Abstract
Lung diseases such as emphysema, interstitial fibrosis, and pulmonary vascular diseases cause significant morbidity and mortality, but despite substantial mechanistic understanding, clinical management options for them are limited, with lung transplantation being implemented at end stages. However, limited donor lung availability, graft rejection, and long-term problems after transplantation are major hurdles to lung transplantation being a panacea. Bioengineering the lung is an exciting and emerging solution that has the ultimate aim of generating lung tissues and organs for transplantation. In this article we capture and review the current state of the art in lung bioengineering, from the multimodal approaches, to creating anatomically appropriate lung scaffolds that can be recellularized to eventually yield functioning, transplant-ready lungs. Strategies for decellularizing mammalian lungs to create scaffolds with native extracellular matrix components vs. de novo generation of scaffolds using biocompatible materials are discussed. Strengths vs. limitations of recellularization using different cell types of various pluripotency such as embryonic, mesenchymal, and induced pluripotent stem cells are highlighted. Current hurdles to guide future research toward achieving the clinical goal of transplantation of a bioengineered lung are discussed.
Keywords: bioengineering, decellularization, pulmonary, tissue engineering, transplantation
lung diseases such as emphysema, chronic obstructive pulmonary disease (COPD), cystic fibrosis, interstitial fibrosis, and pulmonary hypertension cause significant morbidity and mortality worldwide (5). With greater understanding of the mechanisms that underlie these diseases, and development of novel therapeutic approaches, clinical management of these diseases has improved substantially. However, current therapies are not curative and largely rely on preventing exacerbations, blunting symptoms, and slowing progression of pulmonary fibrosis and pulmonary hypertension in particular. Lung disease management relies on the use of multimodal drug therapies, pulmonary and physical rehabilitation, and nontransplant surgical interventions (e.g., lung volume reduction surgery, removal of bullae, placement of stents etc.). Regardless of these advances, the current curative approach for end-stage disease is lung transplantation.
Despite improvements in perioperative management for lung transplantation, a number of limiting issues make the creation of effective alternatives to lung transplantation an urgent, unmet need. By far, the most critical issue is the acute shortage of transplantable donor lungs with unacceptably long wait lists. For example, more than 2,500 patients in the United States alone are on the wait list, with an average wait time of >1 yr (208), and an unfortunately high rate of mortality during the wait period (approximately 15–20 per 100 waitlisted), and deterioration of the recipient's health status in the interim. A separate critical issue is the condition and transplantable status of donor lungs that often suffer edema, atelectasis, or even pneumonia depending on the donor's comorbidities and approaches to preoperative lung handling. Here, long ischemic times that cause lung damage correlate directly with primary graft failure (63). Added to these perioperative issues are reduced outcomes and limited longevity following transplantation due to long-term organ and systemic effects of immune suppression, infections, and problems with graft rejections. Accordingly, effective alternatives to traditional lung transplantation are needed. In addition to whole-lung transplantations, an unmet clinical need exists for strategies to replace large extrapulmonary airways for indications such as tracheal stenosis and tracheobronchial tumors (216). From the vascular perspective, besides artificial grafts, native donor vessels to replace larger pulmonary vessels are also needed (100). Beyond these larger vessels, an obvious need exists to ensure that the structure and function of the blood supply of a transplanted lung matches the functional needs of the organ in terms of ventilation, prevention of edema, and clearance of toxins.
Issues in Lung Bioengineering
There is now a large research and clinical thrust toward engineering of whole organs or their component tissues with a long-term goal of transplanting them into humans. The field of tissue engineering is geared toward developing clinically viable approaches to recreating or enhancing human organs and tissues that recapitulate both the structure and function of the native organ. For example, engineering of replacement skin tissues is now well recognized (68, 187), and there has been substantial progress in other organ systems such as cartilage (45, 54, 107, 159) and bladder (53, 104, 138, 185, 212). With regard to the lung, technologies that mimic lung function such as cardiopulmonary bypass and extracorporeal membrane oxygenation are well developed and routinely used in in-hospital settings, but they suffer from being of short-term, nonambulatory use. Here, despite substantial improvements in the technologies needed for extracorporeal oxygenation and removal of CO2, issues such as coagulation, immunogenicity, and proinflammatory responses limit the long-term use of these technologies. Furthermore, the need for pump-based devices and associated equipment to maintain flow will likely limit miniaturization. In this regard, pumpless technologies with low-resistance diffusion membranes such as the Novalung interventional lung assist device (Novalung, Heilbronn, Germany) are now being explored for use in intensive care settings for removal of CO2 in patients experiencing trauma, infection, and acute respiratory disease syndrome or even as a bridge to lung transplantation (13, 15, 26, 46, 51). However, even though these emerging approaches hold substantial potential for bridging toward transplantation, unlike the increasingly popular ventricular-assist devices that are emerging therapies for treating heart failure, transportable lung-assist devices are still in the research phase (74–76, 188). Thus lung bioengineering is still a nascent field. The questions then become: what is the current status of bioengineered lung tissues, and what are the limitations to bioengineering the lung toward transplantation?
The challenge of cellular heterogeneity.
The native human lung consists of >40 cell types that define its overall structure and functions of maintaining (and appropriately modulating) oxygenation and ventilation; limiting environmental, allergenic, and infectious insults; clearing microbes and toxins; and preventing edema. A progressively branching airway network ends in 200-μm-diameter alveolar sacs that total 70–100 m2 surface area for gas exchange. The airway network intersects a branching pulmonary vascular network to process the entire cardiac output of 5 l/min into capillaries that are separated from alveoli by a thin respiratory membrane. This incredible surface area-to-volume ratio on the one hand is remarkable in terms of gas exchange efficiency, yet it represents a major limitation in the ability to engineer biocompatible materials and recellularization on a large scale that would allow such efficient exchange of oxygen and carbon dioxide. the complex three-dimensional (3-D) anatomical structure of the airways and vasculature accompanied by the regional and functional variations in airway and vascular caliber on the basis of position, oxygen, and ventilation demand and a host of other physiological factors makes bioengineered reproduction of the lung simply challenging. In this regard, some key research areas in lung bioengineering can be identified with the aim of de novo generation of tissues and organs for transplantation where the native organ's structure and function are recapitulated (74, 127, 130, 144, 171, 183, 205). The major themes include 1) the need for scaffolds that mimic lung architecture, allowing for 2) repopularization of such scaffolds with appropriate lung cells that will importantly recapitulate the many aspects of lung function while 3) permitting sufficient vascularization for both maintaining viability of the engineered organ and oxygenation/ventilation, and concurrently 4) minimizing immunogenicity and rejection of the organ.
The challenge of maintaining architecture.
One of the major aspects of lung architecture that make reproduction so difficult is the need for a comparably complex scaffold upon or within which the functional elements of the lung can reside and, importantly, respond to changing demands by maintaining mechanical properties critical for the transplanted lung to function (74, 142, 144, 171, 183, 184, 205). There are now several experimental approaches to generating scaffolds to integrate the airway and vascular structures necessary to make a functional lung, including engineered scaffolds that use synthetic materials and microfluidic networks, and novel fabrication and printing technologies, and as decellularized scaffolds from human or large-animal lungs that use the native extracellular matrix structure for support (Fig. 1). Whether the structural and functional properties of these scaffolds, particularly once recellularized, sufficiently recapitulate those of the native lung is not clear, and represents an area of increasing focus. Some questions within this landscape then become: 1) what components make a good scaffold for recellularization? 2) how do cell-matrix (or more broadly cell-scaffold) interactions influence the organization, structure, and function of cells within the bioengineered lung? and 3) what are the mechanical properties of such scaffolds, and how do they influence the function of the new lung? The current status of research relating to these questions is addressed in the section Materials, Matrix, and Mechanobiology.
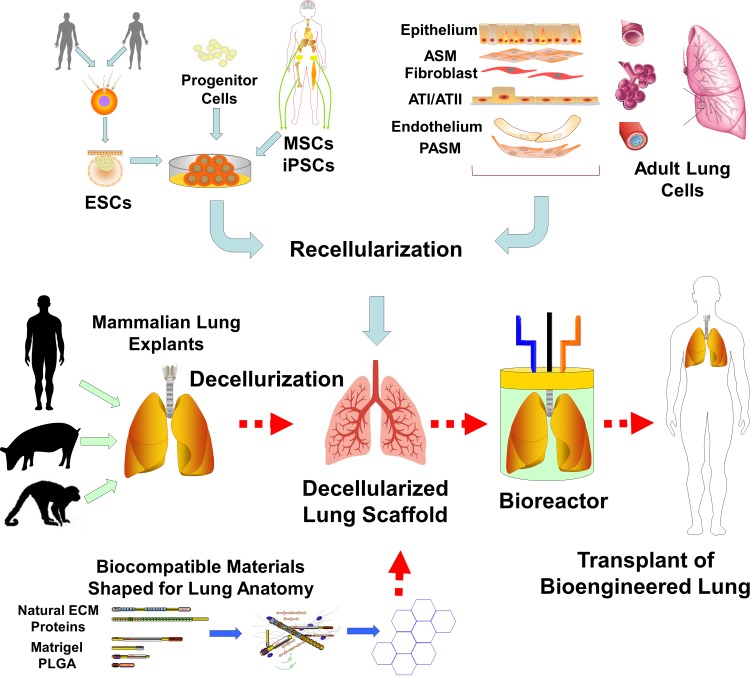
Fig. 1.
Bioengineering the lung. Current thoughts regarding how best to engineer a lung for transplantation in humans involve a 2-pronged approach. The first is to generate an anatomically appropriate lung scaffold, followed by recellularization of the scaffold. One approach would be to chemically and enzymatically decellularize lungs from large mammals, including humans, thus creating a scaffold containing many of the native extracellular matrix (ECM) proteins such as laminin, collagens, and fibronectin in an anatomically appropriate format. The other approach is to use the basic building blocks of the ECM or other biocompatible materials [such as Matrigel and polylactic-coglycolic acid (PLGA)] to generate biocompatible scaffolds that can then be engineered into an anatomical model. Decellularized or acellular scaffolds could then be reseeded with a number of cell types of varying pluripotency such as embryonic stem cells (ESCs), progenitor cells (e.g., those derived from fetal cells), mesenchymal and induced pluripotent stem cells (MSCs and iPSCs, respectively), or even adult lung cells. The idea would be for such cells to eventually entirely occupy the surfaces of the decellularized scaffolds, facilitated by a bioreactor environment with ventilation (mechanical stimuli), perfusion, and additional growth factors and stimuli to generate a functional transplant-ready lung. ASM, airway smooth muscle; PASM, pulmonary artery smooth muscle.
The challenge of recellularization.
As with other bioengineered organs, introduction and management of appropriate cell types to repopulate lung scaffolds represent a separate and evolving challenge as we understand the importance of cell selection; the potential and breadth of their regenerative capacity; their lineage and vintage; the methods for their introduction; the patterns of adherence, growth, migration, and interaction with the local environment including elements of the scaffold itself; their immunogenic potential; their fate; and, importantly, their function. Substantial research has explored the use of autologous primary cells or those with various level of pluripotency [e.g., embryonic stem cells (ESCs), bone marrow-derived and mesenchymal stromal cells (MSCs), and lung-derived progenitor cells, induced pluripotent stem cells (iPSCs)] to seed airway and vascular components in an engineered lung (Fig. 1). In this regard, the current understanding of factors that control stem or progenitor cell adhesion, migration, differentiation, and functionality is still relatively immature. Additionally, an unanswered question is whether mature cells of the lung (or other organs) can be induced to develop pluripotency, or at least regenerative capacity for recellularization. The current status of research, and the potential limitations of the approaches being considered, are explored in the section Recellularization in Lung Bioengineering.
The challenge of mechanobiology.
Rhythmic lung inflation and deflation that accompanies breathing imposes mechanical forces at micro and macro scales that not only tax the cells and matrices of the lung, but also determine and modulate cell growth, survival, and site- and context-appropriate function per se. Similarly, normal blood flow, pulse pressure, and shear forces induced by flowing blood are critical for vascular homeostasis. Added to these passive forces are the active mechanical forces imposed on both airways and vasculature by luminal contraction and relaxation in response to changing demands, and by endogenous and exogenous factors such as local and circulating agonists and antagonists. Although in silico models (79, 83, 99, 132, 148, 196), in vitro and ex vivo systems (59, 92, 103, 108, 118, 176, 193, 202, 203), and whole-organ approaches (94, 143, 205, 206) have been developed to explore the importance of such mechanical forces, further advances in airway, lung parenchyma, and vascular mechanobiology will be critical for bioengineering a lung capable of withstanding the internal and external forces exerted on the transplanted lung within the chest cavity and will be critical to ensuring that lung function occurs without airway collapse, injury, or failure of gas exchange. The challenges to our current understanding of lung mechanobiology and their implications for a bioengineered lung are discussed in Materials, Matrix, and Mechanobiology.
The challenge of vascularization.
Even with appropriate scaffolds and cells to repopulate, one of the major limitations in bioengineering of most organs has been the lack of a vascular system that matches the metabolic and functional needs of the organ being recapitulated. Here, it is critical that a vascular system capable of oxygenating tissue is available and fully functional immediately after transplantation, particularly for the lung. A consistently patent, low-pressure vascular system that mimics the native pulmonary vasculature is needed to respond to blood flow and metabolic demands, and to avoid pulmonary hypertension, right ventricular failure, thromboses, and other complications that are known to occur with allogeneic transplantation. Again, as with the airways, structural integrity in the face of internal and external mechanical forces is important. Thus understanding interactions among mechanical properties of bioengineered vascular structures, hemodynamics, coagulation, and gas exchange is as important as on the airway side of the bioengineered lung. Admittedly, this has been one of the major limitations in lung bioengineering (partly reflecting the complex architecture as well as relationships between airway and vascular function), a topic discussed in Putting It All Together.
The challenge of immunogenicity.
An important consideration (as with allogeneic transplants) is the immunological response of the transplant recipient to the bioengineered lung (102, 131, 189, 190). Regardless of the materials used to engineer a lung, generalized immunosuppression will likely be the initial approach to minimizing rejection, although techniques to enhance acceptance of a bioengineered lung containing specific types of scaffold materials and cell types are obviously a major research focus [e.g., biocompatible materials, novel (ideally personalized) sources for stem cells, and cell differentiation techniques].
Certainly, a multimodal approach involving biocompatible materials and scaffolds, cellularization of implantable structures (especially using stem cells and other cell types with potential for redifferentiation and proliferation), and/or guided regeneration of native tissues forms the basis for the considerable ongoing research and attempts at clinical applications. Such tissue engineering requires synthetic or natural matrices to support and guide cell attachment and growth to eventually recapitulate the native organ's structure and tissue-specific biological functions. However, at the present stage, a bioengineered lung that is ready for transplantation is still a research ideal that faces substantial challenges on many fronts. Nonetheless, the considerable recent and ongoing research in lung bioengineering provides an outstanding platform for understanding basic lung physiology and pathophysiology, for developing and characterizing novel models of lung disease, and for enhancing overall tissue engineering toward transplantation.
Materials, Matrix, and Mechanobiology
A major approach to lung bioengineering has been the use of scaffolds generated by decellularization of whole organs. Decellularization involves removal of native cells from the extracellular matrix (ECM) of tissues to produce a 3-D organ scaffold (7, 10, 28, 37, 57, 70, 125, 171, 184, 205) that ideally retains the 3-D anatomy of the native organ. Whole-organ decellularization using perfusion techniques was initially successfully demonstrated for a whole-heart scaffold (142), and since then for multiple organs including lung, liver, kidney, and pancreas using organs derived from rodents through humans (11, 12, 20, 60, 119, 139–141, 153, 156, 167, 177, 181, 194, 207). Decellularization involves the use of detergents, salts, enzymes, and physical approaches to removing cells while preserving not only ECM composition and organ architecture, but also importantly, ECM bioactivity and mechanics, with <50 ng of dsDNA/mg tissue remaining. A number of decellularization methods have been reported (7, 10, 56, 57, 171, 205), although an optimal decellularization technique is likely organ and context dependent. Given the intricate architecture of the human lung and the importance of the alveolar-capillary interface, this is certainly an advantageous but challenging approach toward developing an elastic scaffold of sufficient surface area for gas transfer.
Decellularization and ECM.
A key aspect of lung decellularization is that native ECM composition must be retained while cellular debris is sufficiently removed. This is a major challenge. For example, several studies have demonstrated that ECM proteins such as collagen, laminin, elastin, and fibronectin can be retained following decellularization, whereas others have reported reduced levels of ECM proteins and important growth factors (1, 27, 152, 162, 217). Here, the protocol for decellularization appears to be important. For example, decellularization of rat lungs using the zwitterionic detergent CHAPS (153) preserves airway and vascular structures with retention of collagen and, to a lesser extent, elastin, whereas glycosaminoglycans (GAGs) are depleted. On the other hand, such matrices can be successfully reseeded with lung epithelial and microvascular endothelial cells and do show gas exchange. In comparison, decellularization with SDS also maintains alveolar and vascular structures and permits recellularization and oxygen exchange (141) and even survival of a rodent transplanted with such a bioengineered lung. However, elastin content is also reduced. On the other hand, decellularization of mouse lungs with Triton X-100 and sodium deoxycholate with DNase (156) allows for retention of collagen, elastin, and GAGs. Furthermore, with this approach, laminin is retained (albeit at a lower level than in the native lung), which would be important for differentiation of alveolar cells following recellularization, and thus eventual barrier function. Conversely, the Triton X-100-based approach found lower levels of GAGs, which may be helpful in reducing adverse immune stimulation, but this remains to be proven. However, the relative advantages of this approach vs. those used by other studies in the overall context of recellularization and eventual function upon transplantation remains to be determined. Such comparisons likely are further driven by the relative roles of different ECM elements in cell attachment, differentiation, migration, immunogenicity, and other aspects of cell-matrix interactions that are in fact not completely understood.
Mechanics and the decellularized lung.
Altered ECM composition and structure affects the strength and mechanics of the remaining scaffold. Decellularization influences matrix stiffness due to both removal of cells and the altered ECM composition, but the effects are not consistent. For example, depending on the method of decellularization, the same organ can show increased tangential modulus and stiffness (142, 218), or minimal to no change (215). Consistently, decellularized lung scaffolds have been evaluated by use of pressure-volume curves (141, 153, 156) and show hysteresis with decreased compliance (increased stiffness), attributed to removal of surfactant and altered ECM (39). However, as mentioned above, the technique of decellularization appears to play a role in the resultant ECM composition.
Modulatory factors in the decellularized scaffold.
Although an important aspect of the ECM is that it provides structural integrity and mechanical stability for the various forces within the lung, it is now clear that the ECM also harbors a number of proteins, enzymes, and growth factors that are important for signaling of epithelia, smooth muscle, nerves, and blood vessels (38, 100, 114, 155). Furthermore, the composition of the ECM changes with age, from early postnatal development through aging. In turn, these ECM components are themselves modulated by diseases such as asthma, COPD, and pulmonary fibrosis (i.e., many of the same diseases that are indications for lung transplantation). Factors such as altered oxygen levels (2, 16, 69, 110, 120, 157, 210, 221, 226), environmental influences (23, 211), and inflammatory and profibrotic mediators (3, 4, 9, 30, 154, 209, 229) can modulate the expression of collagens, fibronectin, elastin. and other important lung ECM components. Indeed, there is now increasing evidence that mechanical forces per se can, on the one hand, influence ECM composition (200), whereas on the other, the ECM influences the cellular properties in the lung (33, 88, 134, 164, 173, 195). Mechanical properties and forces can further modulate cell fate, particularly the differentiation of stem cells on the basis of mechanotransduction (48, 49, 149, 161, 204). Accordingly, the source of the native lung from which a scaffold is derived likely alters the initial conditions under which a lung is subsequently bioengineered. The importance of these initial conditions on the final “product” is still under investigation, given the many permutations and combinations of scaffold materials and cell types being used. Indeed, the relationships between ECM composition and lung cellular properties are themselves being investigated in the context of basic lung biology and alterations in pathophysiological conditions.
At the level of the bronchial epithelium, mechanical forces can be manifest at the luminal level via airflow and ciliary transduction with the additional influence of mucus. However, in addition, mechanical forces can occur through bronchoconstriction as well as the cyclical stretching of the airways that occurs with rhythmic breathing (66). Here, data suggest that mechanical forces can result in activation of the EGF receptor pathway (89) that can then promote airway remodeling: an aspect likely influenced by the ECM (113, 151). Conversely, cyclical stretching of the epithelium can modulate prostanoid synthesis (169). Furthermore, dynamic compressive strain of the epithelial layer that is associated with bronchoconstriction of a circular airway can promote epithelial properties such as solute transport, viral gene delivery (199), and secretion of ECM-modifying proteins such as tissue factor (147). The mechanisms involved in mechanotransduction are still under investigation, but they could include Rho kinase (197), among other factors, which is in turn influenced by the ECM (154).
There is substantial interest in the cell-matrix interactions of airway smooth muscle (ASM) in the context of diseases such as asthma (231) that are likely extensible to the growth of airway cells in a bioengineered or decellularized scaffold. For example, mechanical stretch of human ASM induces TGF-β1 expression through mechanosensitive pathways such as RhoA/ROCK and even PI3/Akt (121). Such effects may be important in the pattern of ASM growth on a decellularized scaffold. Furthermore, molecular pathways such as RhoA are important in maintenance of contractility (96) and may thus play a role beyond recellularization. Here, the matrix stiffness may be relevant. For example, collagen-based hydrogels softer than average airway tissues promote secretion of vascular endothelial growth factor (VEGF) by human ASM cells, whereas stiffer gels stimulate cell proliferation and decrease contractility (i.e., they promote a proliferative cell phenotype) (176). These relationships between extracellular elastic modulus and cellular function are correlated with changes in integrins and other signaling proteins. From a functional standpoint, cyclical forces on the smooth muscle may modulate contractility (65).
Linkages between matrix stiffness and fibroblast properties also appear to be important (103, 108). In human fibroblasts, yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), transcriptional effectors of the Hippo pathway, have been recently reported as key matrix stiffness-regulated coordinators of fibroblast activation and matrix synthesis (103). Here, YAP and TAZ are prominently and preferentially expressed in fibroblasts that populate fibrotic (presumably stiffer) human lungs, but not healthy lung tissue. Both YAP and TAZ are activated when fibroblasts are grown on pathologically stiff matrices but not physiologically compliant matrices. Importantly, suppression of these effectors attenuates matrix synthesis, contraction, and proliferation on pathologically stiff matrices. Together, these results identify YAP and TAZ as mechanoresponsive factors that amplify and sustain fibrosis. Similarly, Rho/ROCK and Mkl1 have been implicated in fibroblast mechanoactivation (77, 179, 233). These emerging albeit limited data show the importance of cell-matrix interactions within the airway wall, which may be important in the context of repopulating a bioengineered scaffold.
Given the importance of mechanical forces of ventilation on the alveoli, understanding mechanotransduction and mechanical forces at the alveolar level is obviously of importance (32, 80, 150, 186, 192, 193), especially if mechanical forces alter ECM generation by alveolar cells. For example, even brief periods of alveolar stretch can induce an inflammatory cascade (227) that can in turn influence alveoli and the pulmonary vasculature. Here, the extent of stretch is likely important (8, 40, 163, 195), and stretch effects may involve a range of pathways including Fas/FasL (93), Rac1 (41), PLA2 (116), GEF-H1 (17), caveolins (222), and Rho kinase (42), with modulating effects of oxygen (78, 164), ROS (31), and inflammation per se (72, 82).
At the vascular level (33, 135, 228), cessation and alterations of blood flow can be mechanosensed by the pulmonary endothelium—an aspect that is highly relevant to conditions such as pulmonary embolism, bypass surgery, and extracorporeal membrane oxygenation, and organ harvest and revascularization in the context of lung transplantation. Cessation of blood flow can be sensed by endothelial caveolae via PECAM-VEGF receptor-VE cadherin, which further leads to cell depolarization, increased intracellular Ca2+, activation of NADPH oxidase, and generation of reactive oxygen and nitrinergic species (33, 224). These cascades alter vascular tone and neovascularization on the one hand, but on the other, they also lead to oxidative injury and/or inflammation and cell death under pathophysiological conditions (33, 224). Although the scaffold will ultimately permit repopulation with a variety of lung cell types to facilitate gas exchange, an important aspect will be endothelial and epithelial barrier function to prevent edema. Here, the role of the ECM per se is not well understood. However, in addition to well-known endothelial barrier signaling pathways, there is recent evidence that the glycocalyx plays a role in barrier functions that may be modulated by mechanical forces (43, 87, 115, 136).
Mechanotransduction can also occur at the level of the vascular smooth muscle; for example, following arterial stiffening in the context of hypertension. Here, the resultant high pulsatility flow can induce endothelial dysfunction (involving decreased eNOS and increased vasoactive mediators and cytokines) that leads to changes in the smooth muscle. In the presence of excitation-contractions, high pulsatility flow results in smooth muscle hypertrophy (and contractile proteins) but not proliferation, whereas mechanical forces on the smooth muscle per se reduce their contractile potential (but do not alter proliferation) (172). Understanding these key events is important not only in the context of allogeneic lung transplantation, but also in the design of vascular scaffolds and networks in the bioengineered lung to ensure proper vascular structure and function.
Although the many elegant studies summarized above have yielded important information on how decellularization techniques influence the resultant scaffold and the potential modulatory factors that influence the resulting scaffold that would be used for eventual recellularization, some fundamental, unanswered questions remain: 1) what is the most effective balance between removal vs. retention of ECM elements in the scaffold in the context of recellularization? 2) perhaps more importantly, how should this balance be determined for eventual recapitulation of lung mechanics and functionality in the recellularized lung? 3) with the assumption that each donor lung (regardless of species) has a somewhat different ECM portfolio that is further modulated by environmental and disease exposure, what is the best approach to individualizing decellularization? and 4) conversely, should decellularization be customized on the basis of the target recipient or the sources from which the cells that will be derived during the recellularization process? Obviously, the answers to these questions lie much further away than the current focus on decellularization, but they will be important to consider for the decellularized lung approach to be eventually useful for a transplantable lung.
Biocompatible Lung Scaffolds
As discussed above, one ideal approach for a bioengineered lung would involve the use of nonimmunogenic, biocompatible materials of appropriate mechanical properties that facilitate recellularization and overall functionality that are key to the transplanted organ. An alternative approach would be degradable biomaterials that facilitate de novo generation, secretion, and deposition of “native” scaffolds by the cells initially introduced into the biomaterial, thus minimizing long-term immunogenicity. Both of these approaches are being explored in the context of lung bioengineering. Several biocompatible and biodegradable scaffolding materials have been explored for the engineering of 3-D lung tissues. Initial and obvious choices are natural ECM proteins such as collagen (29, 71, 145, 146, 191). However, due to its low mechanical properties in loose hydrogel configurations, collagen by itself is not load-bearing unless it is cross-linked or mechanically compressed, which may separately be relevant to the issue of reduced recellularization potential, and reduced flexibility of the scaffold for eventual use. On the other hand, addition of soluble elastin to collagen hydrogels improves the mechanical properties of the resultant scaffold, with further enhancement by seeding with lung fibroblasts that can introduce a variety of ECM proteins that result in stiffness equal to that of a single alveolar wall (≈5 kPa) (44). Such innovative approaches involving a mix of native lung ECM components may provide initial building blocks toward regeneration of new functional lung tissues.
In addition to collagen, Matrigel (101, 123, 124) and other natural polymers (6) have been explored in both in vitro and in vivo studies. Synthetic polymers such as polyglycolic acid (175), polylactic-coglycolic acid (PLGA) (122), and pluronic F-127 are also considered of substantial interest in the context of lung scaffolds (35). An obvious advantage of using such synthetic material is the ability to tailor their chemical and biological properties with additional manipulation to achieve desirable mechanical properties. For example, pluronic-based scaffolds seeded with lung progenitor cells, inject able polymers, and collagen-GAG scaffolds have been used to mimic alveolar structures (6, 34, 35) and allow in vivo implantation (35). However, realization of such approaches toward stable, implantable structures is still investigational. For example, a macroporous matrix from a blend of hydroxyethyl methacrylate-alginate-gelatin has been shown to support human lung epithelial cell proliferation for several weeks and to recruit cells while remaining biocompatible in vivo, but apparently it can also induce infiltration of immune cells (178). Nonetheless, such approaches hold significant potential for reseeding with cells such as alveolar epithelial cells and generating a functioning lung.
In terms of conducting airways, the creation of transplantable trachea has already been demonstrated clinically (106). However, the generation of structurally stable small bronchioles using biocompatible matrices is still under investigation. Here it is increasingly clear that cellular characteristics and responses are likely different in vitro in a 2-D environment as opposed to the 3-D topographies as would exist in vivo. Techniques such as electrospinning are attractive methods for producing 3-D topographies for culturing cells such as ASM because the spun fibers have dimensions that are comparable to those within the ECM of the airway. For example, an electrospun scaffold using nondegradable polyethylene terephthalate has been used to generate the topography of human ASM cells to demonstrate adhesion, alignment, and spindle-shaped morphology, thus generating fully aligned sheets of smooth muscle that demonstrate contractility to bronchoconstriction (126). On the other hand, the idea of initially matrix-free 3-D ASM constructs have also been investigated. For example, microfabricated tissue gauges have been used to develop a 3-D culture model of ASM that is capable of contractility, possesses strong cellular organization, and contains actin stress fibers, particularly with the inclusion of 3T3-fibroblasts (225).
Clearly, compared with the more substantial, albeit preclinical, efforts toward decellularization of mammalian lungs, research on de novo creation of biocompatible lung scaffolds is even more nascent. However, even as this landscape becomes better defined, it would be important to consider two fundamental questions: 1) what is the most appropriate portfolio of ECM and associated proteins required for efficient and effective recellularization? and 2) as with decellularization techniques, how should this portfolio be individualized or customized for recellularization based on cell sources, target recipient, and eventual recapitulation of lung mechanics and functionality?
Recellularization in Lung Bioengineering
Recellularization of a previously cellular, now-decellularized scaffold, or one newly generated using biomaterials involves introducing cells onto and into the matrix; promoting attachment, migration, proliferation; a customization of the ECM environment; and importantly, functionality that mimics the native tissue. A number of studies in different organ systems have now shown that native cells can attach, migrate, and proliferate in decellularized scaffolds (see Refs. 50, 171, and 214 for reviews). For example, transplanted decellularized aortic valves or large-vessel scaffolds can be endothelialized and recellularized by cellular migration upon reseeding. Clinical trials for trachea, bladder, and cardiac valves and vessels have been recently reported (67, 84, 100, 111). However, unlike these simpler organs containing a few cell types in predominance, the complex 3-D structure of the lung and its many functions makes use of decellularized lungs challenging, whereas the large number of cell types with various functions on the other hand also make it a challenge to recellularize in a spatiotemporally and functionally appropriate manner. Nonetheless, decellularized lung scaffolds have been used to understand differentiation and patterning of stem cells and progenitor cells, albeit in small animals. In terms of cell types, the focus to date has been on epithelial cells and endothelialization.
Alveolar epithelial cells.
Given the important functional requirement for a reseeded lung to oxygenate and ventilate, there has been appropriate focus on the alveolar epithelium, which consists of two major types: squamous alveolar epithelial type I (ATI) cells, which occupy a majority of the alveolar surface area in the native lung, and surfactant-producing cuboidal type II (ATII) cells, which occupy a small area but constitute >65% of the epithelial cell population (105). Transformation of ATII cells to ATI cells upon reseeding onto an acellular human alveolar matrix was demonstrated a number of years ago (105). Fetal cells (typically derived from the native organ of interest, and in nonhuman species) have been reported in several studies for tissue bioengineering and seeding of scaffolds (156, 174, 182). An advantage is that fetal cells can retain phenotypic markers as well as their functionality, and, relevant to epithelial and other typically polarized cells, their spatial or compartmental orientation. For example, decellularized rat lung scaffolds seeded with fetal or neonatal rat lung cells are capable of gas exchange (141, 153). Intratracheal seeding of fetal alveolar epithelial cells into decellularized lung (156, 174, 182) has been explored, with the scaffold supporting growth of such fetal ATII cells (156) as evidenced by markers such as pro-SP-C or cytokeratin 18. Thus it appears that the decellularized scaffold retains components necessary to direct the differentiation of cells with proliferative and differentiating capacity. Interestingly, such properties of the scaffold may be tissue-specific in that alveolar progenitor cells seeded onto a liver scaffold do not show lung-specific proteins such as SP-C or aquaporin-5, which are alveolar-type (AT) markers.
It is certainly much easier to acquire adult lung cells (e.g., from surgical resections, transplant recipients, or donor lungs deemed unfit for transplantation), and such adult cells have been explored for their potential for reseeding of scaffolds (alveolar epithelial cells, fibroblasts, and small airway epithelial cells) (131, 137, 194). However, as expected, these adult cells show restricted proliferative capacities and are therefore unlikely to be useful for reseeding large surface areas needed for a bioengineered human lung.
Endothelial cells.
As discussed above, endothelial integrity and function will be key for gas exchange and regulation of lung fluid status, as well as ensuring that sufficient cellular proliferation is maintained to replenish the endothelial barriers. In rat lung scaffolds, use of rat lung microvascular endothelial cell or human umbilical vein endothelial cells (HUVECs) for reseeding the vascular compartment has been shown to result in inadequate and uneven re-endothelialization (141, 153, 182). This can obviously promote thrombosis and is likely insufficient for barrier function.
Embryonic and mesenchymal stem cells.
Stem cells and progenitor cells are of obvious interest on the basis of their ability to expand in culture and differentiate into multiple cell lineages, including ATII, bronchial epithelial, or pulmonary endothelial cells (18, 36, 52, 97, 160). However, the use of stem cells requires extensive knowledge about the spatiotemporal mechanisms underlying lung development, which is relatively well understood in mouse models and to a lesser extent in humans (90, 223), raising the question about the approach to be taken in the use of human-derived stem cells to induce lung cell lineages. Nonetheless, given the cellular and spatial complexity of the lung, it is unlikely that a single lung stem cell is capable of generating all the necessary lineages for a bioengineered lung. Indeed, even in the nonembyronic lung, it is more likely that stem cell or progenitor cell lineages of different regenerative capacities are present in spatially and temporally restricted patterns. A good example is that lung epithelial cells do have the capacity to proliferate and replace lost cells (e.g., during injury caused by environment, mechanical ventilation, etc.) (22, 47). In this regard, adult stem cells are thought to represent a potential cell source for lung repair (14, 24, 81, 106, 109, 130, 133, 168). Furthermore, 3-D scaffolds reseeded with lung progenitors and stem cells, with additional stimuli via growth factors and mechanical forces (simulating those in the native lung) have been shown to induce cellular differentiation and formation of anatomically appropriate lung tissue (122, 166). Human embryonic stem cells (ESCs) seeded onto macaque lung scaffold slices express lung markers (128). Mouse ESC differentiation has been compared between lung scaffolds and hydrogel matrices such as gelfoam, Matrigel, and collagen I (36), and expression of lung markers and scaffold-facilitated cellular differentiation into epithelial and endothelial lineages have been found. However, a problem with ESCs is that the decellularized scaffold may have limited ability to provide all of the necessary cues for differentiation into the range of cell types required. This may be an even greater issue in decellularized scaffolds derived from adult lungs previously exposed to disease or environmental factors that substantially alter the native ECM. On the other hand, predifferentiation of such ESCs can enhance differentiation once seeded onto lung matrix (129). For example, predifferentiation of mouse ESCs into ATII-like cells facilitates their seeding onto decellularized lungs and neovascularization in situ (85). The relationships between ESC differentiation and scaffold properties are still under investigation, and will likely be context-specific in that ESCs or their derivatives may require specific differentiation cues to promote site-specific cell types.
Mesenchymal stem cells.
Mesenchymal stromal, or stem cells (MSCs), are quite appealing in the context of organ engineering on the basis of our current ability to isolate them from peripheral tissues such as bone marrow or the fat of patients, to expand their population, and then redifferentiate them into other cell types in culture. This raises the potential for readministration of autologous MSCs for patient-specific organ engineering (112). However, realization of this potential will be dependent on the ability of MSCs to recapitulate both anatomical and functional features of the different lung cell types. There is now considerable evidence that MSCs play a role in regeneration and repair (61, 62, 81, 91, 112, 201) and in secretion of growth factors, immune modulators, and other factors that support the idea that MSCs may be better taken up in organ scaffolds (158, 198, 220), especially if scaffolds enhance their differentiation (86, 219), presumably through cell-matrix interactions (64, 161, 174, 232). Several studies have shown that MSCs can attach and proliferate in lung scaffolds (39, 117, 131, 137). For example, MSCs derived from human adipose tissue and bone marrow on decellularized rat lungs express markers of pulmonary epithelial cells (117), although the source of the MSCs may play a role in their local behavior such as cell attachment, migration, and proliferation. MSCs within lungs have also been identified that have the ability to express markers of ATI and ATII cells (73, 95, 214). In this regard, a particularly relevant finding is that MSCs may be less finicky toward the scaffold milieu in terms of attachment (19, 21, 170, 180, 213, 217). However, what is not yet established is whether MSCs fully redifferentiate into specific lung cell types within a scaffold and recapitulate the functions of the native cell type.
In addition to ESCs and MSCs, iPSCs have been considered in lung recellularization (55) and repair (14). These cells are generated by reprogramming of somatic cells toward an embryonic state (230) and therefore are highly amenable for the use of patient-derived cells. Recent studies have used iPSCs for lung recellularization and shown that human iPSCs can differentiate to ATII cells that attach and proliferate on decellularized lung matrices while maintaining their cellular phenotype (55, 58).
Immune cells.
An interesting problem that has been scarcely addressed is how to recapitulate the many immune functions of the native lung. Beyond serving as a barrier to the many environmental and biological insults the lung normally faces, lung cells such as the epithelium initiate and modulate immune responses, whereas conversely, the lung hosts a range of immune cells, including dendritic cells, macrophages, and others. Although it is expected that host immune cells would take up residence in an engineered lung and initiate immunity, balancing their effects on maintenance of immune function vs. initiating rejection and dysfunction of the transplanted lung will likely be an important issue to address in the future.
Overall, regardless of the approach taken to providing a scaffold, there is clearly substantial interest and exciting progress in the arena of recellularization techniques utilizing a range of cell types. Whereas the optimal approach to recellularization has yet to be defined, it is indeed possible to ensure attention to some important questions toward realizing a functional recellularized lung: 1) is there a single cell source that will eventually be optimal for repopulating a scaffold with the variety of different cell types needed for functional recapitulation? 2) does the cell source need to be matched to the type of scaffold used (a particularly relevant question if decellularized scaffolds can modulate cellular phenotype and function)? 3) for a scaffold, what are the best routes for recellularization toward functional airways vs. vasculature? and 4) what is best way to balance immunogenicity and host immunity vs. recapitulating the immune function of the native lung?
Putting Things Together
An interesting aspect of recellularization of the lung is that on the one hand a scaffold can be seeded through the airway route, or via the vasculature, but on the other hand limiting cells destined for forming blood vessels to the vasculature rather than to the airway can be a challenge. Several studies have shown that seeding lung cells such as epithelial cells and fibroblasts via the tracheal route and HUVECs via the pulmonary artery allows for reconstitution of alveolar-capillary membranes that can perform gas exchange (25, 141, 153, 156, 182). The ultimate goal is to achieve complete recellularization by distributing cells throughout the branches of the airway and vasculature, which is currently still under investigation. Nonetheless, it is also clear that for clinical success an engineered lung will be dependent on establishing a stable, functional blood supply, especially given that denuded vasculature in scaffolds is highly thrombogenic (11, 140, 165). These issues raise the question of how to best prepare the engineered lung for eventual transplant. Since the first reports of functioning recellularized tissue engineered lungs (141, 153) transplanted into rats showing sustained gas exchange, there has been much excitement regarding the potential of clinically applicable bioengineered lungs. However, the current state of the art in whole-lung bioengineering is admittedly at a proof-of-concept stage, with substantial questions and roadblocks remaining. As highlighted above, issues relating to the type and properties of the lung scaffold and cells for reseeding are particularly important. However, even once these issues are addressed, the question remains as to how to maintain and optimize the engineered lung prior to transplantation. Here, the area of bioreactors has undergone tremendous scientific and technical development, particularly given the incentives for studying lung regenerative strategies in a physiological environment, and for preserving or reconditioning explanted lungs for transplantation. In this regard, bioreactor technology ranges from small-scale apparatuses to study lung cells in vitro and gas exchange, through microfluidic vascular networks mimicking the alveolar-capillary membrane (74–76, 188) to whole-lung bioreactors that facilitate decellularization of the lung by perfusion (thus maintaining vascular structures) followed by recellularization in sterile, closed systems with subsequent ventilation and perfusion (143, 144). However, there are still several technical and logistical hurdles in terms of determining the appropriate parameters for ventilation vs. perfusion based on the scaffolds and cells involved, provision of nutrients, and the eventual functionality of the reseeded lung (143). Here, many of the topics discussed above, such as cell type, scaffold ECM, and mechanical properties, play important roles. Additional issues when it comes to human transplantation will include the need to generate sufficient numbers of appropriate cell types to ensure complete recellularization and inhibited immunogenicity—issues that potentially may be addressable with the use of iPSCs. On the issue of immunogenicity, a particularly challenging roadblock may be the balance between minimizing rejection and resultant failure of the transplanted, bioengineered organ vs. recapitulating and maintaining the inherent immune functions of the lung and its resident cells (or additionally in the context of infectious and environmental exposures).
Conclusion
Although lung bioengineering is admittedly in a nascent stage, there has been significant progress in understanding the important aspects of biocompatible matrices that will allow for reseeding of cells to generate appropriate cell lineages that will adhere, proliferate, migrate, and function in the appropriate locations within the complex anatomy of the lung. Here, use of iPSCs and other cell types may allow for diminished to absent immunogenicity with sufficient cell quantities. Thus, in the foreseeable future, recellularization-based bioengineered lungs may prove to be an effective and alternative clinical therapy for treatment of recalcitrant lung diseases that currently require allogeneic lung transplantation.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grants R01 HL-056470 and HL-088029 to Y. S. Prakash, R01 HL-092961 to D. J. Tschumperlin, and R01 HL-114887 and P01 HL-014985 to K. R. Stenmark.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.S.P., D.J.T., and K.R.S. conception and design of research; Y.S.P., D.J.T., and K.R.S. analyzed data; Y.S.P., D.J.T., and K.R.S. interpreted results of experiments; Y.S.P. prepared figures; Y.S.P. drafted manuscript; Y.S.P., D.J.T., and K.R.S. edited and revised manuscript; D.J.T. and K.R.S. approved final version of manuscript.
REFERENCES
- 1.Akhyari P, Aubin H, Gwanmesia P, Barth M, Hoffmann S, Huelsmann J, Preuss K, Lichtenberg A. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng Part C Methods 17: 915–926, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-β signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol 295: L86–L95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Stanishevsky A, Bulger A, Halloran B, Steele C, Vohra Y, Matalon S. Titanium oxide nanoparticle instillation induces inflammation and inhibits lung development in mice. Am J Physiol Lung Cell Mol Physiol 304: L152–L161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Lung Association. Epidemiology & Statistics (Online). http://www.lungusa.org/finding-cures/our-research/epidemiology-and-statistics-rpts.html. [3 July 2015]
- 6.Andrade CF, Wong AP, Waddell TK, Keshavjee S, Liu M. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol 292: L510–L518, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ. Decellularization for whole organ bioengineering. Biomed Mater 8: 014106, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Arold SP, Bartolak-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 296: L574–L581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HA, Gosens R. β-Catenin signaling is required for TGF-β1-induced extracellular matrix production by airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 301: L956–L965, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13: 27–53, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53: 604–617, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res 173: e11–e25, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Bartosik W, Egan JJ, Wood AE. The Novalung interventional lung assist as bridge to lung transplantation for self-ventilating patients - initial experience. Interact Cardiovasc Thorac Surg 13: 198–200, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Basma H, Gunji Y, Iwasawa S, Nelson A, Farid M, Ikari J, Liu X, Wang X, Michalski J, Smith L, Iqbal J, El Behery R, West W, Yelamanchili S, Rennard D, Holz O, Mueller KC, Magnussen H, Rabe K, Castaldi PJ, Rennard SI. Reprogramming of COPD lung fibroblasts through formation of induced pluripotent stem cells. Am J Physiol Lung Cell Mol Physiol 306: L552–L565, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bein T, Zonies D, Philipp A, Zimmermann M, Osborn EC, Allan PF, Nerlich M, Graf BM, Fang R. Transportable extracorporeal lung support for rescue of severe respiratory failure in combat casualties. J Trauma Acute Care Surg 73: 1450–1456, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 307: L936–L947, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birukova AA, Tian Y, Meliton A, Leff A, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol 302: L965–L975, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop AE, Rippon HJ. Stem cells–potential for repairing damaged lungs and growing human lungs for transplant. Expert Opin Biol Ther 6: 751–758, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Bonenfant NR, Sokocevic D, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, Deng B, Desarno MJ, Ashikaga T, Loi R, Weiss DJ. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials 34: 3231–3245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, Borg ZD, Weiss DJ, Bunnell BA. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A 18: 2437–2452, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 24: 662–670, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Brandsma CA, Timens W, Jonker MR, Rutgers B, Noordhoek JA, Postma DS. Differential effects of fluticasone on extracellular matrix production by airway and parenchymal fibroblasts in severe COPD. Am J Physiol Lung Cell Mol Physiol 305: L582–L589, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Brittan M, Hoogenboom MM, Padfield GJ, Tura O, Fujisawa T, Maclay JD, Macnee W, Mills NL. Endothelial progenitor cells in patients with chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 305: L964–L969, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calle EA, Petersen TH, Niklason LE. Procedure for lung engineering. J Vis Exp 49: 2651, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camboni D, Philipp A, Arlt M, Pfeiffer M, Hilker M, Schmid C. First experience with a paracorporeal artificial lung in humans. ASAIO J 55: 304–306, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Caralt M, Uzarski JS, Iacob S, Obergfell KP, Berg N, Bijonowski BM, Kiefer KM, Ward HH, Wandinger-Ness A, Miller WM, Zhang ZJ, Abecassis MM, Wertheim JA. Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am J Transplant 15: 64–75, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caralt M, Velasco E, Lanas A, Baptista PM. Liver bioengineering: from the stage of liver decellularized matrix to the multiple cellular actors and bioreactor special effects. Organogenesis 10: 250–259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakir J, Page N, Hamid Q, Laviolette M, Boulet LP, Rouabhia M. Bronchial mucosa produced by tissue engineering: a new tool to study cellular interactions in asthma. J Allergy Clin Immunol 107: 36–40, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Al-Alwan L, Audusseau S, Chouiali F, Carlevaro-Fita J, Iwakura Y, Baglole CJ, Eidelman DH, Hamid Q. Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis. Am J Physiol Lung Cell Mol Physiol 306: L132–L143, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L834–L841, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee S, Nieman GF, Christie JD, Fisher AB. Response to letter by Dr. M. S. A. Mohamed (Antagonizing reactive oxygen species during lung perfusion). Am J Physiol Lung Cell Mol Physiol 307: L909, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee S, Nieman GF, Christie JD, Fisher AB. Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol 307: L668–L680, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P, Marsilio E, Goldstein RH, Yannas IV, Spector M. Formation of lung alveolar-like structures in collagen-glycosaminoglycan scaffolds in vitro. Tissue Eng 11: 1436–1448, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Cortiella J, Nichols JE, Kojima K, Bonassar LJ, Dargon P, Roy AK, Vacant MP, Niles JA, Vacanti CA. Tissue-engineered lung: an in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng 12: 1213–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 16: 2565–2580, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 32: 3233–3243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A 18: 1–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davidovich N, Huang J, Margulies SS. Reproducible uniform equibiaxial stretch of precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol 304: L210–L220, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dipaolo BC, Davidovich N, Kazanietz MG, Margulies SS. Rac1 pathway mediates stretch response in pulmonary alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 305: L141–L153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiPaolo BC, Margulies SS. Rho kinase signaling pathways during stretch in primary alveolar epithelia. Am J Physiol Lung Cell Mol Physiol 302: L992–L1002, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dull RO, Cluff M, Kingston J, Hill D, Chen H, Hoehne S, Malleske DT, Kaur R. Lung heparan sulfates modulate Kfc during increased vascular pressure: evidence for glycocalyx-mediated mechanotransduction. Am J Physiol Lung Cell Mol Physiol 302: L816–L828, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunphy SE, Bratt JA, Akram KM, Forsyth NR, El Han AJ. Hydrogels for lung tissue engineering: biomechanical properties of thin collagen-elastin constructs. J Mech Behav Biomed Mater 38: 251–259, 2014. [DOI] [PubMed] [Google Scholar]
- 45.DuRaine GD, Brown WE, Hu JC, Athanasiou KA. Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Ann Biomed Eng 43: 543–554, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A. Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med 35: 945–948, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development 121: 2031–2046, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact 7: 335, 2007. [PubMed] [Google Scholar]
- 50.Figliuzzi M, Remuzzi G, Remuzzi A. Renal bioengineering with scaffolds generated from rat and pig kidneys. Nephron Exp Nephrol 126: 113, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Fischer S, Hoeper MM, Tomaszek S, Simon A, Gottlieb J, Welte T, Haverich A, Strueber M. Bridge to lung transplantation with the extracorporeal membrane ventilator Novalung in the veno-venous mode: the initial Hannover experience. ASAIO J 53: 168–170, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Fishman JM, Lowdell M, Birchall MA. Stem cell-based organ replacements-airway and lung tissue engineering. Semin Pediatr Surg 23: 119–126, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Garriboli M, Radford A, Southgate J. Regenerative medicine in urology. Eur J Pediatr Surg 24: 227–236, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Gaut C, Sugaya K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen Med 10, 665–679, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui L, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123: 4950–4962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem 113: 2217–2222, 2012. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 27: 3675–3683, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Gilpin SE, Ren X, Okamoto T, Guyette JP, Mou H, Rajagopal J, Mathisen DJ, Vacanti JP, Ott HC. Enhanced lung epithelial specification of human induced pluripotent stem cells on decellularized lung matrix. Ann Thorac Surg 98: 1721–1729; discussion 1729, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Godin LM, Vergen J, Prakash YS, Pagano RE, Hubmayr RD. Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. Am J Physiol Lung Cell Mol Physiol 300: L615–L623, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34: 6760–6772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, Dard N, Planes C, Matthay MA, Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 306: L975–L985, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gotts JE, Matthay MA. Mesenchymal stem cells and the stem cell niche: a new chapter. Am J Physiol Lung Cell Mol Physiol 302: L1147–L1149, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Grover FL, Fullerton DA, Zamora MR, Mills C, Ackerman B, Badesch D, Brown JM, Campbell DN, Chetham P, Dhaliwal A, Diercks M, Kinnard T, Niejadlik K, Ochs M. The past, present, and future of lung transplantation. Am J Surg 173: 523–533, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5: 17–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunst SJ. Contractile force of canine airway smooth muscle during cyclical length changes. J Appl Physiol 55: 759–769, 1983. [DOI] [PubMed] [Google Scholar]
- 66.Gunst SJ. Does airway inflation stretch the bronchial mucosal membrane? J Appl Physiol 99: 2059–2060, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Haider H, Lei Y, Ashraf M. MyoCell, a cell-based, autologous skeletal myoblast therapy for the treatment of cardiovascular diseases. Curr Opin Mol Therapeut 10: 611–621, 2008. [PMC free article] [PubMed] [Google Scholar]
- 68.Hall CW, Liotta D, De Bakey ME. Artificial skin. Trans Am Soc Artif Intern Organs 12: 340–345, 1966. [PubMed] [Google Scholar]
- 69.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 303: L711–L719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He M, Callanan A. Comparison of methods for whole-organ decellularization in tissue engineering of bioartificial organs. Tissue Eng Part B Rev 19: 194–208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest 84: 736–752, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Hillman NH, Polglase GR, Pillow JJ, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol 300: L232–L241, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffman AM, Paxson JA, Mazan MR, Davis AM, Tyagi S, Murthy S, Ingenito EP. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells Dev 20: 1779–1792, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoganson DM, Bassett EK, Vacanti JP. Lung tissue engineering. Front Biosci (Landmark Ed) 19: 1227–1239, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Hoganson DM, Pryor HI 2nd, Bassett EK, Spool ID, Vacanti JP. Lung assist device technology with physiologic blood flow developed on a tissue engineered scaffold platform. Lab Chip 11: 700–707, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Hoganson DM, Pryor HI 2nd, Vacanti JP. Tissue engineering and organ structure: a vascularized approach to liver and lung. Pediatr Res 63: 520–526, 2008. [DOI] [PubMed] [Google Scholar]
- 77.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hubmayr RD. Does oxygen tune cellular mechanotransduction? Am J Physiol Lung Cell Mol Physiol 302: L1233–L1234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huh DD. A human breathing lung-on-a-chip. Ann Am Thorac Soc 12, Suppl 1: S42–S44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hussein O, Walters B, Stroetz R, Valencia P, McCall D, Hubmayr RD. Biophysical determinants of alveolar epithelial plasma membrane wounding associated with mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 305: L478–L484, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 303: L967–L977, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ito Y, Correll K, Schiel JA, Finigan JH, Prekeris R, Mason RJ. Lung fibroblasts accelerate wound closure in human alveolar epithelial cells through hepatocyte growth factor/c-Met signaling. Am J Physiol Lung Cell Mol Physiol 307: L94–L105, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jardine B, Bassingthwaighte JB. Modeling serotonin uptake in the lung shows endothelial transporters dominate over cleft permeation. Am J Physiol Lung Cell Mol Physiol 305: L42–L55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jayo MJ, Jain D, Ludlow JW, Payne R, Wagner BJ, McLorie G, Bertram TA. Long-term durability, tissue regeneration and neo-organ growth during skeletal maturation with a neo-bladder augmentation construct. Regen Med 3: 671–682, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, Jaworski DM, Hatton C, Weiss DJ, Finck C. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods 18: 632–646, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang WC, Cheng YH, Yen MH, Chang Y, Yang VW, Lee OK. Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomaterials 35: 3607–3617, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Job KM, Dull RO, Hlady V. Use of reflectance interference contrast microscopy to characterize the endothelial glycocalyx stiffness. Am J Physiol Lung Cell Mol Physiol 302: L1242–L1249, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM, Rennard SI. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol 306: L1006–L1015, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kojic N, Chung E, Kho AT, Park JA, Huang A, So PT, Tschumperlin DJ. An EGFR autocrine loop encodes a slow-reacting but dominant mode of mechanotransduction in a polarized epithelium. FASEB J 24: 1604–1615, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 20: 822–832, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302: L1003–L1013, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 300: C146–C154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kroon AA, Delriccio V, Tseu I, Kavanagh BP, Post M. Mechanical ventilation-induced apoptosis in newborn rat lung is mediated via FasL/Fas pathway. Am J Physiol Lung Cell Mol Physiol 305: L795–L804, 2013. [DOI] [PubMed] [Google Scholar]
- 94.Kuebler WM. Real-time imaging assessment of pulmonary vascular responses. Proc Am Thorac Soc 8: 458–465, 2011. [DOI] [PubMed] [Google Scholar]
- 95.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117: 989–996, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lan B, Deng L, Donovan GM, Chin LY, Syyong HT, Wang L, Zhang J, Pascoe CD, Norris BA, Liu JC, Swyngedouw NE, Banaem SM, Pare PD, Seow CY. Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L1–L10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lane S, Rippon HJ, Bishop AE. Stem cells in lung repair and regeneration. Regen Med 2: 407–415, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol 301: L137–L147, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Levy R, Hill DB, Forest MG, Grotberg JB. Pulmonary fluid flow challenges for experimental and mathematical modeling. Integr Comp Biol 54: 985–1000, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.L'Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts–from research to clinical practice. Nat Clin Pract Cardiovasc Med 4: 389–395, 2007. [DOI] [PubMed] [Google Scholar]
- 101.Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Material Res Part A 79: 963–973, 2006. [DOI] [PubMed] [Google Scholar]
- 102.Lim ML, Jungebluth P, Ajalloueian F, Friedrich LH, Gilevich I, Grinnemo KH, Gubareva E, Haag JC, Lemon G, Sjoqvist S, Caplan AL, Macchiarini P. Whole organ and tissue reconstruction in thoracic regenerative surgery. Mayo Clin Proc 88: 1151–1166, 2013. [DOI] [PubMed] [Google Scholar]
- 103.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu T, Li Y, Chen T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int J Nanomedicine 8: 337–350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lwebuga-Mukasa JS, Ingbar DH, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res 162: 423–435, 1986. [DOI] [PubMed] [Google Scholar]
- 106.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet 372: 2023–2030, 2008. [DOI] [PubMed] [Google Scholar]
- 107.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11: 21–34, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marinkovic A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 303: L169–L180, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin U. Methods for studying stem cells: adult stem cells for lung repair. Methods 45: 121–132, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin YN, Manlove L, Dong J, Carey WA, Thompson MA, Pabelick CM, Pandya HC, Martin RJ, Wigle DA, Prakash YS. Hyperoxia-induced changes in estradiol metabolism in postnatal airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L141–L146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373: 1440–1446, 2009. [DOI] [PubMed] [Google Scholar]
- 112.McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, Fang X, Matthay MA, Lee JW. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol 306: L809–L815, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McGovern T, Risse PA, Tsuchiya K, Hassan M, Frigola G, Martin JG. LTD4 induces HB-EGF-dependent CXCL8 release through EGFR activation in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 299: L808–L815, 2010. [DOI] [PubMed] [Google Scholar]
- 114.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L709–L721, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mehta D, Ravindran K, Kuebler WM. Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 307: L924–L935, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meliton AY, Meng F, Tian Y, Sarich N, Mutlu GM, Birukova AA, Birukov KG. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am J Physiol Lung Cell Mol Physiol 308: L550–L562, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE. Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng Part A 20: 1735–1746, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mih JD, Sharif AS, Liu F, Marinkovic A, Symer MM, Tschumperlin DJ. A multiwell platform for studying stiffness-dependent cell biology. PLoS One 6: e19929, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Farney AC, Stratta RJ, Atala A, Opara EC, Soker S. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials 34: 5488–5495, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mizikova I, Ruiz-Camp J, Steenbock H, Madurga A, Vadasz I, Herold S, Mayer K, Seeger W, Brinckmann J, Morty RE. Collagen and elastin cross-linking is altered during aberrant late lung development associated with hyperoxia. Am J Physiol Lung Cell Mol Physiol 308: L1145–L1158, 2015. [DOI] [PubMed] [Google Scholar]
- 121.Mohamed JS, Boriek AM. Stretch augments TGF-β1 expression through RhoA/ROCK1/2, PTK, and PI3K in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 299: L413–L424, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI, Finck CM. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng 12: 717–728, 2006. [DOI] [PubMed] [Google Scholar]
- 123.Mondrinos MJ, Koutzaki S, Lelkes PI, Finck CM. A tissue-engineered model of fetal distal lung tissue. Am J Physiol Lung Cell Mol Physiol 293: L639–L650, 2007. [DOI] [PubMed] [Google Scholar]
- 124.Mondrinos MJ, Koutzaki SH, Poblete HM, Crisanti MC, Lelkes PI, Finck CM. In vivo pulmonary tissue engineering: contribution of donor-derived endothelial cells to construct vascularization. Tissue Eng Part A 14: 361–368, 2008. [DOI] [PubMed] [Google Scholar]
- 125.Moroni F, Mirabella T. Decellularized matrices for cardiovascular tissue engineering. Am J Stem Cells 3: 1–20, 2014. [PMC free article] [PubMed] [Google Scholar]
- 126.Morris GE, Bridge JC, Eltboli OM, Lewis MP, Knox AJ, Aylott JW, Brightling CE, Ghaemmaghami AM, Rose FR. Human airway smooth muscle maintain in situ cell orientation and phenotype when cultured on aligned electrospun scaffolds. Am J Physiol Lung Cell Mol Physiol 307: L38–L47, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Naderi H, Matin MM, Bahrami AR. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomaterial Appl 26: 383–417, 2011. [DOI] [PubMed] [Google Scholar]
- 128.Nakayama KH, Lee CC, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One 8: e64134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ng SL, Narayanan K, Gao S, Wan AC. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials 32: 7571–7580, 2011. [DOI] [PubMed] [Google Scholar]
- 130.Nichols JE, Cortiella J. Engineering of a complex organ: progress toward development of a tissue-engineered lung. Proc Am Thorac Soc 5: 723–730, 2008. [DOI] [PubMed] [Google Scholar]
- 131.Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S, Ogadegbe M, Mlcak R, Deyo D, Woodson L, McQuitty C, Lick S, Beckles D, Melo E, Cortiella J. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A 19: 2045–2062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nichols JE, Niles JA, Vega SP, Argueta LB, Eastaway A, Cortiella J. Modeling the lung: design and development of tissue engineered macro- and micro-physiologic lung models for research use. Exp Biol Med (Maywood) 239: 1135–1169, 2014. [DOI] [PubMed] [Google Scholar]
- 133.Nishimura R, Nishiwaki T, Kawasaki T, Sekine A, Suda R, Urushibara T, Suzuki T, Takayanagi S, Terada J, Sakao S, Tatsumi K. Hypoxia-induced proliferation of tissue-resident endothelial progenitor cells in the lung. Am J Physiol Lung Cell Mol Physiol 308: L746–L758, 2015. [DOI] [PubMed] [Google Scholar]
- 134.Noble PB, Pascoe CD, Lan B, Ito S, Kistemaker LE, Tatler AL, Pera T, Brook BS, Gosens R, West AR. Airway smooth muscle in asthma: linking contraction and mechanotransduction to disease pathogenesis and remodelling. Pulm Pharmacol Ther 29: 96–107, 2014. [DOI] [PubMed] [Google Scholar]
- 135.Noel J, Wang H, Hong N, Tao JQ, Yu K, Sorokina EM, Debolt K, Heayn M, Rizzo V, Delisser H, Fisher AB, Chatterjee S. PECAM-1 and caveolae form the mechanosensing complex necessary for NOX2 activation and angiogenic signaling with stopped flow in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 305: L805–L818, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O'Callaghan R, Job KM, Dull RO, Hlady V. Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. Am J Physiol Lung Cell Mol Physiol 301: L353–L360, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O'Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, Singh G, Freytes DO, Bacchetta MD, Sonett JR, Vunjak-Novakovic G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg 96: 1046–1055, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Orabi H, Bouhout S, Morissette A, Rousseau A, Chabaud S, Bolduc S. Tissue engineering of urinary bladder and urethra: advances from bench to patients. Sci World J Dec 24: 154564, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Orlando G, Booth C, Wang Z, Totonelli G, Ross CL, Moran E, Salvatori M, Maghsoudlou P, Turmaine M, Delario G, Al-Shraideh Y, Farooq U, Farney AC, Rogers J, Iskandar SS, Burns A, Marini FC, De Coppi P, Stratta RJ, Soker S. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials 34: 5915–5925, 2013. [DOI] [PubMed] [Google Scholar]
- 140.Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, AbouShwareb T, De Coppi P, Wood KJ, Stratta RJ, Atala A, Yoo JJ, Soker S. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg 256: 363–370, 2012. [DOI] [PubMed] [Google Scholar]
- 141.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16: 927–933, 2010. [DOI] [PubMed] [Google Scholar]
- 142.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 14: 213–221, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Panoskaltsis-Mortari A. Bioreactor development for lung tissue engineering. Curr Transplant Rep 2: 90–97, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Panoskaltsis-Mortari A, Weiss DJ. Breathing new life into lung transplantation therapy. Mol Ther 18: 1581–1583, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paquette JS, Moulin V, Tremblay P, Bernier V, Boutet M, Laviolette M, Auger FA, Boulet LP, Goulet F. Tissue-engineered human asthmatic bronchial equivalents. Eur Cell Material 7: 1–11; discussion 11-11, 2004. [DOI] [PubMed] [Google Scholar]
- 146.Paquette JS, Tremblay P, Bernier V, Auger FA, Laviolette M, Germain L, Boutet M, Boulet LP, Goulet F. Production of tissue-engineered three-dimensional human bronchial models. In vitro Cell Dev Biol Anim 39: 213–220, 2003. [DOI] [PubMed] [Google Scholar]
- 147.Park JA, Sharif AS, Shiomi T, Kobzik L, Kasahara DI, Tschumperlin DJ, Voynow J, Drazen JM. Human neutrophil elastase-mediated goblet cell metaplasia is attenuated in TACE-deficient mice. Am J Physiol Lung Cell Mol Physiol 304: L701–L707, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patel B, Gauvin R, Absar S, Gupta V, Gupta N, Nahar K, Khademhosseini A, Ahsan F. Computational and bioengineered lungs as alternatives to whole animal, isolated organ, and cell-based lung models. Am J Physiol Lung Cell Mol Physiol 303: L733–L747, 2012. [DOI] [PubMed] [Google Scholar]
- 149.Pennesi G, Scaglione S, Giannoni P, Quarto R. Regulatory influence of scaffolds on cell behavior: how cells decode biomaterials. Curr Pharm Biotechnol 12: 151–159, 2011. [DOI] [PubMed] [Google Scholar]
- 150.Perlman CE, Wu Y. In situ determination of alveolar septal strain, stress and effective Young's modulus: an experimental/computational approach. Am J Physiol Lung Cell Mol Physiol 307: L302–L310, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Perng DW, Chang KT, Su KC, Wu YC, Chen CS, Hsu WH, Tsai CM, Lee YC. Matrix metalloprotease-9 induces transforming growth factor-beta(1) production in airway epithelium via activation of epidermal growth factor receptors. Life Sci 89: 204–212, 2011. [DOI] [PubMed] [Google Scholar]
- 152.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs 195: 222–231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science 329: 538–541, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Possa SS, Charafeddine HT, Righetti RF, da Silva PA, Almeida-Reis R, Saraiva-Romanholo BM, Perini A, Prado CM, Leick-Maldonado EA, Martins MA, Tibério Ide F. Rho-kinase inhibition attenuates airway responsiveness, inflammation, matrix remodeling, and oxidative stress activation induced by chronic inflammation. Am J Physiol Lung Cell Mol Physiol 303: L939–L952, 2012. [DOI] [PubMed] [Google Scholar]
- 155.Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol 305: L912–L933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A 16: 2581–2591, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qian H, Yang H, Xu W, Yan Y, Chen Q, Zhu W, Cao H, Yin Q, Zhou H, Mao F, Chen Y. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med 22: 325–332, 2008. [PubMed] [Google Scholar]
- 159.Qu D, Mosher CZ, Boushell MK, Lu HH. Engineering complex orthopaedic tissues via strategic biomimicry. Ann Biomed Eng 43: 697–717, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Quan Y, Wang D. Clinical potentials of human pluripotent stem cells in lung diseases. Clin Transl Med 3: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 43: 55–62, 2010. [DOI] [PubMed] [Google Scholar]
- 162.Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, Yuan X, Ding Y. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int 33: 448–458, 2013. [DOI] [PubMed] [Google Scholar]
- 163.Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Lung Cell Mol Physiol 301: L625–L635, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roan E, Wilhelm K, Bada A, Makena PS, Gorantla VK, Sinclair SE, Waters CM. Hyperoxia alters the mechanical properties of alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L1235–L1241, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS One 9: e90406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roomans GM. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur Cell Mater 19: 284–299, 2010. [DOI] [PubMed] [Google Scholar]
- 167.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 20: 2338–2347, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sato T, Nakamura T. Tissue-engineered airway replacement. Lancet 372: 2003–2004, 2008. [DOI] [PubMed] [Google Scholar]
- 169.Savla U, Sporn PH, Waters CM. Cyclic stretch of airway epithelium inhibits prostanoid synthesis. Am J Physiol Lung Cell Mol Physiol 273: L1013–L1019, 1997. [DOI] [PubMed] [Google Scholar]
- 170.Scarritt ME, Bonvillain RW, Burkett BJ, Wang G, Glotser EY, Zhang Q, Sammarco MC, Betancourt AM, Sullivan DE, Bunnell BA. Hypertensive rat lungs retain hallmarks of vascular disease upon decellularization but support the growth of mesenchymal stem cells. Tissue Eng Part A 20: 1426–1443, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotech 3: 43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Scott D, Tan Y, Shandas R, Stenmark KR, Tan W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol Lung Cell Mol Physiol 304: L70–L81, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 173.Setlakwe EL, Lemos KR, Lavoie-Lamoureux A, Duguay JD, Lavoie JP. Airway collagen and elastic fiber content correlates with lung function in equine heaves. Am J Physiol Lung Cell Mol Physiol 307: L252–L260, 2014. [DOI] [PubMed] [Google Scholar]
- 174.Shamis Y, Hasson E, Soroker A, Bassat E, Shimoni Y, Ziv T, Sionov RV, Mitrani E. Organ-specific scaffolds for in vitro expansion, differentiation, and organization of primary lung cells. Tissue Eng Part C Methods 17: 861–870, 2011. [DOI] [PubMed] [Google Scholar]
- 175.Shigemura N, Okumura M, Mizuno S, Imanishi Y, Matsuyama A, Shiono H, Nakamura T, Sawa Y. Lung tissue engineering technique with adipose stromal cells improves surgical outcome for pulmonary emphysema. Am J Respir Crit Care Med 174: 1199–1205, 2006. [DOI] [PubMed] [Google Scholar]
- 176.Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness-modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 308: L1125–L1135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shupe T, Williams M, Brown A, Willenberg B, Petersen BE. Method for the decellularization of intact rat liver. Organogenesis 6: 134–136, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Singh D, Zo SM, Kumar A, Han SS. Engineering three-dimensional macroporous hydroxyethyl methacrylate-alginate-gelatin cryogel for growth and proliferation of lung epithelial cells. J Biomater Sci Polym Ed 24: 1343–1359, 2013. [DOI] [PubMed] [Google Scholar]
- 179.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, Kim KK, Keshamouni VG, White ES, Zhou Y, Higgins PD, Larsen SD, Neubig RR, Horowitz JC. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol 185: 969–986, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sokocevic D, Bonenfant NR, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, Deng B, Desarno MJ, Ashikaga T, Loi R, Hoffman AM, Weiss DJ. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 34: 3256–3269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19: 646–651, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, Ott HC. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg 92: 998–1005, 2011. [DOI] [PubMed] [Google Scholar]
- 183.Song JJ, Ott HC. Bioartificial lung engineering. Am J Transplant 12: 283–288, 2011. [DOI] [PubMed] [Google Scholar]
- 184.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med 17: 424–432, 2011. [DOI] [PubMed] [Google Scholar]
- 185.Song L, Murphy SV, Yang B, Xu Y, Zhang Y, Atala A. Bladder acellular matrix and its application in bladder augmentation. Tissue Eng Part B Rev 20: 163–172, 2014. [DOI] [PubMed] [Google Scholar]
- 186.Spieth PM, Bluth T, Gama De Abreu M, Bacelis A, Goetz AE, Kiefmann R. Mechanotransduction in the lungs. Minerva Anestesiol 80: 933–941, 2014. [PubMed] [Google Scholar]
- 187.Spira M, Fissette J, Hall W, Hardy SB, Gerow FJ. Evaluation of synthetic fabrics as artificial skin grafts to experimental burn wounds. J Biomed Mater Res 3: 213–234, 1969. [DOI] [PubMed] [Google Scholar]
- 188.Sreenivasan R, Bassett EK, Hoganson DM, Vacanti JP, Gleason KK. Ultra-thin, gas permeable free-standing and composite membranes for microfluidic lung assist devices. Biomaterials 32: 3883–3889, 2011. [DOI] [PubMed] [Google Scholar]
- 189.Sueblinvong V, Weiss DJ. Cell therapy approaches for lung diseases: current status. Curr Opin Pharmacol 9: 268–273, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sueblinvong V, Weiss DJ. Stem cells and cell therapy approaches in lung biology and diseases. Transl Res 156: 188–205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sugihara H, Toda S, Miyabara S, Fujiyama C, Yonemitsu N. Reconstruction of alveolus-like structure from alveolar type II epithelial cells in three-dimensional collagen gel matrix culture. Am J Pathol 142: 783–792, 1993. [PMC free article] [PubMed] [Google Scholar]
- 192.Suki B. Assessing the functional mechanical properties of bioengineered organs with emphasis on the lung. J Cell Physiol 229: 1134–1140, 2014. [DOI] [PubMed] [Google Scholar]
- 193.Suki B, Bates JH. Lung tissue mechanics as an emergent phenomenon. J Appl Physiol 110: 1111–1118, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, Yoo JJ. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33: 7756–7764, 2012. [DOI] [PubMed] [Google Scholar]
- 195.Szabari MV, Parameswaran H, Sato S, Hantos Z, Bartolak-Suki E, Suki B. Acute mechanical forces cause deterioration in lung structure and function in elastase-induced emphysema. Am J Physiol Lung Cell Mol Physiol 303: L567–L574, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tawhai M, Clark A, Donovan G, Burrowes K. Computational modeling of airway and pulmonary vascular structure and function: development of a “lung physiome.” Crit Rev Biomed Eng 39: 319–336, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thomas RA, Norman JC, Huynh TT, Williams B, Bolton SJ, Wardlaw AJ. Mechanical stretch has contrasting effects on mediator release from bronchial epithelial cells, with a rho-kinase-dependent component to the mechanotransduction pathway. Respir Med 100: 1588–1597, 2006. [DOI] [PubMed] [Google Scholar]
- 198.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105: 93–98, 2002. [DOI] [PubMed] [Google Scholar]
- 199.Tomei AA, Choe MM, Swartz MA. Effects of dynamic compression on lentiviral transduction in an in vitro airway wall model. Am J Physiol Lung Cell Mol Physiol 294: L79–L86, 2008. [DOI] [PubMed] [Google Scholar]
- 200.Toumpanakis D, Noussia O, Sigala I, Litsiou E, Loverdos K, Zacharatos P, Karavana V, Michailidou T, Magkou C, Zhou Z, Theocharis S, Vassilakopoulos T. Inspiratory resistive breathing induces MMP-9 and MMP-12 expression in the lung. Am J Physiol Lung Cell Mol Physiol 308: L683–L692, 2015. [DOI] [PubMed] [Google Scholar]
- 201.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Alex Mitsialis S, Kourembanas S, Kim CF. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302: L829–L837, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tschumperlin DJ. Physical forces and airway remodeling in asthma. N Engl J Med 364: 2058–2059, 2011. [DOI] [PubMed] [Google Scholar]
- 203.Tschumperlin DJ, Boudreault F, Liu F. Recent advances and new opportunities in lung mechanobiology. J Biomech 43: 99–107, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One 6: e15978, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsuchiya T, Sivarapatna A, Rocco K, Nanashima A, Nagayasu T, Niklason LE. Future prospects for tissue engineered lung transplantation: decellularization and recellularization-based whole lung regeneration. Organogenesis 10: 196–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Uriarte JJ, Nonaka PN, Campillo N, Palma RK, Melo E, de Oliveira LV, Navajas D, Farre R. Mechanical properties of acellular mouse lungs after sterilization by gamma irradiation. J Mech Behav Biomed Mater 40: 168–177, 2014. [DOI] [PubMed] [Google Scholar]
- 207.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16: 814–820, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Valapour M, Paulson K, Smith JM, Hertz MI, Skeans MA, Heubner BM, Edwards LB, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2011 Annual Data Report: lung. Am J Transplant 13, Suppl 1: 149–177, 2013. [DOI] [PubMed] [Google Scholar]
- 209.Van Ly D, Burgess JK, Brock TG, Lee TH, Black JL, Oliver BG. Prostaglandins but not leukotrienes alter extracellular matrix protein deposition and cytokine release in primary human airway smooth muscle cells and fibroblasts. Am J Physiol Lung Cell Mol Physiol 303: L239–L250, 2012. [DOI] [PubMed] [Google Scholar]
- 210.Vogel ER, Britt RD Jr, Trinidad MC, Faksh A, Martin RJ, MacFarlane PM, Pabelick CM, Prakash YS. Perinatal oxygen in the developing lung. Can J Physiol Pharmacol 93: 119–127, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vogel ER, VanOosten SK, Holman MA, Hohbein DD, Thompson MA, Vassallo R, Pandya HC, Prakash YS, Pabelick CM. Cigarette smoke enhances proliferation and extracellular matrix deposition by human fetal airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L978–L986, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vrana NE, Lavalle P, Dokmeci MR, Dehghani F, Ghaemmaghami AM, Khademhosseini A. Engineering functional epithelium for regenerative medicine and in vitro organ models: a review. Tissue Eng Part B Rev 19: 529–543, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, Lathrop MJ, Wallis JD, Daly AB, Lam YW, Deng B, DeSarno MJ, Ashikaga T, Loi R, Weiss DJ. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35: 3281–3297, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, Hoffman AM, Weiss DJ. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18: 895–911, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods 16: 525–532, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Walles T. Tracheobronchial bio-engineering: biotechnology fulfilling unmet medical needs. Adv Drug Deliv Res 63: 367–374, 2011. [DOI] [PubMed] [Google Scholar]
- 217.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 18: 420–432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang B, Borazjani A, Tahai M, Curry AL, Simionescu DT, Guan J, To F, Elder SH, Liao J. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res A 94: 1100–1110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang B, Wang G, To F, Butler JR, Claude A, McLaughlin RM, Williams LN, de Jongh Curry AL, Liao J. Myocardial scaffold-based cardiac tissue engineering: application of coordinated mechanical and electrical stimulations. Langmuir 29: 11109–11117, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA 102: 186–191, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang H, Jafri A, Martin RJ, Nnanabu J, Farver C, Prakash YS, MacFarlane PM. Severity of neonatal hyperoxia determines structural and functional changes in developing mouse airway. Am J Physiol Lung Cell Mol Physiol 307: L295–L301, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang Y, Maciejewski BS, Drouillard D, Santos M, Hokenson MA, Hawwa RL, Huang Z, Sanchez-Esteban J. A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 298: L775–L783, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wedgwood S, Lakshminrusimha S, Schumacker PT, Steinhorn RH. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 309: L196–L203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.West AR, Zaman N, Cole DJ, Walker MJ, Legant WR, Boudou T, Chen CS, Favreau JT, Gaudette GR, Cowley EA, Maksym GN. Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 304: L4–L16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Witsch TJ, Turowski P, Sakkas E, Niess G, Becker S, Herold S, Mayer K, Vadasz I, Roberts JD Jr, Seeger W, Morty RE. Deregulation of the lysyl hydroxylase matrix cross-linking system in experimental and clinical bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 306: L246–L259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Woods SJ, Waite AA, O'Dea KP, Halford P, Takata M, Wilson MR. Kinetic profiling of in vivo lung cellular inflammatory responses to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 308: L912–L921, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys 56: 1–18, 2010. [DOI] [PubMed] [Google Scholar]
- 229.Yoshida S, Minematsu N, Chubachi S, Nakamura H, Miyazaki M, Tsuduki K, Takahashi S, Miyasho T, Iwabuchi T, Takamiya R, Tateno H, Mouded M, Shapiro SD, Asano K, Betsuyaku T. Annexin V decreases PS-mediated macrophage efferocytosis and deteriorates elastase-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol 303: L852–L860, 2012. [DOI] [PubMed] [Google Scholar]
- 230.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007. [DOI] [PubMed] [Google Scholar]
- 231.Zhang W, Gunst SJ. Interactions of airway smooth muscle cells with their tissue matrix: implications for contraction. Proc Am Thorac Soc 5: 32–39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 30: 4021–4028, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest 123: 1096–1108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]