Abstract
Ozone causes airway hyperresponsiveness (AHR) and pulmonary inflammation. Rho kinase (ROCK) is a key regulator of smooth muscle cell contraction and inflammatory cell migration. To determine the contribution of the two ROCK isoforms ROCK1 and ROCK2 to ozone-induced AHR, we exposed wild-type, ROCK1+/−, and ROCK2+/− mice to air or ozone (2 ppm for 3 h) and evaluated mice 24 h later. ROCK1 or ROCK2 haploinsufficiency did not affect airway responsiveness in air-exposed mice but significantly reduced ozone-induced AHR, with a greater reduction in ROCK2+/− mice despite increased bronchoalveolar lavage (BAL) inflammatory cells in ROCK2+/− mice. Compared with wild-type mice, ozone-induced increases in BAL hyaluronan, a matrix protein implicated in ozone-induced AHR, were lower in ROCK1+/− but not ROCK2+/− mice. Ozone-induced increases in other inflammatory moieties reported to contribute to ozone-induced AHR (IL-17A, osteopontin, TNFα) were not different in wild-type vs. ROCK1+/− or ROCK2+/− mice. We also observed a dose-dependent reduction in ozone-induced AHR after treatment with the ROCK1/ROCK2 inhibitor fasudil, even though fasudil was administered after induction of inflammation. Ozone increased pulmonary expression of ROCK2 but not ROCK1 or RhoA. A ROCK2 inhibitor, SR3677, reduced contractile forces in primary human airway smooth muscle cells, confirming a role for ROCK2 in airway smooth muscle contraction. Our results demonstrate that ozone-induced AHR requires ROCK. Whereas ROCK1-dependent changes in hyaluronan may contribute to ROCK1's role in O3-induced AHR, the role of ROCK2 is downstream of inflammation, likely at the level of airway smooth muscle contraction.
Keywords: fasudil, inflammation, bronchoalveolar lavage, hyaluronan, osteopontin
rho-associated coiled-coil-forming kinases (ROCKs) are serine/threonine protein kinases activated by GTP-bound RhoA that may be involved in the pathogenesis of asthma. Many inflammatory mediators associated with asthma cause ROCK activation (1, 32), and ROCKs exert multiple effects that may contribute to asthma. For example, ROCK promotes inflammation and smooth muscle contraction (2, 33, 58, 59), including the ability to promote myosin light-chain (MLC) phosphorylation, an event required for airway smooth muscle contraction (33). Importantly, in mice, both ROCK inhibitors and ROCK insufficiency have been shown to reduce allergen-induced airway hyperresponsiveness (AHR), a canonical feature of asthma (26, 46, 50, 59, 74). Although these data suggest a potential therapeutic role for ROCK inhibitors in the treatment of asthma, the role of ROCK in AHR induced by other asthma triggers has not been established.
The purpose of this study was to examine the role of ROCK in AHR induced by acute exposure to ozone (O3), a common air pollutant produced from automobile exhaust in the presence of sunlight. Exposure to O3, even at concentrations below the US Environmental Protection Agency standard, increases hospital admissions for asthma-associated symptoms (15, 39, 68). O3 causes airway and pulmonary epithelial injury that results in a robust inflammatory response that includes generation of numerous cytokines, chemokines, and other inflammatory moieties, as well as recruitment of neutrophils and macrophages to the lungs (29, 51, 65). O3 also causes AHR both in humans and in animals (12, 16, 55, 57). The ability of O3 to induce both AHR and inflammation may contribute to the role of O3 as an asthma trigger.
Two ROCK isoforms, ROCK1 and ROCK2, have been described (40, 47). Emerging data indicate that these two isoforms may serve different functions depending on the cell type. There are both tissue-specific and subcellular differences in ROCK expression and localization (40, 69, 70). Furthermore, whereas the kinase domains of ROCK1 and ROCK2 are highly homologous, their Rho binding domains are less so, which may account for differences in their regulation (40). Both ROCK1 and ROCK2 deficient mice typically die neonatally, but heterozygous mice (ROCK1+/− and ROCK2+/− mice) are viable (41, 48, 54, 61). In ROCK1+/− mice, pulmonary expression of ROCK1 is ∼50% of wild type (WT) with no change in ROCK2 expression. Similarly, ROCK2 haploinsufficiency reduces pulmonary expression of ROCK2 by ∼50%, with no change in ROCK1 (74).
To examine the role of ROCK1 and ROCK2 in O3-induced AHR, we exposed WT, ROCK1+/−, and ROCK2+/− mice to either room air or O3 (2 ppm for 3 h) and measured airway responsiveness to inhaled aerosolized methacholine 24 h later. We also examined the effect of treatment with the ROCK inhibitor fasudil on airway responsiveness in air and O3-exposed mice. Our results indicate an important role for both ROCK1 and ROCK2 in O3-induced AHR. For ROCK2, this role is largely independent of inflammation.
METHODS
Animals.
This study was approved by the Harvard Medical Area Standing Committee on Animals. ROCK1+/− and ROCK2+/− mice were generated as described previously (41, 49). We mated ROCK1+/− and WT (C57BL/6) mice yielding ROCK1+/− mice and littermate WT controls. Similarly, ROCK2+/− and WT mice were mated to generate ROCK2+/− mice and littermate WT controls for this study. WT mice from the two types of matings were combined into one WT group.
Protocol.
Twenty- to 25-wk-old male WT, ROCK1+/−, and ROCK2+/− mice were exposed to O3 (2 ppm) or room air for 3 h. Twenty-four hours later, mice were anesthetized and instrumented for the measurement of pulmonary mechanics and airway responsiveness. Following these measurements, bronchoalveolar lavage (BAL) was performed, and the lungs were harvested for the preparation of RNA. In another cohort of 11- to 12-wk-old male WT mice, we examined the effect of fasudil, an inhibitor of both ROCK1 and ROCK2, on O3-induced AHR. For these studies, mice were exposed to room air or O3, as described above. Fasudil (3 or 10 mg/kg; LC Laboratories, Woburn, MA) or saline was administered intraperitoneally 24 h after cessation of O3 exposure and 30 min prior to measurements of airway responsiveness. The time of fasudil administration (i.e., after the induction of inflammation by O3) was chosen to allow us to determine whether ROCK inhibition could prevent O3-induced AHR without affecting O3-induced inflammation.
Acute O3 exposure.
Mice were exposed to O3 (2 ppm, 3 h) in individual wire mesh cages inside a stainless-steel and plexiglass chamber, as described previously (56). Other mice were exposed to room air in the same manner in separate but identical chambers. Food and water were removed during the exposures. After exposure, mice were returned to their home cages, and food and water were restored.
Measurement of pulmonary mechanics and airway responsiveness.
Twenty-four hours after air or O3 exposure, mice were anesthetized with pentobarbital sodium (50 mg/kg, Nembutal; Akorn, Lake Forest, IL) and xylazine (7 mg/kg; Lloyd Laboratories, Shenandoah, IA) and instrumented for the measurement of pulmonary mechanics, as described previously (66). To assess airway responsiveness, aerosols of PBS or methacholine dissolved in PBS (increasing in half-log increments from 0.3 to 100 mg/ml) were delivered to the lungs. After each dose was delivered, total lung impedance (ZL) was measured every 15 s for the next 3 min. A parameter estimation model (14) was used to partition ZL into components of Newtonian resistance (Rn) and the coefficients of lung tissue damping (G) and lung tissue elastance (H). Rn largely reflects the conducting airways, whereas G and H assess the small airways and pulmonary parenchyma, including airway closure. Sinusoidal perturbations at a frequency of 2.5 Hz were also applied to determine total lung resistance (RL). To construct dose-response curves to methacholine, we averaged the three highest values of RL, Rn, G, and H obtained after each dose.
Bronchoalveolar lavage.
Once measurements of airway responsiveness were complete, mice were euthanized with an overdose of pentobarbital sodium. BAL was performed as described previously (25). Total BAL cells were counted with a hemacytometer. An aliquot of BAL cells was spun onto glass slides and stained with hematoxylin and eosin, and differential cell counts on at least 300 cells were performed. BAL supernatant was stored at −80°C and subsequently analyzed by ELISA for IL-33, IL-17A, TNFα, KC (CXCL1),monocyte chemoattractant protein-1 (MCP-1), granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein-3 (MIP-3α), and hyaluronic acid (HA) (R & D Systems, Biolegend, eBiosciences, and Echelon Biosciences).
RNA extraction and quantitative PCR.
Total RNA was extracted from left lungs, and cDNA was prepared as described previously (25). Pulmonary mRNA abundance of Grpr was evaluated by quantitative real-time PCR by the SYBR green method (Grpr primers sequences were as follows: sense, tcttctgcacggtcaagtcc; antisense, tacttgctggcatccacagg). The ΔΔCT method was used to assess changes in Grpr expression relative to a housekeeping gene, 36B4 (Rplp0), a ribosomal protein.
Western blotting.
Protein from lungs was extracted in RIPA buffer in the presence of proteases and phosphatase inhibitors. Ten micrograms of protein was separated with a 10% SDS gel, blocked with 5% milk-TBST, and probed with antibodies for ROCK1, ROCK2, RhoA, and β-actin (Cell Signaling Technology, Danvers, MA). The bands were visualized with a digital imager (ChemiGenius, Syngene, UK) and densitometry performed with ImageJ (imagej.nih.gov/ij/).
Traction microscopy.
To measure the effect of ROCK inhibition on contractile force of airway smooth muscle (ASM), we used Fourier transform traction microscopy (FTTM) (6, 43, 62). As described previously (43), we prepared acrylamide hydrogels in glass-bottom 96-well plates (Young's module of hydrogel ∼8 kPa) and coated fluorescent beads and collagen (40 μg/mL) on the hydrogels. Primary human airway smooth muscle (HASM) cells (generously provided by Dr. Reynold Panettieri, University of Pennsylvania) were seeded onto the coated hydrogels to form a confluent monolayer. The cells were serum deprived for 2 days before measurements. Image sets on each well included one phase contrast image of HASM cells and one fluorescent image of beads on the hydrogel surface. Image sets were taken on each well four times: 1) a reference image set prior to cells being seeded; 2) a baseline image set before drugs were added; 3) an image set 1 h after treatment with ROCK inhibitors or vehicle (water); and 4) an image set 1 h after challenge with the agonists histamine, endothelin-1 (ET-1), or bradykinin. After correcting image shifts, traction forces by cells (per unit area) on hydrogel were calculated at three time points: at baseline, after ROCK inhibitors, and after agonist. The average traction at each time point was quantified using root mean square (RMS) traction, and the effect of ROCK inhibition or agonist challenge was quantified using the RMS traction at each time point normalized to baseline RMS traction, which we call “force/response ratio” (43).
Statistical analysis.
The significance of differences in outcome indicators was assessed by factorial ANOVA using exposure (air or O3) genotype or treatment (fasudil) as main effects or for HASM cells using drug treatment (vehicle or ROCK inhibitor) and presence or absence of contractile agonist as main effects. BAL cells were log transformed to conform to a normal distribution. For post hoc comparisons, we used the Fisher least significant difference test. Statistical analyses were carried out using SAS software (SAS Institute, Cary, NC). Results presented are means ± SE. P < 0.05 was considered statistically significant.
RESULTS
O3-induced AHR requires ROCK1 and ROCK2.
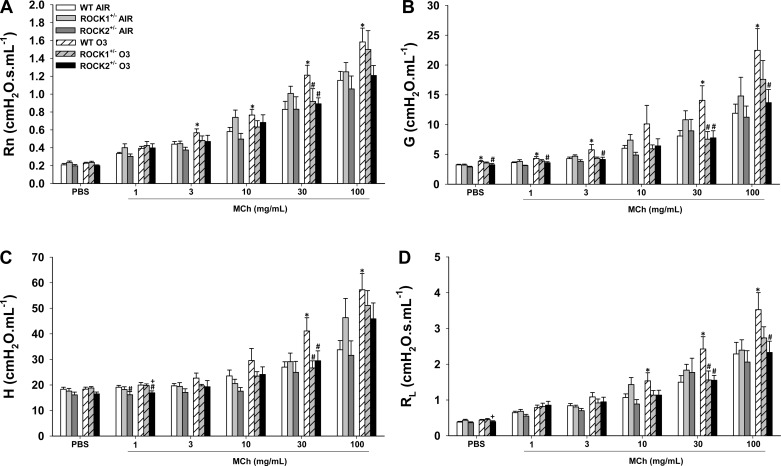
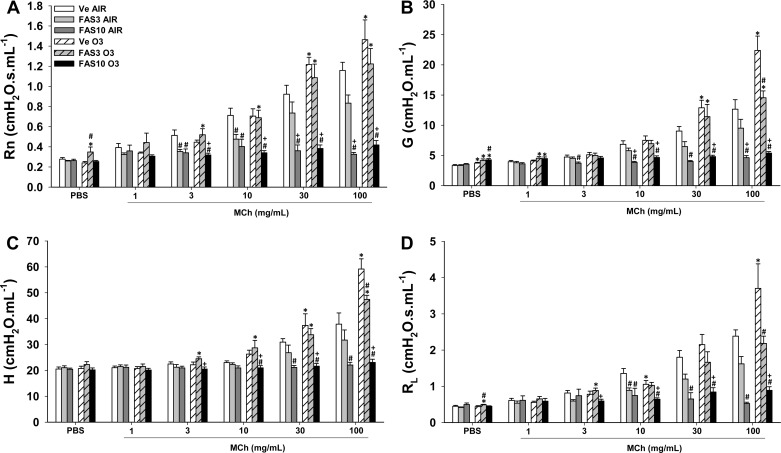
There were no significant differences in the response to methacholine in air-exposed WT, ROCK1+/−, and ROCK2+/− mice. In WT mice, exposure to O3 caused AHR whether RL, Rn, G, or H was used as the index of response (Fig. 1). In contrast, O3 did not induce AHR in either ROCK1+/− or ROCK2+/− mice.
Fig. 1.
Ozone (O3)-induced airway hyperresponsiveness (AHR) is attenuated in Rho-associated coiled-coil-forming kinase (ROCK)1- and ROCK2-haploinsufficient mice. Wild-type (WT), ROCK1+/−, and ROCK2+/− mice were exposed to O3 (2 ppm for 3 h) or to room air and airway responsiveness was measured 24 h later. Newtonian resistance (Rn; A), the coefficient of lung tissue damping (G; B), the coefficient of lung tissue elastance (H; C), and lung resistance (RL; D). Results are means ± SE of data from 5 to 12 mice/group. *P < 0.05 vs. air-exposed mice; #P < 0.05 vs. WT O3-exposed mice; +P < 0.05 vs. ROCK1+/−.
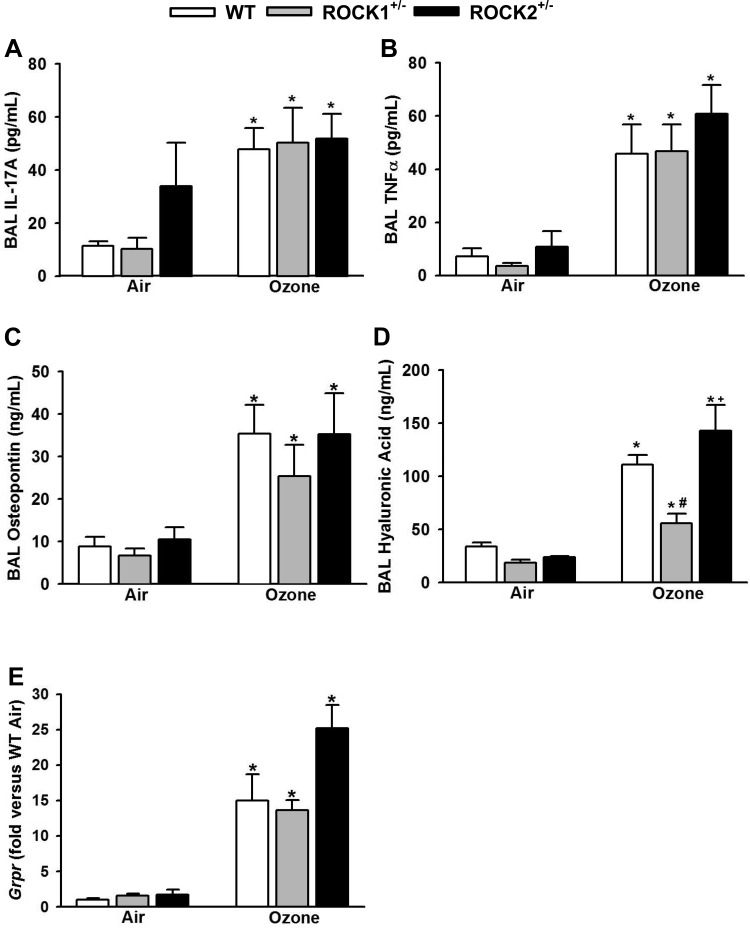
Because IL-17A, TNFα, and osteopontin have each been reported to play a role in O3-induced AHR in WT mice (5, 8, 45, 56, 67), we measured BAL IL-17A, TNFα, and osteopontin in these mice. There was a robust increase in BAL concentrations of all three moieties in O3 vs. air exposed mice (Fig. 2, A–C), but O3-induced increases in BAL IL-17A, TNFα, and osteopontin were similar in WT, ROCK1+/−, and ROCK2+/− mice. Fragmentation of the matrix glycoprotein hyaluronan (HA) has also been reported to contribute to O3-induced AHR in WT mice (11). O3-induced increases in BAL HA were not different in WT and ROCK2+/− mice (Fig. 2D). However, BAL HA was significantly lower in O3-exposed ROCK1+/− than in WT and ROCK2+/− mice. O3 increases the pulmonary expression of Grpr (73), the receptor for gastrin-releasing peptide (GRP), a peptide found in pulmonary neuroendocrine cells (21). GRP has the capacity to contract airway smooth muscle (34), and others have reported that signaling through GRPR is required for O3-induced AHR in WT mice (73). Consequently, we measured pulmonary mRNA abundance of Grpr by RT-PCR. O3 caused a robust increase in the mRNA abundance of Grpr in WT mice, but there was no impact of either ROCK1 or ROCK2 haploinsufficiency on Grpr mRNA abundance (Fig. 2E). Indeed, there was a trend, albeit non significant, for increased Grpr in O3-exposed ROCK2+/− mice.
Fig. 2.
Effect of ROCK1- and ROCK2-haploinsufficient mice on factors contributing to O3-induced AHR in WT mice. Bronchoalveolar lavage (BAL) concentrations of IL-17A (A), TNFα (B), osteopontin (C), hyaluronic acid (HA; D), and pulmonary mRNA abundance of Grpr (E) and in WT, ROCK1+/−, and ROCK2+/− mice exposed to O3 (2 ppm for 3 h) or air and studied 24 h later. Results are means ± SE of data from 4 to 11 mice/group. *P < 0.05 vs. air, #P < 0.05 vs. WT mice with same exposure, and +P < 0.05 vs. ROCK1+/− mice with same exposure.
O3-induced inflammation and injury in ROCK haploinsufficient mice.
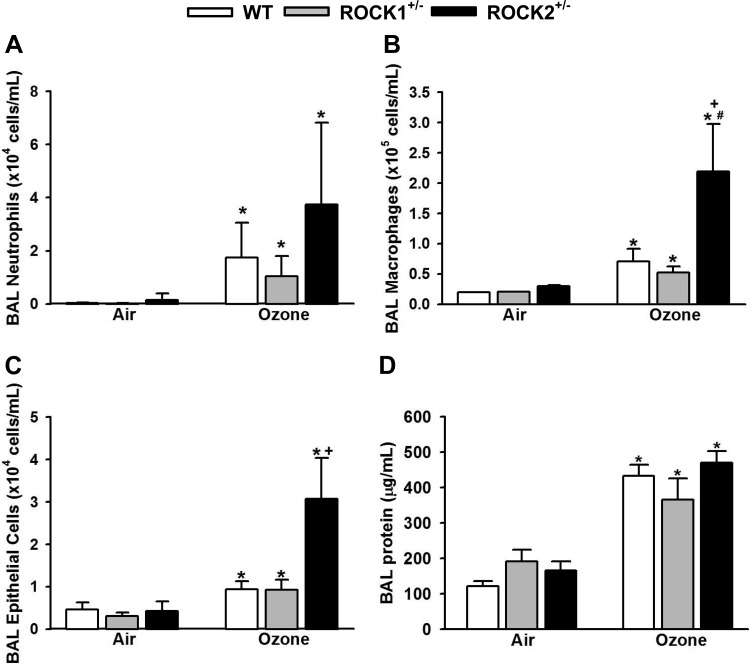
In WT mice, O3 increased BAL neutrophils, macrophages, epithelial cells, and protein (Fig. 3), as described previously (23, 30). There were no significant differences in BAL neutrophils, macrophages, epithelial cells, or protein in ROCK1+/− vs. WT mice exposed to O3. However, in ROCK2+/− mice, BAL macrophages and epithelial cells were significantly greater than in WT or ROCK1+/− mice (Fig. 3, B and C). A similar trend was observed for BAL neutrophils (Fig. 3A).
Fig. 3.
Effect of ROCK1 and ROCK2 haploinsufficiency on O3-induced inflammation and injury. BAL neutrophils (A), macrophages (B), epithelial cells (C), and protein (D) in WT, ROCK1+/−, and ROCK2+/− mice exposed to O3 (2 ppm for 3 h) or air and studied 24 h later. Results are means ± SE of data from 4 to 11 mice/group. *P < 0.05 vs. air; #P < 0.05 vs. WT mice with same exposure; +P < 0.05 vs ROCK1+/− mice with same exposure.
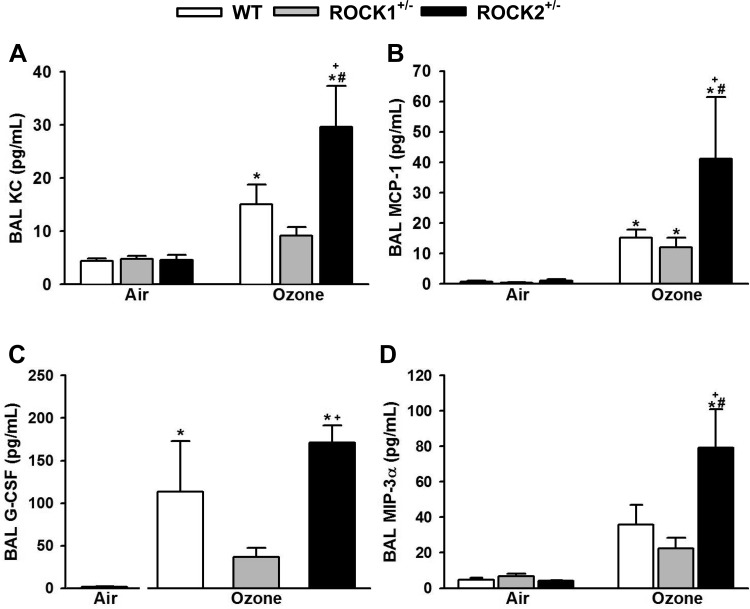
O3-induced increases in the neutrophil chemotactic factor KC (CXCL1) and the neutrophil survival factor G-CSF have been shown to contribute to O3-induced increases in BAL neutrophils (22, 25, 27), whereas MCP-1 is required for O3-induced increases in BAL macrophages (72). The chemokine MIP-3α also has the capacity to participate in neutrophil recruitment (36) and is induced by O3 (67). O3-induced increases in BAL KC, MCP-1, G-CSF, and MIP-3α were not different in WT and ROCK1+/− mice (Fig. 4). However, BAL KC, MCP-1, and MIP-3α were each greater in ROCK2+/− than in WT mice exposed to O3 (Fig. 4, A, B, and D), consistent with the greater increases in BAL macrophages and neutrophils in the ROCK2+/− mice (Fig. 3).
Fig. 4.
Effect of ROCK1 and ROCK2 haploinsufficiency on O3-induced increases in BAL chemokines and growth factors. BAL KC (A), monocyte chemoattractant protein-1 (MCP-1; B), granulocyte colony-stimulating factor (G-CSF; C), and MIP-3α (D) in WT, ROCK1+/−, and ROCK2+/− mice exposed to O3 (2 ppm for 3 h) or air and studied 24 h later. Results are means ± SE of data from 4 to 11 mice/group. *P < 0.05 vs. air; #P < 0.05 vs. WT mice with same exposure; +P < 0.05 vs. ROCK1+/− mice with same exposure.
Fasudil abrogates O3-induced AHR.
Because our data indicated a role for ROCK on O3-induced AHR and also that this role was independent of O3-induced inflammation, we examined the effect of the ROCK1/ROCK2 inhibitor fasudil on O3-induced AHR. Fasudil was administered 30 min before measurements of airway responsiveness, i.e., after the induction of inflammation and injury by O3. In air-exposed mice, fasudil treatment caused a dose-dependent reduction in airway responsiveness, regardless of which measurement of lung function (RL, Rn, G, or H) was used to assess responsiveness (Fig. 5). Indeed, at 10 mg/kg fasudil, the response to methacholine was largely abolished. Fasudil (10 mg/kg) also abolished O3-induced AHR, and even at 3 mg/kg there was a substantive reduction in both G and H at the highest methacholine concentration in O3-exposed mice. As expected, given that fasudil was administered just before measurements of AHR (i.e., 24 h after O3 exposure), fasudil had no effect on BAL inflammatory cells or BAL protein (data not shown).
Fig. 5.
Effect of the ROCK inhibitor fasudil on O3-induced AHR. WT mice were exposed to O3 (2 ppm for 3 h) or air. Twenty-four hours later, mice were treated with fasudil (3 or 10 mg/kg ip) or PBS, and airway responsiveness was measured. Rn (A), G (B), H (C), and RL (D). Results are means ± SE of data from 5 to 6 mice/group. *P < 0.05 vs. air-exposed mice with the same treatment; #P < 0.05 vs. PBS-treated mice with same exposure; +P < 0.05 vs. 3 mg/kg.
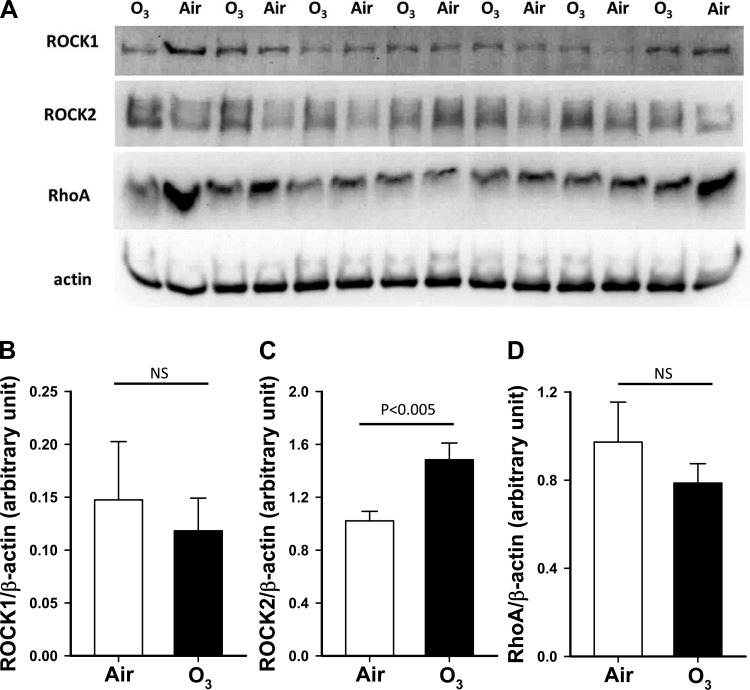
Ozone increases pulmonary expression of ROCK2 but not ROCK1 or RhoA. Twenty-four hours after cessation of air or O3 exposure, lungs of WT mice were harvested, and total lung protein was prepared for Western blotting. Compared with air, O3 exposure had no effect on either ROCK1 or RhoA expression (Fig. 6). In contrast, O3 exposure caused a significant, ∼50% increase in pulmonary expression of ROCK2.
Fig. 6.
Effect of O3 on pulmonary expression of ROCK1, ROCK2, and RhoA. A: Western blot showing ROCK1, ROCK2, RhoA, and β-actin expression. Protein was extracted from lungs of wild-type mice exposed to air or O3 (2 ppm for 3 h). B–D: densitometry for ROCK1 (B), ROCK2 (C), and RhoA (D) normalized for β-actin expression. Results are mean ± SE for 7 mice/group. Statistics were performed on log-transformed data to conform to a normal distribution.
ROCK2 inhibition decreases contractile force of HASM cells.
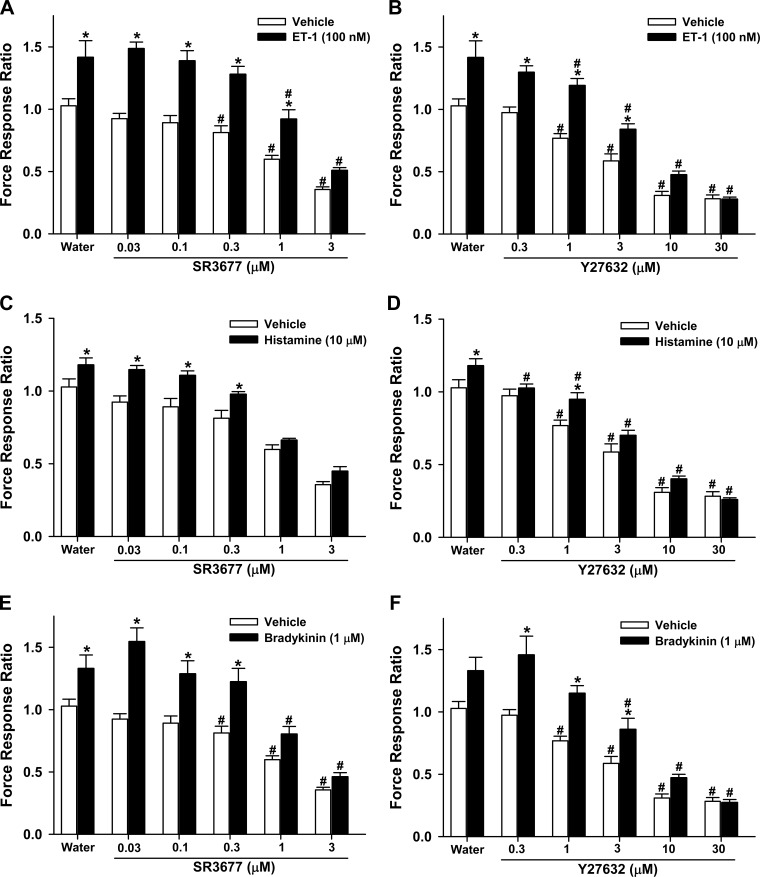
Our data indicate that reductions in O3-induced AHR in ROCK+/− mice may be attributable to decreases in inflammatory moieties induced by O3 (Figs. 2 and 4). However, in ROCK2+/− mice, reduced O3-induced AHR was observed despite elevations in inflammatory cells and mediators (Figs. 2–4). The data suggest that ROCK2-mediated events are downstream of O3-induced inflammation and/or injury. Although ROCK is known to contribute to ASM contraction (4, 7, 13, 18, 35, 37, 71), the specific role of the ROCK2 isoform has not been established. Consequently, we examined the effect of a ROCK2 inhibitor on contractile force of HASM cells using FTTM. HASM cells in culture exhibit substantial contractile forces that can be reduced by relaxant agonists such as isoproterenol (63). In this study, we found that the pan ROCK inhibitor Y27632 and the specific ROCK2 inhibitor SR3677 also caused dose-dependent reductions in contractile forces (vehicle-treated cells in Fig. 7). These effects of Y27632 are consistent with previously reported effects of this pan ROCK inhibitor on baseline stiffness in HASM cells, a surrogate for force generation (4). In cells not pretreated with ROCK inhibitors (water-treated cells in Fig. 7), the contractile agonists ET-1, histamine, and bradykinin each significantly increased HASM contractile forces by 20–40% of baseline values. Both Y27632 and SR3677 caused a dose-dependent reduction in contractile forces in cells stimulated with all three contractile agonists (Fig. 7), and at the higher concentrations of these inhibitors, differences between vehicle and contractile agonist-stimulated cells were no longer significant.
Fig. 7.
Effect of ROCK inhibition in human airway smooth muscle (HASM) cells. Traction forces normalized to baseline values were assessed in HASM cells treated with various doses of the ROCK2 inhibitor SR3677 (A, C, and E), the pan-ROCK inhibitor Y27632 (B, D, and F), or an equal volume of water (the vehicle used to dissolve SR3677 and Y27632) and challenged with endothelin-1 (ET-1; A and B), histamine (C and D), or bradykinin (E and F) or vehicle. Data are from 2 HASM cell donors and are expressed as means ± SE of n = 9–21 wells/group for ET-1 and histamine, and n = 6–21 wells/group for bradykinin. *P < 0.05 vs. vehicle; #P < 0.05 vs. water control.
DISCUSSION
Our data indicate that ROCK is required for O3-induced AHR (Fig. 1); compared with WT mice, O3-induced AHR was attenuated in both ROCK1+/− and ROCK2+/− mice. Moreover, despite the fact that ROCK can play a role in immune and inflammatory cell migration and activation (9, 52, 53, 60), our data indicate that the role of ROCK2 in O3-induced AHR is independent of such effects; O3-induced AHR was reduced in ROCK2+/− mice, even though inflammatory cells and mediators were largely either unchanged or actually increased in these mice (Figs. 2–4). Furthermore, O3-induced AHR was reduced when WT mice were treated with the ROCK1/2 inhibitor fasudil (Fig. 5), even though fasudil was administered after the induction of inflammation and injury by O3. The data are consistent with a role for ROCK2 in O3-induced AHR that is downstream of inflammation and injury, likely at the level of airway smooth muscle contraction. In contrast, ROCK1-mediated changes in hyaluronic acid (Fig. 2D) and/or other inflammatory mediators (Fig. 2) may also contribute to ROCK1-dependent effects on O3-induced AHR.
To assess the individual contributions of each ROCK isoform, we used ROCK1- and ROCK2-haploinsufficient mice, as described previously (26, 49, 74). These mice have an approximate 50% reduction in pulmonary expression of the affected ROCK isoform with normal expression of other isoforms (74). Nevertheless, despite retention of the other isoform, O3-induced AHR was not observed in either ROCK1+/− or ROCK2+/− mice, indicating that both ROCK1 and ROCK2 play independent roles in the induction of AHR after O3. In contrast, we observed no difference in airway responsiveness among WT, ROCK1+/−, and ROCK2+/− mice after air exposure (Fig. 1), indicating that in this setting, either the presence of one ROCK isoform compensates for the reduction in expression of the other or a 50% reduction in expression of one ROCK isoform is insufficient to affect responsiveness. The observation that in air-exposed mice inhibition of both ROCK isoforms with fasudil did reduce airway responsiveness (Fig. 5) suggests an essential role for ROCK in ASM contraction even in the absence of asthma triggers. Such a role is also supported by the demonstration of marked reductions in contractile force of HASM cells treated with ROCK inhibitors (Fig. 7).
Fragmentation of the extracellular matrix protein hyaluronan by oxidative stress has been reported to contribute to O3-induced AHR in WT mice (11). We did observe a significant reduction in HA in O3-exposed ROCK1+/− vs. WT mice (Fig. 2D), and it is conceivable that this reduced HA contributed to the reduced O3-induced AHR observed in these mice. However, despite the profound reductions in O3-induced AHR in ROCK2+/− mice (Fig. 1), we observed no reduction in BAL HA in these mice, nor were BAL concentrations of TNFα, IL-17A, and osteopontin or pulmonary mRNA abundance of Grpr reduced in ROCK2+/− vs. WT mice (Fig. 2). Each of these inflammatory moieties has been reported to play a role in O3-induced AHR in WT mice (5, 45, 56, 73). Consequently, the ability of ROCK2 haploinsufficiency to reduce O3-induced AHR is not the result of changes in these moieties. Others have suggested that activation of mast cells occurs after acute O3 exposure (31), and we have previously reported a role for ROCK1 in mast cell activation in the setting of allergen sensitization and challenge (26). However, we were unable to detect any mast cell protease/tryptase-1 (mMCP-1), a marker of mast cell activation, in BAL fluid after O3 exposure (data not shown), suggesting that the effects of ROCK on mast cells also fail to account for the role of ROCK isoforms in O3-induced AHR. Consistent with these observations, Noviski et al. (42) reported no effect of mast cell deficiency on O3-induced AHR in mice.
Instead, ROCK2 may act within ASM cells. ROCK1 and ROCK2 are ubiquitously expressed (47), and Western blotting of protein extracts from cultured murine tracheal smooth muscle cells indicates that these cells do express ROCK2 as well as ROCK1 (Liao JK and Shore SA, unpublished observations). Others have demonstrated the importance of ROCK activation for smooth muscle cell contractility both in vitro and in vivo (28, 33, 59, 74). Indeed, we have previously reported reduced contractile responses to methacholine in tracheal rings from ROCK1+/− and ROCK2+/− vs. WT mice (26). Exposure of ASM cells to G protein-coupled receptor agonists like methacholine results in MLC phosphorylation and consequent ASM cell contraction (33). This process is reversed by MLC dephosphorylation, which is catalyzed by myosin light-chain phosphatase (MLCP). ROCK negatively regulates MLCP thus augmenting and prolonging contraction (28, 64), but can also have additional effects on the cytoskeleton that may promote or sustain contraction (35). Given the effects of a ROCK2 inhibitor on force generation in HASM cells (Fig. 7), it is evident that ROCK2 can participate in airway smooth muscle contraction. Indeed, the observation that the ROCK2 inhibitor, SR3677, was nearly as effective as the pan ROCK inhibitor, Y27632, at reducing cellular contractile forces (Fig. 7) suggests a dominant role for ROCK2 over ROCK1 in ASM contraction, though we cannot rule out the possibility of some nonspecific effects of SR3677 on ROCK1, at least at the higher concentrations of this inhibitor.
The observation that airway responsiveness is reduced in ROCK1+/− and ROCK2+/− vs. WT mice exposed to O3, but not in ROCK1+/− or ROCK2+/− vs. WT mice exposed to air (Fig. 1), suggests that there may be differences in the extent of ROCK1/2 activation in air vs. O3-exposed mice. Such differences could be the result of either alterations in the expression of these ROCK isoforms in relevant cell types or O3-induced effects on factors inducing their activation. With respect to expression, we did observe increased pulmonary expression of ROCK2 in O3- vs. air-exposed mice (Fig. 6). ROCKs are ubiquitously expressed (47), and we do not know the lung cell type(s) in which ROCK2 increases after O3 exposure. Numerous inflammatory mediators are released following O3 exposure (Figs. 2 and 4) and could contribute to changes in ROCK2 expression in the lungs. For example, others have reported that IL-17A has the capacity to induce ROCK2 expression in murine airway smooth muscle cells (32). Hence, it is conceivable that release of IL-17A after O3 (Fig. 2A) accounts for the observed changes in ROCK2 expression (Fig. 6). However, initial experiments using anti-IL17A vs. isotype antibody treatment of mice prior to O3 exposure indicated no effect of anti-IL-17A on lung ROCK2 expression (data not shown). Whether other inflammatory mediators induced by O3 (Fig. 2 amd 4) have the capacity to induce ROCK2 expression remains to be established; we also do not know whether the observed changes in ROCK2 expression (Fig. 6) are actually required for the ability of this ROCK isoform to affect O3-induced AHR. For example, ROCK1 also contributed to O3-induced AHR (Fig. 1) without evidence of any change in ROCK1 expression (Fig. 6), although it is conceivable that there were undetectable changes in ROCK1 in some cell type that makes up only a small portion of total lung protein.
With respect to ROCK activation, binding of GTP-bound RhoA to ROCK is the primary mechanism for ROCK activation (3, 10, 19). RhoA-GDP binds to the regulatory domain of ROCK, thereby opening the catalytic domain of ROCK permitting its phosphorylation of downstream targets such as the myosin-binding subunit of MLCP (20, 28, 38). Both ROCK1 and ROCK2 contain Rho-binding sites (10, 40). Pulmonary RhoA expression was not affected by O3 (Fig. 6), but it is possible that RhoA-GTP was affected. Many proinflammatory mediators cause binding of RhoA to GDP, thus inducing ROCK activation (19, 38). For example, TNFα, osteopontin, and GRPR expression were all increased after O3 exposure (Fig. 2). Each of these moieties has the capacity to induce ROCK activation (17, 24, 44). Given the likely role for ASM in ROCK2-dependent effects on O3-induced AHR (see above), it is also possible that O3 acts in some way to augment the ability of methacholine to induce ROCK activation in ASM and thus potentiate airway responsiveness.
Whereas O3-induced AHR was suppressed in ROCK2+/− and ROCK1+/− vs. WT mice, neither ROCK1 nor ROCK2 haploinsufficiency attenuated O3-induced recruitment of neutrophils or macrophages to the lungs (Fig. 3). Indeed, BAL macrophages were actually elevated in ROCK2+/− vs. WT mice (Fig. 3B), and there was a similar trend for BAL neutrophils (Fig. 3A). BAL epithelial cells were also increased in ROCK2+/− vs. WT mice, suggesting greater airway injury in the ROCK2+/− mice, which might be expected to increase inflammatory cell recruitment. There was also greater release of chemotactic factors such as KC and MCP-1 into BAL fluid in ROCK2+/− vs. WT mice (Fig. 4, A and B), which is consistent with greater epithelial cell injury in the ROCK2+/− mice. Exactly how ROCK protects against such injury is not yet known, but it is conceivable that ROCK2 is required for the migration and/or proliferation of airway epithelial cells necessary for repair of the damaged epithelium. In any event, despite greater injury and inflammation in ROCK2+/− mice (Fig. 3), O3-induced AHR was reduced in these mice, indicating that the role of ROCK2 in promoting O3-induced AHR is downstream of the inflammatory response. The observation that the ROCK1/ROCK2 inhibitor fasudil also reduced airway responsiveness in O3-exposed animals also suggests that the effects of ROCK that promote AHR are downstream of O3-induced inflammation; fasudil was administered just before measurements of AHR at a time when inflammation was already well established. Fasudil also reduces AHR induced by allergen sensitization and challenge (26, 59) at least in part as a result of the effects on ROCK activation at the level of airway smooth muscle contraction (26).
In conclusion, our results demonstrate that O3-induced AHR requires ROCK. Whereas ROCK1 dependent changes in the fragmentation of HA may contribute to its role in O3-induced AHR, our data are consistent with the ASM being the site of action of ROCK2. ROCK also plays an important role in allergen-induced AHR (26, 59, 74). The ROCK inhibitor fasudil is in clinical trials in the US for the treatment of other diseases, and ROCK1 and ROCK2 selective inhibitors are also being developed (26). Greater understanding of the specific roles of ROCK1 and ROCK2 in other types of asthma, including obesity-related asthma and asthma triggered by viral infections, may ultimately permit the use of these agents as pan-phenotype therapeutics for asthma without unwanted side effects due to cardiovascular and other systemic effects.
GRANTS
This study was supported by National Institutes of Health Grants HL-091933, ES-013307, and ES-000002. J. K. Liao was also supported by HL-052233, and J. A. Mathews was supported by F32-ES-022556.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.I.K., C.Y.P., J.K.L., and S.A.S. conception and design of research; D.I.K., J.A.M., C.Y.P., Y.C., G.H., and A.P.W. performed experiments; D.I.K., J.A.M., C.Y.P., G.H., and S.A.S. analyzed data; D.I.K., J.A.M., C.Y.P., and S.A.S. interpreted results of experiments; D.I.K. and J.A.M. prepared figures; D.I.K., C.Y.P., and S.A.S. drafted manuscript; D.I.K., J.A.M., C.Y.P., A.P.W., J.K.L., and S.A.S. edited and revised manuscript; D.I.K., J.A.M., C.Y.P., Y.C., G.H., A.P.W., J.K.L., and S.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Drs. Jin-Ah Park and Jennifer Mitchel in our department for help with Western blots.
REFERENCES
- 1.Adachi T, Vita R, Sannohe S, Stafford S, Alam R, Kayaba H, Chihara J. The functional role of rho and rho-associated coiled-coil forming protein kinase in eotaxin signaling of eosinophils. J Immunol 167: 4609–4615, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science 271: 648–650, 1996. [DOI] [PubMed] [Google Scholar]
- 4.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol 283: C792–C801, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Barreno RX, Richards JB, Schneider DJ, Cromar KR, Nadas AJ, Hernandez CB, Hallberg LM, Price RE, Hashmi SS, Blackburn MR, Haque IU, Johnston RA. Endogenous osteopontin promotes ozone-induced neutrophil recruitment to the lungs and airway hyperresponsiveness to methacholine. Am J Physiol Lung Cell Mol Physiol 305: L118–L129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cazzola M, Calzetta L, Rogliani P, Lauro D, Novelli L, Page CP, Kanabar V, Matera MG. High glucose enhances responsiveness of human airways smooth muscle via the Rho/ROCK pathway. Am J Respir Cell Mol Biol 47: 509–516, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-α receptors. Am J Physiol Lung Cell Mol Physiol 280: L537–L546, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cronshaw DG, Owen C, Brown Z, Ward SG. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J Immunol 172: 7761–7770, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa K, Fujita A, Ishizaki T, Saito Y, Narumiya S. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem 271: 23022–23028, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 12.Gordon T, Venugopalan CS, Amdur MO, Drazen JM. Ozone-induced airway hyperreactivity in the guinea pig. J Appl Physiol 57: 1034–1038, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Hanazaki M, Yokoyama M, Morita K, Kohjitani A, Sakai H, Chiba Y, Misawa M. Rho-kinase inhibitors augment the inhibitory effect of propofol on rat bronchial smooth muscle contraction. Anesth Analg 106: 1765–1771, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985) 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Holtzman MJ, Cunningham JH, Sheller JR, Irsigler GB, Nadel JA, Boushey HA. Effect of ozone on bronchial reactivity in atopic and nonatopic subjects. Am Rev Respir Dis 120: 1059–1067, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Holtzman MJ, Fabbri LM, Skoogh BE, O'Byrne PM, Walters EH, Aizawa H, Nadel JA. Time course of airway hyperresponsiveness induced by ozone in dogs. J Appl Physiol 55: 1232–1236, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Hunter I, Cobban HJ, Vandenabeele P, MacEwan DJ, Nixon GF. Tumor necrosis factor-alpha-induced activation of RhoA in airway smooth muscle cells: role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol Pharmacol 63: 714–721, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Iizuka K, Yoshii A, Samizo K, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. A major role for the rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br J Pharmacol 128: 925–933, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 15: 1885–1893, 1996. [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett 404: 118–124, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DE, Georgieff MK. Pulmonary neuroendocrine cells. Their secretory products and their potential roles in health and chronic lung disease in infancy. Am Rev Respir Dis 140: 1807–1812, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol 288: L61–L67, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Johnston RA, Theman TA, Terry RD, Williams ES, Shore SA. Pulmonary responses to acute ozone exposure in fasted mice: effect of leptin administration. J Appl Physiol 102: 149–156, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kang CG, Han HJ, Lee HJ, Kim SH, Lee EO. Rho-associated kinase signaling is required for osteopontin-induced cell invasion through inactivating cofilin in human non-small cell lung cancer cell lines. Bioorg Med Chem Lett 25: 1956–1960, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, Wurmbrand AP, Jastrab J, Hug C, Umetsu DT, Shore SA. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol 188: 4558–4567, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasahara DI, Ninin FM, Wurmbrand AP, Liao JK, Shore SA. Abrogation of airway hyperresponsiveness but not inflammation by Rho kinase insufficiency. Clin Exp Allergy 42: 457–470, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA Jr, Zangrilli J, Haczku A. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy 63: 438–446, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Kleeberger SR, Bassett DJ, Jakab GJ, Levitt RC. A genetic model for evaluation of susceptibility to ozone-induced inflammation. Am J Physiol Lung Cell Mol Physiol 258: L313–L320, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Kleeberger SR, Levitt RC, Zhang LY. Susceptibility to ozone-induced inflammation. II. Separate loci control responses to acute and subacute exposures. Am J Physiol Lung Cell Mol Physiol 264: L21–L26, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Kleeberger SR, Seiden JE, Levitt RC, Zhang LY. Mast cells modulate acute ozone-induced inflammation of the murine lung. Am Rev Respir Dis 148: 1284–1291, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, Sheppard D. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 18: 547–554, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 272: 12257–12260, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Lach E, Haddad EB, Gies JP. Contractile effect of bombesin on guinea pig lung in vitro: involvement of gastrin-releasing peptide-preferring receptors. Am J Physiol Lung Cell Mol Physiol 264: L80–L86, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Lan B, Deng L, Donovan GM, Chin LY, Syyong HT, Wang L, Zhang J, Pascoe CD, Norris BA, Liu JC, Swyngedouw NE, Banaem SM, Pare PD, Seow CY. Force maintenance and myosin filament assembly regulated by Rho-kinase in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 308: L1–L10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Burns AR, Miller SB, Smith CW. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J 25: 2659–2668, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu C, Zuo J, Janssen LJ. Regulation of airway smooth muscle RhoA/ROCK activities by cholinergic and bronchodilator stimuli. Eur Respir J 28: 703–711, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15: 2208–2216, 1996. [PMC free article] [PubMed] [Google Scholar]
- 39.McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res 80: 110–121, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 392: 189–193, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest 118: 1632–1644, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noviski N, Brewer JP, Skornik WA, Galli SJ, Drazen JM, Martin TR. Mast cell activation is not required for induction of airway hyperresponsiveness by ozone in mice. J Appl Physiol 86: 202–210, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Park CY, Zhou EH, Tambe D, Chen B, Lavoie T, Dowell M, Simeonov A, Maloney DJ, Marinkovic A, Tschumperlin DJ, Burger S, Frykenberg M, Butler JP, Stamer WD, Johnson M, Solway J, Fredberg JJ, Krishnan R. High-throughput screening for modulators of cellular contractile force. Integr Biol (Camb). In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel M, Kawano T, Suzuki N, Hamakubo T, Karginov AV, Kozasa T. Galpha13/PDZ-RhoGEF/RhoA signaling is essential for gastrin-releasing peptide receptor-mediated colon cancer cell migration. Mol Pharmacol 86: 252–262, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med 205: 385–393, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Possa SS, Charafeddine HT, Righetti RF, da Silva PA, Almeida-Reis R, Saraiva-Romanholo BM, Perini A, Prado CM, Leick-Maldonado EA, Martins MA, Tiberio Ide F. Rho-kinase inhibition attenuates airway responsiveness, inflammation, matrix remodeling, and oxidative stress activation induced by chronic inflammation. Am J Physiol Lung Cell Mol Physiol 303: L939–L952, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4: 446–456, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36: 2251–2257, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation 112: 2959–2965, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaafsma D, Bos IS, Zuidhof AB, Zaagsma J, Meurs H. The inhaled Rho kinase inhibitor Y-27632 protects against allergen-induced acute bronchoconstriction, airway hyperresponsiveness, and inflammation. Am J Physiol Lung Cell Mol Physiol 295: L214–L219, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ueki IF, Leikauf GD, Goetzl EJ, Boushey HA. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol 60: 1321–1326, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Shimada H, Rajagopalan LE. Rho-kinase mediates lysophosphatidic acid-induced IL-8 and MCP-1 production via p38 and JNK pathways in human endothelial cells. FEBS Lett 584: 2827–2832, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem 285: 12536–12542, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 168: 941–953, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shore SA, Johnston RA, Schwartzman IN, Chism D, Krishna Murthy GG. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J Appl Physiol 92: 1019–1028, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 164: 602–607, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Silverman F. Asthma and respiratory irritants (ozone). Environ Health Perspect 29: 131–136, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simoes RL, Fierro IM. Involvement of the Rho-kinase/myosin light chain kinase pathway on human monocyte chemotaxis induced by ATL-1, an aspirin-triggered lipoxin A4 synthetic analog. J Immunol 175: 1843–1850, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Taki F, Kume H, Kobayashi T, Ohta H, Aratake H, Shimokata K. Effects of Rho-kinase inactivation on eosinophilia and hyper-reactivity in murine airways by allergen challenges. Clin Exp Allergy 37: 599–607, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Tharaux PL, Bukoski RC, Rocha PN, Crowley SD, Ruiz P, Nataraj C, Howell DN, Kaibuchi K, Spurney RF, Coffman TM. Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells. J Immunol 171: 96–105, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 23: 5043–5055, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nat Physics 5: 426–430, 2009. [Google Scholar]
- 63.Wang N, Tolić-Nørrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenović D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282: C606–C616, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res 104: 531–540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werthamer S, Schwarz LH, Carr JJ, Soskind L. Ozone-induced pulmonary lesions. Severe epithelial changes following sublethal doses. Arch Environ Health 20: 16–21, 1970. [DOI] [PubMed] [Google Scholar]
- 66.Williams AS, Kasahara DI, Verbout NG, Fedulov AV, Zhu M, Si H, Wurmbrand AP, Hug C, Ranscht B, Shore SA. Role of the adiponectin binding protein, T-cadherin (cdh13), in allergic airways responses in mice. PLoS One 7: e41088, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams AS, Mathews JA, Kasahara DI, Chen L, Wurmbrand AP, Si H, Shore SA. Augmented pulmonary responses to acute ozone exposure in obese mice: roles of TNFR2 and IL-13. Environ Health Perspect 121: 551–557, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams LK, Langley R, Howell RJ. Ozone. The good, the bad, and the ugly. N C Med J 61: 84–89, 2000. [PubMed] [Google Scholar]
- 69.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170: 443–453, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell 18: 66–75, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshii A, Iizuka K, Dobashi K, Horie T, Harada T, Nakazawa T, Mori M. Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization. Am J Respir Cell Mol Biol 20: 1190–1200, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol Lung Cell Mol Physiol 274: L39–L46, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Zhou S, Potts EN, Cuttitta F, Foster WM, Sunday ME. Gastrin-releasing peptide blockade as a broad-spectrum anti-inflammatory therapy for asthma. Proc Natl Acad Sci USA 108: 2100–2105, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Zhu M, Liu PY, Kasahara DI, Williams AS, Verbout NG, Halayko AJ, Fedulov A, Shoji T, Williams ES, Noma K, Shore SA, Liao JK. Role of Rho kinase isoforms in murine allergic airway responses. Eur Respir J 38: 841–850, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]