Abstract
Neovascularization, the formation of new blood vessels, requires multiple processes including vascular leak, migration, and adhesion. Endosomal proteins, such as Rabs, regulate trafficking of key signaling proteins involved in neovascularization. The novel endosome protein, p18, enhances vascular endothelial (VE)-cadherin recycling from early endosome to cell junction to improve pulmonary endothelial barrier function. Since endothelial barrier integrity is vital in neovascularization, we sought to elucidate the role for endosome proteins p18 and Rab4, Rab7, and Rab9 in the process of vessel formation within the pulmonary vasculature. Overexpression of wild-type p18 (p18wt), but not the nonendosomal-binding mutant (p18N39), significantly increased lung microvascular endothelial cell migration, adhesion, and both in vitro and in vivo tube formation. Chemical inhibition of mTOR or p38 attenuated the proneovascularization role of p18wt. Similar to the effect of p18wt, overexpression of prorecycling wild-type (Rab4WT) and endosome-anchored (Rab4Q67L) Rab4 enhanced neovascularization processes, whereas molecular inhibition of Rab4, by using the nonendosomal-binding mutant (Rab4S22N) attenuated VEGF-induced neovascularization. Unlike p18, Rab4-induced neovascularization was independent of mTOR or p38 inhibition but was dependent on p18 expression. This study shows for the first time that neovascularization within the pulmonary vasculature is dependent on the prorecycling endocytic proteins Rab4 and p18.
Keywords: endocytosis, endothelium, mTOR, p38, neovascularization, LAMTOR, pulmonary
neovascularization, the de novo formation of new blood vessels, or angiogenesis, the outgrowth of existing vessels, are vital processes in embryonic development and adult repair and remodeling. The formation of blood vessels, in response to angiogenic factors such as VEGF, requires vascular leakage prior to endothelial cell proliferation, migration, and adhesion. The transition of these initial sprouts into mature, stable endothelium involves the formation of cell-cell junctions and association with smooth muscle cells and pericytes. A defining characteristic of these newly formed vessels is tight intercellular junctions, such as adherens junctions formed by homotypic vascular endothelial (VE)-cadherin interactions, to maintain vascular permeability (12). The class II phosphatidylinositol-3-kinase, PI3K-C2α, regulates VE-cadherin trafficking from the trans-Golgi network to the cell surface concomitant with enhanced angiogenic potential of the vasculature (51). We have previously demonstrated that the novel endosomal adaptor protein p18 tightens the endothelial barrier through enhanced recycling of VE-cadherin to the adherens junction (6). In addition to this role at the early endosome, p18 also anchors the late endosome/lysosome to act as a scaffold for the Rag-GTPase complex (30, 38, 44). In this cellular location, p18 facilitates activation of mTORC1 and downstream MEK/MAPK signaling proteins (30, 46); however, the barrier protective effects of p18 are independent of mTOR/MAPK activity (6). Although p18 is known to regulate vascular permeability through trafficking at the early endosome, a role for the endosomal protein in neovascularization has not yet been studied.
Mediators of endosomal trafficking are known to play a key role in neovascularization (22, 23), for example, the family of endocytic master regulators, Rab GTPases. Although over 60 Rab GTPases have been identified, only Rabs 1–9, 11, 13–15, 22, and 30 have been identified in endothelial cells (11, 45). Rab GTPases vary in spatial distribution. For example, prorecycling Rab4 is responsible for rapid recycling of endosomal cargo to the plasma membrane (10). Conversely, cargo destined for degradation is delivered to the lysosome directly via the Rab7-dependent late endosome or indirectly from the trans-Golgi network via Rab9-mediated trafficking (13, 28). Knockdown of the prorecycling Rab4 decreased VEGF/VEGFR-mediated cell migration and tube formation in human umbilical vein endothelial cells (22, 24). Recently, Okon et al. (33) demonstrated a VEGF-independent role for Rab7-mediated lysosomal degradation in attenuating tumor angiogenesis and growth in lung adenocarcinomas. Thus regulation of endocytic trafficking, via Rab GTPases, can play a key role in modulating the angiogenic process.
Within the bronchial circulation, structural changes in vessels have been observed; however, evidence for new vessel formation in the adult pulmonary circulation is limited (34, 40). Pulmonary neovascularization has been demonstrated in animal models including chronic Pseudomonas aeruginosa infections (17), metastatic diseases (31), the compensatory growth postpneumonectomy surgery (5, 27), and pulmonary hypertension (40). Tube formation in the pulmonary vasculature is also important in healthy individuals to renew regions of injured endothelium; indeed, pulmonary microvascular endothelial cells display high expression and activity of proliferative factors (43). Although the vasculogenic capacity of pulmonary microvascular endothelial cells has been previously established (2, 7), the mechanisms responsible for these adaptations are not well understood.
In the present study, we investigated the role of endocytic proteins p18, Rab4, Rab7, and Rab9 in the regulation of neovascularization in the pulmonary endothelium. Using endosome-locked and nonendosomal-binding mutants or chemical inhibitors, we further studied whether the vasculogenic process is mediated through endosomal trafficking or mTOR/MAPK-dependent mechanisms. We demonstrate that p18 and Rab4 collaborate to regulate neovascularization through two distinctive pathways and propose that manipulation of these proteins may regulate vessel formation in the pulmonary vasculature in settings of aberrant neovascularization.
MATERIALS AND METHODS
Cell lines and reagents.
All materials were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Rat lung microvascular endothelial cells (LMVEC) (Vec Technologies, Rensselaer, NY) were maintained in MCDB-131 (no. 120114, Vec Technologies) and used between passages 3 and 11.
The vector encoding wild-type p18 (GFP-p18wt) and mutant p18, lacking endosome-binding region (p18N39), were a kind gift from Shigeyuki Nada (30, 44) (Department of Oncogene Research, Osaka University). The vectors encoding wild-type (Rab4WT), dominant positive (Rab4Q67L), and dominant negative (Rab4S22N) Rab4 were a kind gift from Mary McCaffrey (Cell and Molecular Biology, University College Cork). The vectors encoding dominant negative Rab7 (Rab7T22N, no. 44826) and Rab9 (Rab9S21N, no. 12664) were purchased from Addgene. Phosphorylated green fluorescent protein (pGFP-C1) was obtained from Clontech (no. 608-1, Mountain View, CA); antibody for von Willebrand factor (vWF) was purchased from Dako (Carpinteria, CA); p18 siRNA was obtained from Orignene (no. SR513910, Rockville, MD).
Antibodies directed against phosphorylated MEK1/2 (S218/S222; no. sc-7995-R), MEK (no. sc-436), p70 (no. sc-8418), Rab4 (no. sc-271982), and actin (no. sc-1615) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Green fluorescent protein (GFP) antibody (no. A11120) and human recombinant VEGF (no. PHC9394) were purchased from Invitrogen (no. A11122, Grand Island, NY). Phosphorylated mTOR (S2448; no. 2971S), p70 (T389; no. 9205S), p38 (T180/Y182; no. 9215S), ERK1/2 (T202/Y204; no. 9101), unphosphorylated mTOR (no. 2972S), p38 (no. 9212S) and ERK1/2 (no. 9102S), p18 (c11orf59, no. 8975S), Rab7 (no. 9367S) and Rab9 (no. 5118P) antibodies, and ERK1/2 inhibitor U0126 (no. 9903) were all purchased from Cell Signaling (Beverly, MA). SB203580 (no. S8307), rapamycin (no. R0395), and vinculin antibody (no. V9131) were purchased from Sigma (St. Louis, MO). Polyjet reagent (no. SL100688) and Matrigel Basement Membrane Matrix (no. 356230) were obtained from SignaGen (Gaithersburg, MD) and BD Biosciences (San Jose, CA), respectively.
Transient transfection.
Rat LMVEC were transiently transfected with plasmid cDNA-encoding GFP, p18WT, p18N39, Rab4WT, Rab4Q67L, Rab4S22N, Rab7T22N, and Rab9S21N by using Polyjet reagent, as per manufacturer's instructions. Alternatively, LMVEC were transfected with p18 siRNA duplex (300 nM) by using Amaxa Nucleofector technology (Lonza), as per manufacturer's instructions. Overexpression of cDNA and knockdown via siRNA was assessed by fluorescence microscopy and immunoblot analysis.
Cell proliferation assay.
Transfected LMVEC were plated for 24 h to reach ∼60% confluence. Cells were then quiesced in MCDB-131 media with 1% FBS for 24 h, followed by treatment with VEGF (50 ng/ml) for a further 24 h. LMVEC were then counted and assessed by immunoblot analysis.
Immunoblot analysis.
LMVEC were lysed with radio immunoprecipitation assay buffer and subjected to immunoblot analysis performed on 10% SDS-PAGE gels by using primary antibody dilutions of 1:1,000, except vinculin (1:5,000) and secondary antibody dilutions of 1:5,000.
In vitro tube formation.
Transfected LMVEC were plated onto Matrigel-coated dishes and plates were incubated for 48 h at 37°C, 5% CO2. Transfected cells were treated with 50 ng/ml VEGF for 6 h. Images of tube formation were captured at ×10 magnification by using a Nikon Eclipse TE2000-U microscope. The number of junctions and segments and segment length was calculated by using Angiogenesis Analyzer software in Image J. An average from 2 to 3 wells was assessed to represent an n = 1.
Cell adhesion assay.
Transfected LMVEC were pretreated p38 inhibitor SB203580 (10 nM, 30 min) followed by replating onto gelatin-coated plates. Following pretreatment with SB203580, cells were treated with VEGF (50 ng/ml) or rapamycin (10 nM) for 2 h. LMVEC were then rinsed with PBS to remove nonadherent cells, and adherence to gelatin was assessed by using methyl thiazolyl tetrazolium (MTT) assay by quantifying the reduction of solube yellow tetrazolium dye into insoluble purple formazan. Cells were incubated with MTT (5 mg/ml) for 4 h at 37°C, followed by the addition of 0.04–0.1 N HCl in isopropanol for 15 min. The absorbance was measured by using a microplate reader (GenTech) at a wavelength of 570 nm with background readings at 630 nm and 690 nm.
Cell migration assay.
After 48 h, transfected LMVEC were scratched by using a pipette tip and followed by pretreatment with SB203580 (10 nM, 30 min) and treatment with VEGF (50 ng/ml) or rapamycin (10 nM) for 6 h. Cell migration was monitored at 2-h time intervals following the initial wound, and images were captured at ×10 magnification on a Nikon Eclipse TE2000-U microscope. Cell migration was assessed by using MiToBo analyzer software in Image J. An average from 2 to 3 wells was assessed to represent an n = 1.
In vivo de novo tube formation.
Transfected LMVEC (1.5 × 106) were suspended in 100 μl of cooled MCDB-131 media and 250 μl of unpolymerized Matrigel at 4°C (to maintain the mixture in a fluid phase). The mixture was injected subcutaneously (two plugs per animal) into anaesthetized (isofluorane inhalation 2.5%) Sprague-Dawley rats by using a 23-gauge needle. The injected mixture polymerizes at body temperature and becomes a plug following subcutaneous contact. At 4 days after injection, Matrigel plugs were excised from the subcutaneous regions of anesthetized animals (inhalation anesthesia 2.5%) and immersion fixed in 4% paraformaldehyde. Fixed Matrigel plugs were dehydrated in ethanol, embedded in paraffin, cut in 5-μm sections, and stained with hematoxylin and eosin or vWF for light microscopy analysis. Images of stained sections were captured via Aperio slide scanner (Leica), and the number of tubes containing red blood cells (blood vessels) within the gel were counted. Blood vessels at the periphery of the gel in close proximity to the surrounding tissue or embedded within native tissue anywhere in the gel were not included for quantification. Results from each separate plug were considered n = 1.
Statistical analysis.
Experimental number (n) is presented for each experiment. For three or more groups, variance was assessed by using Bartlett's test with data sets not reaching significance studies by Kruskal-Wallis test followed by Dunn's test. For all other data sets, differences among the means were tested for significance in all experiments by ANOVA with Tukey's range significance difference test. For two groups, variance in data sets was assessed by using Mann-Whitney test followed by t-test. Data are presented as means ± SD. Significance was reached when P < 0.05.
RESULTS
Tethered to the endosome, p18 promotes the vasculogenic process.
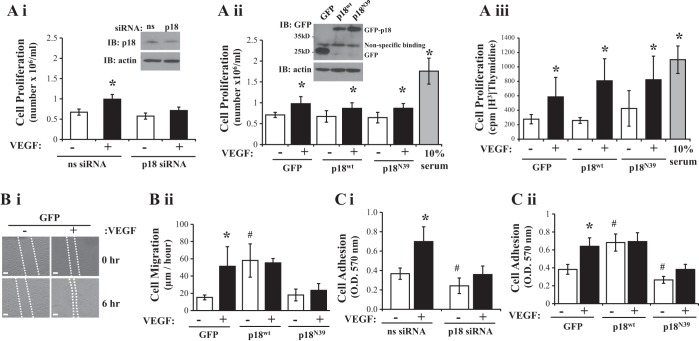
Recently, we have demonstrated that the novel endosomal adaptor protein p18 regulates endothelial barrier function through enhanced recycling of VE-cadherin to the plasma membrane (6). Thus we sought to assess whether p18 regulates key processes involved in neovascularization by p18 knockdown using specific siRNA in LMVEC. Densitometry quantification showed that p18 expression in LMVEC lysate, following siRNA knockdown, was significantly decreased by 49% compared with scrambled, nonspecific (ns) siRNA (ns siRNA: 2.64 ± 0.31 r.u.; p18 siRNA: 1.34 ± 0.54 r.u.). Although we noted that p18 protein suppression exerts no effect on endothelial cell proliferation at baseline, compared with nonspecific siRNA (Fig. 1Ai), we did observe an attenuation of VEGF-induced cell proliferation following p18 knockdown (Fig. 1Ai). To further understand the effect of p18 on cell proliferation, we overexpressed cDNA-encoding GFP-conjugated wild-type p18 (p18wt) or a nonendosomal-binding mutant (p18N39) or GFP control in LMVEC (Fig. 1Aii, inset). Densitometry quantification showed that equivalent levels of GFP were expressed in LMVEC transiently transfected with p18wt (0.49 ± 0.14 r.u.), p18N39 (0.51 ± 0.21 r.u.), and GFP (0.55 ± 0.14 r.u.) cDNA. We observed increased cell proliferation in response to VEGF treatment; however, no significant changes in cell proliferation were noted in cells overexpressing p18wt or p18N39 compared with GFP (Fig. 1, Aii and Aiii). We next studied the effect of p18 on cell migration and adhesion. VEGF treatment significantly increased cell migration, assessed by wound-healing assay, (Fig. 1B and Supplementary Fig. S1) and adhesion to gelatin (Fig. 1C) in GFP-expressing endothelial cells. Overexpression of p18wt significantly increased cell migration (Fig. 1Bii) and adhesion (Fig. 1Ci) in the absence of stimulants, whereas p18N39 overexpression had significantly reduced VEGF-induced cell migration (Fig. 1Bii) and cell adhesion (Fig. 1Cii) compared with GFP-overexpressing cells. Interestingly, siRNA knockdown of p18 significantly reduced cell adhesion, under baseline and VEGF-treated conditions, compared with nonsilencing siRNA (Fig. 1Ci).
Fig. 1.
p18 expression affects endothelial cell adhesion and migration. Equivalent numbers of lung microvascular endothelial cells (LMVEC) were transiently transfected with p18 (Ai) or nonsilencing siRNA (Ci) or green fluorescent protein (GFP), p18wt, or p18N39 cDNA (Aii, Aiii, Bii, and Cii). A: following 24 h, cells were serum starved with MCDB 131 media containing 1% FBS and, after a further 24 h, cells were treated with VEGF (50 ng/ml) or incubated with MCDB 131 media containing 10% FBS (full serum) for 24 h, and cells were counted. Lysates were assessed for p18 knockdown (Ai) or overexpression (Aii) by Western blot analysis (insets). Representative blots are shown. Alternatively, cell proliferation was assessed by [H3]-thymidine uptake and incorporation (Aiii). B: following 48 h, cell migration was measured by wound-healing assay in the presence and absence of VEGF (50 ng/ml) up to 6 h. Images were captured, a representative image for GFP is shown (Bi), and data was analyzed by using MiToBo analyzer in ImageJ and expressed as distance migrated (μm) per hour (Bii). C: following 48 h, p18 knockdown (Ci) or overexpressing (Cii) cells were replated onto gelatin-coated dishes and treated with VEGF (50 ng/ml) for 2 h. Cells that adhered to gelatin were measured by methyl thiazolyl tetrazolium (MTT) assay (O.D.570nm). All images were captured at ×10 magnification, scale bar 20 μm. Data are presented as means ± SD; n = 4 (A), n = 4–5 (B), n = 3–6 (C); *P < 0.05 vs. vehicle, #P < 0.05 vs. GFP or nonsilencing siRNA.
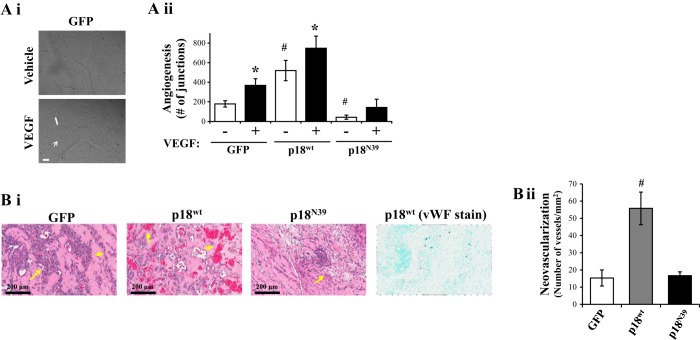
We next studied the effect of endosomal p18 on tube formation in vitro and in vivo. GFP-overexpressing cells, plated onto Matrigel extracellular matrix, displayed a significant increase in vessel formation following exposure to VEGF (Fig. 2Ai), as assessed by number of junctions (Fig. 2Aii) and number of segments and length of segments (data not shown). In the absence of VEGF, overexpression of p18wt significantly increased vessel formation, compared with GFP-overexpressing cells (Fig. 2Aii). Interestingly, VEGF induced a further increase in number of junctions in cells overexpressing p18wt (Fig. 2A). Conversely, p18N39 overexpression significantly decreased vessel formation in both the presence and absence of VEGF (Fig. 2A). In vivo measurements of neovascularization, using Matrigel plugs injected into rats, were quantified as the number of vessels containing blood cells. Immunohistochemical analysis of von Willebrand factor (vWF) demonstrated that regions containing blood cells were surrounded by endothelial cells (vWF-positive) (Fig. 2Bi). Matrigel plugs containing cells overexpressing p18wt cDNA demonstrated significantly increased number of vessels compared with plugs combined with GFP-overexpressing cells (Fig. 2B). Furthermore, the number of vessels formed in Matrigel plugs with cells overexpressing p18N39 was similar to the GFP-overexpressing plugs (Fig. 2B).
Fig. 2.
p18 promotes tube formation in vitro and in vivo. Equivalent numbers of LMVEC were transiently transfected with GFP, p18wt, or p18N39 cDNA. A: transfected cells were plated directly onto Matrigel-coated wells for 48 h. Cells were then treated with VEGF (50 ng/ml) for a further 6 h, and images of branching were captured (Ai) and in vitro vessel formation was quantified by using Angiogenesis Analyzer in ImageJ, expressed as number of junctions formed (arrow) per field of view (Aii). Images were captured at ×10 magnification, scale bar 20 μm. B: de novo vessel formation, by transfected endothelial cells, was examined by using in vivo Matrigel plugs in rats. Vessel formation was determined by examining presence of blood vessels containing red blood cells within the Matrigel plug. Plugs were removed 4 days after injection, formalin-fixed, sliced in paraffin, and stained with hematoxylin and eosin. Images were captured by using Aperio ScanScope CS (Bi, left) and number of vessels per field of view were quantified (Bii). Staining with von Willebrand factor (vWF) antibody demonstrated that the appearance of red blood cells coincided with formation of blood vessels within the plug (Bi, right). Images were captured at ×20 magnification, scale bar 200 μm. Arrows demonstrate examples of neovasculogenesis within plugs. Data are presented as means ± SD; n = 4 (A), n = 5 (B); *P < 0.05 vs. vehicle, #P < 0.05 vs. GFP.
This data demonstrates a role for p18 in the migration, adhesion and in vitro and in vivo tube formation capability of LMVEC. We further show that the role of p18 on these vasculogenic processes is dependent on its ability to bind to the endosome.
Through activation of mTOR and p38, p18 regulates neovascularization.
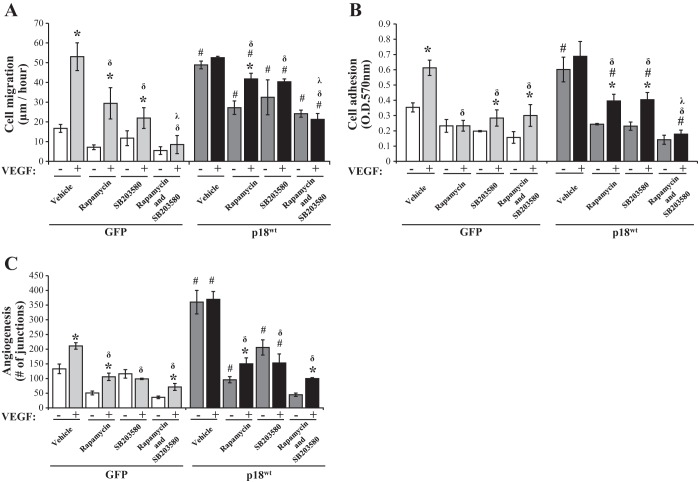
We have previously demonstrated that p18 overexpression upregulated mTOR and p38, but not ERK, activities (6). To understand the mechanism through which p18 regulates the vasculogenic process, our next set of experiments studied whether p18 mediates cell migration (Fig. 3A), adhesion (Fig. 3B), and tube formation (Fig. 3C) through mTOR- or p38-dependent pathways. Following exposure to the chemical p38 inhibitor, SB203580, or mTOR inhibitor, rapamycin, cell migration, adhesion, and tube formation in GFP-overexpressing cells were significantly decreased under basal conditions and VEGF exposure (Fig. 3). The MEK/ERK inhibitor, U0126, had no effect on baseline or VEGF-induced cell adhesion in p18wt overexpressing endothelial cells (data not shown). Overexpression of p18wt maintained higher levels of cell migration, cell adhesion, and tube formation, irrespective of inhibitor treatment, than cells overexpressing GFP. However, addition of both rapamycin and SB203580 significantly reduced p18wt-induced cell migration and adhesion in both the presence and absence of VEGF in an additive manner (Fig. 3, A and B). Interestingly, the addition of both inhibitors did not further downregulate p18wt-induced tube formation in either the presence or absence of VEGF (Fig. 3C). These data demonstrate that p18 enhances endothelial cell migration and adhesion in LMVEC through both p38- and mTOR-dependent pathways. We further demonstrate that p18-mediated tube formation is dependent on either a p38- or mTOR-dependent pathway.
Fig. 3.
p18 regulates the angiogenic process through activation of mTOR and p38. Equivalent numbers of LMVEC were transiently transfected with GFP or p18wt cDNA. A: following 48 h, cell migration was measured by wound-healing assay in the presence or absence of SB203580 (10 nM, 30 min) pretreatment followed by exposure to rapamycin (10 nM) and VEGF (50 ng/ml) up to 6 h. Images were captured and data was analyzed by using MiToBo analyzer in ImageJ and expressed as the rate of cell migration (μm) per hour. B: following 48 h, cells were replated onto gelatin-coated dishes and pretreated in the presence or absence of SB203580 (10 nM, 30 min) followed by treatment with rapamycin (10 nM) and VEGF (50 ng/ml) for 2 h. Cells adhered to gelatin were measured by MTT assay (O.D.570nm). C: transfected cells were plated directly onto Matrigel-coated wells for 48 h. Cells were then preexposed to SB203580 (10 nM, 30 min) followed by treatment with rapamycin (10 nM) and VEGF (50 ng/ml) for a further 6 h, images of tube formation were captured, and in vitro vessel formation was assessed by using Angiogenesis Analyzer in ImageJ and expressed as number of junctions formed. Data are presented as means ± SD; n = 4 (A), n = 4–5 (B), n = 6 (C); *P < 0.05 vs. vehicle for VEGF, #P < 0.05 vs. GFP, δP < 0.05 vs. VEGF with no treatment (rapamycin or SB203580), ʎP < 0.05 vs. VEGF with treatment (rapamycin or SB203580).
Prorecycling Rab4 activation regulates neovascularization in LMVEC.
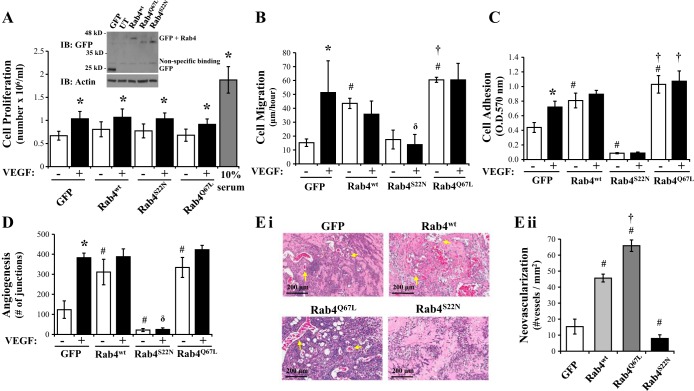
As p18 has been shown to promote recycling of early endosome cargo to the cell surface, we next used mutants of the prorecycling Rab GTPases Rab4 to test the effect of enhanced endosome recycling on vasculogenic endpoints. LMVEC were transiently transfected with cDNA-encoding GFP-conjugated wild-type Rab4 (Rab4WT), dominant active, GTPase-deficient Rab4 (Rab4Q67L), which remains anchored to the endosome, or the dominant negative, GDP-locked Rab4 (Rab4S22N), which is unable to bind the endosome (29). Densitometry quantification showed that equivalent levels of GFP were expressed in LMVEC transiently transfected with Rab4WT (0.38 ± 0.21 r.u.), Rab4Q67L (0.57 ± 0.22 r.u.), Rab4S22N (0.71 ± 0.3 r.u.), and GFP (0.44 ± 0.18 r.u.) cDNA. Cell proliferation was significantly increased in cells exposed to VEGF for 24 h (Fig. 4A); however, overexpression of Rab4WT, Rab4Q67L, or Rab4S22N exerted no effect on cell proliferation compared with cells overexpressing GFP (Fig. 4A). A significant increase in the rate of endothelial basal cell adhesion and migration was observed in cells overexpressing Rab4wt and Rab4Q67L, but not Rab4S22N, compared with GFP-overexpressing cells (Fig. 4, B and C). Interestingly, VEGF-induced increases in the rate of migration and adhesion in GFP-overexpressing cells was not observed in cells overexpressing any of the Rab4 constructs (Fig. 4, B and C). We next studied the effect of Rab4 activity on tube formation in vitro (Fig. 4D). GFP-overexpressing cells displayed a significant increase in vessel formation following exposure to VEGF, as assessed by number of junctions (Fig. 4D). In the absence of VEGF, overexpression of Rab4wt and Rab4Q67L increased number of junctions formed, whereas Rab4S22N significantly decreased tube formation (Fig. 4D).
Fig. 4.
Rab4 activity regulates the angiogenic process in LMVEC. Equivalent numbers of LMVEC were transiently transfected with GFP, Rab4WT, Rab4Q67L, or Rab4S22N cDNA. A: following 24 h, cells were counted (as Fig. 1A) and lysates were assessed for Rab4 overexpression by Western blot analysis (inset). A representative blot is shown. B: following 48 h, cell migration was measured by wound-healing assay in the presence and absence of VEGF and migration (μm) of cells per hour was assessed (as Fig. 1B). C: following 48 h, cell adhesion to gelatin was measured by MTT assay (O.D.570nm) (as Fig. 1C). D: transfected cells were plated directly onto Matrigel-coated wells for 48 h. Cells were then treated with VEGF (50 ng/ml) for a further 6 h, and in vitro vessel formation, expressed as number of junctions formed, was assessed by using Angiogenesis Analyzer in ImageJ (as Fig. 2A). E: de novo vessel formation of transfected LMVEC was examined by using in vivo Matrigel plugs. Neovasculogenesis was determined by examining presence of blood vessels containing red blood cells within the Matrigel (as Fig. 2B). Images were captured using Aperio ScanScope CS (Ei) and number of vessels was quantified (Eii). Images were captured at ×10 magnification, scale bar 200 μm. Arrows demonstrate examples of neovasculogenesis within plugs as determined by presence of red blood cells within vessels. Data are presented as means ± SD; n = 4 (A), n = 3–5 (B), n = 5–6 (C), n = 4 (D), n = 5 (E); *P < 0.05 vs. vehicle, #P < 0.05 vs. GFP, δP < 0.05 vs. VEGF, †P < 0.05 vs. Rab4wt.
With in vivo measurements of neovascularization, we noted a significantly increased number of vessels formed with cells overexpressing Rab4WT compared with plugs combined with GFP-overexpressing cells (Fig. 4E). Furthermore, the number of vessels formed was further increased in Matrigel plugs combined with cells overexpressing Rab4Q67L (Fig. 4E).
Similar to p18 data, these results demonstrate a role for the prorecycling endocytic regulator, Rab4, in the migration, adhesion, and in vitro and in vivo tube formation capability of the pulmonary vasculature. We further show that activation of Rab4, and thus enhanced endosome binding, is important to neovascularization.
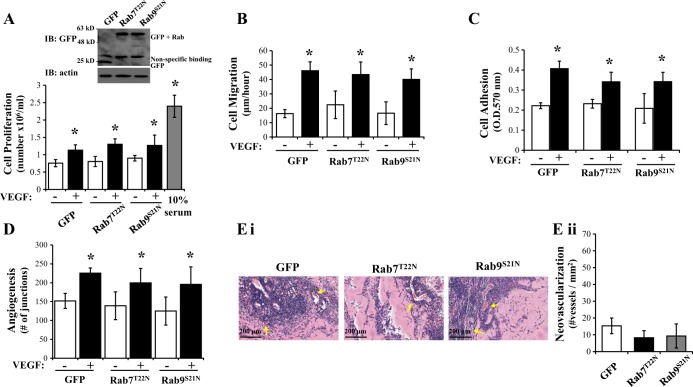
Inactivation of Rab7 and Rab9 exerts no effect on VEGF-induced angiogenic processes in LMVEC.
We next sought to understand whether the effect of Rab4 on neovascularization was observed with any other Rab GTPases. We utilized GFP-conjugated mutants of the prodegradative Rab7 and Rab9 which are GDP-locked, dominant negative mutants Rab7T22N and Rab9S21N, and thus cannot bind the endosome and traffic toward the trans-Golgi apparatus for sorting (Rab9) or lysosome for degradation (Rab7) (13, 28). Densitometry quantification showed that equivalent levels of GFP were expressed in LMVEC transiently transfected with Rab7T22N (1.55 ± 0.92 r.u.), Rab9S21N (0.95 ± 0.24 r.u.), and GFP (0.98 ± 0.16 r.u.) cDNA. Overexpression of either Rab7T22N or Rab9S21N exerted no effect on cell proliferation in the presence or absence of VEGF compared with cells overexpressing GFP (Fig. 5A). Similarly, overexpression of Rab7T22N or Rab9S21N had no effect on cell migration, adhesion, or tubule formation compared with cells overexpressing GFP (Fig. 5, B–D). Further studies of in vivo tube formation demonstrate no change in number of vessels formed in Matrigel plugs combined with cells overexpressing Rab7T22N or Rab9S21N compared with GFP (Fig. 5E).
Fig. 5.
Inactivation of Rab7 and Rab9 exerts no effect on VEGF-induced angiogenic processes in LMVEC. Equivalent numbers of LMVEC were transiently transfected with GFP, Rab7T22N, or Rab9S21N cDNA. A: following 24 h, cells were counted (as Fig. 1A) and lysates were assessed for Rab4 overexpression by Western blot analysis (inset). A representative blot is shown. B: following 48 h, cell migration was measured by wound-healing assay in the presence and absence of VEGF and migration (μm) of cells per hour was assessed (as Fig. 1B). C: following 48 h, cell adhesion to gelatin was measured by MTT assay (O.D.570nm) (as Fig. 1C). D: transfected cells were plated directly onto Matrigel-coated wells for 48 h. Cells were then treated with VEGF (50 ng/ml) for a further 6 h and in vitro vessel formation, expressed as number of junctions formed, was assessed by using Angiogenesis Analyzer in ImageJ (as Fig. 2A). E: de novo vessel formation of transfected LMVEC was examined by using in vivo Matrigel plugs. Neovasculogenesis was determined by examining presence of blood vessels containing red blood cells within the Matrigel (as Fig. 2B). Images were captured using Aperio ScanScope CS (Ei) and number of vessels was quantified (Eii). Images were captured at ×10 magnification, scale bar 200 μm. Arrows demonstrate examples of neovasculogenesis within plugs as determined by presence of red blood cells within vessels. Data are presented as means ± SD; n = 4 (A), n = 3–5 (B), n = 5–6 (C), n = 4 (D), n = 5–6 (E); *P < 0.05 vs. vehicle.
These data suggest that modulation of prodegradative Rab GTPases, Rab7 and Rab9, exert no effect on the vasculogenic process in pulmonary endothelial cells. Thus enhanced recycling of endosomes, as opposed to attenuation of trafficking to the lysosome, is likely to be responsible for Rab4-mediated neovascularization in the pulmonary vasculature.
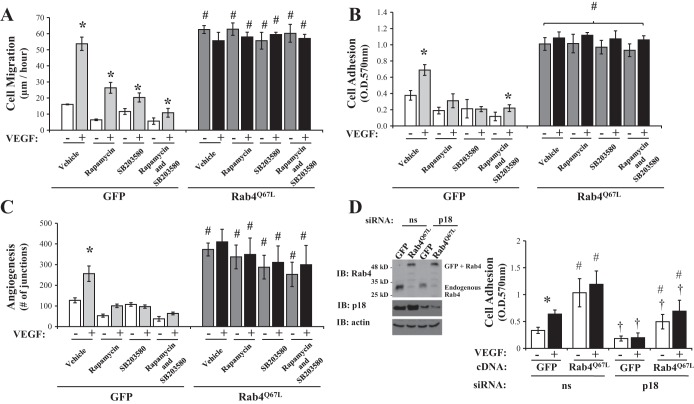
Rab4-mediated neovascularization is independent of mTOR/p38 signaling.
Although overexpression of Rab4WT enhanced endothelial cell migration, adhesion, and tube formation, Rab4Q67L overexpression caused significantly higher levels of adhesion and migration; therefore, our final studies sought to understand whether enhanced neovascularization, mediated by dominant active Rab4Q67L, is also dependent on mTOR- and p38-signaling, as noted with p18. As previously observed (Fig. 3), chemical inhibition of mTOR (rapamycin) and p38 (SB203580) significantly reduced endothelial cell migration (Fig. 6A), adhesion (Fig. 6B), and in vitro tube formation (Fig. 6C) in GFP-overexpressing cells, both in the presence and absence of VEGF. However, no significant changes in these parameters were observed in endothelial cells overexpressing Rab4Q67L (Fig. 6). These data demonstrate that, unlike p18, the role of Rab4 activity on vasculogenic processes is independent of mTOR and p38 signaling.
Fig. 6.
Rab4 regulates the angiogenic process independent of mTOR and p38 activation. Equivalent numbers of LMVEC were transiently transfected with GFP or Rab4Q67L cDNA. A: following 48 h, cell migration was measured by wound-healing assay in the presence or absence of SB203580 (10 nM, 30 min) preexposure followed by treatment with rapamycin (10 nM) and VEGF (50 ng/ml) up to 6 h. Images were captured and data was analyzed by using MiToBo analyzer in ImageJ and expressed as migration (μm) per hour. B: following 48 h, cells were replated onto gelatin-coated dishes and treated in the presence or absence of SB203580 (10 nM, 30 min) followed by treatment with rapamycin (10 nM) and VEGF (50 ng/ml) for 2 h. Cells adhered to gelatin were measured by MTT assay (O.D.570nm). C: transfected cells were plated directly onto Matrigel-coated wells for 48 h. Cells were then preexposed to SB203580 (10 nM, 30 min) followed by treatment with rapamycin (10 nM) and VEGF (50 ng/ml) for a further 6 h, images of tube formation were captured, and in vitro vessel formation was assessed by using Angiogenesis Analyzer in ImageJ and expressed as number of junctions formed. D: at 24 h prior to cDNA transfection, LMVEC were transiently transfected with p18 or nonsilencing siRNA. After a further 48 h, cells were replated onto gelatin-coated dishes and treated in the presence or absence of VEGF (50 ng/ml) for 2 h. Cells adhered to gelatin were measured by MTT assay (O.D.570nm). Data are presented as means ± SD; n = 4 (A), n = 4–5 (B), n = 6 (C); *P < 0.05 vs. vehicle, #P < 0.05 vs. GFP, †P < 0.05 vs. nonsilencing siRNA.
Coordination of endothelial cell function by p18 and Rab4.
Lastly, we sought to understand whether p18 regulates the vasculogenic process through mediating Rab4-dependent endosomal recycling. Cell adhesion, as an endpoint of angiogenesis, was assessed in LMVEC following simultaneous siRNA knockdown of p18 and overexpression of Rab4Q67L. At baseline conditions, knockdown of p18 expression significantly blunted adhesion in Rab4Q67L-overexpressing cells (Fig. 6D). This effect was also noted in the setting of VEGF stimulation. Thus p18 is downstream of Rab4-mediated effects on endothelial adhesion and possibly neovascularization.
DISCUSSION
Pulmonary vascular function is mediated through barrier integrity, vessel growth, and stabilization. The novel endocytic adaptor protein, p18, enhances barrier integrity and attenuates lung injury-induced vascular leak (6). Vessel growth and stabilization is dependent on the formation of a tight endothelial barrier; therefore, in the present study, we examined whether p18 modulates the process of neovasclarization. We demonstrate p18-mediated vessel formation required both the ability of p18 to bind the endosome and mTOR/MAPK signaling downstream of the late endosome/lysosome. We further demonstrate that the endosome recycling regulator Rab4, but not Rab7 or Rab9, enhances vessel formation through an mTOR/MAPK-independent mechanism. In addition, we show that Rab4 effect on endothelial cell function is dependent upon p18 protein expression. Our studies establish a role for endosome-associated p18 protein and Rab4 GTPase, and subsequent downstream signaling from the endosome, in the process of neovascularization within the pulmonary vasculature.
Vasculogenesis is the de novo formation of the vascular network through differentiation and growth of blood vessels from endothelial progenitor cells primarily observed during development (4, 36). Neovascularization is the development of new blood vessels in tissue and organs (2, 50). In contrast, angiogenesis is the expansion of the vascular network through formation of capillaries from preexisting blood vessels, important in remodeling and repair (3, 35). Angiogenesis requires sprouting of new vessels, a process involving endothelial cell proliferation and migration, followed by cell adhesion and tube formation (1, 9). Our studies focus on both the angiogenic and neovascularization processes in the pulmonary vasculature. We demonstrate that endosomal p18 and Rab4 enhance the capacity of pulmonary microvascular endothelial cells to migrate, adhere, form new vessels in vitro, and form new blood vessel networks from preexisting vessels in vivo. Another key component of the angiogenic and vasculogenic process is endothelial cell proliferation, permitting the growth of new blood vessels prior to tube formation. Following vessel formation, the endothelium returns to a quiescent, stable state. In the present study, we observe that knockdown of p18 expression attenuates VEGF-induced cell proliferation, whereas overexpression of the protein exhibits no effect. Interestingly, Jopling et al. (24) showed that Rab4 depletion, but not Rab11, affected VEGF-induced endothelial proliferation, possibly by changing the rate of VEGFR2 recycling to the cell surface. Thus it is possible that whereas p18 depletion affects VEGF signaling by diminished VEGFR2 at the surface, p18 overexpression may not significantly increase the level of the receptor at the cell surface and result in an enhanced proliferation. Future work would need to be done to determine the impact of p18 altered expression on VEGF signaling; however, studies to understand the physiological function of p18 are difficult, as p18 knockout mice are embryonic lethal, dying during embryonic stage 7 by undergoing growth arrest with severe defects in endosome/lysosome organization and membrane protein transport (30).
Endosome-related proteins can regulate the angiogenic process. Yoshioka et al. (51) demonstrated that knockdown of the endosome-binding phosphatidylinositol 3-kinase isoform, PI3K-C2α, impairs tube formation and cell migration, thus reducing hindlimb angiogenesis following ischemic injury. Our studies focus on the novel endosome adaptor protein p18 and the small GTPase endocytic regulators Rab 4, Rab7, and Rab9. Previous studies have demonstrated a role for Rab-mediated recycling of VEGF receptor (VEGFR2) in regulating blood vessel formation in zebrafish and human umbilical vein endothelial cells (HUVECs) (23, 24, 42). Binding of VEGF-A to VEGFR2 stimulates tyrosine kinase activity, autophosphorylation, and signaling to downstream proteins regulating migration, vascular permeability, cell survival, and proliferation (41, 49). In unstimulated cells, VEGFR2 was shown to be predominantly located in recycling endosomes identified by Rab4 and Rab5 (14). Our data demonstrate that, independently of VEGF stimulation, the prorecycling Rab4 enhanced endothelial angiogenic processes in the pulmonary vasculature. It is therefore possible that Rab4 promotes VEGFR2 recycling at baseline conditions to such an extent that VEGF exposure, resulting in further stimulation of the VEGFR2 pathway, had no further effect on the angiogenic process. Likewise, nonendosomal-binding Rab4 mutant, Rab4S22N, was not influenced by VEGF stimulation, suggesting blunted recycling of the VEGFR2 in response to Rab4 inhibition.
Although we have previously demonstrated a role for p18 in recycling of VE-cadherin from the early endosome to the cell surface (6), a role for p18 in VEGFR2 trafficking or angiogenic processes has not previously been elucidated. Our present studies indicate that p18 associated with the endosome enhances endothelial cell migration, adhesion, and tube formation. In p18-depleted neuroblastoma cells, Guillaumot et al. (16) observed increased LDL uptake, which is associated with enhanced VEGFR2 internalization and degradation, and decreased angiogenesis in isolated HUVECs (21). It is therefore possible that p18 enhances the angiogenic process through reduced LDL uptake and subsequent blunting of VEGF-VEGFR2 signaling. Alternatively, Hoshino et al. (18, 19) noted enhanced p18 expression in metastatic melanomas associated with RhoA-dependent invasion and migration of tumor cells. Thus p18 may modulate the angiogenic process in the pulmonary microvasculature through signaling of invasive factors such as MMPs. We and others have previously demonstrated that p18 anchors the Rag-GTPase complex to the late endosome/lysosome, facilitating increased activation of p38 and mTOR (6, 30). Both p38 and mTOR activity correlates with proangiogenic signaling (37, 47). VEGF binding to VEGFR2 induces phosphorylation of the MAPKs p38 and ERK1/2 (15) and p70, the downstream substrate of mTOR activity (20, 25). In the present study, p18-mediated endothelial cell migration, adhesion, and tube formation is dependent on its ability to bind the endosome and activity of p38 and mTOR, but not ERK1/2. The role of p18 on enhanced VE-cadherin trafficking and endothelial barrier integrity is independent of mTOR and p38 activity (6). Thus p18 appears to regulate pulmonary vascular function through two differing pathways: signaling from the late endosome and trafficking of the early endosome.
In addition to regulation of receptor recycling, p18- and Rab4-mediated endocytosis may also affect intercellular junctional and cell-extracellular matrix structures, hence impacting the angiogenic and neovascularization potential of the cells. Indeed, we recently demonstrated that p18 regulates VE-cadherin surface expression in lung endothelial cells in response to edemagenic agents (3). Others have shown that tight junction proteins, such as zonal occludin-1 (ZO-1), junctional adhesion molecules, and claudins, affect endothelial function (8, 32, 48). ZO-1 was recently identified to serve to regulate tight junctions via acting on adherens junctions and actin cytoskeleton formation which, in turn, influenced endothelial cell migration and angiogenesis (48). Focal adhesion turnover is also required for endothelial cell migration, angiogenesis, and adhesion. In settings of disrupted endocytosis, where LAMTOR2, LAMTOR3, or dynamin are deleted, the rate of focal adhesion turnover is reduced, resulting in the formation of large endosomal structures, delayed cell migration, and increased accumulation of stress fibers (26, 39). Thus it is possible that p18 and Rab4 may promote the stability or regulation of tight junctions, or affect focal adhesion dynamics, resulting in enhanced endothelial cell function.
It is known that p18 binds to lysosomes and regulates lysosome biogenesis, but p18 also acts as a scaffold for signaling pathways mediated by mTOR and MEK (30, 38). In addition, VEGF induces angiogenesis through a multitude of pathways, including signaling via mTOR and MAPK. Thus it is not surprising that we observed p18-mediated neovascularization which was sensitive to mTOR and p38 inhibitors. However, although p18 appears to function downstream of Rab4, and through mTOR and p38, to regulate endothelial neovascularization, Rab4 itself appears to act through signaling to alternate pathways, other than mTOR or p38. Another possibility is that Rab4 promotes neovascularization primarily by recycling key angiogenic growth factor receptors, integrins, and intercellular junctional proteins hence enhancing endothelial cell function. Further studies are needed to understand which angiogenic factors may be recycled by Rab4.
Taken together, our studies demonstrate a key role for the endosomal recycling proteins p18 and Rab4 in the regulation of vessel formation within the pulmonary vasculature. We also show that VEGF may regulate endothelial angiogenic processes through both p18 and Rab4 association at the endosome. Our data further demonstrates that p18-mediated, but not Rab4-mediated, neovascularization is regulated through both mTOR/p38 signaling. Finally, we provide evidence that Rab4 effects are dependent upon p18 expression. Thus we propose that modulation of p18 and Rab4 may represent novel therapeutic targets to promote vessel formation in settings of disrupted pulmonary angiogenesis.
GRANTS
This material is the result of work supported with resources and the use of facilities at the Providence Veterans Affairs Medical Center. Support for this work was provided by National Heart, Lung, and Blood Institute Grant R01 HL067795, American Heart Association Grant 10GRNT4160055, COBRE National Institute of General Medical Sciences Award 5P20 GM103652, and the University Medicine Foundation of Rhode Island Hospital (to E. Harrington). H. Chichger was supported by American Heart Association Grant 13POST16860031. M. (Myers) Stark was supported by National Heart, Lung, and Blood Institute Grant R25 HL088992 and the Leadership Alliance. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.C. and E.O.H. conception and design of research; H.C., J.B., H.D., and M.S. performed experiments; H.C., J.B., H.D., and M.S. analyzed data; H.C., M.S., and E.O.H. interpreted results of experiments; H.C. and E.O.H. prepared figures; H.C. and E.O.H. drafted manuscript; H.C. and E.O.H. edited and revised manuscript; H.C., J.B., H.D., M.S., and E.O.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Diego Alvarez, University of South Alabama, in aiding the preparation of the in vivo tube formation experiments.
REFERENCES
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol 294: L419–L430, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389–395, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Chamoto K, Gibney BC, Ackermann M, Lee GS, Lin M, Konerding MA, Tsuda A, Mentzer SJ. Alveolar macrophage dynamics in murine lung regeneration. J Cell Physiol 227: 3208–3215, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chichger H, Duong H, Braza J, Harrington EO. p18, a novel adaptor protein, regulates pulmonary endothelial barrier function via enhanced endocytic recycling of VE-cadherin. FASEB J 29: 868–881, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark J, Alvarez DF, Alexeyev M, King JA, Huang L, Yoder MC, Stevens T. Regulatory role for nucleosome assembly protein-1 in the proliferative and vasculogenic phenotype of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 294: L431–L439, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Cooke VG, Naik MU, Naik UP. Fibroblast growth factor-2 failed to induce angiogenesis in junctional adhesion molecule-A-deficient mice. Arterioscler Thromb Vasc Biol 26: 2005–2011, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature 438: 937–945, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA 93: 9559–9564, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leeuw HP, Koster PM, Calafat J, Janssen H, van Zonneveld AJ, van Mourik JA, Voorberg J. Small GTP-binding proteins in human endothelial cells. Br J Haematol 103: 15–19, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16: 209–221, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol 131: 1435–1452, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gampel A, Moss L, Jones MC, Brunton V, Norman JC, Mellor H. VEGF regulates the mobilization of VEGFR2/KDR from an intracellular endothelial storage compartment. Blood 108: 2624–2631, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gong C, Xu C, Ji L, Wang Z. A novel semi-synthetic andrographolide analogue A5 inhibits tumor angiogenesis via blocking the VEGFR2-p38/ERK1/2 signal pathway. Biosci Trends 7: 230–236, 2013. [PubMed] [Google Scholar]
- 16.Guillaumot P, Luquain C, Malek M, Huber AL, Brugiere S, Garin J, Grunwald D, Regnier D, Petrilli V, Lefai E, Manie SN. Pdro, a protein associated with late endosomes and lysosomes and implicated in cellular cholesterol homeostasis. PLoS One 5: e10977, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins N, McLoughlin P. The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis? J Anat 201: 335–348, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino D, Koshikawa N, Seiki M. A p27(kip1)-binding protein, p27RF-Rho, promotes cancer metastasis via activation of RhoA and RhoC. J Biol Chem 286: 3139–3148, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino D, Tomari T, Nagano M, Koshikawa N, Seiki M. A novel protein associated with membrane-type 1 matrix metalloproteinase binds p27(kip1) and regulates RhoA activation, actin remodeling, and matrigel invasion. J Biol Chem 284: 27315–27326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong W, Kim J, Bazer FW, Song G. Stimulatory effect of vascular endothelial growth factor on proliferation and migration of porcine trophectoderm cells and their regulation by the phosphatidylinositol-3-kinase-AKT and mitogen-activated protein kinase cell signaling pathways. Biol Reprod 90: 50, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jin F, Hagemann N, Brockmeier U, Schafer ST, Zechariah A, Hermann DM. LDL attenuates VEGF-induced angiogenesis via mechanisms involving VEGFR2 internalization and degradation following endosome-trans-Golgi network trafficking. Angiogenesis 16: 625–637, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Jones MC, Caswell PT, Moran-Jones K, Roberts M, Barry ST, Gampel A, Mellor H, Norman JC. VEGFR1 (Flt1) regulates Rab4 recycling to control fibronectin polymerization and endothelial vessel branching. Traffic 10: 754–766, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol 18: 549–557, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Jopling HM, Odell AF, Pellet-Many C, Latham AM, Frankel P, Sivaprasadarao A, Walker JH, Zachary IC, Ponnambalam S. Endosome-to-plasma membrane recycling of VEGFR2 receptor tyrosine kinase regulates endothelial function and blood vessel formation. Cells 3: 363–385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BS, Park JY, Kang HJ, Kim HJ, Lee J. Fucoidan/FGF-2 induces angiogenesis through JNK- and p38-mediated activation of AKT/MMP-2 signalling. Biochem Biophys Res Commun 450: 1333–1338, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Lee MY, Skoura A, Park EJ, Landskroner-Eiger S, Jozsef L, Luciano AK, Murata T, Pasula S, Dong Y, Bouaouina M, Calderwood DA, Ferguson SM, De Camilli P, Sessa WC. Dynamin 2 regulation of integrin endocytosis, but not VEGF signaling, is crucial for developmental angiogenesis. Development 141: 1465–1472, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin M, Chamoto K, Gibney BC, Lee GS, Collings-Simpson D, Houdek J, Konerding MA, Tsuda A, Mentzer SJ. Angiogenesis gene expression in murine endothelial cells during post-pneumonectomy lung growth. Respir Res 12: 98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J 12: 677–682, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaffrey MW, Bielli A, Cantalupo G, Mora S, Roberti V, Santillo M, Drummond F, Bucci C. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett 495: 21–30, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nada S, Hondo A, Kasai A, Koike M, Saito K, Uchiyama Y, Okada M. The novel lipid raft adaptor p18 controls endosome dynamics by anchoring the MEK-ERK pathway to late endosomes. EMBO J 28: 477–489, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nannmark U, Johansson BR, Bryant JL, Unger ML, Hokland ME, Goldfarb RH, Basse PH. Microvessel origin and distribution in pulmonary metastases of B16 melanoma: implication for adoptive immunotherapy. Cancer Res 55: 4627–4632, 1995. [PubMed] [Google Scholar]
- 32.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161: 653–660, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okon IS, Coughlan KA, Zou MH. Liver kinase B1 expression promotes phosphatase activity and abrogation of receptor tyrosine kinase phosphorylation in human cancer cells. J Biol Chem 289: 1639–1648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravnic DJ, Konerding MA, Pratt JP, Wolloscheck T, Huss HT, Mentzer SJ. The murine bronchopulmonary microcirculation in hapten-induced inflammation. J Thorac Cardiovasc Surg 133: 97–103, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Risau W. Mechanisms of angiogenesis. Nature 386: 671–674, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 11: 73–91, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15: 2169–2177, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiefermeier N, Scheffler JM, de Araujo ME, Stasyk T, Yordanov T, Ebner HL, Offterdinger M, Munck S, Hess MW, Wickstrom SA, Lange A, Wunderlich W, Fassler R, Teis D, Huber LA. The late endosomal p14-MP1 (LAMTOR2/3) complex regulates focal adhesion dynamics during cell migration. J Cell Biol 205: 525–540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schraufnagel DE. Monocrotaline-induced angiogenesis. Differences in the bronchial and pulmonary vasculature. Am J Pathol 137: 1083–1090, 1990. [PMC free article] [PubMed] [Google Scholar]
- 41.Scott A, Mellor H. VEGF receptor trafficking in angiogenesis. Biochem Soc Trans 37: 1184–1188, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Solodushko V, Fouty B. Proproliferative phenotype of pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 292: L671–L677, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Soma-Nagae T, Nada S, Kitagawa M, Takahashi Y, Mori S, Oneyama C, Okada M. The lysosomal signaling anchor p18/LAMTOR1 controls epidermal development by regulating lysosome-mediated catabolic processes. J Cell Sci 126: 3575–3584, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi Y, Nada S, Mori S, Soma-Nagae T, Oneyama C, Okada M. The late endosome/lysosome-anchored p18-mTORC1 pathway controls terminal maturation of lysosomes. Biochem Biophys Res Commun 417: 1151–1157, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Tang Y, Vater C, Jacobi A, Liebers C, Zou X, Stiehler M. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br J Pharmacol 171: 2440–2456, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 208: 821–838, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S, Toy K, Ingle G, Zlot C, Williams PM, Fuh G, Li B, de Vos A, Gerritsen ME. Vascular endothelial growth factor-induced genes in human umbilical vein endothelial cells: relative roles of KDR and Flt-1 receptors. Arterioscler Thromb Vasc Biol 22: 1797–1803, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Yao W, Firth AL, Sacks RS, Ogawa A, Auger WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA, Jamieson SW, Rubin LJ, Yuan JX. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L870–L878, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, Du W, Qi X, Asanuma K, Sugihara K, Aki S, Miyazawa H, Biswas K, Nagakura C, Ueno M, Iseki S, Schwartz RJ, Okamoto H, Sasaki T, Matsui O, Asano M, Adams RH, Takakura N, Takuwa Y. Endothelial PI3K-C2alpha, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med 18: 1560–1569, 2012. [DOI] [PubMed] [Google Scholar]