxidative stress.
High NaCl increases mitochondrial reactive oxygen species in vivo and in cell culture (38, 39). Prkcb produces mitochondrial reactive oxygen species.
Protease.
Otud7a cleaves ubiquitin.
Signaling molecules.
Myct1 mediates many of the known phenotypic features associated with Myc, including promotion of apoptosis, alteration of morphology, enhancement of anchorage-independent growth, tumorigenic conversion, promotion of genomic instability, and inhibition of hematopoietic differentiation; Pde1a is a cyclic nucleotide phosphodiesterase that regulates intracellular cyclic nucleotide concentrations; Rgs4 inhibits signal transduction by increasing the GTPase activity of G protein alpha subunits thereby driving them into their inactive GDP-bound form; Chrna1 is a channel gated by acetylcholine; bdkrb2 is a receptor for bradykinin that associates with G proteins to stimulate a phosphatidylinositol-calcium second messenger system; Arrb1 is a cofactor in the beta-adrenergic receptor kinase-mediated desensitization of beta-adrenergic receptors.
Steroid hormones.
Cyp11a1 protein localizes to the mitochondrial inner membrane and catalyzes the conversion of cholesterol to pregnenolone, the first and rate-limiting step in the synthesis of the steroid hormones.
Transport.
Rhag is part of a red blood cell membrane channel that transports ammonium and carbon dioxide.
DISCUSSION
Functions of NFAT5 Targets Upregulated after NaCl Is Elevated for Only 24 h
Much of what is known about the effects of hypertonicity is based on numerous studies of elevating NaCl in cell culture or native tissues for minutes to hours (reviewed in Refs. 2, 11). Initially, there is rapid osmotic efflux of water, causing the cells to shrink. Then, within minutes to hours the cells swell back toward their original volume because of uptake of salts, accompanied by osmotic influx of water (so called “regulatory volume increase” or RVI). The ion transport systems that are activated include Na+/H+ exchangers, Na+,K+,2Cl− cotransporters, and cation channels. Since the immediate activation of these transporters is posttranslational, RNA-Seq cannot detect it. RVI restores cell volume, but it does so at the expense of continuous elevation intracellular ionic strength, which in itself is detrimental (36). While this is occurring, NFAT5 becomes activated, increasing transcription of genes whose protein products either synthesize compatible organic osmolytes or transport existing ones into the cells. The compatible organic osmolytes restore normal cellular function since they are not detrimental, and, as they accumulate, they in effect replace the excess inorganic ions. Our RNA-Seq measurement after 24 h exposure of WT-MEFs high NaCl detects genes involved in accumulation of the organic osmolytes, e.g., Slc6a12, which codes for a glycine betaine transporter, and Akr1b1, which codes for aldose reductase, an enzyme that catalyzes production of sorbitol from glucose. Other proteins known to contribute to accumulation of compatible organic osmolytes are the taurine transporter, Taut (coded for by Slc6a6), and SMIT (coded for by Slc5a3), a sodium/myo-inositol cotransporter. Although high NaCl increases both Slc6a6 and Slc5a3 in WT-MEFs, their FDRs do not meet our stringent criteria for significance (FDR < 0.05).
Other categories of NFAT5 targets that we identify as upregulated after 24 h of high NaCl in WT-MEFs, code for “heat shock proteins,” “keratinization of epidermis,” “cytoskeleton,” and “immune function”. The hypertonicity-induced elevation of intracellular ionic strength that occurs both before and during RVI causes aggregation of proteins and interferes with their function (36). Heat shock proteins ameliorate these adverse effects temporarily while the compatible organic osmolytes accumulate. Hypertonicity also induces rapid F-actin polymerization and remodeling of the actin cytoskeleton in a fashion that varies between cell types and whose role is not entirely clear (2). The recognized effectors of the remodeling act posttranslationally, but our identification of NFAT5 regulated targets involved in “keratinization of epidermis” and “cytoskeleton” suggests possible additional transcriptional effects. Finally, although the role of NFAT5 in “immune function” was previously known (9), our RNA-Seq analysis reveals NFAT5 targets in addition to those previously recognized.
Functions of NFAT5 Targets Upregulated after Adaptation to High NaCl
Does NFAT5 target any special genes after adaptation to high NaCl? That question arises because there are some tissues, most notably the renal medulla, in which interstitial NaCl normally is chronically elevated. After WT-MEFs adapt to chronic high NaCl, NFAT5 remains active in WT-MEFs, but at a lower level than after only 24 h of high NaCl (Fig. 1A). Despite lesser NFAT5 activity, expression of many NFAT5 targets that increased after 24 h of high NaCl remains elevated (Fig. 1, B and D; Table 4). In addition, after adaptation to high NaCl, we detect increased expression of numerous NFAT5 targets not identified after only 24 h of high NaCl (Table 4). The NFAT5 targets that we identify only after adaptation to high NaCl fall into numerous categories (listed above in results). Genes in some of those categories point to the specific NFAT5 targets involved in previously recognized functions of NFAT5 and suggest additional or secondary functions. Tinag and Pax2 are involved in nephrogenesis, which is defective in NFAT5-null mice (21). Eya2 is involved in DNA repair, which is inhibited by high NaCl (4). Muc1 codes for a mucous that coats and protects epithelial cells. Adamtsl1 and Adamtsl2 function in the extracellular matrix. Fabp4, Rora, Cmklr1, and Fabp5 are involved in lipid metabolism. Trhr2, Myct1, Pde1a, Rgs4, Bdkrb2, and Arrb1 are signaling molecules. Cyp11a1 is an enzyme involved in synthesis of steroid hormones. Finally, NFAT5 upregulates the K+ ion transporters Kcnh5 and Kcnk10 in WT-MEFs adapted to high NaCl in contrast to the previously recognized ion transporters upregulated after NaCl is elevated for only 24 h, namely the Na+-coupled transporters (Slc6a7 and Slc1a3), a Na+ channel (Scn11a) and a Cl− transporter (Clca5).
Proposed Model for Synergy between NFAT5 and IRF1
In WT cells both NFAT5 and IRF1 motifs are enriched in the promoter regions of upregulated genes, suggesting synergy between the two factors in promoting target expression. The transcript levels of the same gene set would not be induced in the null cells due to the lack of a functional NFAT5 (Fig. 2B). On the other hand, downregulated genes identified in WT cells are enriched for the IRF1 but not the NFAT5 motif. It is plausible that recruitment of IRF1 to NFAT5 targets may reduce the IRF1 pool available for other biding sites and ultimately lead to a decreased expression of the respective genes. The putative mechanism is specific for WT cells since IRF1 would not be titrated out by NFAT5 in the null cells (Fig. 2C). Lastly, we speculate that IRF1, in the absence of NFAT (e.g., in null cells), may have its own binding specificity and promote gene expression of a subset of genes. It may explain why the IRF1 motif is also modestly overrepresented in gene specifically upregulated in null cells under the adapted condition (Fig. 2D). In this respect we previously observed (14) that JUN and FOS bind to an AP-1 site near osmotic response elements and contribute to NFAT5 activation of its target genes.
Novel Categories of NFAT5 Targets Newly Identified by RNA-Seq
We call special attention to several of the categories of NFAT5 targets newly identified by RNA-Seq: enzymes involved in metabolism of sulfur, gastrointestinal function and lipid metabolism; components of epidermis, muscle, nerve, and cell junctions; extracellular matrix; and blood clotting. These categories suggest directions for further investigation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.I., J.Z., M.B.B., and J.D.F. conception and design of research; Y.I. performed experiments; Y.I., W.Y., J.Z., M.B.B., and J.D.F. analyzed data; Y.I., W.Y., J.Z., M.B.B., and J.D.F. interpreted results of experiments; Y.I., W.Y., and M.B.B. prepared figures; Y.I., W.Y., J.Z., M.B.B., and J.D.F. drafted manuscript; Y.I., W.Y., J.Z., M.B.B., and J.D.F. edited and revised manuscript; Y.I., W.Y., J.Z., M.B.B., and J.D.F. approved final version of manuscript.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J Biol Chem 283: 7309–7313, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chu Y, Corey DR. RNA sequencing: platform selection, experimental design, and data interpretation. Nucleic Acid Ther 22: 271–274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dmitrieva NI, Cai Q, Burg MB. Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci USA 101: 2317–2322, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esensten JH, Tsytsykova AV, Lopez-Rodriguez C, Ligeiro FA, Rao A, Goldfeld AE. NFAT5 binds to the TNF promoter distinctly from NFATp, c, 3 and 4, and activates TNF transcription during hypertonic stress alone. Nucleic Acids Res 33: 3845–3854, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrow MA, Kim EY, Wolinsky SM, Sheehy AM. NFAT and IRF proteins regulate transcription of the anti-HIV gene, APOBEC3G. J Biol Chem 286: 2567–2577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101: 10673–10678, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grady CR, Knepper MA, Burg MB, Ferraris JD. Database of osmoregulated proteins in mammalian cells. Physiol Rep 2: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halterman JA, Kwon HM, Wamhoff BR. Tonicity-independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). Am J Physiol Cell Physiol 302: C1–C8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 115: 2989–2997, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193–277, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Huang dW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: role in osmoprotective transcriptional regulation. Proc Natl Acad Sci USA 103: 8882–8887, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irarrazabal CE, Williams CK, Ely MA, Birrer MJ, Garcia-Perez A, Burg MB, Ferraris JD. Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J Biol Chem 283: 2554–2563, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Ito T, Asakura K, Tougou K, Fukuda T, Kubota R, Nonen S, Fujio Y, Azuma J. Regulation of cytochrome P450 2E1 under hypertonic environment through TonEBP in human hepatocytes. Mol Pharmacol 72: 173–181, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ko BC, Turck CW, Lee KW, Yang Y, Chung SS. Purification, identification, and characterization of an osmotic response element binding protein. Biochem Biophys Res Commun 270: 52–61, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Lee SD, Choi SY, Lim SW, Lamitina ST, Ho SN, Go WY, Kwon HM. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: organic osmolyte-dependent and -independent pathways. Am J Physiol Renal Physiol 300: F707–F715, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Kaz AM, Kanngurn S, Welsch P, Morris SM, Wang J, Lutterbaugh JD, Markowitz SD, Grady WM. NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer. PLoS Genet 9: e1003552, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maallem S, Wierinckx A, Lachuer J, Kwon MH, Tappaz ML. Gene expression profiling in brain following acute systemic hypertonicity: novel genes possibly involved in osmoadaptation. J Neurochem 105: 1198–1211, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mak MC, Lam KM, Chan PK, Lau YB, Tang WH, Yeung PK, Ko BC, Chung SM, Chung SK. Embryonic lethality in mice lacking the nuclear factor of activated T cells 5 protein due to impaired cardiac development and function. PLoS One 6: e19186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 96: 2538–2542, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montmayeur JP, Valius M, Vandenheede J, Kazlauskas A. The platelet-derived growth factor beta receptor triggers multiple cytoplasmic signaling cascades that arrive at the nucleus as distinguishable inputs. J Biol Chem 272: 32670–32678, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Nahm O, Woo SK, Handler JS, Kwon HM. Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am J Physiol Cell Physiol 282: C49–C58, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Ni T, Tu K, Wang Z, Song S, Wu H, Xie B, Scott KC, Grewal SI, Gao Y, Zhu J. The prevalence and regulation of antisense transcripts in Schizosaccharomyces pombe. PLoS One 5: e15271, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettiford SM, Herbst R. The protein tyrosine phosphatase HePTP regulates nuclear translocation of ERK2 and can modulate megakaryocytic differentiation of K562 cells. Leukemia 17: 366–378, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J 17: 7337–7350, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramskold D, Wang ET, Burge CB, Sandberg R. An abundance of ubiquitously expressed genes revealed by tissue transcriptome sequence data. PLoS Comput Biol 5: e1000598, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos BC, Chevaile A, Hebert MJ, Zagajeski J, Gullans SR. A combination of NaCl and urea enhances survival of IMCD cells to hyperosmolality. Am J Physiol Renal Physiol 274: F1167–F1173, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Schafer C, Gehrmann T, Richter L, Keitel V, Kohrer K, Haussinger D, Schliess F. Modulation of gene expression profiles by hyperosmolarity and insulin. Cell Physiol Biochem 20: 369–386, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Su PH, Lin YW, Huang RL, Liao YP, Lee HY, Wang HC, Chao TK, Chen CK, Chan MW, Chu TY, Yu MH, Lai HC. Epigenetic silencing of PTPRR activates MAPK signaling, promotes metastasis and serves as a biomarker of invasive cervical cancer. Oncogene 32: 15–26, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem 281: 25416–25424, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Vogel R, Garten A, Klammt J, Barnikol-Oettler A, Kiess W. Activation of Erk1/2 phosphorylation but not of Akt/Pkb through an inducible CSF1R/IRR-receptor construct in INS-1E beta-cells. Arch Physiol Biochem 116: 128–136, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol 22: 5753–5760, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222, 1982. [DOI] [PubMed] [Google Scholar]
- 37.Yasuoka H, Hsu E, Ruiz XD, Steinman RA, Choi AM, Feghali-Bostwick CA. The fibrotic phenotype induced by IGFBP-5 is regulated by MAPK activation and egr-1-dependent and -independent mechanisms. Am J Pathol 175: 605–615, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Ferraris JD, Burg MB. Mitochondrial reactive oxygen species contribute to high NaCl-induced activation of the transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 290: F1169–F1176, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Ferraris JD, Cai Q, Agarwal A, Burg MB. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 289: F377–F385, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Gallazzini M, Burg MB, Ferraris JD. Contribution of SHP-1 protein tyrosine phosphatase to osmotic regulation of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 7072–7077, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Wang H, Burg MB, Ferraris JD. High NaCl-induced inhibition of PTG contributes to activation of NFAT5 through attenuation of the negative effect of SHP-1. Am J Physiol Renal Physiol 305: F362–F369, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.