Abstract
The health care burden of ST elevation myocardial infarction (STEMI) in India is enormous. Yet, many patients with STEMI can seldom avail timely and evidence based reperfusion treatments. This gap in care is a result of financial barriers, limited healthcare infrastructure, poor knowledge and accessibility of acute medical services for a majority of the population. Addressing some of these issues, STEMI India, a not-for-profit organization, Cardiological Society of India (CSI) and Association Physicians of India (API) have developed a protocol of “systems of care” for efficient management of STEMI, with integrated networks of facilities. Leveraging newly-developed ambulance and emergency medical services, incorporating recent state insurance schemes for vulnerable populations to broaden access, and combining innovative, “state-of-the-art” information technology platforms with existing hospital infrastructure, are the crucial aspects of this system. A pilot program was successfully employed in the state of Tamilnadu. The purpose of this article is to describe the framework and methods associated with this programme with an aim to improve delivery of reperfusion therapy for STEMI in India. This programme can serve as model STEMI systems of care for other low-and-middle income countries.
Keywords: STEMI reperfusion, Systems of care, Framework for a national strategy
1. Introduction
India has the highest burden of Acute Coronary Syndrome (ACS) patients in the world.1 Patients in India who suffer from ACS are younger (56.3 years) and have a higher proportion (>60.6%) of ST-elevation myocardial infarction (STEMI) than patients in developed countries.1 It is estimated that more than 3 million STEMI occurs every year in India.1 Since most of these patients are poor, less likely to get evidence-based treatments, and have greater 30-day mortality, reducing delays in access to hospital care and ensuring provision of affordable treatments could reduce morbidity and mortality.
This article attempts to lay down a framework for a National STEMI Program in India. Although this framework is national in its perspective, implementation will be made by individual states in conjunction with local communities. Attempts to run this in units smaller than a state (e.g., a few hospitals in a town) are fraught with the danger of this program not being inclusive and a substantial number of STEMI patients within the same geography being unable to benefit from this program.
While each state may make certain modifications based on their local needs, infrastructure and medical personnel availability, the basic framework, based on the experience of the Tamilnadu Pilot STEMI program, would be the ideal way forward.
2. Outline
-
1.
Why is there a need for a STEMI System of Care in India?
-
2.
Structure of the STEMI System of care
-
3.
Partners
-
4.
Infrastructure requirement
-
5.
Management structure
-
6.
Audit and quality improvement
-
7.
Timelines for implementation
3. Why is there a need for a STEMI system of care in India?
Despite all its recent and substantial economic advances, many people in India remain poor. Over 450 million Indians currently live at or below the poverty line, earning less than US$1.25 a day.2 Developing countries such as India, therefore, have many urgent public health needs to address, such as nutrition, sanitation and housing, as well as childhood vaccination and other preventive services. On the surface, these challenges make acute reperfusion therapy in STEMI patients appear less of a priority, a concern more relevant for affluent countries and healthcare systems. However, coronary artery disease (CAD) is a major contributor of death and disability in India, and its overall prevalence has risen dramatically over the past two decades.3 Approximately 3–4% of Indians in rural areas and 8–10% in urban areas have CAD.3 Moreover, Indians are more likely to develop CAD at younger ages during an individual's working years, and as a result, there is an extremely high loss of potentially productive years of life in India. Among working-age adults (35–64 years old), nearly 18 million productive years of life are expected to be lost from CAD by 2030, a number more than nine times higher than expected in the USA.4 This pattern of disease has substantial implications for India's growing workforce and economy. Another reason for concern is the growth of CAD among poor and middle-class Indians, when once it was considered a disease of the wealthy.5 Reasons for this include the potential relationship between fetal or childhood under nutrition and the subsequent development of cardiovascular risk factors; a disproportionate use of tobacco products among the poor; and less access to preventive services and medical care when compared with wealthier patients.6–8 Not surprisingly, recent studies suggest that poor patients with CAD in India appear to be at greater risk of acute presentations of CAD and have worse outcomes following such events. The most complete data about contemporary trends in STEMI patients come from CREATE, a large clinical registry of acute coronary syndrome patients from 89 large hospitals in 10 regions and cities across India.9 Among the more than 20 000 patients enrolled in CREATE, over 60% had STEMI, a proportion that is substantially higher than in North American and European registries. STEMI patients also were younger and had a lower socioeconomic status when compared with non- STEMI patients. The median time from the onset of symptoms to hospital arrival was 300 min in STEMI patients, again more than double the delay reported in developed countries. Finally, approximately 60% received fibrinolytic therapy and only 8% underwent percutaneous coronary intervention (PCI) during their hospitalisation, suggesting substantial room for improvement in the use of acute reperfusion therapy.10
All this clearly indicates the urgent need to develop a system for STEMI care in India.
4. Structure of the STEMI system of care
There are three ways of reperfusion in STEMI. The earliest studies examined thrombolytics, initially with streptokinase and subsequently with tissue plasminogen activator (TPA) and its analogues. A meta-analysis of thrombolytics showed that this was a good way of reperfusion with improved outcomes across subsets except in the elderly and those delayed beyond 12 h of symptom onset.11,12 Subsequently multiple studies have shown the superiority of primary PCI – both in terms of efficacy and mortality. USA and Europe have used this as the basis for developing a STEMI system of care. Although these systems are effective, they are resource intensive and this approach pre-supposes the availability of a fairly evenly distributed cath lab density coupled with a good emergency medical services (EMS) system and physical infrastructure for transportation. Data from National Interventional Council as depicted in Table 1, shows that there is a steady increase in the number of primary PCI done in India though the percentage remains the same (Table 1).13 However, still only a small minority of STEMI patients receive this modality of reperfusion.9
Table 1.
Interventions in Acute MI-Coronary Intervention data for the year 2012.13
| 2009 | 2010 | 2011 | 2012 | |
|---|---|---|---|---|
| Total no. of primary PCI | 5584 | 14271 | 20541 | 21343 |
| % of Total interventions | 9.79% | 12.15% | 13.48% | 12.04% |
Multiple studies have subsequently shown that a strategy of routine and systematic catheterization, with PCI if indicated, within 24 h of thrombolysis reduces the rate of re-infarction and is superior to the widely prevalent approach of thrombolysis followed by cath only for demonstrable ischemia – a strategy now called the Pharmaco-invasive strategy.14 The recent STREAM data15 and the Indian data from the STEP PAMI study16 showed that the pharmaco-invasive strategy compared well with primary PCI in reducing overall morbidity and mortality.
Based on this evidence, STEMI India has developed a strategy of combining primary PCI and the Pharmaco-invasive strategy of reperfusion to produce a coherent framework for developing a STEMI system of care suitable for India.17,18 The recommended timelines for the management of STEMI are given in Fig. 1.
-
1.
Primary PCI is advocated for patients located close to catheterization laboratories – mostly patients in urban areas with short transportation times to hospitals with 24/7 primary PCI capabilities.
-
2.
Patients in rural areas, with long transportation times to PCI capable hospitals, will utilise the Pharmaco-invasive strategy-of thrombolysis followed by catheterization and PCI if indicated, within 3–24 h of thrombolysis.
Fig. 1.
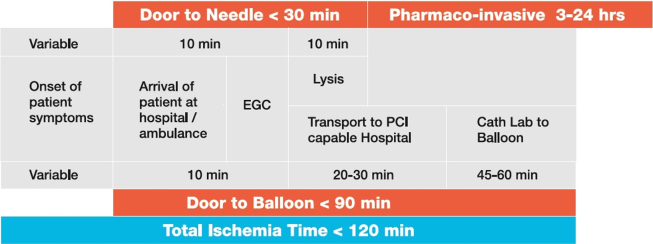
Recommended timelines for management of STEMI. FMC – First Medical Center, PHC-Primary HJealth Center.
The pilot Kovai Erode Study10 and the subsequent Pilot Tamilnadu STEMI program19 have shown the feasibility of combining the two strategies of primary PCI and the pharmaco-invasive strategy.
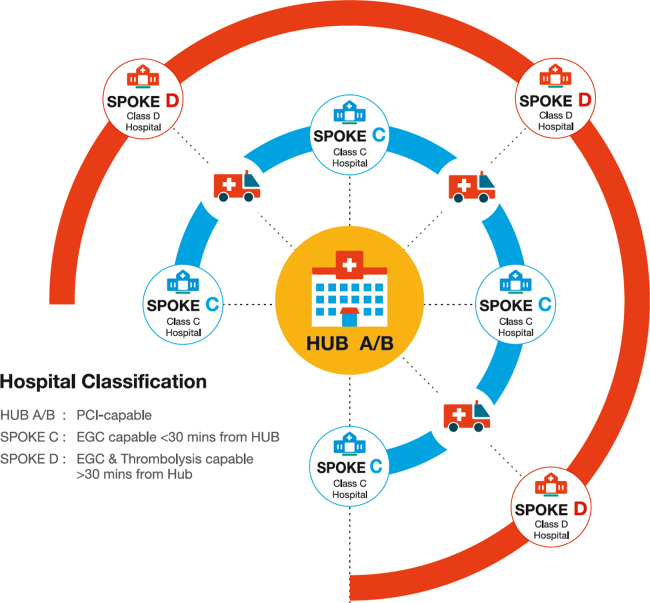
The architecture of this system is based on a ‘hub’ and ‘spoke’ model as depicted in Fig. 2, with each unit being called a STEMI cluster. Each cluster is made up of two types of hub hospitals, Class ‘A’ and Class ‘B’ hospitals and two types of spoke hospitals, Class ‘C’ and Class ‘D’ hospitals.
Fig. 2.
Architecture of an ST-elevation myocardial infarction cluster.
Class A hospital is a hub hospital with 24 h primary PCI capability. The aim is to perform primary PCI at Class A hospitals with a door to balloon time of less than 90 min. Patients transferred from a linked spoke hospital would also have their catheterization and PCI done here between 3 and 24 h of thrombolysis.
Class B hospital is also a hub hospital, however, the catheterization laboratory does not function 24/7. Patients would have primary PCI during regular working hours but may be thrombolysed during other times and then take the pharmacoinvasive approach with catheterization performed the next day. Spoke hospitals linked to the Class B hospital will thrombolyse patients and the transfer them here for further management.
Class C hospital is a spoke hospital located within 30 min transportation time of the hub hospital. Patients self-presenting to these hospitals will have ECG's done, STEMI confirmed and then transferred to the hub hospital for primary PCI.
Class D Hospital is a hospital located beyond 30 min transportation time of a hub hospital where a patient with STEMI is thrombolysed, stabilised and then transported to the hub hospital within 3–24 h for catheterization and PCI, if indicated.
Standardized protocols describing the expected care delivery for a STEMI patient have been designed. These are available from STEMI India. Different protocols have been implemented depending on the setting of care – i.e. emergency medical services, rural spoke hospitals, and PCI-capable hub hospitals.
However, the care at each of these locations is largely standardized to meet best practices. Protocols will be simple and straightforward to eliminate complexity which can lead to confusion during emergency care situations. Local adoptions of these standardized protocols will require education of healthcare providers. Protocols should be translated into local languages to increase their usefulness to patients and local healthcare providers but the core essence of the processes recommended should be maintained.
5. Partners
For the National STEMI program to be successful there has to be a clear partnership between the various key stakeholders.
-
1.The state government: Any STEMI program will require the support and involvement of the government. Social insurance to cover the below poverty line population, ambulance services and participation of the government hospitals in a STEMI program is crucial to its success as all these are controlled and facilitated by the state government. Furthermore, funding for the program will come from the health budget of each state government. The other important areas that the State Government, in consultation with the other stakeholders such as STEMI India, CSI and API, would be involved in would be as follows:
-
a.Legislation to accredit STEMI hospitals and prescribe minimum training, infrastructure and manpower requirements to handle STEMI patients
-
b.Legislation for EMS to bypass non STEMI hospitals and transport patients to STEMI accredited hospitals for management
-
c.Regulate new STEMI hospitals so that there is an even distribution of STEMI hospitals across a geographical area. This could be similar to the ‘certificate of need’ legislation in the USA. This would encourage new centres in inadequately served areas and discourage allocation of resources in other areas.
-
a.
-
2.
State wide ambulance network. The GVK EMRI ambulance service now exists in 15 states and union territories. It has a state wide presence and is an efficient and tested service. It is critical that an ambulance system similar to GVK EMRI be available for inter-hospital transfer.
-
3.
Cardiological Society of India (CSI): All the hub hospitals with catheterization laboratories and a significant number of thrombolytic spokes would have members of the CSI as the heads of the cardiology or medical departments. Each state has a state branch of the CSI and their involvement in the planning, development and running of the state program will be crucial for its success.
-
4.
Association Physicians of India (API): A significant proportion of the thrombolytic spoke hospitals are managed by physicians and not by cardiologists. Involvement of the state branch of the API is also therefore very essential.
-
5.
STEMI India will lead the national program by setting the national strategy and facilitating its implementation in different states. The Tamilnadu Pilot STEMI project tools, e.g., the protocols and manuals have already been developed and tested; technical knowhow and training programs are also available and can be used. However these may be modified, when required based on local needs, in consultation with the local state partners.
-
6.
Public: Engagement with the public to educate patients about symptoms of concern with STEMI and the availability of these services will need to be considered by states that begin to roll out these programs.
6. Infrastructure requirements
For the program to be successful, certain basic infrastructure has to be in place across the state. Each state desirous of starting a STEMI program is required to be well equipped before employing a state-wide program.
-
1.
Facility and manpower mapping: This is a basic pre-requisite to estimate the number centres capable of PCI or thrombolysis. It is also important to have data on the availability of number of cardiologists/physicians, 24/7 catheterization laboratory, and number of intensive care beds in each centre.
-
2.
Geographic mapping: This is necessary to decide on the size of each STEMI cluster and plan out the linkages. The number of spokes assigned to a hub would depend on the population covered, facilities for primary PCI and number of cardiologist available at that hub. Smaller hubs would have less spokes attached to them and would be smaller STEMI clusters. While it is desirable to have a minimum number of primary PCI in a hospital to qualify as a hub hospital, these criteria may need to be relaxed in poorly served areas. An appropriate decision should be made by the governing body in each state based on local need and resources.
-
3.
STEMI incidence and management data: This is important information that is required prior to implementing a STEMI program. A 3–4 month pre-implementation data collection from the hub and spoke hospitals would help to understand the current case load and treatment practices. This would help in planning the program as well as estimating the resource and manpower allocation requirements for a program.
-
4.
Availability of state-wide health insurance to take care of the vulnerable sections of the population. This insurance should take care of thrombolysis, Pharmaco-invasive management and primary PCI. The ‘Tamilnadu comprehensive health insurance scheme’ can be used as a model to cover these basic and necessary services.
-
5.
State wide ambulance service. The GVK EMRI Ambulance system is now available in 15 states of the country. The ambulance services function as an independent entity in each state and this is the model that, we believe, should be replicated.
-
6.
Project management team: Each state should develop a project management team responsible for administering the state's program on a day-to-day basis. Membership on the project management team should be multidisciplinary and will involve physicians, nurses, emergency personnel, information technology, and others as appropriate for each state. The project management team is responsible for program administration, STEMI protocol implementation, identification of local operational challenges, data collection, and reporting of performance as well as outcomes. The project management team also helps adoption of the program with a particular focus on bringing it to local population. This may involve local outreach into both urban and rural areas and also communication of the STEMI protocols and program in local languages. The project management team serves as the first contact for healthcare providers.
7. Management structure
The state wide STEMI program should be run by a governing body consisting of.
-
1.
State government
-
2.
Chief Operating Officer (COO) of the state ambulance services
-
3.
Representative of the state CSI
-
4.
Representative of the state API
-
5.
Insurance agency running the state insurance scheme and
-
6.
STEMI India
-
7.
Public representative
The governing body delegates the operations of the project to the project management team.
Members of the project management team include the following:
-
•
Project director
-
•
Physicians
-
•
Nurses
-
•
STEMI coordinator
-
•
Emergency personnel
-
•
Information technology
-
•
Biomedical engineer
-
•
Data analyst/statisticians
8. Audit and quality improvement
All centers participating in the STEMI program need to be committed to adequate data collection and reporting. Data collection should ideally be in real-time (i.e. at the time of patient care) and electronic with rapid transmission to centralized databases. Timely reporting of aggregate data should be submitted for review, both at the system level and also locally at each site of care. All centers should commit to accuracy of data collection to reflect the true nature of the patient care delivered. Results of data are best used to drive quality improvements in performance and punitive actions should be avoided.
A quality improvement (QI) committee may be formed for each region which would oversee the quality improvement activities such as conduct of periodic review meetings at predefined intervals to assess the effectiveness of the program as an ongoing process. The datasets that would be utilized for the assessment of quality improvement are also to be predefined and fulfill the goals to be achieved through this program. The overall care, outcomes and any scope for improvement could be assessed and discussed. Any issues identified are to be addressed with suggested corrective action plans and recommendations.
9. Timelines for implementation
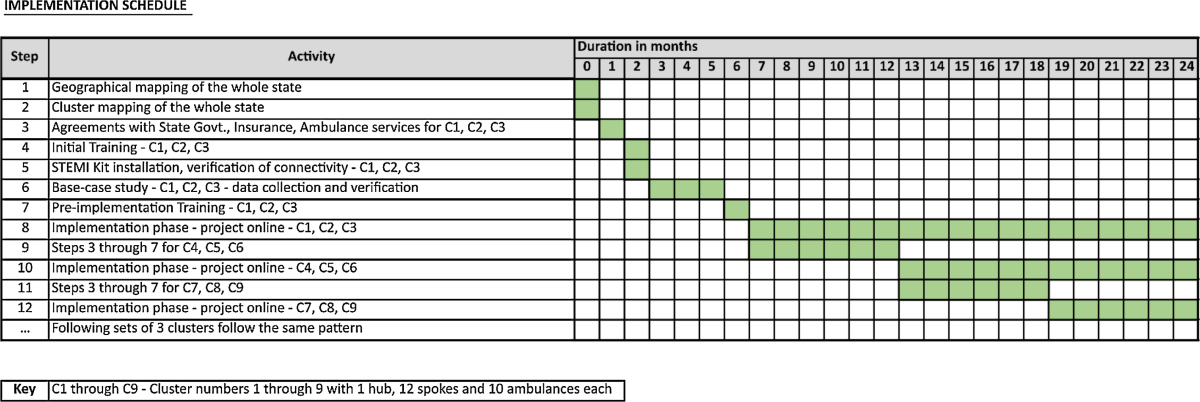
Implementation should occur in a systematic, step-wise fashion with specific attention to the required steps. Clinical deployment can occur in a staggered manner with 3–4 clusters initially and gradually more clusters can be added on to cover the whole state. The suggested timelines for implementation of a National STEMI framework are given in Table 2.
Table 2.
Timelines for implementation of National STEMI framework.
10. Conclusion
STEMI management in India evidently require organized systems of care to improve key processes. This “systems of care” for STEMI management will create new opportunities to deliver adequate reperfusion therapies in India by addressing various clinical, logistical and societal factors. As in other countries, effective management of STEMI at the community level in India will require executing proven treatment protocols along with efficient and rapid inter hospital transfer within coordinated hospital networks. Encouraged by the success of these protocols implemented in the state of Tamilnadu, we propose extension of this program to include the rest of the country. This approach is particularly worthwhile as it leverages unique public and private partnerships, technological innovation in monitoring devices, an expanding ambulance system, and novel strategies for reperfusion therapy and early invasive risk stratification. If successful, this type of network may be extended to other low and middle-income countries.
Conflicts of interest
The authors have none to declare.
References
- 1.National Commission on Macroeconomics and Health . Ministry of Health and Family Welfare, Government of India; Delhi, India: 2005. Burden of Disease in India.http://www.who.int/macrohealth/action/NCMH_Burden%20of%20disease(2%20Sep%202005).pdf Accessed 20.07.11. [Google Scholar]
- 2.Revised Poverty Estimates: What does this mean for India? http://www.worldbank.org.in/WBSITE/EXTERNAL/COUNTRIES/SOUTHASIAEXT/INDIAEXTN/0,con entMDK:21880804wpagePK:141137wpiPK:141127wtheSitePK:295584,00.html Accessed 20.07.11.
- 3.Ghaffar A., Reddy K.S., Singhi M. Burden of non-communicable diseases in South Asia. BMJ. 2004;328:807–810. doi: 10.1136/bmj.328.7443.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leeder S., Raymond S., Greenberg H. Columbia University; New York, USA: 2004. A Race Against Time: The Challenge of Cardiovascular Disease in Developing Economies.http://www.earthinstitute.columbia.edu/news/2004/images/Race against time_FINAL_051104.pdf Accessed 20.07.11. [Google Scholar]
- 5.Jeemon P., Reddy K.S. Social determinants of cardiovascular disease outcomes in Indians. Indian J Med Res. 2010;132:617–622. doi: 10.4103/0971-5916.73415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillman M.W. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rani M., Bonu S., Jha P. Tobacco use in India: prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control. 2003;12:e4. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramaraj R., Alpert J.S. Indian poverty and cardiovascular disease. Am J Cardiol. 2008;102:102–106. doi: 10.1016/j.amjcard.2008.02.104. [DOI] [PubMed] [Google Scholar]
- 9.Xavier D., Pais P., Devereaux P.J. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 10.Alexander T, Mehta S, Mullasari A et al. Systems of care for ST-elevation myocardial infarction in India: is it time? Hearthttp://dx.doi.org/10.1136/heartjnl-2011–301009. [DOI] [PubMed]
- 11.Armstrong P.W., Collen D., Antman E. Fibrinolysis for acute myocardial infarction. Circulation. 2003;107:2533–2537. doi: 10.1161/01.CIR.0000072930.64775.DC. [DOI] [PubMed] [Google Scholar]
- 12.Sabatine M.S., Cannon C.P., Gibson C.M. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 13.Data Presented at National Interventional Council. April 2013. [Calcutta] [Google Scholar]
- 14.Antman E.M., Van de Werf F. Pharmacoinvasive therapy. Circulation. 2004;109:2480–2486. doi: 10.1161/01.CIR.0000128736.57259.48. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong P.W., Gershlick A.H., Goldstein P. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2013 Apr 11;368:1379–1387. doi: 10.1056/NEJMoa1301092. [DOI] [PubMed] [Google Scholar]
- 16.Victor S.M., Subban V., Alexander T. A prospective, observational, multicentre study comparing tenecteplase facilitated PCI versus primary PCI in Indian patients with STEMI (STEPP-AMI) Open Heart. 2014 Aug 20;1:e000133. doi: 10.1136/openhrt-2014-000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalal J.J., Alexander T., Banerjee P.S. 2013 consensus statement for early reperfusion and pharmaco-invasive approach in patients presenting with chest pain diagnosed as STEMI (ST elevation myocardial infarction) in an Indian setting. J Assoc Physicians India. June 2014;62:13–23. [PubMed] [Google Scholar]
- 18.Banerjee A.K., Kumar S. Guidelines for management of acute myocardial infarction. J Assoc Physicians India. 2011 Special issue, 59:37–42. [PubMed] [Google Scholar]
- 19.Alexander T., Victor S.M., Mullasari A.S. Protocol for a prospective, controlled study of assertive and timely reperfusion for patients with ST-segment elevation myocardial infarction in Tamil Nadu: the TN-STEMI programme. BMJ Open. 2013;3:e003850. doi: 10.1136/bmjopen-2013-003850. [DOI] [PMC free article] [PubMed] [Google Scholar]