Abstract
Gram-positive cocci are emerging causes of spontaneous bacterial peritonitis (SBP), especially in patients with healthcare-associated infections. We report the case of a 68-year-old man with hepatitis C virus and alcohol-related cirrhosis who developed SBP due to methicillin-resistant Staphylococcus aureus treated with daptomycin. We discuss the potential role of daptomycin in this setting with a review of the literature about the use of daptomycin in primary or secondary bacterial peritonitis.
Key words: Daptomycin, cirrhosis, methicillinresistant Staphylococcus aureus, spontaneous bacterial peritonitis
Introduction
Spontaneous bacterial peritonitis (SBP) is a common bacterial infection in patients with cirrhosis and ascites. Its mortality is still high, accounting for 20-25% with early diagnosis and treatment.1 In recent years, a change in epidemiology of bacteria causing SBP has been observed in patients with long-term norfloxacin prophylaxis consisting in the emergence of quinolone-resistant bacteria as well as Gram-positive cocci, and further studies have documented an increasing frequency of Gram-positive cocci in patients with culture-positive SBP.2
Antibiotic options for multi-drug resistant (MDR) Gram-positive cocci are limited, and may lead to undesired toxic effects especially in cirrhotic patients with thrombocytopenia, renal failure, or hepato-renal syndrome. Herein we describe a case of methicillin-resistant Staphylococcus aureus (MRSA) SBP that was successfully treated with daptomycin and discuss the potential role of this antibiotic in intra-abdominal infections in patients with cirrhosis.
Case Report
A 68-year-old man with a history of HCV and alcohol-related cirrhosis (Child-Pugh stage C) was admitted for confused mental state, increased abdominal volume, edema of the lower limbs, and deterioration of general conditions lasting for several days. The patient had been hospitalized the previous month for ascites and decompensated cirrhosis, and had undergone paracentesis. Up to two weeks prior to the last hospitalization he was on ciprofloxacin 500 mg BID. Allergy to tetracycline was documented.
Upon admission the patient was in poor clinical conditions: blood pressure 100/50 mmHg, heart rate 100 beats per minute, respiratory rate 20 breaths per minute, with stupor, flapping tremor, significant bilateral ankle edema with positive fovea sign, and jaundice. There was abdominal distension, pain on deep palpation on the lower and lateral quadrants, and negative Blumberg’s sign. Blood gas analysis revealed respiratory and metabolic alkalosis; laboratory analysis showed total leukocytes of 11.7×109/L with neutrophilia, and thrombocytopenia (platelet count of 39×109/L); creatinine was 1.8 mg/dL (estimated creatinine clearance of 48 mL/min), hyperbilirubinemia (total 5.2 mg/dL, direct 2.9 mg/dL), increased INR (1.7), and hyperammonemia. The patient was treated with diuretics and ceftriaxone (2 g IV OD), and admitted to the internal medicine department.
After admission the patient’s conditions worsened with deterioration of encephalopathy, fever of 38°C, and an increase in creatinine (2.5 mg/dL) and C-reactive protein (120 mg/L; normal value <5 g/L). Blood and urine cultures were initiated. Explorative paracentesis detected the presence of polymorphonuclear neutrophils (910/mm3). Ceftriaxone therapy was replaced with meropenem (1 g IV BID; dose adjusted for creatinine clearance). Despite antibiotic therapy, fever persisted with hypotension and signs of sepsis. On day 4 after admission microbiological results of ascites fluid showed MRSA. The complete antibiogram of the isolate is shown in Table 1. After infectious disease consultation, daptomycin 6 mg/kg/day was initiated, considering the high MIC of vancomycin and the concomitant presence of renal insufficiency and thrombocytopenia. The informed consent was obtained from the patient.
Table 1.
Antibiogram of Staphylococcus aureus isolate.
| Antibiotic | Min. inhibitory concentration | Resistant/sensitive |
|---|---|---|
| Amoxicillin/clavulanic acid | ≥32 | R |
| Ampicillin/sulbactam | 32 | R |
| Fusidic acid | ≥32 | R |
| Cefoxitin screening | Positive | + |
| Quinupristin/dalfopristin | 0.25 | S |
| Gentamycin | 0.25 | S |
| Linezolid | 1 | S |
| Imipenem | 1 | S |
| Oxacillin | ≥4 | R |
| Rifampicin | <0.25 | S |
| Teicoplanin | 4 | S |
| Trimethoprim/sulfamethoxazole | <10 | S |
| Vancomycin | 2 | S |
| Tigecycline | 1 | S |
After initiation of anti-MRSA therapy there was progressive clinical improvement with defervescence, reduction of ammonemia, improvement of encephalopathy, and a decrease in abdominal volume and peripheral edema. The patient was treated with daptomycin for 12 days with progressive normalization of C-reactive protein; an increase of CPK and/or impairment of renal function were not recorded during the treatment. The patient was then discharged and referred to an ambulatory clinic.
Discussion
Chronic liver disease and cirrhosis are among the most common causes of acquired immunodeficiency, and patients with cirrhosis are at high risk for infections. In particular, bacterial infections represent the most frequent complications and dangerous complications in cirrhotic patients, especially in advanced stages of disease. It has been estimated that 30-50% of patients with cirrhosis present with a bacterial infection upon hospital admission or will develop it during recovery, with a mortality rate of around 25%.1
There is evidence for a progressive change in the epidemiology of chronic infections with an increasing prevalence of infections caused by multi-drug resistant (MDR) pathogens, and during last decades Gram-positive MDR bacteria have been becoming widespread in patients with either nosocomial or healthcare-associated (HCA) SPB. Campillo and colleagues were the first to report such increases in a prospective study on 194 consecutive episodes of SBP and 119 episodes of bacteremia: seventy percent of all infections were caused by Gram-positive bacteria, with S. aureus being the most prevalent pathogen (MRSA in 24.8% of cases); multivariate analysis showed that staphylococci were significantly associated with mortality [odds ratio (OR) 2.84, 95%CI 1.42-5.69, P=0.003].3 It is interesting to note that in our case the patient developed SBP caused by MRSA after a previous treatment of few weeks earlier with ciprofloxacin, which confirms the favorable effect of fluoroquinolones in selection of MRSA and other methicillin-resistant staphylococci.
A prospective study by our group on a cohort of hospitalized cirrhotic patients revealed that 41% of HCA infections are caused by MDR bacteria, and that Gram-positive bacteria (S. aureus followed by CNS and enterococci) are the primary cause of nosocomial SBP. Moreover, the frequent failure of empirical therapy with 3rd generation cephalosporins such as cefotaxime or ceftriaxone was seen, along with an association between the development of nosocomial infection and the number of procedures the patient has undergone and overcrowding of hospital rooms.4
The predisposing mechanism of SBP is intestinal bacterial translocation, which allows bacteria present in the intestinal microflora to reach the ascites via mesenteric lymphatic vessels (or sometimes even via the bloodstream resulting in secondary bacteremia). Several factors can predispose the cirrhotic patient to bacterial translocation through increased mucosal permeability, frequent alterations in immune function especially in the advanced stages of disease, bacterial overgrowth caused by concomitant therapies, and frequent intestinal bleeding. The pathophysiology of spontaneous bacterial peritonitis is shown in Figure 1.
Figure 1.
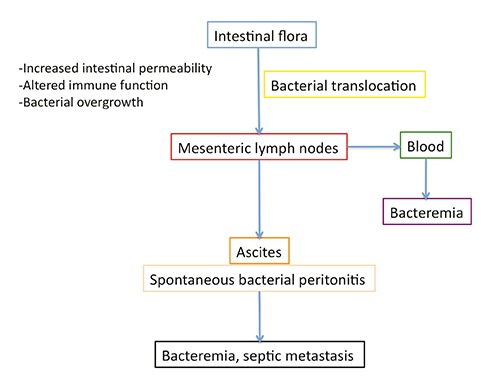
Pathophysiological mechanism of spontaneous bacterial peritonitis.
It is important for the clinician and gastroenterologist to consider nosocomial and HCA infections distinct from community infections: while in the latter a standard approach with 3rd generation cephalosporins seem justified, for nosocomial and HCA infections broad spectrum antibiotic therapy seems necessary that includes MRSA and Gram-negative MDR and XDR. Currently, vancomycin, which for years been the chief antibiotic for infection by MRSA, has limits in terms of in vitro sensitivity, toxicity, and overall management of patients. In fact, during the last decade several epidemiological studies have documented an increase in the mean MICs of vancomycin in clinical isolates of S. aureus, a phenomenon known as MIC creep. It has been demonstrated that the efficacy of vancomycin is directly correlated with the ability to achieve levels in blood and at the site of infection that are 10-15 × the MIC in order to obtain an adequate pharmacokinetic/pharmacodynamic target (ratio AUC/MIC). However, it is nearly impossible to reach such a target (and therefore have clinical efficacy) if the isolate has an MIC that is >1 mg/L since the concentrations of vancomycin needed to reach this level would invariably lead to renal insufficiency.5
A possible alternative is linezolid, which is adequately concentrated at the biliary level; however, it may be associated with myelotoxicity, and thrombocytopenia is frequent in cirrhotic patients with Child-Pugh stage C. In the clinical case described, it was problematic to use vancomycin due to the elevated MIC of the MRSA isolate (2 mg/L) and the presence of renal insufficiency. At the same time, linezolid did not seem to be indicated due to the presence of marked thrombocytopenia (39×109/L). Moreover, the patient was allergic to tetracycline, and thus tigecycline was contraindicated. Given the above, daptomycin was chosen. Daptomycin does not undergo hepatic metabolism, and dose adjustments are needed only in severe renal insufficiency (creatinine clearance <35 mL/min). There are some data on the use of daptomycin in intra-peritoneal infections. In an experimental mouse model of peritonitis caused by MRSA, Mortin et al. showed that daptomycin had greater and more rapid anti-bacterial activity than vancomycin or linezolid.6 Daptomycin has also been studied in patients undergoing peritoneal dialysis and pharmacokinetic data showed a good activity of daptomycin and optimal levels of the drug in peritoneal fluid.7 Moreover, Tascini et al. reported the drug to be effective at a dose of 8 mg/kg/day in the treatment of biliary-tract infections, and the drug reached significant concentrations both in bile and serum.8 In Table 2 is reported the review of literature about use of daptomycin in primary or secondary bacterial peritonitis.9-16 Recently, Piano et al. reported as the empiric use of daptomycin in association with meropenem was more effective than ceftazidime in the antibiotic treatment of nosocomial SBP due to Enterococcus spp.9
Table 2.
Review of literature about use of daptomycin in primary or secondary bacterial peritonitis.
| First author | Type of article | N° of patiens | Type of infection | Etiology | Dosage of daptomycin | Outcome |
|---|---|---|---|---|---|---|
| Burklein10 | Case report | 1 | Secondary peritonitis | Enterococcus faecium | 4 mg/kg/day | Cure |
| Huen11 | Case report | 2 | Peritonitis associated with peritoneal dyalisis | VR-Enterococcus faecium; VR-Enterococcus faecium | IP infusion with a loading dose of 100 mg/L, continued to 20 mg/L: IP infusion of 4 mg/L | Cure |
| Khadzhynov12 | Case report | 1 | Peritonitis associated with peritoneal dyalisis | Staphylococcus capitis | 5 mg/Kg every 48 hours | Cure |
| Hassoun13 | Case report | 1 | Peritonitis associated with peritoneal dyalisis | VR-Enterococcus faecium | IP 15 mg/kg once weekly | Cure |
| Bahte14 | Case report | 1 | Peritonitis associated with peritoneal dyalisis | Probably Staphylococcus aureus | 7 mg/kg intraperitoneally | Cure |
| Lin15 | Case report | 1 | Peritonitis associated with peritoneal dyalisis | MRSA | IP infusion with a loading dose of 100 mg/L, continued to 20 mg/L plus rifampin | Cure |
| Gilmore16 | Case report | 1 | Peritonitis associated with peritoneal dyalisis | Micrococcus spp + Enterococcus spp | IP infusion of 40 mg/in 2 L | Cure |
| Piano9 | Randomized clinical trial | 16 | Nosocomial SBP | Enterococcal species | 6 mg/Kg/day plus meropenem | Cure:13 patients Failure: 3 patients |
| Current case | Case report | 1 | SBP | MRSA | 6 mg/kg/day | Cure |
VR, vancomycin-resistant; IP, intraperitoneal; MRSA, methicillin-resistant Staphylococcus aureus; SBP, spontaneous bacterial peritonitis.
The approved dose of daptomycin is 6 mg/kg/day for bacteremia, although there is convincing evidence that higher doses are needed in critical patients with sepsis.17,18 In fact, patients with severe sepsis or in septic shock have several dysfunctions in the acute phase that can significantly reduce the serum levels of daptomycin. It may thus be necessary to increase the dose to 10 mg/kg/day or use a fixed dose such as 750 mg/day (doses should be reduced as soon as the patient achieves hemodynamic compensation).17 However, in the presence of reduced glomerular filtration the dose of daptomycin in cirrhotic patients should be carefully monitored, dosing the levels of the drug if possible, and reserving high doses only for critical cases.
Conclusions
We describe a case of SBP caused by MRSA in a cirrhotic patient with Child-Pugh stage C that was successfully treated with daptomycin. Additional clinical and pharmacokinetic data is needed regarding the available treatment options, including daptomycin. Considering its spectrum of activity of activity, tolerability, and preliminary results on concentrations at the sites of infection, daptomycin may be an option of interest in this setting.
Acknowledgements
Editorial assistance was provided by Health Publishing & Services, Milan, Italy and supported by Novartis Farma SpA.
References
- 1.de Mattos AA, Costabeber AM, Lionço LC, Tovo CV. Multi-resistant bacteria in spontaneous bacterial peritonitis: A new step in management? World J Gastroenterol 2014;20:14079-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulou A, Papadopoulos N, Eliopoulos DG, et al. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int 2013;33:975-81. [DOI] [PubMed] [Google Scholar]
- 3.Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis 2002;35:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol 2010;8:979-85. [DOI] [PubMed] [Google Scholar]
- 5.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 2012;54:755-71. [DOI] [PubMed] [Google Scholar]
- 6.Mortin LI, Li T, Van Praagh AD, et al. Rapid bactericidal activity of daptomycin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus peritonitis in mice as measured with bioluminescent bacteria. Antimicrob Agents Chemother 2007;51:1787-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khadzhynov D, Joukhadar C, Peters H. Plasma and peritoneal dialysate levels during daptomycin therapy for peritonitis. Am J Kidney Dis 2009;53:911-2. [DOI] [PubMed] [Google Scholar]
- 8.Tascini C, Di Paolo A, Polillo M, et al. Case report of a successful treatment of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and MRSA/vancomycin-resistant Enterococcus faecium cholecystitis by daptomycin. Antimicrob Agents Chemother 2011;55:2458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piano S, Fasolato S, Salinas F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized controlled clinical trial. Hepatology 2015. Jun 18 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Burklein D, Heyn J, Kirchhoff C, et al. Analysis of plasma and peritoneal fluid concentrations of daptomycin in a patient with Enterococcus faecium peritonitis. Int J Antimicrob Agents 2008;32:369-71. [DOI] [PubMed] [Google Scholar]
- 11.Huen SC, Hall I, Topal J, et al. Successful use of intraperitoneal daptomycin in the treatment of vancomycin-resistant enterococcus peritonitis. Am J Kidney Dis 2009;54:538-41. [DOI] [PubMed] [Google Scholar]
- 12.Khadzhynov D, Joukhadar C, Peters H. Plasma and peritoneal dialysate levels during daptomycin therapy for peritonitis. Am J Kidney Dis 2009;53:911-2. [DOI] [PubMed] [Google Scholar]
- 13.Hassoun AA, Coomer RW, Mendez-Vigo L. Intraperitoneal daptomycin used to successfully treat vancomycin-resistant enterococcus peritonitis. Perit Dial Int 2009;29:671-3. [PubMed] [Google Scholar]
- 14.Bahte SK, Bertram A, Burkhardt O, et al. Therapeutic serum concentrations of daptomycin after intraperitoneal administration in a patient with peritoneal dialysis-associated peritonitis. J Antimicrob Chemother 2010;65:1312-4. [DOI] [PubMed] [Google Scholar]
- 15.Lin SY, Ho MW, Liu JH, et al. Successful salvage of peritoneal catheter in unresolved methicillin-resistant Staphylococcus aureus peritonitis by combination treatment with daptomycin and rifampin. Blood Purif 2011;32:249-52. [DOI] [PubMed] [Google Scholar]
- 16.Gilmore JF, Kim M, LaSalvia MT, Mahoney MV. Treatment of enterococcal peritonitis with intraperitoneal daptomycin in a vancomycin-allergic patient and a review of the literature. Perit Dial Int 2013;33:353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falcone M, Russo A, Cassetta MI, et al. Variability of pharmacokinetic parameters in patients receiving different dosages of daptomycin: is therapeutic drug monitoring necessary? J Infect Chemother 2013;19:732-9. [DOI] [PubMed] [Google Scholar]
- 18.Falcone M, Russo A, Venditti M, et al. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2013;57:1568-76. [DOI] [PubMed] [Google Scholar]


