Abstract
Objective: To correlate the highest percentage core involvement (HPCI) and corresponding tumor length (CTL) on systematic 12-core biopsy (SBx) and targeted magnetic resonance imaging/transrectal ultrasonography (MRI/TRUS) fusion biopsy (TBx), with total MRI prostate cancer (PCa) tumor volume (TV).
Patients and Methods: Fifty patients meeting criteria for active surveillance (AS) based on outside SBx, who underwent 3.0T multiparametric prostate MRI (MP–MRI), followed by SBx and TBx during the same session at our institution were examined. PCa TVs were calculated using MP-MRI and then correlated using bivariate analysis with the HPCI and CTL for SBx and TBx.
Results: For TBx, HPCI and CTL showed a positive correlation (R2=0.31, P<0.0001 and R2=0.37, P<0.0001, respectively) with total MRI PCa TV, whereas for SBx, these parameters showed a poor correlation (R2=0.00006, P=0.96 and R2=0.0004, P=0.89, respectively). For detection of patients with clinically significant MRI derived tumor burden greater than 500 mm3, SBx was 25% sensitive, 90.9% specific (falsely elevated because of missed tumors and extremely low sensitivity), and 54% accurate in comparison with TBx, which was 53.6% sensitive, 86.4% specific, and 68% accurate.
Conclusions: HPCI and CTL on TBx positively correlates with total MRI PCa TV, whereas there was no correlation seen with SBx. TBx is superior to SBx for detecting tumor burden greater than 500 mm3. When using biopsy positive MRI derived TVs, TBx better reflects overall disease burden, improving risk stratification among candidates for active surveillance.
Introduction
Prostate cancer (PCa) tumor volume (TV) has been directly correlated with histopathologic stage and biochemical recurrence after radical prostatectomy (RP), and therefore is an important prognostic factor.1,2 During initial evaluation, tumor quantification with percent of positive cores and/or core length involvement on prostate biopsy is used to infer burden of disease and subsequently risk stratify patients. The percent of tumor involvement in positive cores and core tumor length on systematic transrectal ultrasonography (TRUS)-guided prostate biopsy (SBx) have been correlated with TV as well as pathologic and oncologic outcomes.3–8 Accurate assessment of core involvement by percent core and core length is therefore critical in stratifying PCa patients, particularly those being considered for active surveillance (AS) versus local and/or definitive treatment.
Advances in multiparametric prostate magnetic resonance imaging (MP-MRI) have allowed for better visualization of the intraprostatic anatomy and identification of potentially malignant lesions. MP-MRI derived TV is positively correlated with and accurately predicts histopathologic TV from RP specimens, making it a reliable surrogate for PCa TV calculation.9–11 The targeted MRI/TRUS fusion biopsy (TBx) platform directly targets and more accurately samples intraprostatic lesions.12–14 This improved targeting may allow for better assessment of tumor burden and more accurate risk stratification in patients with PCa when compared with 12-core SBx.
The aim of this study was to determine the correlation of highest percentage core involvement and corresponding core tumor length, obtained with SBx and TBx, with total MRI PCa TV.
Patients and Methods
Study population
This prospective study assessing TBx with electromagnetic tracking at the National Cancer Institute (NCI) of the National Institutes of Health (NIH) was approved by the Institutional Review Board (ClinicalTrials.gov identifier: NCT00102544). Patients were prospectively enrolled and provided written informed consent. Patients who underwent MP-MRI with SBx and TBx at the NCI between January 2007 and June 2013 were reviewed and retrospectively analyzed.
We identified 823 patients who had MP-MRI with subsequent SBx and TBx during the study period. Fifty of these patients had a diagnosis of PCa and met criteria for AS, based on outside SBx, defined by clinical stage T1c disease, PSA density <0.15 ng/mL, Gleason score ≤6, two or fewer biopsy cores with cancer, and a maximum of 50% involvement of any core with cancer.15 These patients were included for analysis. Patient demographics, prebiopsy prostate-specific antigen (PSA) level, PSA density, number of cancer-positive lesions, lesion diameter, and total PCa TV were noted.
MP-MRI and segmentation
Diagnostic MP-MRI using a 3.0T MRI scanner (Achieva; Philips Healthcare, Andover, MA) with a 16-channel cardiac surface coil (SENSE; Philips Healthcare) positioned over the pelvis and an endorectal coil (BPX-30; Medrad Inc., Pittsburgh, PA) was performed. Tri-planar T2 weighted (T2W), axial diffusion weighted (DW) imaging, axial dynamic contrast-enhanced (DCE) and three-dimensional MR spectroscopy were conducted according to protocol as described previously.16 Images underwent blinded centralized radiologic evaluation by two genitourinary radiologists (BT and PLC) with 8 and 14 respective years of experience with MP-MRI. Screen-positive lesions were identified and assigned PCa suspicion scores (low, moderate, and high) based on the number of positive imaging sequences for each individual lesion in accordance with previously described criteria.14
A commercial research software platform (iCAD, Nashua, NH) was used to measure greatest diameter, manually segment, and calculate the TV for each identified lesion on MRI, blinded to the clinical and histopathologic data. TV calculation was standardized by summation of MRI TVs from biopsy positive lesions determined on TBx. For tumor segmentation on MRI, T2W MRI, apparent diffusion coefficient maps of DW-MRI and DCE-MRI sequences were integrated and used in combination. The final regions of interest, however, were drawn on T2W MRI for targeting during fusion biopsy.
Biopsy protocol
After baseline MP-MRI study, patients with identified lesions suspicious for PCa (stratified based on the validated NIH scoring system) subsequently underwent prostate biopsy.14 All patients underwent SBx, in which the operator was blinded to the suspicious lesions that were identified on MRI. During the same biopsy session, the patients also had a TBx using the prebiopsy MP-MRI images, which were segmented, registered, and fused with the TRUS images using electromagnetic tracking. For each suspicious lesion, a core was taken in the axial and sagittal planes targeting the center of the lesion.12,17 All biopsies underwent blinded centralized histopathologic evaluation by a single genitourinary pathologist (MJM).
Study design
The highest percentage of a positive core and the corresponding core length in centimeters, for both SBx and TBx, was determined for each patient. Only biopsy results that corresponded temporally with the MP-MRI study used for lesion segmentation were evaluated for this study, irrespective of multiple MP-MRI studies or repeated biopsy sessions. Based on the TBx positive lesions, the individual TVs calculated were summated to obtain a total PCa TV for each patient. Results from SBx and TBx were also compared to assess the detection of MRI derived tumor burden greater than 500 mm3, a previously identified threshold for low volume disease.1 The biopsy parameter used was the highest percentage core involvement at a threshold of 50%, a value commonly used in multiple AS criteria.18
Statistical analysis
Descriptive statistics were used to report patient demographics. Bivariate analysis was used to determine the empirical relationship, for both SBx and TBx, between the highest percentage core involvement and corresponding tumor length and the total PCa TV. The correlative R2 value for each of these was also determined. JMP Pro v.10.0 (SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results
Patient-level characteristics of the cohort are outlined in Table 1. The mean age of the patient population was 61.4 years, mean prebiopsy PSA level was 5.34 ng/mL, and the mean prebiopsy PSA density was 0.12 ng/mL2. The mean number of PCa lesions based on TBx was 1.38 lesions and mean lesion diameter was 1.1 cm.
Table 1.
Demographics, Multiparametric Prostate Magnetic Resonance Imaging and Biopsy Findings of 50 Men with Prostate Cancer Meeting Criteria for Active Surveillance
| Characteristics | P-Value |
|---|---|
| Number of men | 50 |
| Age, year, mean±SD | 61.4±7.7 |
| PSA, ng/mL, mean±SD | 5.34±2.6 |
| PSA density, ng/mL2, mean±SD | 0.12±0.07 |
| Highest cancer suspicion score on MP-MRI, No. (%)a | |
| Low | 9 (18) |
| Moderate | 32 (64) |
| High | 9 (18) |
| Biopsy parameters | Mean±SD |
| Number of lesions biopsied | 2.6±1.4 |
| Number of positive lesions (on targeted biopsy) | 1.38±0.6 |
| Lesion diameter of positive lesions, cm | 1.1±0.5 |
| Total positive tumor volume, mm3 | 968.3±1078.6 |
| Highest % core involvement (on systematic 12-core biopsy) | 22.5±22 |
| Highest % core tumor length (on systematic 12-core biopsy), cm | 0.33±0.32 |
| Highest % core involvement (on targeted biopsy) | 34.6±27.4 |
| Highest % core tumor length (on targeted biopsy), cm | 0.49±0.43 |
Based on appearance of suspicious lesions on four different magnetic resonance imaging parameters as noted in Methods.
PSA=prostate-specific antigen; SD=standard deviation; MP-MRI=multiparametric prostate magnetic resonance imaging.
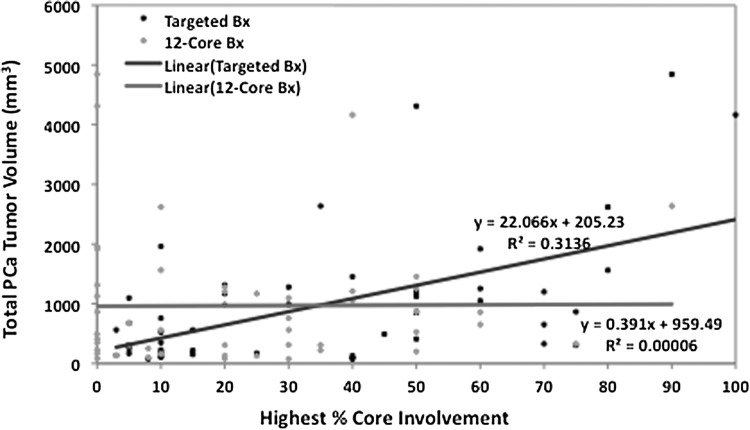
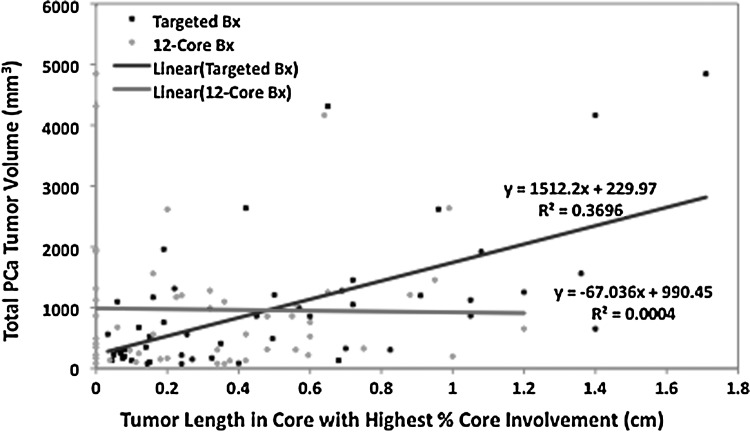
The mean total PCa TV for the cohort was 968.3 mm3 (Table 1). TBx diagnosed PCa in 50 patients and SBx diagnosed PCa in 37 patients, resulting in a cancer detection rate of 100% and 74%, respectively. Figure 1 illustrates the linear regression model presenting the correlation of the highest percentage core involvement to total PCa TV for SBx and TBx. For the highest percentage core involvement, TBx showed a positive correlation (R2=0.31) whereas SBx showed a poor correlation (R2=0.00006) with total PCa TV (P<0.0001 and P=0.96, respectively). Figure 2 illustrates the linear regression model presenting the correlation of the tumor length in the core with the highest percentage involvement to total PCa TV for SBx and TBx. For the tumor length of the highest percentage core, TBx showed a positive correlation (R2=0.37) whereas SBx showed a poor correlation (R2=0.0004) with total PCa TV (P<0.0001 and P=0.89, respectively).
FIG. 1.
Correlation of highest percentage core involvement to total positive tumor volume for systematic 12-core biopsy and targeted magnetic resonance imaging/transrectal untrasonography fusion biopsy (Bx). PCa=prostate cancer.
FIG. 2.
Correlation of tumor length in core with highest percentage core involvement to total positive tumor volume for systematic 12-core biopsy and targeted magnetic resonance imaging/transrectal untrasonography fusion biopsy (Bx).
Of particular clinical relevance is the ability of the biopsy core to reflect clinically significant PCa disease burden. Tables 2a and 2b compare SBx and TBx parameters for the detection of tumor burden greater than 500 mm3. SBx core percentage did not demonstrate any association with tumor burden greater than 500mm3 (P=0.26) whereas TBx did demonstrate a significant association (P=0.0067). For detecting tumor burden greater than 500 mm3, SBx highest percentage core involvement greater than 50% yielded a sensitivity, specificity, and accuracy of 25%, 90.9%, and 54%, respectively. TBx yielded a sensitivity, specificity, and accuracy of 53.6%, 86.4%, and 68%, respectively. The positive predictive value (PPV) and negative predictive value (NPV) for SBx were 77.8% and 48.8% respectively; for TBx, the PPV was 83.3% and NPV was 59.4% (Table 3).
Table 2.
Comparison of Biopsy Results from (A) Systematic 12-Core Biopsy and (B) Targeted Magnetic Resonance Imaging/Transrectal Ultrasonography Fusion Biopsy for Detection of Tumor Burden Greater Than 500 mm3
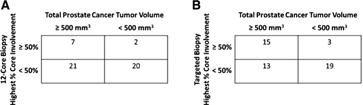 |
Numbers in table represent “n.”
Table 3.
Diagnostic Accuracy and Predictive Values of Systematic 12-Core Biopsy and Targeted Magnetic Resonance Imaging/Transrectal Ultrasonography Fusion Biopsy for Detection of Tumors Greater Than 500 mm3
| Biopsy method | Sens (%) | Spec (%) | Accuracy (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| Systematic 12-core biopsy | 25 | 90.9 | 54 | 77.8 | 48.8 |
| Targeted MRI/TRUS fusion biopsy | 53.6 | 86.4 | 68 | 83.3 | 59.4 |
Sens=sensitivity; Spec=specificity; PPV=positive predictive value; NPV=negative predictive value; MRI/TRUS=magnetic resonance imaging/transrectal ultrasonography.
Discussion
Tumor volume is an important prognostic factor in patients with PCa and is associated with histopathologic stage, Gleason grade, lymph node metastasis, and disease progression.1,19–21 Prostate tumor burden is determined directly from RP specimens or inferred indirectly from SBx, having limited relevance for patients desiring other forms of treatment or who are not surgical candidates. One critical manner in which urologists determine burden of disease preintervention is percentage core involvement and tumor length determined on prostate biopsy. The current study demonstrated that percent core involvement and corresponding tumor length from TBx were significantly better predictors of total MRI PCa TV than the same parameters derived from SBx.
Previous studies, using systematic TRUS-guided prostate biopsies, have demonstrated the correlation of the percentage of tumor involvement in positive cores and core tumor length with PCa TV, pathologic stage, and/or biochemical recurrence.3–8,22–25 In these studies, patient populations were not limited to AS candidates who have a lower disease burden and likely account for the differences in our findings. In addition, lesions sampled with TRUS-guided biopsy were largely within the more accessible posterior peripheral zone and did not account for lesion location.
Given that TBx can find cancer outside of the established template, it is understandable that SBx would tend to yield results that correlate poorly with TV, whereas TBx would be expected to yield more reliable predictions of TV. TV from radical prostatectomy specimens is difficult to ascertain because it is derived from linear measurements at selected levels of permanent pathology sections. MP-MRI based TV is a reliable surrogate for calculating PCa TV, because volumetric measurements have been positively correlated with and accurately predict actual whole mount histopathologic TVs.
Turkbey and associates9 retrospectively evaluated 135 patients, 65% of whom had index tumors with Gleason score ≤3+4, and reported a correlation coefficient of 0.401 (P<0.00001) for T2W MRI, DW MRI, and DCE MRI at 3T used in combination.9 Similarly, Mazaheri and colleagues10 reported a correlation coefficient of 0.6 for T2W MRI-DW MRI at 1.5T in a cohort of 45 patients, 76% of whom had a Gleason score ≤3+4 on final pathologic evaluation. Total PCa TV was used for analysis because it best represents total burden of disease in each patient.
Poulos and coworkers19 demonstrated that the highest percentage of adenocarcinoma at any biopsy site correlated poorly with TV in RP specimens that were whole mount sectioned and analyzed using the grid method (R2=0.041, P=0.012). Similarly, our data confirmed that for SBx, the highest percentage core involvement and corresponding tumor length had poor correlation with total PCa TV. Furthermore, SBx missed the diagnosis of PCa in 26% of the patients, whose disease was ultimately diagnosed on TBx. The poor correlation of highest percent core and corresponding tumor length with total PCa TV persisted when these 13 patients were removed from the analysis (R2=0.077, R2=0.056, respectively; data not shown).
The current standard of care TRUS-guided prostate biopsy is not a sufficiently accurate means of sampling PCa, resulting in significant pathologic upgrading of low-grade disease when compared with RP specimens.26,27 Although office based, TRUS-guided prostate biopsy inadequately samples the peripheral zone and neglects anterior lesions.28 Of the 15 tumors that were missed by SBx in our cohort, 8 were located in the posterior peripheral zone, 6 in the anterior central gland, and 1 in the anterior peripheral zone.
Advances in MP-MRI have allowed for the identification of tumor foci within the prostate and the fusion of MRI with real-time TRUS using computer registration, which allows for the office-based targeted sampling of suspicious prostate lesions.29 The use of this TBx platform has been shown to improve detection of PCa lesions, clinically significant disease, and improve detection in patients with previous negative TRUS biopsy.12,14,27,30–35 The use of the TBx platform may therefore represent a more accurate means to detect and characterize cancer and thus alter the treatment of patients with PCa.
In the AS population where disease burden dictates the treatment, it is essential to have the most accurate estimate of volume, and it is reasonable to assume that TBx may provide a novel pathway to achieve this. Correlation of TRUS biopsy parameters with TV have been reported, but limited data exist with MRI-directed sampling. In our cohort, the highest percentage core involvement and corresponding tumor length on TBx showed a positive correlation with total PCa TV.
Patients with low-risk PCa are assigned a high probability of clinically insignificant disease, defined as PCa volume less than 500 mm3.1 We also sought to determine the correlation of SBx and TBx highest percent core measurements with this established threshold. Targeted biopsy was more sensitive and accurate than SBx in detecting tumor burden greater than 500 mm3 and can therefore better identify patients with clinically significant, larger tumors. Standard SBx appeared to be more specific than TBx for identifying such patients; however, this outcome is confounded by the increased sampling error as evidenced by the 13 missed cancers on SBx. Seven (54%) of these 13 patients had total PCa TVs greater than 500 mm3, 5 of whom had core involvements ≥50% on TBx. Thus, specificity comes at the cost of sensitivity with missed significant cancers.
Based on these results, TBx significantly outperformed SBx for the prediction of total PCa TV and in the detection of tumor burden greater than 500 mm3. Having accurate predictors of TV can allow for more confidence when counseling patients regarding AS, focal therapy, radiotherapy, or surgery. This is, however, most important in patients being considered for AS, because correct risk stratification of these patients will allow for the most appropriate course of action in regard to continuing or starting surveillance versus planning for intervention. We propose using MP-MRI and a MRI/TRUS fusion platform as a complementary means of assessing TV and overall disease burden in patients with PCa. As this literature continues to expand, the use and integration of these techniques into current screening and treatment guidelines will be better delineated.
A limitation of this study is that only patients who had MP-MRI visible lesions were subsequently biopsied and included for this study. Although representing a small population of patients, those who had tumors not visible on MP-MRI were therefore excluded from this study. Tumors not visible on MRI tend to be more likely clinically insignificant, and there is a low prevalence of cancer in this subgroup of patients.36 In addition, all of the patients were enrolled in a prospective trial investigating the use of MP-MRI and performance of the TBx; however, the data were collected and analyzed retrospectively and therefore subject to bias.
Finally, given that all of the patients in the cohort were being followed by AS, whole mount specimens could not be used for tumor volume measurements. Unfortunately, to our knowledge, no study has looked at the correlation of MP-MRI TVs to histopathologic TVs solely in an AS population; however, several studies have shown MRI derived TVs to be an appropriate surrogate for TV calculation in cohorts with a broad disease burden. In addition, MRI derived TVs are more applicable to AS patients or any patient who has not undergone definitive treatment.
Conclusions
The highest percentage core involvement and corresponding tumor length on TBx positively correlates with total MRI PCa TV, in contrast to SBx, which shows a poor correlation. Targeted biopsy is superior to SBx for detecting tumor burden greater than 500 mm3. When using biopsy positive MRI derived TVs, targeted biopsy better reflects overall disease burden and may aid in improving risk stratification among candidates for AS.
Abbreviations Used
- AS
active surveillance
- DCE
dynamic contrast-enhanced
- DW
diffusion weighted
- MP-MRI
multiparametric prostate magnetic resonance imaging
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- NPV
negative predictive value
- PCa
prostate cancer
- PPV
positive predictive value
- PSA
prostate-specific antigen
- RP
radical prostatectomy
- SBx
systematic 12-core biopsy
- TBx
targeted magnetic resonance imaging/ultrasound fusion biopsy
- TRUS
transrectal ultrasonography
- T2W
T2 weighted
- TV
tumor volume
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field.
This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71(Suppl 3):933–938 [DOI] [PubMed] [Google Scholar]
- 2.Nelson BA, Shappell SB, Chang SS, et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int 2006;97:1169–1172 [DOI] [PubMed] [Google Scholar]
- 3.Sebo TJ, Bock BJ, Cheville JC, et al. The percent of cores positive for cancer in prostate needle biopsy specimens is strongly predictive of tumor stage and volume at radical prostatectomy. J Urol 2000;163:174–178 [PubMed] [Google Scholar]
- 4.Grossklaus DJ, Coffey CS, Shappell SB, et al. Prediction of tumour volume and pathological stage in radical prostatectomy specimens is not improved by taking more prostate needle-biopsy cores. BJU Int 2001;88:722–726 [DOI] [PubMed] [Google Scholar]
- 5.Brimo F, Vollmer RT, Corcos J, et al. Prognostic value of various morphometric measurements of tumour extent in prostate needle core tissue. Histopathology 2008;53:177–183 [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Csathy GS, Dorey F, Aronson WJ. Percent prostate needle biopsy tissue with cancer is more predictive of biochemical failure or adverse pathology after radical prostatectomy than prostate specific antigen or Gleason score. J Urol 2002;167:516–520 [DOI] [PubMed] [Google Scholar]
- 7.Dietrick DD, McNeal JE, Stamey TA. Core cancer length in ultrasound-guided systematic sextant biopsies: A preoperative evaluation of prostate cancer volume. Urology 1995;45:987–992 [DOI] [PubMed] [Google Scholar]
- 8.Egevad L, Norberg M, Mattson S, et al. Estimation of prostate cancer volume by multiple core biopsies before radical prostatectomy. Urology 1998;52:653–658 [DOI] [PubMed] [Google Scholar]
- 9.Turkbey B, Mani H, Aras O, et al. Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 2012;188:1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazaheri Y, Hricak H, Fine SW, et al. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: Correlation with pathologic tumor volume. Radiology 2009;252:449–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baco E, Ukimura O, Rud E, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: Correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol 20145;67:787–794 [DOI] [PubMed] [Google Scholar]
- 12.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186:1281–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011;186:1818–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013;190:1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994;271:368–374 [PubMed] [Google Scholar]
- 16.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology 2010;255:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong CW, Rais-Bahrami S, Walton-Diaz A, et al. Comparison of magnetic resonance imaging and ultrasound (MRI-US) fusion-guided prostate biopsies obtained from axial and sagittal approaches. BJU Int 2015;115:772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol 2011;29:2185–2190 [DOI] [PubMed] [Google Scholar]
- 19.Poulos CK, Daggy JK, Cheng L. Prostate needle biopsies: Multiple variables are predictive of final tumor volume in radical prostatectomy specimens. Cancer 2004;101:527–532 [DOI] [PubMed] [Google Scholar]
- 20.Bostwick DG, Graham SD, Jr., Napalkov P, et al. Staging of early prostate cancer: A proposed tumor volume-based prognostic index. Urology 1993;41:403–411 [DOI] [PubMed] [Google Scholar]
- 21.Ochiai A, Troncoso P, Chen ME, et al. The relationship between tumor volume and the number of positive cores in men undergoing multisite extended biopsy: Implication for expectant management. J Urol 2005;174:2164–2168 [DOI] [PubMed] [Google Scholar]
- 22.Villamon-Fort R, Martinez-Jabaloyas JM, Soriano-Sarria P, et al. Percentage of cancer in prostate biopsies as prognostic factor for staging and postoperative biochemical failure after radical prostatectomy. Urol Int 2007;78:328–333 [DOI] [PubMed] [Google Scholar]
- 23.Rubin MA, Bassily N, Sanda M, et al. Relationship and significance of greatest percentage of tumor and perineural invasion on needle biopsy in prostatic adenocarcinoma. Am J Surg Pathol 2000;24:183–189 [DOI] [PubMed] [Google Scholar]
- 24.Park EA, Lee HJ, Kim KG, et al. Prediction of pathological stages before prostatectomy in prostate cancer patients: Analysis of 12 systematic prostate needle biopsy specimens. Int J Urol 2007;14:704–708 [DOI] [PubMed] [Google Scholar]
- 25.Ravery V, Chastang C, Toublanc M, et al. Percentage of cancer on biopsy cores accurately predicts extracapsular extension and biochemical relapse after radical prostatectomy for T1-T2 prostate cancer. Eur Urol 2000;37:449–455 [DOI] [PubMed] [Google Scholar]
- 26.Cohen MS, Hanley RS, Kurteva T, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: The Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol 2008;54:371–381 [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012;188:2152–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothwax JT, George AK, Wood BJ, Pinto PA. Multiparametric MRI in biopsy guidance for prostate cancer: Fusion-guided. BioMed Res Int 2014;2014:439171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastinehad AR, Turkbey B, Salami SS, et al. Improving detection of clinically significant prostate cancer: Magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J Urol 2014;191:1749–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raskolnikov D, George AK, Rais-Bahrami S, et al. Multiparametric magnetic resonance imaging and image-guided biopsy to detect seminal vesicle invasion by prostate cancer. J Endourol 2014;28:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raskolnikov D, Rais-Bahrami S, George AK, et al. The role of image-guided biopsy targeting in patients with atypical small acinar proliferation. J Urol 2015;193:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakir NA, George AK, Siddiqui MM, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol 2014;192:1642–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George AK, Pinto PA, Rais-Bahrami S. Multiparametric MRI in the PSA screening era. BioMed Res Int 2014;2014:465816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 2012;188:1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]