Abstract
Patients who survive traumatic brain injury (TBI) are often faced with persistent memory deficits. The hippocampus, a structure critical for learning and memory, is vulnerable to TBI and its dysfunction has been linked to memory impairments. Protein kinase RNA-like ER kinase regulates protein synthesis (by phosphorylation of eukaryotic initiation factor 2 alpha [eIF2α]) in response to endoplasmic reticulum (ER) stressors, such as increases in calcium levels, oxidative damage, and energy/glucose depletion, all of which have been implicated in TBI pathophysiology. Exposure of cells to guanabenz has been shown to increase eIF2α phosphorylation and reduce ER stress. Using a rodent model of TBI, we present experimental results that indicate that postinjury administration of 5.0 mg/kg of guanabenz reduced cortical contusion volume and decreased hippocampal cell damage. Moreover, guanabenz treatment attenuated TBI-associated motor, vestibulomotor, recognition memory, and spatial learning and memory dysfunction. Interestingly, when the initiation of treatment was delayed by 24 h, or the dose reduced to 0.5 mg/kg, some of these beneficial effects were still observed. Taken together, these findings further support the involvement of ER stress signaling in TBI pathophysiology and indicate that guanabenz may have translational utility.
Key words: : CHOP, ER stress, hippocampus, phosphatase, TBI
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability among young adults. One of the most disruptive symptoms experienced by TBI patients who survive their injuries are persistent memory deficits.1–3 These deficits include declarative memory and working memory dysfunction that are caused by damage to the hippocampus.4–11 Problems with memory can interfere with post-TBI recovery, rehabilitation, and living an independent life. Both clinical and experimental studies have shown that hippocampal neurons are particularly vulnerable to TBI, and loss of these neurons can continue for weeks to months after moderate-to-severe TBI.12,13 The link between hippocampal neuronal damage and memory impairments has been established both in human TBI patients and in rodent TBI models using hippocampal-dependent learning and memory tasks.14–16 However, no clinically proven neuroprotective therapy to reduce neuronal damage and improve learning and memory has been identified.17–19
Endoplasmic reticulum (ER) function is critical for maintaining intracellular calcium homeostasis, lipid biosynthesis, protein folding, and transport. Protein folding within the ER is highly sensitive to Ca2+ levels, redox states, and nutrient status.20–22 Altered levels of these molecules, as well as increased rate of protein synthesis and inflammation, can trigger ER stress.23 Unfolded protein response (UPR) is a collection of three signaling pathways (protein kinase RNA-like ER kinase [PERK], inositol-requiring enzyme-1 [IRE1], and activating transcription factor 6 [ATF6]) that are activated by ER stressors and serve to restore ER function and provide a conducive environment for proper protein folding.24,25 PERK is a single-pass mitochondria-associated ER membrane protein kinase that plays a principal role in restoring ER homeostasis.26,27 Activated PERK phosphorylates its downstream target, eukaryotic initiation factor 2 alpha (eIF2α), leading to a global reduction in protein synthesis that provides the ER with an opportunity to correct protein folding. However, if UPR signals are unable to resolve ER stress (e.g., when the stressor persists or is intense), eIF2α increases expression of the proapoptotic protein, CIEBP homologous protein (CCAAT) element-binding protein homologous protein (CHOP), leading to cell death.28–31
A number of studies have shown that TBI can cause oxidative stress, energy/glucose depletion, inflammatory response, and altered pH and calcium levels, all of which can induce ER stress.32–36 Consistent with this, a recent study has shown that controlled cortical impact (CCI) injury activates the PERK-eIF2α signaling pathway in the cortex.37 In this study, we report that administration of docosahexaenoic acid (DHA) to injured animals reduced the activation of this pathway and improved motor function. However, because DHA has been reported to target multiple mechanisms, it is unclear whether the observed beneficial effects resulted from a direct action on PERK-eIF2α. Further, the hippocampus, a structure within the temporal lobe, is highly vulnerable to TBI, with damage/dysfunction of this structure shown to underlie TBI-associated learning and memory dysfunction.4–10 At present, it is not known whether ER stress occurs in the hippocampus post-TBI, nor has it been examined whether lessening ER stress can improve learning and memory post-TBI. Guanabenz was recently identified as an inhibitor of growth arrest and DNA damage-inducible protein 34 (an eIF2α phosphatase), and its exposure to HeLa cells increases eIF2α phosphorylation.38 In the present study, we administered guanabenz post-CCI and compared the motor and cognitive function of drug-treated versus vehicle-treated injured rats. After the completion of behavioral testing, histopathological changes in both groups were compared to assess neuronal protection.
Methods
Materials
Male Sprague-Dawley rats (275–300 g) were purchased from Charles River Laboratories (Wilmington, MA). Guanabenz was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies (Abs) for phospho-eIF2α (p-eIF2α; Ser51) and total eIF2α were purchased from Cell Signaling Technology (Danvers, MA), and the Ab for ß-actin was obtained from Sigma-Aldrich. Abs for NeuN and microtubule-associated protein 2 (MAP2) were purchased from Millipore (Billerica, MA).
Production of cortical impact injury and drug administration
Surgical procedures were approved by the institutional animal care and use committee and were conducted in accord with the recommendations provided in the Guide for the Care and Use of Laboratory Animals. Protocols were designed to minimize pain and discomfort during the injury procedure and recovery period. An electromagnet-driven CCI device was used to cause brain injury, as previously described.9,39,40 Briefly, animals were anesthetized using 5% isofluorane with a 1:1 O2/N2O mixture, mounted on a stereotaxic frame, and a midline incision was made. Bilateral 6-mm craniectomies were produced midway between the bregma and lambda with the medial edges of the craniectomies 1-mm lateral to the midline. Although lateral CCI has been demonstrated to cause axonal damage, a key pathological component of TBI, this damage is predominantly found in the ipsilateral hemisphere. It has been shown that the presence of bilateral craniectomies shifts intracranial strain distribution to the contralateral hemisphere and causes increased contralateral axonal damage.41,42 As such, bilateral Craniectomies were chosen in order to increase the likelihood of contralateral hippocampal damage. Rats received a single impact (2.5-mm deformation) on the right parietal lobe with an impact velocity of 5 m/sec. Core body temperature was maintained at 37–38°C by use of a heating pad. Animals were given time to recuperate in a warming chamber before being returned to their home cages. Animals were weighed daily postinjury for the first 3 days, then weekly thereafter.
Primary neuronal culture and drug treatment
Cortical tissues from embryonic Sprague-Dawley rats (E17–E19) were dissected and dissociated, as described previously.43 Neurons were plated at a density of approximately 650,000 cells per well in 24-well poly-d-lysine-coated plastic culture dishes and maintained in a humidified CO2 incubator at 37°C. Cells were cultured for 7 days in neurobasal medium supplemented with 2 mM of glutaMAX, 2% B-27, and penicillin-streptomycin before use. A stock solution of guanabenz (20 mM) was prepared in sterile water, then diluted in conditioned media before addition to cells. Cells were incubated for 2 h, after which they were placed on ice, washed with ice-cold phosphate-buffered saline (PBS), and lysed in 60°C sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Resultant samples were sonicated to disrupt DNA and centrifuged at 20,000g for 30 min. Supernatant solution was removed and used for western blot analysis. Experiments were repeated three times.
Tissue sample preparation
At the indicated time points postinjury, hippocampal tissues were quickly removed while submerged in ice-cold artificial cerebral spinal fluid (10 mM of HEPES [pH 7.2], 1.3 mM of NaH2PO4, 3 mM of KCl, 124 mM of NaCl, 10 mM of dextrose, 26 mM of NaHCO3, and 2 mM of MgCl2) containing phosphatase inhibitors (2 mM of sodium fluoride, 2 mM of sodium molybdate, and 1 mM of sodium orthovanadate). Tissues were homogenized (20 strokes) in 10 volumes of a buffer containing 10 mM of Tris (pH 7.4), 1 mM of ethylene glycol tetraacetic acid (EGTA), 1 mM of ethylenediaminetetraacetic acid (EDTA), 0.5 mM of dithiothreitol (DTT), phosphatase inhibitors (0.1 μM of okadaic acid and 1 mM of sodium orthovanadate), and protease inhibitors (1 mM of phenylmethylsulfonyl fluoride [PMSF] and 10 μg/mL of leupeptin) using a motorized Teflon-glass homogenizer. Samples were immediately aliquoted and frozen at −80°C.
Western blotting
To examine whether TBI causes ER stress, rats were injured then euthanized at 3 h, 24 h, or 3 days postinjury (n=4/time point) and hippocampi removed and homogenized (20 strokes) in 10 volumes of a buffer containing 10 mM of Tris (pH 7.4), 1 mM of EGTA, 1 mM of EDTA, 0.5 mM of DTT, phosphatase inhibitors (0.1 μM of okadaic acid and 1 mM of sodium orthovanadate), and protease inhibitors (1 mM of PMSF and 10 μg/mL of leupeptin) using a motorized Teflon-glass homogenizer. Hippocampal tissue extracts were sonicated (5 pulses of 1 sec each) using a Sonics Vibracell sonicator (Sonics & Materials, Inc., Newtown, CT) and a 0.4-mm-diameter probe. The amount of protein in each sample was determined by a Micro BCA assay (Thermo Scientific, Waltham, MA) using bovine serum albumin (BSA) as the standard. Samples were denatured at 95°C for 3 min in 1×NuPage SDS sample buffer (Invitrogen, Carlsbad, CA). Equal amounts of protein were loaded, electro-phoresced, and transferred to Immobilon-P membranes (Millipore) using the NOVEX X-Cell II system (Invitrogen, Burlingame, CA) and the buffers provided by the vendor. Activation of ER stress was evaluated by examining phosphorylation of eIF2α on Ser51, the site of PERK phosphorylation. Total eIF2α and B-actin were used as loading controls. Membranes were blocked overnight in 5% BSA in Tris-buffered saline with 0.05% Tween-20 (TBST), followed by a 3-h incubation in primary Abs (0.1–0.5 μg/mL in TBST containing 2% BSA) at room temperature. Membranes were then washed in TBST and incubated at room temperature with alkaline phosphatase–conjugated secondary Abs for 1 h, as recommended by the vendor (Vector Laboratories, Burlingame, CA). After extensive washing, immunoreactivity was detected using a chemiluminescence system and quantified using ImageJ software (freely available through the National Institutes of Health [NIH], Bethesda, MD).
Drug preparation and administration
Guanabenz was dissolved in sterile saline to a concentration of either 2.5 (for the 5.0-mg/kg dose) or 0.25 mg/mL (for the 0.5-mg/kg dose). For testing the influence of guanabenz on cognitive function post-TBI, injured animals were assigned to one of four groups: 1) vehicle; 2) 5.0 mg/kg of guanabenz initiated 30 min postinjury; 3) 0.5 mg/kg of guanabenz initiated 30 min postinjury; or 4) 5.0 mg/kg of guanabenz initiated 24 h postinjury. The 5.0-mg/kg dose was based upon previous studies that demonstrated that guanabenz can reduce ER stress in mice,44,45 whereas the 0.5-mg/kg dose reflects the U.S. Food and Drug Administration (FDA)-approved dose range for guanabenz. All animals were injected for a total of 3 days, with the stock drug solution diluted such that a consistent volume was administered and a single vehicle group could be prepared as a control. This was done in order to minimize the number of animals required for the study. Injury and injections were staggered such that all animals were tested in the motor and cognitive tasks at the same time postinjury.
Assessment of motor function
All behavioral tests were conducted by an experimenter blind to the treatment groups. A vestibulomotor (beam balance) and a motor skill task (paw placement) were used to determine animals' motor performance on days 1–3 post-treatment, as described previously.39,46–48 For beam balance, rats were preassessed by placing them on a narrow wooden beam (1.5 cm wide) and measuring the duration they remained on the beam for up to 60 sec. Animals were given repeated training until capable of balancing on the beam for the entire 60-sec period for three consecutive trials. Postinjury, animals were given three daily trials during which the length of time spent on the beam was recorded. Paw placement was evaluated by placing the animal on a wire grid (opening size of 2×2cm) and counting the number of foot faults out of a total of 50 steps. A foot fault was defined as when a front paw missed and appeared below the plane of the grid. Paw placement was repeated three times to give an average daily score.
Assessment of cognitive function
Rats were tested for their cognitive performance using the novel object recognition (NOR) task, the standard hidden platform version of the Morris water maze (MWM), and a spatial reversal learning task as described previously.40,49–51 All animals had recovered from the TBI-associated motor dysfunction before performing the cognitive testing. The NOR task assesses recognition memory, which has been previously shown to be impaired post-TBI.52 The NOR task relies on the behavior that a rat will spend more time exploring a novel object than it does an object with which it is familiar.53 In this task, animals were pre-exposed to a testing chamber (100×100 cm box), after which two identical objects were introduced and the rat allowed to explore them for a period of 10 min. Twenty-four hours later, the rat was placed back into the training chamber as done for training; only one of the objects was replaced by a new object that had a different shape, but the same color and relative size. Time spent exploring the novel and familiar objects was recorded during the 4-min testing session with the difference in time spent exploring the novel object used as a measure of memory. For water maze testing, animals were given four consecutive training trials per day for 6 days with an intertrial interval of 4 min. If the animal failed to locate the platform within 60 sec on any given trial, it was led there by the experimenter. Twenty-four hours after the final day of training, rats were tested for their spatial memory using a probe trial in which the platform was removed and the animal allowed to search for a period of 60 sec. Forty-eight hours after completion of training, animals were tested in a reversal learning task. Reversal learning is a type of cognitive flexibility that allows an animal to update learned information in order to make an appropriate behavioral response that is opposite to that previously learned. This form of learning has been demonstrated to be more sensitive to subtle hippocampal damage than initial spatial learning.54,55 The platform was moved 180 degrees from its original location and rats given four trials to learn the new location within the maze. All other procedures as well as the extramaze cues remained identical to those used in the standard hidden platform training. Movement within the maze was monitored using a video camera linked to tracking software (Ethovision; Noldus Information Technology, Leesbury, VA).
Contusion volume measurement
After completion of the behavioral studies, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with PBS followed by 4% paraformaldehyde (PFA). Brains were removed, postfixed overnight in perfusant, then cryoprotected in a 30% sucrose solution. Cortical tissue loss was estimated essentially as described previously by experimenters kept blind with respect to the treatment groups.56 In brief, cryosections (40-μm thickness) spanning the rostral-caudal extent of the injured cortex were selected and stained with cresyl violet by an experimenter given only the animal's identifier code. Images of the resultant slides were then used for tissue loss measurement by a second experimenter. The area of cortical tissue loss for each section was carefully outlined using Adobe Illustrator, with the area of the resultant outlines quantified by ImageJ software (NIH). Contusion volume was calculated using the equation A1(0.5 * X1)+A2[(0.5 * X1)+(0.5 * X2)]+An-1[(0.5 * Xn-2)+(0.5 * Xn-1)]+An(0.5 * Xn-1), where A is the area (mm2) of the contusion for each slice and X is the distance (mm) between two sequential slices. Once contusion volume had been calculated for each animal, the blind code was broken and group differences assessed.
Immunohistochemistry
For post-TBI histological evaluation, additional tissue sections generated during measurement of cortical contusion volume were used for immunohistochemistry (IHC). Free-floating slices were incubated overnight in primary Ab (0.5–1.0 μg/mL) in Tris-buffered saline containing 2% BSA, 2.5% normal goat serum, and 0.25% Triton X-100. After extensive washing, immunoreactivity was detected using species-specific secondary Abs coupled to Alexa Fluors. These fluorescently labeled tissue sections were evaluated by a blind observer to identify any potential differences between treatment groups. Fluorescent intensity (FI) measurements for MAP2 disruptions were made in four nonoverlapping regions (100×100 μm) per section from both the damaged and nondamaged hippocampus. FI measurements from the damaged area were divided by the FI measurements in the undamaged hippocampus to determine the degree of immunoreactivity reduction.
Statistical analysis
For evaluation of behavioral data, repeated-measures analyses of variance (ANOVAs; two-way or one-way, as appropriate) and t-tests were utilized to determine statistical differences. For two-way ANOVAs, either group main effects or interactions of group and trial were used to determine statistical differences. Holm-Sidak's method for multiple comparisons post-hoc test was used to determine data points with significant differences. Western blot results and contusion volume data were evaluated using a one-way ANOVA followed by post-hoc t-tests to determine data points with significant differences. For data that did not pass a Shapiro-Wilk's normality test, appropriate nonparametric analysis was performed. Data were considered significant at p<0.05 and are presented as mean±standard error of the mean.
Results
Traumatic brain injury increases the phosphorylation of eukaryotic initiation factor 2 alpha in the hippocampus
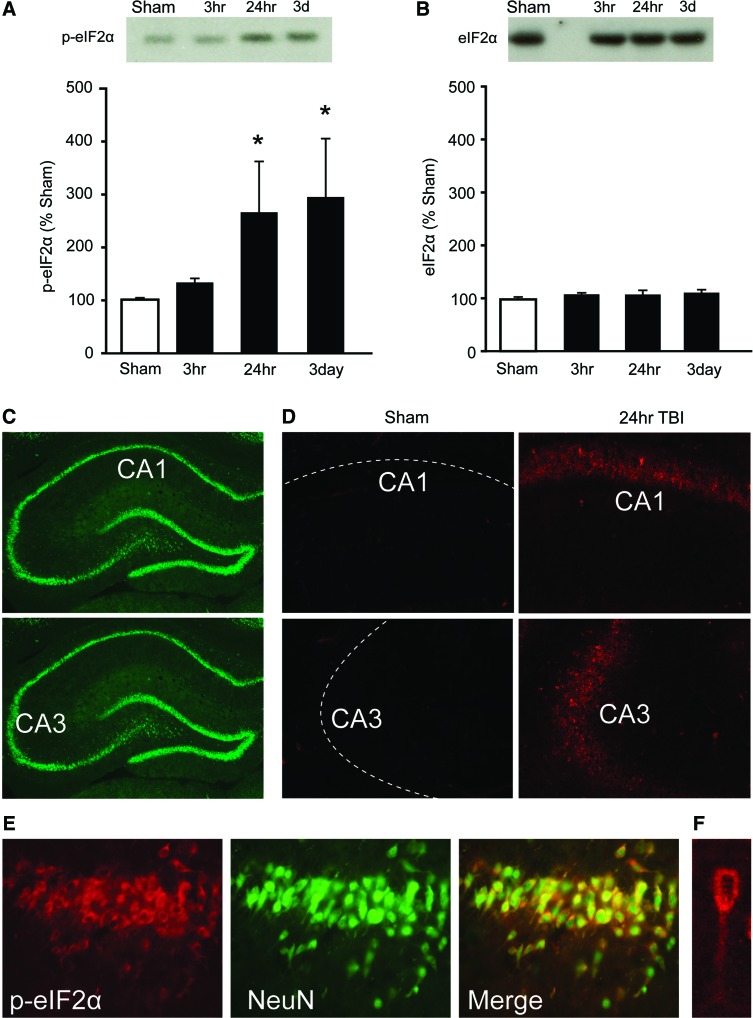
To determine whether brain injury activates eIF2α (a marker of ER stress) in the hippocampus, western blots using hippocampal tissue extracts were performed to examine levels of phosphorylated eIF2α. Figure 1A shows a representative picture of a western blot for phospho-eIF2α and summary data indicating that eIF2α phosphorylation is significantly increased by 24 h and 3 days post-TBI (Kruskal-Wallis' one-way ANOVA on ranks, H=10.39; p=0.016). In contrast, total levels of eIF2α did not change as a result of brain injury (one-way ANOVA, F(3,12)=0.88; p=0.48; Fig. 1B).
FIG. 1.
Traumatic brain injury (TBI) increases eIF2α phosphorylation in the hippocampus. Representative western blots and summary data (n=4/group) showing (A) phospho-eIF2α and (B) total eIF2α immunoreactivity at different time points postinjury. (C) Images of a NeuN-immunostained hippocampus showing relative positions of the CA1 and CA3 photomicrographs that are shown in (D). (D) Representative photomicrographs of phospho-eIF2α immunoreactivity in the CA1 and CA3 subfields in a sham and a 24-h post-TBI animal. Dotted lines indicate relative positions of neuronal cell layers. (E) Phospho-eIF2α immunoreactivity (red) in the hippocampus was found to colocalize with NeuN (green), indicating activation in neurons. The high-magnification image shown in (F) demonstrates cytosolic and dendritic localization of phospho-eIF2α in a CA1 pyramidal neuron. Data are presented as the mean±standard error of the mean. *p<0.05. p-eIF2α, phospho-eukaryotic initiation factor 2 alpha; TBI, traumatic brain injury; CA, cornu ammonis. Color image is available online at www.liebertpub.com/neu
To examine the cell types in which p-eIF2α was expressed, IHC was performed using tissue sections obtained from animals 24 h postinjury or sham operation. Figure 1C shows pictures of a hippocampal section from an uninjured animal immunostained with an Ab against the neuronal marker, NeuN. Figure 1D shows representative photomicrographs of tissue sections containing the dorsal hippocampus immunostained with the p-eIF2α Ab. In sham-operated animals, no signal could be detected in either the cornu ammonis (CA)1 or CA3 subfields. Dotted lines indicate the position of the neuronal layers. By 24 h postinjury, numerous cells expressing p-eIF2α could be observed in neuronal layers of both the CA1 and CA3 subfields of the ipsilateral hippocampus. Similar increases were observed in the granular cells of the dentate gyrus (data not shown). Consistent with its localization in the neuronal layers of the hippocampus, Figure 1E shows that p-eIF2α immunoreactivity (red) is predominately located within neurons, as indicated by colocalization (yellow) with the neuronal marker, NeuN (green). The high magnification image shows that p-eIF2α immunoreactivity can be observed in both the soma and apical dendrite of a CA1 pyramidal neuron (Fig. 1F).
Guanabenz treatment increases eukaryotic initiation factor 2 alpha phosphorylation in primary neuronal cultures
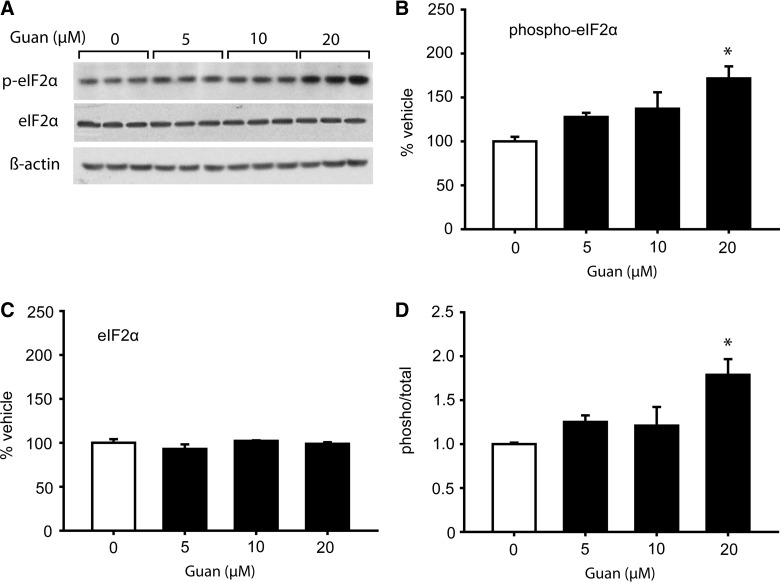
To examine whether guanabenz can increase eIF2α phosphorylation in neurons, cultured primary neurons were exposed to increasing concentrations of the drug. Representative western blots shown in Figure 2A indicate that 20 μM of guanabenz increased eIF2α phosphorylation, with no apparent change in total eIF2α or ß-actin. Quantification of immunoreactive bands indicated that guanabenz significantly increased eIF2α phosphorylation (one-way ANOVA, F(3,8)=6.11; p=0.018), but not total eIF2α (one-way ANOVA, F(3,8)=1.29; p=0.341; Fig. 2B,C). When the phosphorylation/total ratio was calculated for each sample, a significant increase (one-way ANOVA, F(3,8)=6.60; p=0.015) was detected at the 20 μM dose. Encouraged by these results, we next tested the consequences of guanabenz treatment in brain-injured animals.
FIG. 2.
Guanabenz increases eIF2α phosphorylation in cultured primary neurons. Primary neuronal cultures were treated with increasing concentrations of guanabenz. Two hours later, cells were lysed in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, sonicated, and equal volumes run in SDS-PAGE gels. (A) Pictures of western blots showing relative immunoreactivities of phospho-eIF2α (Ser 51), total eIF2α, and ß-actin in culture extracts. Quantification of the immunoreactive bands indicated that guanabenz increased (B) phosphorylation of eIF2α, but had no effect on (C) total eIF2α levels. Calculation of the phospho/total ratio indicated a significant increase in eIF2α phosphorylation at the 20-μM dose. Data are presented as the mean±standard error of the mean. *p<0.05. peIF2α, phospho-eukaryotic initiation factor 2 alpha. Guan, Guanabenz.
Post-traumatic brain injury administration of guanabenz improves motor and cognitive function
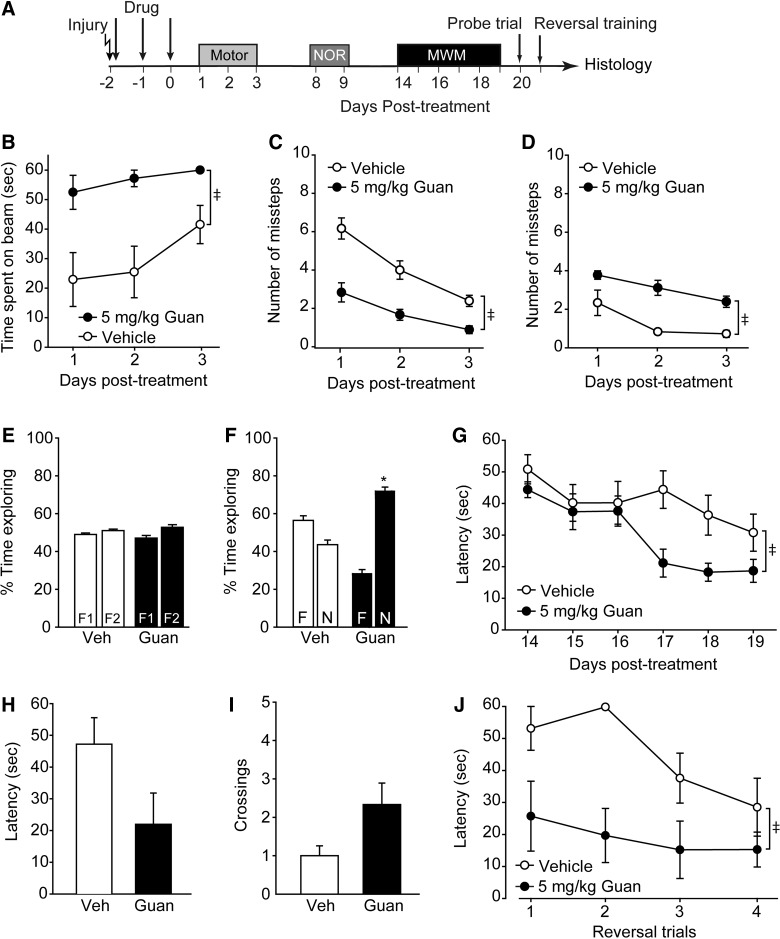
To examine whether guanabenz can influence outcome post-TBI, independent cohorts of rats (n=12) were injured and then randomly divided into two groups. Acute neurological responses (e.g., recovery of pain reflexes and righting response) were not significantly different between the two groups (data not shown). Beginning 30 min postinjury, one group was injected (intraperitoneal) with 5 mg/kg of guanabenz whereas the other received an equal volume of saline (Fig. 3A). Vestibulomotor and motor functions were assessed on days 1–3 post-treatment using the beam balance and foot-fault tasks, respectively. Figure 3B shows that vestibulomotor function was significantly improved as a result of postinjury guanabenz administration (group main effect, F(1,10)=15.07; p=0.003), evidenced by an improved ability to remain on a balance beam (Fig. 3B). When tested for their motor function using the foot-fault task, injured rats treated with guanabenz made significantly fewer contra- (interaction of group and trial, F(2,20)=36.94; p<0.001) and ipsilateral (group main effect, F(1,10)=31.40; p<0.001) forelimb foot faults than did vehicle-treated controls, suggesting improved motor function (Fig. 3C,D).
FIG. 3.
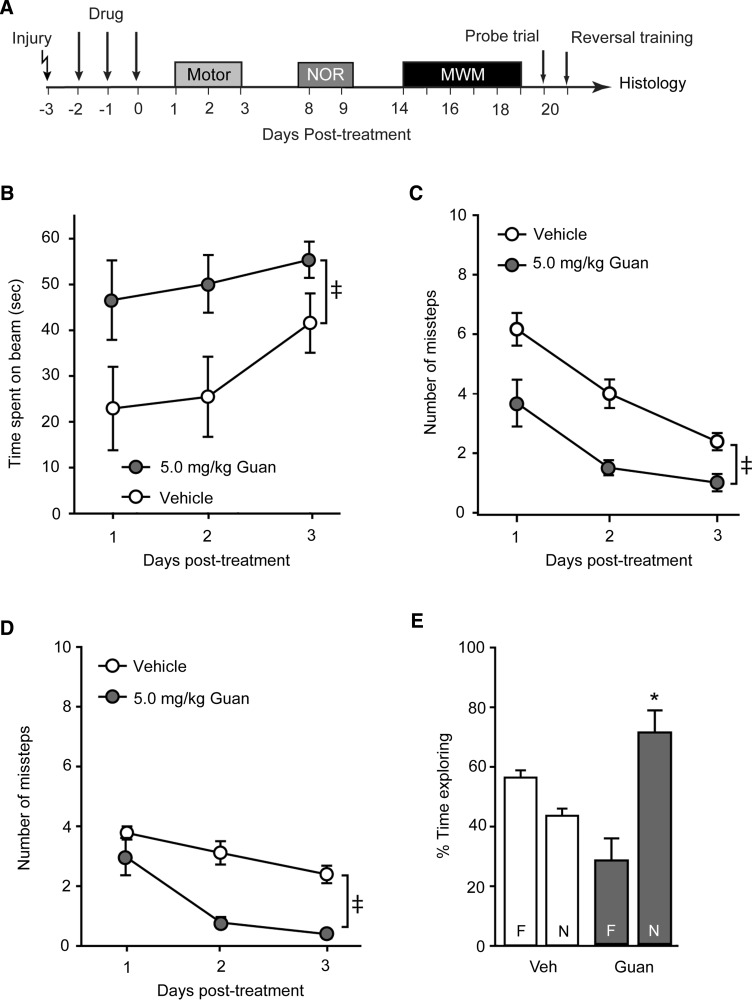
Postinjury administration of guanabenz improves vestibulomotor, motor and cognitive function. (A) Controlled cortical impact animals were treated starting 30 min postinjury with either 5.0 mg/kg of guanabenz or vehicle (n=6/group), then tested for motor and cognitive abilities. Rats treated with guanabenz had improved (B) vestibulomotor function, as indicated by improved ability to balance on a balance beam, and motor function, as indicated by reduced (C) contra- and (D) ipsilateral foot faults in a paw placement task. (E) Percent time exploring two objects during the familiarization period of the novel object recognition (NOR) task. (F) Rats treated with guanabenz had improved recognition memory, evidenced by increased time exploring the novel object in the NOR task. (G) Guanabenz-treated rats acquired the position of hidden platform in the Morris water maze (MWM) task significantly faster than vehicle-treated injured controls. Spatial memory testing did not reveal any significant differences in the two groups in terms of (H) latency to the hidden platform location or (I) the number of platform crossings. (J) When the platform was moved to a new location to test reversal learning, guanabenz-treated rats learned the new platform location significantly faster than vehicle-treated injured controls. Data are presented as the mean±standard error of the mean. ‡Significant difference by two-way repeated measures analysis of variance. *p<0.05. Veh, vehicle; Guan, Guanabenz.
On day 8 post-treatment, animals were trained in the NOR task by exposing them to two identical objects (F1 and F2). Figure 3E shows that, during training, both groups spent equivalent amounts of time exploring the two objects. On day 9, one of the objects was randomly chosen and replaced with a novel object that was similar in color and size, but different in shape. Vehicle-treated injured animals had recognition memory dysfunction, as indicated by equivalent times spent exploring the novel (N) and the familiar (F) objects (Fig. 3F). By comparison, injured rats treated with 5 mg/kg of guanabenz spent significantly more time exploring the novel object, suggesting improved recognition memory (Student's t-test, t=9.631; df=10; p<0.001). Spatial learning was assessed using the hidden platform version of the MWM on days 14–19 post-treatment. Figure 3G shows that, by comparison to vehicle-treated injured animals, guanabenz-treated rats had improved performance in the water maze task (group main effect, F(1,10)=6.075; p=0.033). When spatial memory was tested 24 h after training using a probe trial, the guanabenz-treated animals had reduced latency to find the hidden platform (Fig. 3H; Student's t-test, t=1.958; df=10; p=0.079) and crossed the hidden platform with greater frequency (Fig. 3I; Student's t-test, t=−2.169; df=10; p=0.055), although these measures did not reach statistical significance. When tested in a reversal learning paradigm, animals treated with 5 mg/kg of guanabenz postinjury quickly learned the new position of the hidden platform (group main effect, F(1,10)=18.234; p=0.002), indicating enhanced reversal learning (Fig. 3J). By comparison, vehicle-treated injured animals failed to effectively learn the new platform position.
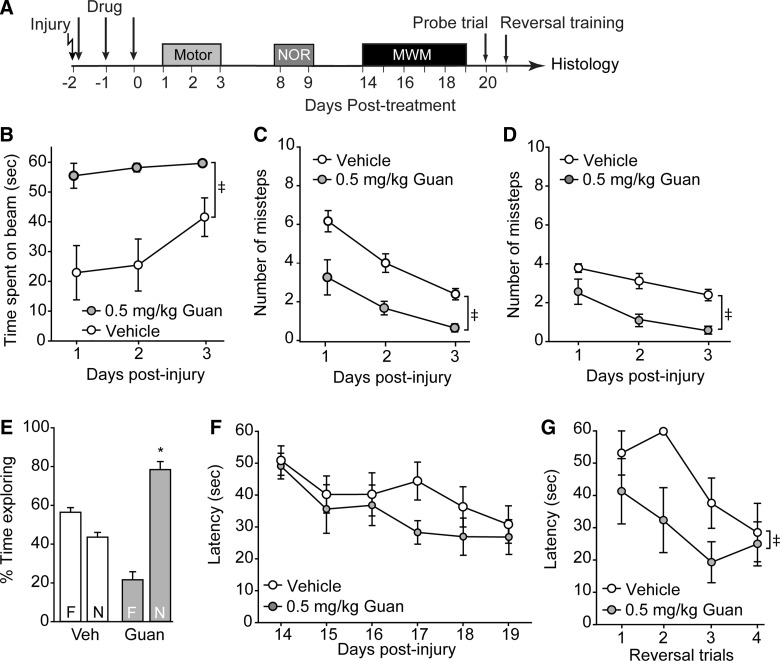
Low-dose guanabenz improves motor function and recognition memory
The timeline for injury and behavioral testing is shown in Figure 4A. Figure 4 shows that both vestibulomotor (Fig. 4B; beam balance: group main effect, F(1,10)=15.070; p=0.003) and motor (Fig. 4C, contralateral foot faults: group main effect, F(1,10)=17.153, p=0.002; Fig. 4D, ipsilateral foot faults: group main effect, F(1,10)=20.778, p=0.001) performances were significantly improved in the animals treated with 0.5 mg/kg of guanabenz, a dose within the FDA-approved range. Recognition memory was also found to be improved, as indicated by increased exploration of the novel object relative to the familiar one in the NOR task (Fig. 4E; p<0.001). Although acute postinjury administration of 0.5 mg/kg of guanabenz did not significantly improve initial learning in the MWM task (Fig. 4F; group main effect, F(1,10)=2.313; p=0.159), cognitive flexibility was enhanced, as indicated by a significant improvement in the reversal learning paradigm (Fig. 4G; group main effect, F(1,10)=5.584; p=0.040).
FIG. 4.
Low-dose guanabenz improves outcome after traumatic brain injury. (A) Controlled cortical impact animals were treated starting 30 min postinjury with either 0.5 mg/kg of guanabenz or vehicle (n=6/group), then tested for their motor and cognitive abilities. Rats treated with low-dose guanabenz had improved performance in the (B) beam balance task and displayed fewer (C) contra- and (D) ipsilateral foot faults than vehicle-treated injured animals. (E) Recognition memory, but not (F) spatial learning, was improved as a result of low-dose guanabenz treatment. (G) Reversal learning was significantly improved in injured rats treated with 0.5 mg/kg of guanabenz. Please note that the vehicle-treated group is the same as that shown in Figure 3. Data are presented as the mean±standard error of the mean. ‡Significant difference by two-way repeated measures analysis of variance. *p<0.05. NOR, novel object recognition; MWM, Morris water maze; Veh, vehicle; Guan, Guanabenz.
Delayed administration of guanabenz remains effective in reducing traumatic brain injury–associated motor and recognition memory dysfunction
To test whether guanabenz remained effective if the initiation time point was delayed, groups of rats were injured using the CCI device and received 5.0 mg/kg of guanabenz starting 24 h postinjury (Fig. 5A). Figure 5B shows that, when these animals were tested using the beam balance task, a significant improvement was observed compared to simultaneously tested vehicle-treated injured controls (group main effect, F(1,10)=5.068; p=0.048). Motor function was also found to be significantly improved after delayed guanabenz administration, with both the number of contra- (Fig. 5C; group main effect, F(1,10)=19.867; p=0.001) and ipsilateral (Fig. 5D; group main effect, F(1,10)=49.133; p<0.001) foot faults being significantly reduced. Delayed guanabenz administration also resulted in improved recognition memory (Fig. 5E; p=0.002), but did not improve either spatial learning (group main effect, F(1,10)=1.346; p=0.273) or reversal learning (group main effect, F(1,10)=4.630; p=0.057) tested using the water maze task (data not shown).
FIG. 5.
Delayed administration of guanabenz improves vestibulomotor, motor, and recognition memory after traumatic brain injury. (A) Controlled cortical impact animals were treated starting 24 h postinjury with either 5.0 mg/kg of guanabenz or vehicle (n=6/group), then tested for their motor and cognitive abilities. When treatment initiation was delayed, guanabenz remained effective at decreasing (B) vestibulomotor deficits tested using the beam balance task. Both (C) contra- and (D) ipsilateral foot faults were reduced in animals receiving delayed guanabenz treatment. (E) When tested for recognition memory, rats receiving guanabenz starting 24 h postinjury had a significant preference for the novel object, indicating improved recognition memory. Vehicle-treated animals did not remember the familiar object, spending equivalent amounts of time exploring it and the novel object. Please note that the vehicle-treated group is the same as that shown in Figure 3. Data are presented as the mean±standard error of the mean. ‡Significant difference by two-way repeated measures analysis of variance. *p<0.05. NOR, novel object recognition; MWM, Morris water maze; Veh, vehicle. Guan, Guanabenz.
Postinjury treatment with guanabenz offers neuroprotection
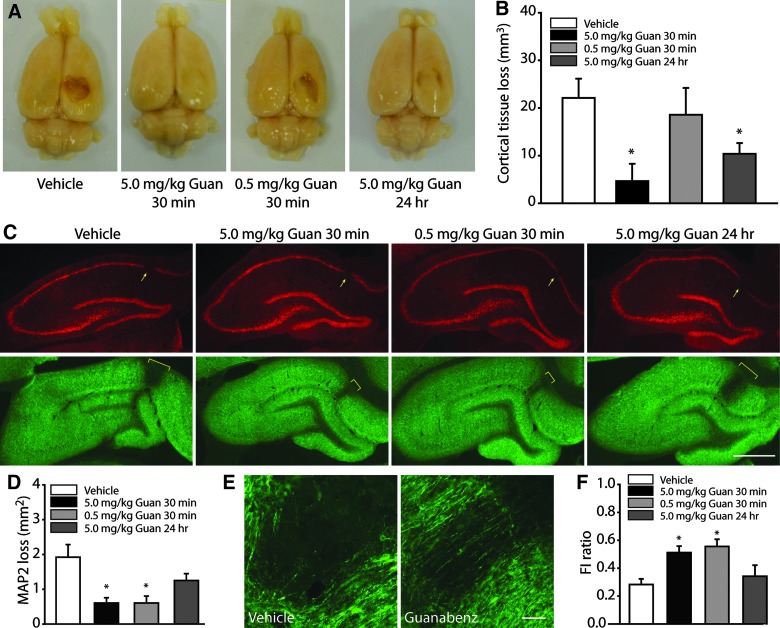
After completion of behavioral assessments, animals were transcardially perfused with PFA and brains removed for histological evaluation. Figure 6A shows representative photographs of brains from injured animals treated with vehicle, 5.0 mg/kg of guanabenz administered starting 30 min postinjury, 0.5 mg/kg of guanabenz initiated 30 min postinjury, or 5.0 mg/kg of guanabenz starting 24 h postinjury. Cortical tissue loss can be easily seen on the ipsilateral hemisphere in both the vehicle- and 0.5-mg/kg guanabenz-treated animals. This loss is apparently reduced in rats treated with 5.0 mg/kg of guanabenz, with both acute and delayed administration preserving cortical tissue. When tissue sections from these animals were used for contusion volume measurements, a significant difference across groups was observed. Post-hoc analysis revealed that this difference was owing to a statistically significant decrease in cortical contusion volume in rats treated with 5.0 mg/kg of guanabenz (Fig. 6B).
FIG. 6.
Guanabenz reduces cortical tissue loss and improves hippocampal integrity after traumatic brain injury. (A) Representative pictures of brains removed from injured animals treated with vehicle, 5.0 mg/kg of guanabenz initiated 30 min postinjury, 0.5 mg/kg of guanabenz started 30 min postinjury, and delayed administration of 5.0 mg/kg of guanabenz. (B) Summary data showing that 5.0 mg/kg, but not 0.5 mg/kg, of guanabenz significantly reduced cortical tissue loss in controlled cortical impact rats. (C) Representative photomicrographs showing that an absence in NeuN immunoreactivity (arrows) was detected in the cornu ammonis 1 subfield of the ipsilateral hippocampus in all groups, suggesting neuronal death. Accompanying this loss, disrupted microtubule-associated protein 2 (MAP2) immunoreactivity was detected (marked by yellow brackets), a finding indicative of dendritic loss/damage. Scale bar=1 mm. (D) Quantification of the area of MAP2 disruption revealed that animals treated with guanabenz 30 min postinjury, but not 24 h postinjury, had reduced dendritic damage. (E) High-magnification images showing that, in vehicle-treated injured animals, an almost complete loss of MAP2-positive dendrites occurred in the area of dendritic damage. By comparison, in an animal treated with 5.0 mg/kg of guanabenz 30 min postinjury, some sparred fibers can be seen. Scale bar=100 μm. (F) Quantification of relative MAP2 immunoreactivity (fluorescent intensity; FI) in the damaged versus healthy regions of the hippocampus revealed that animals treated acutely postinjury with either 5.0 mg/kg or 0.5 mg/kg of guanabenz had preserved dendrites within the area of damage. Delayed guanabenz treatment had a damaged/healthy FI ratio for MAP2 that was not significantly different than that calculated for vehicle-treated injured controls. Data are presented as the mean±standard error of the mean. *p<0.05. Guan, Guanabenz. Color image is available online at www.liebertpub.com/neu
Figure 6C shows that although all animals displayed neuronal loss in the CA1 subfield (as indicated by an absence of NeuN immunoreactivity; arrows) that was associated with a disruption in MAP2 immunoreactivity, the degree of dendritic loss appeared to be reduced in animals treated with guanabenz initiated 30 min postinjury. To quantify this, the extent of MAP2 disruption was outlined and the resultant area calculated. Figure 6D shows that there was a significant reduction in the area of dendritic damage in both the 5.0- and 0.5-mg/kg guanabenz-treated groups when the treatment was initiated 30 min postinjury. This protective effect was lost when treatment initiation was delayed to 24 h. In addition to a reduction in the area of dendritic damage, there was an apparent sparing of dendrites within the damaged region in response to guanabenz treatment. The high magnification images presented in Figure 6E show that virtually no MAP2-positive dendrites could be seen in the area of dendritic loss in the vehicle-treated animal. In contrast, several dendrites can be observed within the area of damage in the guanabenz-treated rat. When the FI of the MAP2 signal within the area of damage was compared to unaffected regions of the hippocampus (FI ratio: FI damaged/FI healthy), vehicle-treated animals had a 75% reduction in MAP2 immunoreactivity (Fig. 6F). In contrast, both groups of animals treated with guanabenz 30 min postinjury had preserved MAP2 immunoreactivity within the area of damage, giving rise to FI ratios of approximately 50%.
Discussion
Death and/or damage to neurons in the hippocampus, a brain structure involved in learning and memory storage, is thought to underlie some of the cognitive impairments that result from a TBI. Although it has been appreciated for years that a single TBI can trigger neurochemical changes that can give rise to both acute and progressive neurodegeneration, the molecular mechanisms that underlie TBI-triggered neurodegeneration have not been fully elucidated. In the present study, we examined the involvement of the PERK-eIF2α pathway, an arm of the UPR, in TBI pathology and cognitive outcome. Our results revealed three key findings: 1) TBI increases the phosphorylation of the PERK target, eIF2α, in the hippocampus of brain-injured animals; 2) postinjury administration of guanabenz, an inhibitor of eIF2α phosphatase, reduces neuronal damage, decreases contusion volume, and improves both motor performance and learning and memory; and 3) when the drug administration is delayed to 24 h after injury, some improvements in cognitive function can still be observed. Taken together, our results support the findings by Begum and colleagues, that ER stress contributes to cortical cell loss post-TBI and extends these observations to demonstrate that ER stress is an underlying mechanism by which TBI causes hippocampal damage and cognitive dysfunction.37
Three UPR signals (PERK, IRE1, and ATF6) are activated in response to altered Ca2+ levels, redox states, nutrient status, and inflammation, all of which are altered by TBI.32–35 PERK is rapidly activated in response to ER stressors and acts to inhibit global protein synthesis. Under normal physiological conditions, the chaperone, 78 kDa glucose-regulated protein (Grp78; a.k.a. binding immunoglobulin protein), binds to the ER luminal domain of PERK, keeping PERK in an inactive state.57 Accumulation of unfolded/misfolded proteins within the ER causes translocation of Grp78 away from PERK, after which PERK is transphosphorylated and activated.58 Activated PERK phosphorylates eIF2α (on serine 51), which results in the translational inhibition of messenger RNAs (mRNAs) containing a 7-methylguanosine 5′-cap. Unaffected by this translational block are mRNAs that use a non-cap-mediated translation mechanism, such as Grp78, and the transcription factor, ATF4.59,60 IRE1 is an endoribonuclease and a kinase that is inactive when bound to Grp78. Translocation of Grp78 in response to ER stressors leads to oligomerization and activation of IRE1.61 Active IRE1 removes a 26-base intron from Xbp1 mRNA, allowing translation of functional X-box binding protein 1 (Xbp1) protein. Xbp1 is a transcription factor that increases the expression of a class of genes whose protein products facilitate protein folding and degradation of misfolded proteins by ER stress-activated degradation.30,62 Similar to PERK and IRE1, the transcription factor, ATF6, is retained in the ER by Grp78. ER stressors cause Grp78 to dissociate, allowing ATF6 to move to the Golgi apparatus, where it is cleaved by the protease, S1P, to generate an active ATF6.63 ATF6 increases the transcription of genes, including Xbp1, to resolve ER stress.64,65 When ER stress cannot be mitigated, PERK increases CHOP expression and induces apoptosis.25 CHOP is a key proapoptotic transcription factor that down-regulates survival factors, such as B-cell lymphoma 2 (Bcl-2), while promoting the transcription of proapoptotic proteins, such as Bcl2-interacting mediator of cell death, leading to cell death.66 Thus, ER stress responses can be either pro-cell survival or pro-cell death, depending on the cellular context and the degree of ER stress signal activation.
Begum and colleagues have reported that CCI activates the PERK-eIF2α-ATF4 pathway in the injured cortex, resulting in a sustained activation between days 3 and 21 postinjury.37 Our results indicate that CCI also increases the phosphorylation of eIF2α in the hippocampus, with significant increases observed as early as 24 h postinjury. Given that activation of eIF2α attempts to mitigate ER stress, we reasoned that further increasing eIF2α phosphorylation would be beneficial. Consistent with this premise, inhibition of eIF2α phosphatase activity by guanabenz reduced hippocampal neuronal damage and improved hippocampus-dependent learning and memory. The neuroprotective effect of guanabenz is further supported by a recent study that indicated that guanabenz treatment enhances neuronal survival and prolongs the life span of mutant superoxide dismutase 1 mice.67,68 Further, salubrinal, a chemical inhibitor of eIF2α phosphatase, has also been shown to decrease excitotoxicity of neurons in culture and reduce accumulation of α-synuclein oligomers in a transgenic mouse model of Parkinson's disease.69,70 In addition to reducing learning and memory dysfunction, guanabenz significantly improved vestibulomotor and motor function in brain-injured animals. These findings are consistent with those of Begum and colleagues, who have reported improved performance in beam balance, beam walking, and fine motor function in injured animals treated with DHA, effects associated with reduced CHOP expression.37
In order to assess the translational significance of postinjury guanabenz treatment, we tested a dose of guanabenz comparable to that recommended by the FDA (0.5 mg/kg), and in separate animals, delayed the treatment initiation by 24 h. Low-dose guanabenz treatment (starting 30 min postinjury) improved both motor and cognitive function in brain-injured animals. Cognitive improvements were observed in both recognition memory and in reversal learning, effects consistent with those seen at the 5.0-mg/kg dose. When the treatment was delayed, however, the influence of the drug on postinjury spatial learning was lost. This suggests that delayed guanabenz treatment may not be effectively reducing hippocampal damage. Consistent with this, we observed that acute, but not delayed, administration of guanabenz was associated with a significant preservation of MAP2 immunoreactivity, suggesting less dendritic damage. However, we cannot rule out that a longer administration routine for guanabenz may have improved spatial learning even at the 24-h delay, given that Begum and colleagues reported that eIF2α phosphorylation persists for up to 21 days postinjury in the cortex.37 Interestingly, both acute and delayed guanabenz treatment improved recognition memory. A recent study has shown that the novel object recognition task depends on the function of the entorhinal cortex.71 Although we did not observe any overt neuronal loss or dendritic damage in either the ipsi- or contralateral entorhinal cortex (data not shown), we cannot rule out any influences guanabenz treatment may have had on the function of these neurons in the absence of observable neuroprotection.
To date, guanabenz is the only FDA-approved drug that has been shown to inhibit eIF2α phosphatase.38 Although our results support further translational testing of guanabenz as a treatment of TBI, it may have certain limitations. For example, as an antihypertensive, guanabenz may decrease blood flow to the injured brain. Previous clinical and experimental studies have shown that postinjury hypotension can exacerbate tissue damage.72,73 For example, a recent study by Robertson and colleagues has reported that a 60-min period of hypotension (MAP reduced to 40 mm Hg), when initiated up to 1 h postinjury, significantly increases cortical contusion volume.72 Although we have observed that guanabenz can cause a similar reduction in blood pressure in uninjured anesthetized animals (data not shown), both acute (30 min postinjury) and delayed (24 h postinjury) administration of 5.0 mg/kg of guanabenz reduced cortical tissue loss. Although the longer delay did not offer significant hippocampal protection, determination of the therapeutic window may help to maximize neuroprotection while minimizing adverse influences associated with postinjury mean arterial pressure (MAP) reduction. In addition to inhibiting eIF2α phosphatase, guanabenz is known to act as an alpha 2 adrenergic receptor agonist.74 Given that examination of the influence of postinjury alpha 2 agonism on outcome has been limited,75 the contribution of this mechanism to the beneficial effects of guanabenz we observed cannot be excluded. Finally, guanabenz has documented sedative effects. In our experiments, the sedative effects of guanabenz occurred within 30 min of injection and persisted for 4–6 h. As shown in Figure 3A, drug administration was discontinued 24 h preceding motor skills testing, and thus no animals were sedated during behavioral testing. However, because it has been shown that postinjury anesthesia can either exacerbate or improve TBI outcome,76 it remains unclear how the sedative effect of guanabenz impacted the results of this study. Additional studies are necessary to dissect the contribution of each of these mechanisms to the overall neuroprotection, motor, and cognitive improvements observed as a result of postinjury guanabenz treatment.
Acknowledgments
This project was made possible by funds provided to P.K.D. by the NIH (NS087149), the Gillson-Longenbaugh Foundation, and the TIRR Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Lew H.L., Poole J.H., Guillory S.B., Salerno R.M., Leskin G., and Sigford B. (2006). Persistent problems after traumatic brain injury: the need for long-term follow-up and coordinated care. J. Rehabil. Res. Dev. 43, vii–vix [DOI] [PubMed] [Google Scholar]
- 2.McAllister T.W. (1992). Neuropsychiatric sequelae of head injuries. Psychiatr. Clin. North Am. 15, 395–413 [PubMed] [Google Scholar]
- 3.Cernak I., and Noble-Haeusslein L.J. (2010). Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 30, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeves T.M., Lyeth B.G., Phillips L.L., Hamm R.J., and Povlishock J.T. (1997). The effects of traumatic brain injury on inhibition in the hippocampus and dentate gyrus. Brain Res. 757, 119–132 [DOI] [PubMed] [Google Scholar]
- 5.Lyeth B.G., Gong Q.Z., Shields S., Muizelaar J.P., and Berman R.F. (2001). Group I metabotropic glutamate antagonist reduces acute neuronal degeneration and behavioral deficits after traumatic brain injury in rats. Exp. Neurol. 169:191–199 [DOI] [PubMed] [Google Scholar]
- 6.Prins M.L., and Hovda D.A. (1998). Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. J. Neurotrauma 15, 799–811 [DOI] [PubMed] [Google Scholar]
- 7.Kline A.E., Yan H.Q., Bao J., Marion D.W., and Dixon C.E. (2000). Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci. Lett. 280, 163–166 [DOI] [PubMed] [Google Scholar]
- 8.Atkins C.M., Falo M.C., Alonso O.F., Bramlett H.M., and Dietrich W.D. (2009). Deficits in ERK and CREB activation in the hippocampus after traumatic brain injury. Neurosci. Lett. 459, 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992) Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 10.Piao S., Lee S.H., Kim H., Yum S., Stamos J.L., Xu Y., Lee S.J., Lee J., Oh S., Han J.K., Park B.J., Weis W.I., and Ha N.C. (2008). Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS One 3, e4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 12.Colicos M.A., Dixon C.E., and Dash P.K. (1996). Delayed, selective neuronal death following experimental cortical impact injury in rats: possible role in memory deficits. Brain Res. 739, 111–119 [DOI] [PubMed] [Google Scholar]
- 13.Titus D.J., Sakurai A., Kang Y., Furones C., Jergova S., Santos R., Sick T.J., and Atkins C.M. (2013). Phosphodiesterase inhibition rescues chronic cognitive deficits induced by traumatic brain injury. J. Neurosci. 33, 5216–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milner B., Squire L.R., and Kandel E.R. (1998). Cognitive neuroscience and the study of memory. Neuron 20, 445–468 [DOI] [PubMed] [Google Scholar]
- 15.Smith C.J., Johnson B.N., Elkind J.A., See J.M., Xiong G., and Cohen A.S. (2012). Investigations on alterations of hippocampal circuit function following mild traumatic brain injury. J. Vis. Exp. (69), e4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochanek A.R., Kline A.E., Gao W.M., Chadha M., Lai Y., Clark R.S., Dixon C.E., and Jenkins L.W. (2006). Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev. Neurosci. 28, 410–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoica B., Byrnes K., and Faden A.I. (2009). Multifunctional drug treatment in neurotrauma. Neurotherapeutics 6, 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Arrastia R., Kochanek P.M., Bergold P., Kenney K., Marx C.E., Grimes C.J., Loh L.T., Adam L.T., Oskvig D., Curley K.C., and Salzer W. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K.K., Larner S.F., Robinson G., and Hayes R.L. (2006). Neuroprotection targets after traumatic brain injury. Curr. Opin. Neurol. 19, 514–519 [DOI] [PubMed] [Google Scholar]
- 20.van der Vlies D., Makkinje M., Jansens A., Braakman I., Verkleij A.J., Wirtz K.W., and Post J.A. (2003). Oxidation of ER resident proteins upon oxidative stress: effects of altering cellular redox/antioxidant status and implications for protein maturation. Antioxid. Redox. Signal 5, 381–387 [DOI] [PubMed] [Google Scholar]
- 21.Chakrabarti A., Chen A.W., and Varner J.D. (2011). A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 108, 2777–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei Y., Thompson M.D., Cohen R.A., and Tong X. (2013). Endoplasmic reticulum stress and related pathological processes. J. Pharmacol. Biomed. Anal. 1, 1000107. [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhari N., Talwar P., Parimisetty A., Lefebvre , d’Hellencourt C., and Ravanan P. (2014). A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell Neurosci. 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J.H., Li H., Yasumura D., Cohen H.R., Zhang C., Panning B., Shokat K. M., Lavail M.M., and Walter P. (2007). IRE1 signaling affects cell fate during the unfolded protein response. Science. 318, 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin J.H., Li H., Zhang Y., Ron D., and Walter P. (2009). Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4, e4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teske B.F., Wek S.A., Bunpo P., Cundiff J.K., McClintick J.N., Anthony T.G., and Wek R.C. (2011). The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol. Biol. Cell. 22, 4390–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hetz C. (2012). The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 [DOI] [PubMed] [Google Scholar]
- 28.Sano R., and Reed J.C. (2013). ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 1833, 3460–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutkowski D.T., Arnold S.M., Miller C.N., Wu J., Li J., Gunnison K.M., Mori K., Sadighi Akha A.A., Raden D., and Kaufman R.J. (2006). Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J.H., Li H., Yasumura D., Cohen H.R., Zhang C., Panning B., Shokat K.M., Lavail M.M., and Walter P. (2007). IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheuner D., Patel R., Wang F., Lee K., Kumar K., Wu J., Nilsson A., Karin M., and Kaufman R.J. (2006). Double-stranded RNA-dependent protein kinase phosphorylation of the alpha-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J. Biol. Chem. 281, 21458–21468 [DOI] [PubMed] [Google Scholar]
- 32.Ji J., Kline A.E., Amoscato A., Samhan-Arias A.K., Sparvero L.J., Tyurin V.A., Tyurina Y.Y., Fink B., Manole M.D., Puccio A.M., Okonkwo D.O., Cheng J.P., Alexander H., Clark R.S., Kochanek P.M., Wipf P., Kagan V.E., and Bayir H. (2012). Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 15, 1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark R.S., Nathaniel P.D., Zhang X., Dixon C.E., Alber S.M., Watkins S.C., Melick J.A., Kochanek P.M., and Graham S.H. (2007). boc-Aspartyl(OMe)-fluoromethylketone attenuates mitochondrial release of cytochrome c and delays brain tissue loss after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 27, 316–326 [DOI] [PubMed] [Google Scholar]
- 34.Sullivan P.G., Springer J.E., Hall E.D., and Scheff S.W. (2004). Mitochondrial uncoupling as a therapeutic target following neuronal injury. J. Bioenerg. Biomembr. 36, 353–356 [DOI] [PubMed] [Google Scholar]
- 35.Pandya J.D., Nukala V.N., and Sullivan P.G. (2013). Concentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parameters. Front. Neuroenergetics. 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larner S.F., Hayes R.L., and Wang K.K. (2006). Unfolded protein response after neurotrauma. J. Neurotrauma 23, 807–829 [DOI] [PubMed] [Google Scholar]
- 37.Begum G., Kintner D., Liu Y., Cramer S.W., and Sun D. (2012). DHA inhibits ER Ca2+release and ER stress in astrocytes following in vitro ischemia. J. Neurochem. 120, 622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsaytler P., Harding H.P., Ron D., and Bertolotti A. (2011). Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 [DOI] [PubMed] [Google Scholar]
- 39.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 40.Dash P.K., Moore A.N., and Dixon C.E. (1995). Spatial memory deficits, increased phosphorylation of the transcription factor CREB, and induction of the AP-1 complex following experimental brain injury. J. Neurosci. 15, 2030–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao H., Yang K.H., King A.I., and Yang K. (2010). Computational neurotrauma—design, simulation, and analysis of controlled cortical impact model. Biomech. Model. Mechanobiol. 9, 763–772 [DOI] [PubMed] [Google Scholar]
- 42.Meaney D.F., Ross D.T., Winkelstein B.A., Brasko J., Goldstein D., Bilston L.B., Thibault L.E., and Gennarelli T.A. (1994). Modification of the cortical impact model to produce axonal injury in the rat cerebral cortex. J. Neurotrauma 11, 599–612 [DOI] [PubMed] [Google Scholar]
- 43.Tsvetkov A.S., Arrasate M., Barmada S., Ando D.M., Sharma P., Shaby B.A., and Finkbeiner S. (2013). Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat. Chem. Biol. 9, 586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Popko B., Tixier E., and Roos R.P. (2014). Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 71, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang H.Q., Ren M., Jiang H.Z., Wang J., Zhang J., Yin X., Wang S.Y., Qi Y., Wang X.D., and Feng H.L. (2014). Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience 277, 132–138 [DOI] [PubMed] [Google Scholar]
- 46.Dixon C.E., Kraus M.F., Kline A.E., Ma X., Yan H.Q., Griffith R.G., Wolfson B.M., and Marion D.W. (1999). Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 14, 285–294 [PubMed] [Google Scholar]
- 47.Hamm R.J., White-Gbadebo D.M., Lyeth B.G., Jenkins L.W., and Hayes R.L. (1992) The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurgery 31, 1072–1077 [DOI] [PubMed] [Google Scholar]
- 48.Dash P.K., Orsi S.A., Zhang M., Grill R.J., Pati S., Zhao J., and Moore A.N. (2010) Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One 5, e11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prins M.L., and Hovda D.A. (1998). Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. J. Neurotrauma 15, 799–811 [DOI] [PubMed] [Google Scholar]
- 50.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 51.Lyeth B.G., Jenkins L.W., Hamm R.J., Dixon C.E., Phillips L.L., Clifton G.L., Young H.F., and Hayes R.L. (1990). Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 526, 249–258 [DOI] [PubMed] [Google Scholar]
- 52.Prins M.L., Hales A., Reger M., Giza C.C., and Hovda D.A. (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 32, 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ennaceur A., and Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 31, 47–59 [DOI] [PubMed] [Google Scholar]
- 54.Whishaw I.Q., and Tomie J. (1997). Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 7:361–370 [DOI] [PubMed] [Google Scholar]
- 55.Pouzet B., Welzl H., Gubler M.K., Broersen L., Veenman C.L., Feldon J., Rawlins J.N., and Yee B.K. (1999). The effects of NMDA-induced retrohippocampal lesions on performance of four spatial memory tasks known to be sensitive to hippocampal damage in the rat. Eur. J. Neurosci. 11, 123–140 [DOI] [PubMed] [Google Scholar]
- 56.Sullivan P.G., Bruce-Keller A.J., Rabchevsky A.G., Christakos S., Clair D.K., Mattson M.P., and Scheff S.W. (1999). Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 19, 6248–6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., and Ron D. (2000). Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 [DOI] [PubMed] [Google Scholar]
- 58.Walter P., and Ron D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 59.Lee A.S. (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35, 373–381 [DOI] [PubMed] [Google Scholar]
- 60.Luo S., Baumeister P., Yang S., Abcouwer S.F., and Lee A.S. (2003). Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 278, 37375–37385 [DOI] [PubMed] [Google Scholar]
- 61.Ron D., and Hubbard S.R. (2008). How IRE1 reacts to ER stress. Cell 132, 24–26 [DOI] [PubMed] [Google Scholar]
- 62.Olivari S., Galli C., Alanen H., Ruddock L., and Molinari M. (2005). A novel stress-induced EDEM variant regulating endoplasmic reticulum-associated glycoprotein degradation. J. Biol. Chem. 280, 2424–2428 [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Shen J., Arenzana N., Tirasophon W., Kaufman R.J., and Prywes R. (2000). Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 275, 27013–27020 [DOI] [PubMed] [Google Scholar]
- 64.Yoshida H., Matsui T., Yamamoto A., Okada T., and Mori K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 [DOI] [PubMed] [Google Scholar]
- 65.Wu J., Rutkowski D.T., Dubois M., Swathirajan J., Saunders T., Wang J., Song B., Yau G.D., and Kaufman R.J. (2007). ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 [DOI] [PubMed] [Google Scholar]
- 66.McCullough K.D., Martindale J.L., Klotz L.O., Aw T.Y., and Holbrook N.J. (2001). Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tribouillard-Tanvier D., Beringue V., Desban N., Gug F., Bach S., Voisset C., Galons H., Laude H., Vilette D., and Blondel M. (2008). Antihypertensive drug guanabenz is active in vivo against both yeast and mammalian prions. PLoS One 3, e1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L., Popko B., Tixier E., and Roos R.P. (2014). Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 71, 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sokka A.L., Putkonen N., Mudo G., Pryazhnikov E., Reijonen S., Khiroug L., Belluardo N., Lindholm D., and Korhonen L. (2007). Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 27, 901–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colla E., Jensen P.H., Pletnikova O., Troncoso J.C., Glabe C., and Lee M.K. (2012). Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J. Neurosci. 32, 3301–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson D.I., Langston R.F., Schlesiger M.I., Wagner M., Watanabe S., and Ainge J.A. (2013). Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 23, 352–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Navarro J.C., Pillai S., Cherian L., Garcia R., Grill R.J., and Robertson C.S. (2012). Histopathological and behavioral effects of immediate and delayed hemorrhagic shock after mild traumatic brain injury in rats. J. Neurotrauma 29, 322–334 [DOI] [PubMed] [Google Scholar]
- 73.Hemerka J.N., Wu X., Dixon C.E., Garman R.H., Exo J.L., Shellington D.K., Blasiole B., Vagni V.A., Janesko-Feldman K., Xu M., Wisniewski S.R., Bayir H., Jenkins L.W., Clark R.S., Tisherman S.A., and Kochanek P.M. (2012). Severe brief pressure-controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J. Neurotrauma 29, 2192–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holmes B., Brogden R.N., Heel R.C., Speight T.M., and Avery G.S. (1983). Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs 26, 212–229 [DOI] [PubMed] [Google Scholar]
- 75.McAllister T.W., McDonald B.C., Flashman L.A., Ferrell R.B., Tosteson T.D., Yanofsky N.N., Grove M.R., and Saykin A.J. (2011). Alpha-2 adrenergic challenge with guanfacine one month after mild traumatic brain injury: altered working memory and BOLD response. Int. J. Psychophysiol. 82, 107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Statler K.D., Alexander H., Vagni V., Dixon C.E., Clark R.S., Jenkins L., and Kochanek P.M. (2006). Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J. Neurotrauma 23, 97–108 [DOI] [PubMed] [Google Scholar]