Abstract
A survey of Anaplasma species in small ruminants is still lacking in North African countries. In this study, the presence of A. phagocytophilum, A. phagocytophilum–related species, and A. ovis was investigated in a total of 563 healthy small ruminants (303 goats and 260 sheep), from 25 randomly selected flocks sampled in Tunisia. Anaplasma spp. and A. ovis overall infection rates were 95.0% and 93.8% in sheep and 69.6% and 65.3% in goats, respectively. A. phagocytophilum was not detected in any of tested animals. A total of 20 sheep (7.7%) and 144 goats (47.5%) were infected by Anaplasma strains genetically related to A. phagocytophilum. Both in sheep and goats A. ovis prevalence was higher in adults (≥2 years) than in young (<2 years) subjects (p = 0.001 and 0.002 for goats and sheep, respectively). In sheep, A. ovis prevalence was higher in ewes with respect to rams (p = 0.010). The A. ovis infection rate was significantly lower in goats of the local breed (p = 0.049) and it was higher in goats infested by ticks than in not infested animals (p = 0.005). Genetic analysis of the msp4 gene of A. ovis indicated the presence of strains shared by Tunisian sheep and goats. Sequence analysis and phylogenetic studies on the basis of the 16S rRNA gene provided evidence for the circulation of at least two different potentially novel species genetically related to A. phagocytophilum in Tunisian small ruminants. These findings cause concern about specificity of serological tests used for detection of A. phagocytophilum in ruminants and provide additional information for elucidating pathogenesis and molecular epidemiology of A. phagocytophilum and related species.
Key Words: : Anaplasma ovis, A. phagocytophilum and related species, Small ruminants, Molecular prevalence, 16S rRNA and msp4 genes, Tunisia
Introduction
Bacterial species of the genus Anaplasma are obligate intracellular bacteria that cause animal and human anaplasmosis, a disease widely distributed in tropical, subtropical, and temperate regions (Kocan et al. 2003). The disease impact on veterinary and public health can be significant, because at least one Anaplasma species has a zoonotic nature and the six species of this genus cause economic losses to farmers and in general to animal production (Woldehiwet 2010, Zobba et al. 2014).
Among Anaplasma species, A. ovis, which infects sheep, goat, and deer, is an obligate intraerythrocytic bacterium and the causative agent ovine anaplasmosis (Friedhoff 1997). In small ruminants, infection is usually subclinical; occasionally, it can be severe with hemolytic anaemia, hemoglobinuria, and fever (Barry and Van Niekerk 1990, Stoltsz 1994, Hornok et al. 2007). In addition, A. ovis infection may predispose to other infectious and/or parasitic diseases that aggravate an animal's condition, occasionally leading to death (Kocan et al. 2004).
A. phagocytophilum infects neutrophil granulocytes of many animal species and human (Dumler et al. 2001). In ruminants, it causes tick-borne fever (TBF) (Stuen 2007, Woldehiwet 2010), with the most common symptoms including high fever, anorexia, dullness, and milk yield decrease (Tuomi 1967, Woldehiwet 2010). In Japan, strains closely related to A. phagocytophilum, have been detected in cattle, in sika deer, and in some ticks species infesting ruminants (Ixodes persulcatus, Ixodes ovatus, Haemaphysalis megaspinosa) (Ohashi et al. 2005, Jilintai et al. 2009, Yoshimoto et al. 2010). On the basis of multigene analyses using 16S rRNA, groEL, and gltA genes, these strains were placed in a distinct monophyletic cluster (Ybañez et al. 2012a). The absence of clinical signs in infected animals and the different related tick vectors (respect to A. phagocytophilum) provide additional evidence for species designation of these potential novel Anaplasma strains initially found in Japan (Jilintai et al. 2009, Yoshimoto et al. 2010, Ybañez et al. 2012a). More recently in China, Kang et al. (2014) identified Anaplasma sp. strains in Hyalomma asiaticum ticks infesting sheep, which differ from the Japanese strains (Anaplasma sp. Japan) and all other classified Anaplasma species. These latter strains generated a distinct clade in phylogenetic trees on the basis of the 16S rRNA, gltA, and groEL genes, suggesting the presence of a new Anaplasma species genetically related to A. phagocytophilum in ticks infesting ruminants in China.
To date, molecular studies in Tunisia showed the occurrence of A. phagocytophilum in both animal hosts (dogs and horses) and ticks (Ixodes ricinus, Hyalomma scupense, and Hyalomma marginatum) (Sarih et al. 2005, M'ghirbi et al. 2009, 2012). A. phagocytophilum infection has also been reported in Tunisian horses and dromedaries by serology (Ben Said et al. 2013, 2014). Recently, A. ovis has been detected and characterized in sheep from northern and central Tunisia (Belkahia et al. 2014). The aim of this study was to investigate A. ovis and A. phagocytophilum and related species in small ruminants from five localities belonging to two governorates in northern Tunisia.
Materials and Methods
Blood sampling, tick collection, and DNA extraction
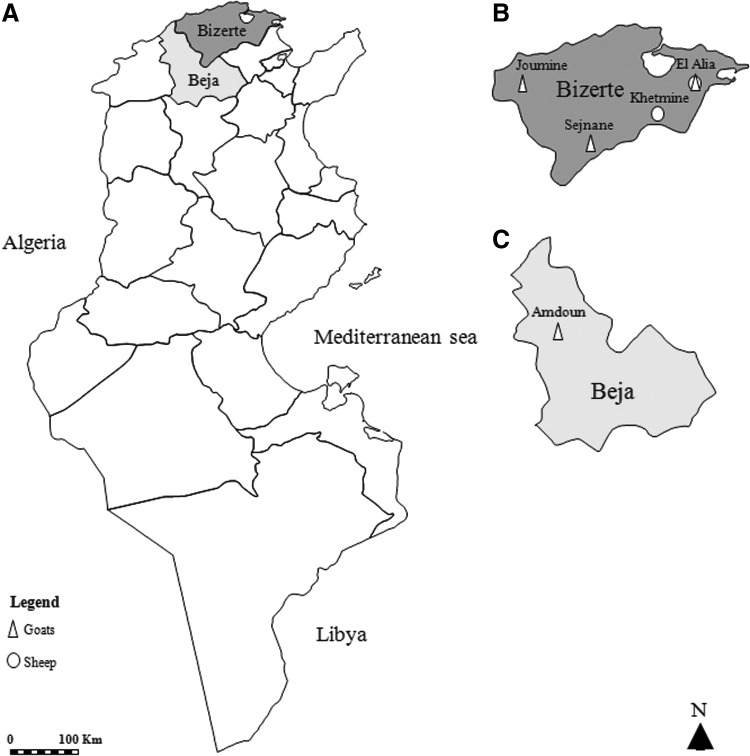
A cross-sectional study was carried out in five localities of northern Tunisia (Fig. 1). El Alia (37°16′N; 10°03′E) and Khetmine (37°16′N; 9°99′E) fall in the subhumid bioclimatic zone with average annual rainfall of 400 mm, whereas Joumine (36°92′N; 9°38′E), Sejnane (37°15′N; 9°23′E), and Amdoun (36°76′N; 9°08′E) are characterized by humid climate with an average annual rainfall of 650 mm. Between 2011 and 2013, a total of 563 samples were collected from 303 healthy goats (233 does and 70 bucks) and 260 healthy sheep (210 ewes and 50 rams). Goat samples originated from 16 herds located in Sejnane (n = 3), El Alia (n = 4), and Joumine (n = 5) belonging to the Bizerte governorate and in Amdoun (n = 4, Beja governorate). Sheep samples were taken from nine herds located in El Alia (n = 4) and Khetmine (n = 5) in the governorate of Bizerte.
FIG. 1.
Localities studied. (A) Geographical position of the governorates of Bizerte and Beja in Tunisia. (B) Position of localities sampled in the governorate of Bizerte. (C) Sampling area in the governorate of Beja.
For each animal, blood samples were collected in EDTA tubes from the jugular vein; gender, breed, and approximate age were noted. Goats belonged to three breeds: Local breed (275), Alpine (23), and Maltese (5). Sheep belonged to six breeds: Barbarine (118), Noire de Thibar (82), Queue Fine de l'Ouest (10), Merino (2), Sicilo-sarde (1), and crossbreeds (47). Sex ratio (male:female) was, respectively, 0.3 and 0.24 in goats and sheep, whereas mean age was 3.9 ± 1.7 years in goats and 4.9 ± 2.0 in sheep. All small ruminants were divided into three age groups for females and two age groups for males. DNA was extracted from 300 μL of each blood sample with Wizard® Genomic DNA Purification Kit (Promega, Madison, WI) according to the manufacturer's instructions. Ticks were collected manually from each sampled animal, placed in tubes containing 70% ethanol, and morphologically identified using the taxonomical key of Walker et al. (2013).
Detection of A. phagocytophilum and related species
Nested PCR was performed with outer primers EE1 and EE2 and inner primers SSAP2f and SSAP2r to amplify a 641-bp sequence of the 16S rRNA gene (Liu et al. 2012). According to Ybañez et al. (2012a), inner primers allow the detection of A. phagocytophilum and related species (Table S1) (Supplementary Data are available at www.liebertpub/vbz/). For specific detection of A. phagocytophilum, positive 16S rRNA samples were tested by hemi-nested PCR using outer primers EphplgroEL-F and EphplgroEL-R, and inner primers EphplgroEL-F and EphgroEL-R amplifying 573 bp sequence of the groEL gene (Alberti et al. 2005; Table S1). Each reaction was performed in a final volume of 50 μL containing 0.125 U/μL Taq DNA polymerase (Biobasic Inc, Canada), 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 2 μL of genomic DNA, and 0.5 μM primers. Thermal cycling reactions were performed in an automated DNA thermal cycler (Techne Flexigene, Cambridge, UK). One microliter of each amplicon was used for PCR reaction with specific primers at the same conditions as for the first PCR. PCR products were electrophoresed in 1% agarose gels containing 0.5 μg/mL of ethidium bromide. Distilled water and DNA samples positive to A. phagocytophilum (Zobba et al. 2014) were used as negative and positive controls in each PCR experiment.
Detection of A. ovis
To detect A. ovis in sheep samples, loop-mediated isothermal amplification (LAMP) reactions were performed using a set of six primers targeting the msp4 gene (Ma et al. 2011; Table S1). Reaction conditions and thermal profiles were as described by Belkahia et al. (2014). Negative and positive controls were included in all runs. After amplification, LAMP products were detected by electrophoresis in 1.5% agarose gel and either visualized under a ultraviolet (UV) light after staining with ethidium bromide or by visual inspection of tubes after adding 1 μL of 1000× SYBR Green I (Cambrex BioScience, USA). A. ovis infection in goat samples was detected by single PCR with the AovisMSP4Fw- and AovisMSP4Rev-specific primers designed by Torina et al. (2012). Each PCR reaction contained a mix of 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.125 U/μL Taq DNA polymerase (Biobasic Inc., Canada), 2 μL (1–10 ng) of DNA, 0.5 μM primers (Table S1), and milliQ sterile water to a total volume of 50 μL. Distilled water and DNA extracted from A. ovis were used as negative and positive controls, respectively. Thermal cycling reactions were performed as described by Torina et al. (2012). Genotyping of the A. ovis msp4 gene was performed by amplifying positive sheep and goat samples with A. ovis–specific LAMP and PCR reactions using MSP45 and MSP43 primers (de la Fuente et al. 2005, 2007; Table S1). Amplification was performed as described previously (de la Fuente et al. 2005, 2007, Belkahia et al. 2014). PCR products were electrophoresed in 1.5% agarose gel.
DNA sequencing and phylogenetic analysis
Selected positive PCR products from primers SSAP2f/SSAP2r and MSP45/MSP43 of A. phagocytophilum and/or related species, and A. ovis, respectively, were purified with the GF-1 Ambi Clean Kit (Vivantis, USA) according to manufacturer's instructions. Purified DNA fragments were sequenced in both directions, using the same primers as for the PCR amplifications (Table S1). The reactions were performed using a conventional Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Applied Biosystems, Foster City, CA) and an ABI3730XL automated DNA sequencer by Macrogen Europe (Amsterdam, The Netherlands). The chromatograms were evaluated with Chromas Lite v. 2.01. The DNAMAN software (v. 5.2.2; Lynnon Biosoft, Quebec, Canada) was used to perform multiple sequence alignment of 16S rRNA and msp4 sequences and to translate nucleotide to amino acid msp4 sequences. BLAST analysis of GenBank was used to assess the level of similarity with previously reported sequences (http://blast.ncbi.nlm.nih.gov/; Altschul et al. 1997). Neighbor-joining (NJ) phylogenetic trees were constructed using the DNAMAN software based on the Saitou and Nei (1987) distance method with bootstrap analysis of 1000 reiterations.
GenBank accession numbers
The 16S rRNA partial sequences of Anaplasma sp. strains related to A. phagocytophilum Aplike1GGo1-3 and Aplike2GGo1 isolated in goats have been deposited in GenBank under accession numbers KM285226, KM285227, KM285229, and KM285228, respectively. The 16S rRNA partial sequences of Anaplasma sp. strains related to A. phagocytophilum AplikeGOv1-3 isolated in sheep have been deposited in GenBank under accession numbers KM285230–KM285232. The msp4 partial sequences of A. ovis strains AoGOv1–5 found in sheep and the AoGGo1 genotype found in goat have been deposited in GenBank under accession numbers KM285218-22 and KM285217, respectively.
Statistical analysis
Exact confidence intervals (CI) for prevalence rates at the 95% level were calculated. Comparison of the prevalence of Anaplasma species in sheep and goats according to risk factors, localities, and governorates, and comparison of rates and infestation prevalence of each tick species found in goats and sheep were performed with Epi Info 6.01 (Centers for Disease Control and Prevention, Atlanta, GA), using the chi-squared test and Fisher exact test with a threshold value of 0.05. To consider any confusion factor, a chi-squared Mantel–Haenszel test was performed.
Results
Parasitological data
A total of 1223 engorged and semiengorged ticks were collected from 563 small ruminants (Table S2). Specifically, 919 ticks were found on 241 (92.69%) out of 260 sampled sheep and belonged to three Rhipicephalus species. R. turanicus (52.77%) and R. sanguineus (43.96%) were the dominant tick species, followed by R. annulatus (3.26%) (p < 0.001). Also, 304 adult ticks were collected from 113 (37.29%) out 303 goats and were ascribed to four species. R. turanicus (79.93%) and R. bursa (14.47%) were the dominant species, followed by R. sanguineus (4.93%) and a few Hyalomma excavatum ticks (0.65%) (p < 0.001).
Prevalence of Anaplasma species
The overall infection rates of Anaplasma spp. and A. ovis in goats were 69.6% and 65.3%, respectively. In sheep, infection rates were 95.0% (Anaplasma spp.) and 93.8% (A. ovis) (Table S1). A total of 144 goats (47.5%) and 20 sheep (7.7%) were found positive to A. phagocytophilum and/or related species by 16S rRNA PCR, but specific detection of A. phagocytophilum conducted by targeting the groEL gene showed that none of these animals were positive to this zoonotic species. In goats, A. ovis infection rate was significantly lower in young (55.6%) than adult goats (72.9%) (p = 0.001) and in local breed (63.6%) compared to other breeds (82.1%) (p = 0.049). Goats infested by ticks (75.2%) were more infected by A. ovis than noninfested animals (59.5%) (p = 0.005) (Table 1). In sheep, A. ovis prevalence was higher in ewes (95.7%) than in rams (86.0%) (p = 0.010) and in adults (>2 years) (96.4%) than in young sheep (≤2 years) (55.7%) (p = 0.002) (Table 1).
Table 1.
Molecular Prevalence of Anaplasma ovis According to Gender, Age, Breed, and Tick Infestation of Goats and Sheep
| Goats | Sheep | |||||
|---|---|---|---|---|---|---|
| Risk factor | Number | Positive (% ± CIa) | p value | Number | Positive (% ± CIa) | p value |
| Gender | ||||||
| Male | 70 | 44 (62.9 ± 0.11) | 0.617 | 50 | 43 (86.0 ± 0.10) | 0.010* |
| Female | 233 | 154 (66.1 ± 0.06) | 210 | 201 (95.7 ± 0.03) | ||
| Age | ||||||
| <2 years | 133 | 74 (55.6 ± 0.08) | 0.001* | 63 | 54 (85.7 ± 0.09) | 0.002* |
| ≥2 years | 170 | 124 (72.9 ± 0.07) | 197 | 190 (96.4 ± 0.02) | ||
| Breed | ||||||
| Local/Barbarineb | 275 | 175 (63.6 ± 0.06) | 0.049* | 118 | 113 (95.8 ± 0.04) | 0.056 |
| Other breedsc | 28 | 23 (82.1 ± 0.14) | 142 | 127 (89.4 ± 0.05) | ||
| Tick infestation | ||||||
| Infested | 113 | 85 (75.2 ± 0.08) | 0.005* | 244 | 229 (93.9 ± 0.03) | 0.986 |
| Not infested | 190 | 113 (59.5 ± 0.07) | 16 | 15 (93.8 ± 0.12) | ||
| Total | 303 | 198 (65.3 ± 0.05) | 260 | 244 (93.8 ± 0.03) | ||
CI, 95% confidence interval.
Local for goats and Barbarine for sheep.
Other breeds are Alpine and Maltese for goats and Noire de Thibar, Queue fine de l'Ouest, Merinos, Sicilo-sarde, and crossbred for sheep.
Significance at the p < 0.05 level.
A. ovis msp4 genotypes
A. ovis infection was confirmed by sequencing of 719 bp of the msp4 gene (84.4% of the gene size) from 20 randomly selected positive goat samples (five from each sampling region) and six positive sheep samples (three from each sampling region). Alignment of these sequences revealed five different genotypes (AoGOv1–5) in sheep (GenBank acc. nos. KM285218–KM285222) and one genotype (AoGGo1) in goats (GenBank acc. no. KM285217) differed from each other in five nucleotide positions. Each nucleotide change conferred an amino acid variation, except in nucleotide position 467 (Table 2). The msp4 gene sequences obtained in this study shared 99.6–100% and 98.7–100% nucleotides and amino acids similarity, respectively. The AoGOv1 and AoGGo1 genotypes were 100% identical to the GBK2 genotype (GenBank acc. no. KC432642) from Tunisia, and to the genotype III represented by the “Italy20” A. ovis strain from Sicilian sheep (GenBank acc. no. AY702923, Table 2). The AoGOv5 genotype revealed 100% homology with the GBK1 genotype (GenBank acc. no. KC432641) from Tunisia, and the genotype II represented by the “Italy147” A. ovis strain from Sicilian sheep (GenBank acc. no. AY702924) (Table 2). Three novel A. ovis msp4 genotypes (AoGOv2 to AoGOv4) were identified (Table 2). Phylogenetic analysis revealed that the two new genotypes AoGOv3 and AoGOv4 clustered with the A. ovis Panagcy strain found in human samples from Cyprus (GenBank acc. no. FJ460443); and the new genotype AoGOv2 clustered with the genotype III represented by the “Italy20” A. ovis strain from Sicilian sheep (GenBank acc. no. AY702923).
Table 2.
Nucleotide and Amino Acid Differences among msp4 Sequences (719 bp) from Anaplasma ovis Strains
| Host | Variant | Sample symbola | Country | GenBankb | msp4 nucleotide positions (amino acid positions)c | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 230 (77) | 244 (83) | 470 (157) | 476 (159) | 532 (178) | ||||||
| Sheep | Italy 147 | NId | Italy | AY702924 | G (R) | A (S) | C (A) | C | C (L) | de la Fuente et al. (2005) |
| Italy 20 | NId | Italy | AY702923 | * | * | T (V) | * | * | de la Fuente et al. (2005) | |
| GBK1 | B1; B2; K2; K4 | Tunisia | KC432641 | * | * | * | * | * | Belkahia et al. (2014) | |
| GBK2 | B4; K3 | Tunisia | KC432642 | * | * | T (V) | * | * | Belkahia et al. (2014) | |
| GB3 | B3 | Tunisia | KC432643 | T (I) | * | T (V) | * | * | Belkahia et al. (2014) | |
| GK1 | K1 | Tunisia | KC432644 | T (I) | * | T (V) | * | A (I) | Belkahia et al. (2014) | |
| AoGOv1 | Kh1; Kh2 | Tunisia | KM285218 | * | * | T (V) | * | * | Present study | |
| AoGOv2 | Al1 | Tunisia | KM285219 | G (R) | G (G) | T (V) | * | * | Present study | |
| AoGOv3 | Al2 | Tunisia | KM285220 | T (I) | * | T (V) | A | * | Present study | |
| AoGOv4 | Al3 | Tunisia | KM285221 | G (R) | * | T (V) | A | * | Present study | |
| AoGOv5 | Kh3 | Tunisia | KM285222 | * | * | * | * | * | Present study | |
| Goat | AoGGo1 | Al1–Al5; Sj1–Sj5; Jm1–Jm5; Am1–Am5 | Tunisia | KM285217 | * | * | T (V) | * | * | Present study |
B1–B4 and K1–K4 sheep samples were collected from El Alia (Bizerte governorate) and Sbikha (Kairouan governorate), respectively, by Belkahia et al. (2014). Al1–Al3 and Kh1–Kh3 sheep samples were collected from El Alia (Bizerte governorate) and Khetmine (Bizerte governorate), respectively, in the present study. Al1–Al5, Sj1–Sj5, Jm1–Jm5, and Am1–Am5 goat samples were collected from El Alia, Sejnane, Joumine, and Amdoun, respectively.
GenBank accession number of the variant.
Numbers represent the nucleotide position starting at translation initiation codon Adenine. Conserved nucleotide positions with respect to the Italy 147 strain, Sicily (Italy) are indicated with asterisks (de la Fuente et al., 2005).
NI, not indicated.
Amino acid changes are indicated between parentheses with single letter code.
Amino acids: R, arginine; I, isoleucine; S, serine; G, glycine; V, valine; A, alanine; L, leucine. Nucleotides: T, thymine; C, cytosine; G, guanine; A, adenine.
Anaplasma sp. 16S rRNA genotypes
Sequencing of 599 bp (41.8%) of the 16S rRNA gene obtained from 16 randomly selected positive goat samples (seven, four, three, and two samples from Joumine, Sejnane, Amdoun, and El Alia, respectively) and 10 positive sheep samples (six and four samples from Khetmine and El Alia, respectively) confirmed the infection by Anaplasma strains genetically related to A. phagocytophilum. Alignment of these sequences revealed four genotypes from goats (Aplike1GGo1 to Aplike1GGo3 and Aplike2GGo1; KM285226, KM285227, KM285229, and KM285228, respectively) and three from sheep (Aplike1GOv1 to Aplike1GOv3; KM285230–KM285232, respectively). Except for the Aplike2GGo1 sequence, all 16S rRNA sequences obtained in this study shared 99.3–100% nucleotide similarity and differed from each other in five nucleotide positions (four substitutions and one deletion; Tables 3 and 4). The Aplike1GGo1 and Aplike1GOv1 genotypes were 100% identical to the Clone 1 genotype of Anaplasma sp. isolated from deer in Japan (JN055357) (Tables 3 and 4). The Aplike2GGo1 genotype shared 98.3–98.7% identity with all other revealed sequences and was 99.7% and 99.2% identical to the KS8 genotype of Anaplasma sp. from Japanese sheep recorded as A. phagocytophilum (AB96720) and the BL099-6 genotype of Anaplasma sp. isolated from H. asiaticum tick infesting sheep in China (KJ410247), respectively (Table 4).
Table 3.
Nucleotide Diversity among 16S rRNA Sequences from Anaplasma Strains Closely Related to A. phagocytophilum (599 bp)
| Host or vector | Genotypea | Country | GenBankb | 16S rRNA nucleotide positionsc | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 795 | 802 | 824 | 827 | 880 | 983 | 1081 | 1085 | 1092 | 1120 | 1209 | 1211 | 1212 | 1223 | 1231 | 1263 | |||||
| Deer | Clone 1 | Japan | JN055357 | C | T | G | T | A | A | G | — | C | C | T | T | C | G | G | C | Ybañez et al. (2012a) |
| Goat | Aplike1GGo1 | Tunisia | KM285226 | * | * | * | * | * | * | * | — | * | * | * | * | * | * | * | * | Present study |
| Aplike1GGo2 | Tunisia | KM285227 | * | * | * | * | * | * | * | T | * | T | * | * | * | * | * | * | Present study | |
| Aplike1GGo3 | Tunisia | KM285229 | * | * | * | * | C | * | * | T | * | T | * | * | * | * | * | * | Present study | |
| Sheep | Aplike1GOv1 | Tunisia | KM285230 | * | * | * | * | * | * | * | — | * | * | * | * | * | * | * | * | Present study |
| Aplike1GOv2 | Tunisia | KM285231 | * | * | C | * | * | * | * | — | * | * | * | * | * | * | * | * | Present study | |
| Aplike1GOv3 | Tunisia | KM285232 | * | * | C | * | * | * | * | — | * | * | * | * | * | A | * | * | Present study | |
| Hyalomma asiaticum | BL099-6 | China | KJ410247 | T | A | * | * | * | G | A | T | T | * | C | C | * | * | A | T | Kang et al. (2014) |
| Goat | Aplike2GGo1 | Tunisia | KM285228 | * | A | * | C | * | G | * | T | T | * | C | C | T | * | * | T | Present study |
Conserved nucleotide positions are indicated with asterisks.
Nucleotides: T, thymine; C, cytosine; G, guanine; A, adenine.
Jm1–Jm8, Sj1–Sj4, Am1–Am3, and Al1–Al2 Anaplasma sp.–positive goat samples were collected from Joumine (Bizerte governorate), Sejnane (Bizerte governorate), Amdoun (Beja governorate), and El Alia (Bizerte governorate) localities, respectively.
Al1–Al4 and Kh1–Kh6 Anaplasma sp.–positive sheep samples were collected from El Alia (Bizerte governorate), and Khetmine (Bizerte governorate), localities, respectively.
The Aplike1GGo1 genotype was isolated from Jm1, Jm2, Jm4–Jm7, Sj1, Sj3, Sj4, Am1–Am3, al1 goat samples; the Aplike1GGo2 genotype was isolated from Jm3 and Jm8 goat samples; the Aplike1GGo3 genotype was isolated from Al2 goat sample; the Aplike1GOv1 genotype was isolated from Al1-4, Kh1, and Kh4–6 sheep samples; the AplikeGOv2 genotype was isolated from Kh2 sheep sample; the AplikeGOv3 genotype was isolated from Kh3 sheep sample, and the Aplike2GGo1 genotype was isolated from Sj2 goat sample.
GenBank accession number.
Numbers represent the nucleotide position with respect to the HZ strain from USA for A. phagocytophilum (GenBank acc. no. NC_007797).
Table 4.
Comparison of 16S rRNA Sequences (599 pb) from Anaplasma Strains Genetically Related to A. phagocytophilum Isolated from Tunisian Small Ruminants and Other Anaplasma Species Found in GenBank
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A. sp (Aplike1GGo1) | 100 | |||||||||||||||
| 2 | A. sp (Aplike1GGo2) | 99.8 | 100 | ||||||||||||||
| 3 | A. sp (Aplike1GGo3) | 99.7 | 99.8 | 100 | |||||||||||||
| 4 | A. sp (Aplike1GOv1) | 100 | 99.8 | 99.7 | 100 | ||||||||||||
| 5 | A. sp (Aplike1GOv2) | 99.8 | 99.7 | 99.5 | 99.8 | 100 | |||||||||||
| 6 | A. sp (Aplike1GOv3) | 99.7 | 99.5 | 99.3 | 99.7 | 99.8 | 100 | ||||||||||
| 7 | A. sp (Clone 1) | 100 | 99.8 | 99.7 | 100 | 99.8 | 99.7 | 100 | |||||||||
| 8 | A. sp (Aplike2GGo1) | 98.7 | 98.5 | 98.3 | 98.7 | 98.5 | 98.3 | 98.7 | 100 | ||||||||
| 9 | A. sp (KS8) | 98.7 | 98.5 | 98.3 | 98.7 | 98.5 | 98.3 | 98.7 | 99.7 | 100 | |||||||
| 10 | A. sp (BL099-6) | 98.5 | 98.3 | 98.2 | 98.5 | 98.3 | 98.2 | 98.5 | 99.2 | 99.5 | 100 | ||||||
| 11 | A. p (HN) | 98.9 | 98.9 | 98.8 | 98.9 | 98.8 | 98.7 | 98.9 | 97.0 | 97.5 | 97.7 | 100 | |||||
| 12 | A. pl (Okinawa) | 98.8 | 98.8 | 98.7 | 98.8 | 98.7 | 98.5 | 98.8 | 97.3 | 97.3 | 97.5 | 99.2 | 100 | ||||
| 13 | A. o (Jingtai) | 97.7 | 97.8 | 97.7 | 97.7 | 97.5 | 97.3 | 97.7 | 97.3 | 97.3 | 97.5 | 97.5 | 97.7 | 100 | |||
| 14 | A. m (Lushi) | 97.3 | 97.5 | 97.3 | 97.3 | 97.2 | 97.0 | 97.3 | 97.0 | 97.0 | 97.2 | 97.2 | 97.3 | 99.7 | 100 | ||
| 15 | A. c (CC) | 97.0 | 97.2 | 97.0 | 97.0 | 96.8 | 96.7 | 97.0 | 96.7 | 96.7 | 96.8 | 96.8 | 97.0 | 99.3 | 99.0 | 100 | |
| 16 | A. b (YX4) | 97.0 | 97.2 | 97.0 | 97.0 | 96.8 | 96.7 | 97.0 | 96.5 | 96.5 | 96.3 | 96.8 | 96.3 | 95.7 | 95.7 | 95.7 | 100 |
The numbers represent the nucleotide identity rates found between the sequences.
A. sp, (Aplike1GGo1–3 and Aplike2GGo1), Anaplasma sp. isolated from Tunisian goats (AplikeGGo1–3 strains, GenBank acc. nos. KM285226, KM285227, KM285229, and KM285228, respectively); A. sp, (AplikeGOv1–3), Anaplasma sp. isolated from Tunisian sheep (AplikeGOv1–3 strains, GenBank acc. nos. KM285230–KM285232, respectively); A. sp (Clone 1), Anaplasma sp. related to A. phagocytophilum found on Japanese deer (Clone 1, GenBank acc. no. JN055357); A. sp (KS8), Anaplasma sp. isolated from Chinese sheep registered as A. phagocytophilum (KS8 isolate, GenBank acc. no. KJ782385); A. sp (BL099-6), Anaplasma sp. isolated from Hyalomma asiaticum tick infested Japanese ruminants (BL099-6 isolate, GenBank acc. no. KJ410247); A. p (HN strain), A. phagocytophilum strain isolated from Chinese rodent (HN strain, GenBank acc. no. KC470064); A. pl (Okinawa), A. platys isolate found on Japanese dog (Okinawa isolate, GenBank acc. no. AY077619); A. o (Jingtai), A. ovis isolate found on Chinese goat (Jingtai isolate, GenBank acc. no. AJ633049); A. m (Lushi), A. marginale isolate found on Chinese cattle (Lushi isolate, GenBank acc. no. AJ633048); A. c (CC), A. centrale strain isolated from Italian cattle (CC strain, GenBank acc. no. EF520686); A. b (G49), A. bovis isolate found on Chinese goat (G49 isolate, GenBank acc. no. JN558824).
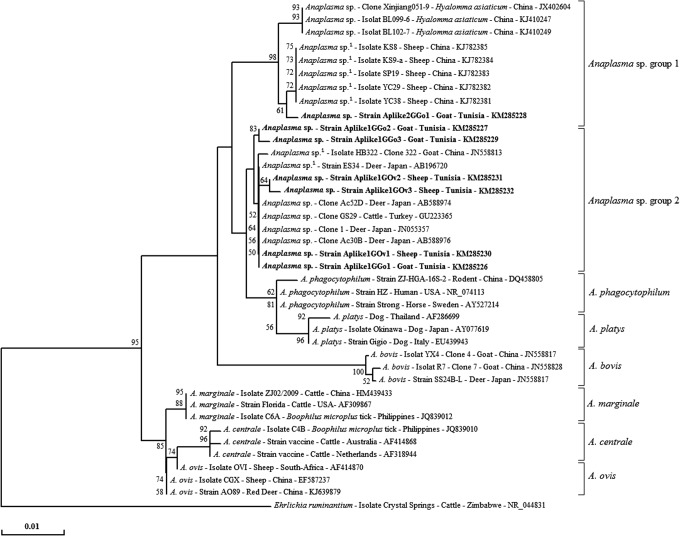
16S rRNA gene–based phylogenetic trees revealed that all Anaplasma sp. strains from this study clustered independently from A. phagocytophilum strains isolated in human, horse and rodents (NR_074113, AY527214, and DQ458805, respectively). The Aplike2GGo1 genotype clustered in Anaplasma sp. group 1 with Anaplasma sp. isolates found in Chinese sheep (KJ782381–KJ782385, registered as A. phagocytophilum) and Anaplasma sp. isolates detected in H. asiaticum tick infesting Chinese ruminants (JX402604, KJ410247, and KJ410249). All other genotypes clustered in Anaplasma sp. group 2 with Anaplasma sp. (Japan) strains isolated from deer (AB96720, registered as A. phagocytophilum, JN055357, AB588974, and AB588976), Anaplasma sp. strain isolated from cattle in Turkey (GU223365) and Anaplasma sp. strain isolated from Chinese goat (JN558817, deposited in the GenBank as A. phagocytophilum; Fig. 3). Nucleotide sequence identities, calculated by comparing sequences obtained in this study versus those of other Anaplasma species, were 97.0–99.0% with A. phagocytophilum, 97.3–98.8% with A. platys, 97.3–97.8% with A. ovis, 97.0–97.5% with A. marginale, 96.7–97.2% with A. centrale, and 96.5–97.2% A. bovis.
FIG. 3.
Neighbor-joining tree based on the alignment of partial A. phagocytophilum 16S rRNA sequences (599 bp) obtained in this study with selected sequences representative of the Anaplasma genus. Bootstrap values (1000 replicates) are indicated in each node (only percentages greater than 50% are shown). Sequences obtained in this study are indicated in bold and marked with asterisks. The host or vector, the strain or isolate name, the country of origin and the GenBank accession number are indicated. 1Anaplasma sp. deposited in the GenBank as A. phagocytophilum.
Discussion
Although several reports showing the presence of anaplasmosis in North Mediterranean small ruminants are available (de la Fuente et al. 2005, Torina et al. 2008a,b, Torina and Caracappa 2012, Zobba et al. 2014), similar studies are lacking in most of the South Mediterranean countries such as Tunisia, where surveys are limited to the detection of zoonotic A. phagocytophilum strains in horses, dogs, and ticks (Sarih et al. 2005, M'ghirbi et al. 2009, 2012).
In this study, we investigated the molecular epidemiology of selected Anaplasma species in small ruminants from northern Tunisia. Results clearly indicate evidence of A. ovis infection in sheep and goats sampled in the study area (Table S1). In sheep, A. ovis prevalence (overall 93.8%, minimum 91.4% in El Alia, maximum 96.4% in Khetmine) was similar to the prevalence reported by de la Fuente et al. (2005) in Italy (87%) and by Renneker et al. (2013) in Portugal (82.5%). It was higher than that observed in Sudan (41.7%; Renneker et al. 2013), Turkey (31.4%; Renneker et al. 2013), and Senegal (11.5%; Djiba et al. 2013). Overall prevalence in goats (65.3%; range, 44.4–78.8%) was similar to the prevalence reported by Altay et al. (2014) in Turkey (66.4%), lower than that estimated in Angola (100%; Kubelová et al. 2012), and higher than that observed in China (15.3–25.6%; Liu et al., 2012, Chi et al., 2013). The higher prevalence of A. ovis in sheep, also reported in Italy (Torina et al. 2008a,b, 2010, Torina and Caracappa 2012), may be the result of the different distribution of A. ovis tick vectors in goats and sheep sampling localities, which fall into different bioclimatic zones.
The analysis of A. ovis msp4 sequences revealed a single genotype (AoGGO1) infecting all selected goats and five different genotypes infecting sheep (Table 2). The goat genotype is identical to the AoGOv1 genotype found in two infected sheep, whereas each one of the other sheep genotypes was found in a single sheep. In contrast, Liu et al. (2012) suggest that A. ovis msp4 genotypes may be different among sheep and goats. Strains found in Tunisian sheep were classified in three clusters, indicating multiple introductions of genetically distinct A. ovis strains in the studied regions. The two new genotypes, namely AoGOv3 and AoGOv4, were classified with A. ovis Panagcy strain (FJ460443) in an independent cluster (Chochlakis et al. 2010; Fig. 2). In agreement with Belkahia et al. (2014), comparison of msp4 sequences isolated from sheep from El Alia and Khetmine suggests that geographic location is not the origin of genetic diversity of the A. ovis msp4 gene.
FIG. 2.
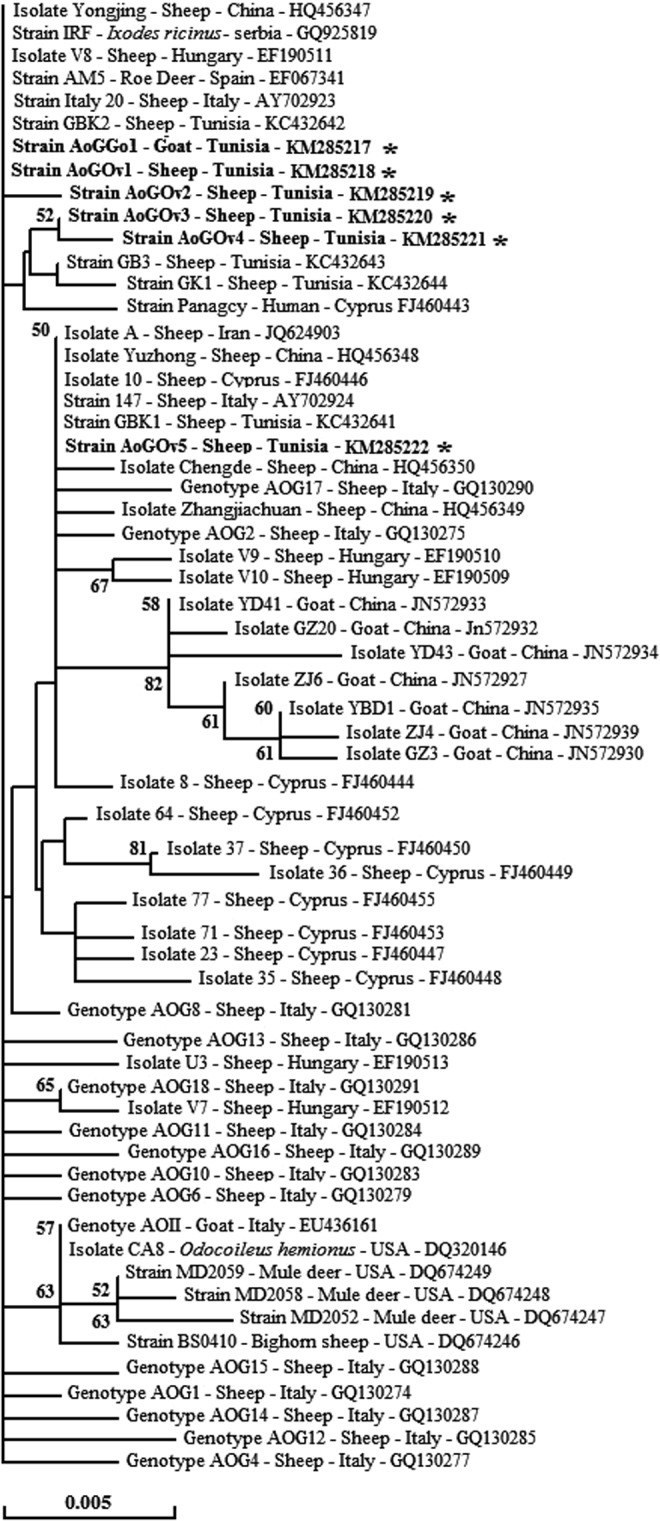
Representative neighbor-joining tree based on multiple sequence alignments of the partial msp4 nucleotide sequences (719 bp) of Tunisian strains with strains isolated in the Mediterranean area. Numbers in nodes represent the percentage of 1000 bootstrap reiterations supporting the nodes (only percentages greater than 50% are shown). The host or vector, strain or isolate identification, country of origin, and GenBank accession number are indicated in the tree for each sequence. Sequences newly obtained in this study are highlighted in bold and marked with asterisks.
The A. ovis infection rate was significantly higher in adult goats (>2 years) compared to young goats (<2 years). This could be explained by the fact that adult goats were more exposed to tick infestation because they went through more tick seasons. Similarly, due to different exposures of rams and ewes to tick infestation, A. ovis prevalence in sheep was significantly higher in ewes than rams. Indeed, almost two-thirds of ewes were more than 4 years old and grazed on natural pastures, a trend reported by Belkahia et al. (2014). A local goat breed was less infected by A. ovis than other goat breeds, this difference possibly being due to genetic resistance of local breeds to hemopathogens.
Goats infested by ticks were statistically more infected by A. ovis than those free of ticks. Our result is consistent with that reported in sheep situated in northern Tunisia infested by Rhipicephalus spp. (Belkahia et al. 2014). Notably, the most dominant tick species was R. turanicus in both small ruminants, which is in agreement with other Tunisian surveys (Bouattour et al. 1999, Bouattour 2002, Darghouth 2004). Some of identified tick species, such as R. turanicus, R. bursa, and R. sanguineus, have been proposed previously as vectors of A. ovis in Mediterranean countries (de la Fuente et al. 2005, Torina et al. 2008a, Aktas et al. 2009). Aktas et al. (2009) reported the presence of A. ovis DNA in the salivary glands of R. sanguineus collected from Turkey. Torina et al. (2008a) and de la Fuente et al. (2005) demonstrated that R. turanicus is the main vector of A. ovis in Sicily (Italy). Until now, the vectors of A. ovis are still unknown in Tunisia; thus, further studies are needed to identify the main vectors of this bacterium.
PCR results based on the groEL gene demonstrate the absence of A. phagocytophilum in Tunisian small ruminants. This finding is in agreement with those reported in the North Mediterranean area by Torina et al. (2008a) and Zobba et al. (2014). On the basis of the 16S rRNA gene, DNA fragments of Anaplasma species were detected in small ruminants in northern Tunisia. According to the cutoff value (99.0%) for species delineation (Adékambi et al. 2008), all genotypes obtained in this study can be potentially classified in two Anaplasma species different from all classified Anaplasma species, as sequence identities varied from 98.8% to 96.5% when comparing them to the six Anaplasma species officially recognized worldwide and varied from 98.7% to 98.3% between the Aplike2GGo1 genotype and all other genotypes identified in the present study.
On the one hand, genotypes from Aplike1GGo1 to Aplike1GGo 3 and from Aplike1GOv1 to Aplike1GOv 3 were 99.7–100% identical to Anaplasma sp. (Japan) (JN055357), considered as an unclassified novel species closely related to A. phagocytophilum by Ybañez et al. (2012a). Although previous reports described this potentially novel species as A. phagocytophilum, Ybañez et al. (2012a,b, 2013) suggested that the A. phagocytophilum strains initially found in Japan are distinct from those of the A. phagocytophilum strains in other countries. This Anaplasma species closely related to A. phagocytophilum has been detected earlier in Japanese ruminants and ticks (Ohashi et al. 2005, Jilintai et al. 2009, Yoshimoto et al. 2010, Ybañez et al. 2012a), but the presence of this species in small ruminants in Tunisia suggests that infection is not dependent on the geographical area but probably on the host. Instead, the Aplike2GGo1 genotype was 99.2% identical to Anaplasma sp. detected in H. asiaticum tick-infested Chinese ruminants (JX402604, KJ410247, and KJ410249) considered as another potentially novel species closely related to A. phagocytophilum by Kang et al. (2014).
In phylogenetic trees based on the 16S rRNA gene, genotypes of Anaplasma species obtained in this study were consistently placed in two divergent clusters relatively distant from the A. phagocytophilum strains infecting humans, dogs, horses, and rodents. In addition, these novel A. phagocytophilum–like strains are associated to different possible tick vectors (R. turanicus, R. sanguineus, R. annulatus, and H. excavatum) with respect to A. phagocytophilum strains (I. pacificus and I. scapularis for American strains and I. ricinus for European strains; Richter et al. 1996, Telford et al. 1996, Petrovec et al. 1997, Woldehiwet 2010). Furthermore, infected small ruminants exhibited no clinical signs of active disease, as also reported for similar strains by Jilintai et al. (2009) and Yoshimoto et al. (2010). All of these findings suggest the circulation of at least two potential novel species genetically related to A. phagocytophilum in small ruminants in Tunisia.
In conclusion, molecular identification of potential novel Anaplasma species increases concerns about the specificity of serological tests routinely used in ruminants for diagnosis of anaplasmosis and provides additional information for the pathogenesis and molecular epidemiology of A. phagocytophilum and related species. Further studies are needed to characterize strains better by more discriminative genes and to identify the main tick vectors implicated in the transmission of these potentially novel Anaplasma species in Tunisia.
Supplementary Material
Acknowledgments
This work was supported by the Laboratoire d'épidémiologie d'infections enzootiques des herbivores en Tunisie (LR02AGR03), funded by the Ministry of Higher Education, Scientific Research, and Information and Communication Technologies of Tunisia, the research project Epidémiologie de maladies bactériennes à transmission vectorielle des herbivores (06-680-0029), which was funded by the Ministry of Agriculture of Tunisia, and the research project “PS1.3.023–RESTUS” funded by the European Neighbourhood and Partnership Instrument (ENPI)–Transboundary Cooperation (TC)–Italy–Tunisia 2007–2013. The authors would like to thank Drs. Leïla Sayeh, Hichem Talhaoui, Rabeh Bouazizi, and Samir Touil, and to acknowledge their technicians for their help and their contribution in facilitating the access to the farms. They are thankful to Pr. Ali Bouattour for his assistance in tick identification.
Author Disclosure Statement
No competing financial interests exist.
References
- Adékambi T, Shinnick TM, Raoult D, Drancourt M. Complete rpoB gene sequencing as a suitable supplement to DNA–DNA hybridization for bacterial species and genus delineation. Int J Syst Evol Microbiol 2008; 58:1807–1814 [DOI] [PubMed] [Google Scholar]
- Aktas M, Altay K, Dumanli N, Kalkan A. Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol Res 2009; 104:1243–1248 [DOI] [PubMed] [Google Scholar]
- Alberti A, Zobba R, Chessa B, Addis MF, et al. . Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl Environ Microbiol 2005; 71:6418–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altay K, Dumanli N, Aktas M, Özubek S. Survey of Anaplasma infections in small ruminants from east part of Turkey. Kafkas Universitesi Veteriner Fakültesi Dergisi 2014; 20:1–4 [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, et al. . Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 1997; 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DM, Van Niekerk CH. Anaplasmosis in improved Boer goats in South Africa artificially infected with Anaplasma ovis. Small Rumin Res 1990; 3:191–197 [Google Scholar]
- Belkahia H, Ben Said M, El Hamdi S, Yahiaoui M, et al. . First molecular identification and genetic characterization of Anaplasma ovis in sheep from Tunisia. Small Rumin Res 2014; 121:404–410 [Google Scholar]
- Ben Said M, Belkahia H, Sayahi L, Aloui M, et al. . Première étude sérologique de la prévalence d'Anaplasma phagocytophilum chez le dromadaire (Camelus dromedarius) en Tunisie. Bull Soc Pathol Exot 2013; 107:1–6 [DOI] [PubMed] [Google Scholar]
- Ben Said M, Belkahia H, Héni MM, Bouattour A, Ghorbel A, et al. . Seroprevalence of Anaplasma phagocytophilum in well-maintained horses from Northern Tunisia. Trop Biomed 2014; 31:432–440 [PubMed] [Google Scholar]
- Bouattour A. Clé dichotomique et identification des tiques (Acari: Ixodidae), parasites du bétail au Maghreb. Arch Inst Pasteur Tunis 2002; 79:43–50 [PubMed] [Google Scholar]
- Bouattour A, Darghouth MA, Daoud A. Distribution and ecology of ticks (Acari: Ixodidae) infesting livestock in Tunisia: An overview of eighth years field collections. Parassitologia 1999; 41:5–10 [PubMed] [Google Scholar]
- Chi Q, Liu Z, Li Y, Yang J, et al. . Development of a real-time PCR assay for detection and quantification of Anaplasma ovis infection. Transbound Emerg Dis 2013; 60:119–124 [DOI] [PubMed] [Google Scholar]
- Chochlakis D, Ioannou I, Tselentis Y, Psaroulaki A. Human anaplasmosis and Anaplasma ovis variant. Emerg Infect Dis 2010; 16:1031–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darghouth MA. Piroplasmids of livestock in Tunisia. Arch Inst Pasteur Tunis 2004; 81:21–25 [PubMed] [Google Scholar]
- de la Fuente J, Torina A, Caracappa S, Tumino G, et al. . Serologic and molecular characterization of Anaplasma species infection in farm animals and ticks from Sicily. Vet Parasitol 2005; 133:357–362 [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Atkinson MW, Naranjo V, Fernández de Mera IG, et al. . Sequence analysis of the msp4 gene of Anaplasma ovis strains. Vet Microbiol 2007; 119:375–381 [DOI] [PubMed] [Google Scholar]
- Djiba ML, Mediannikov O, Mbengue M, Thiongane Y, et al. . Survey of Anaplasmataceae bacteria in sheep from Senegal. Trop Anim Health Prod 2013; 45:1557–1561 [DOI] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, et al. . Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 2001; 51:2145–2165 [DOI] [PubMed] [Google Scholar]
- Friedhoff KT. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997; 39:99–109 [PubMed] [Google Scholar]
- Hornok S, Elek V, de la Fuente J, Naranjo V, et al. . First serological and molecular evidence on the endemicity of Anaplasma ovis and A. marginale in Hungary. Vet Microbiol 2007; 122:316–322 [DOI] [PubMed] [Google Scholar]
- Jilintai NS, Hayakawa D, Suzuki M, Hata H, et al. . Molecular survey for Anaplasma bovis and Anaplasma phagocytophilum infection in cattle in a pasture and where sika deer appear in Hokkaido, Japan. Jpn J Infect Dis 2009; 62:73–75 [PubMed] [Google Scholar]
- Kang YJ, Diao XN, Zhao GY, Chen MH, et al. . Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol Biol 2014; 14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Rikihisa Y, Lin Q, Isogai E, et al. . Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol 2006; 72:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan KM, de la Fuente J, Guglielmone AA, Meléndez RD. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin Microbiol Rev 2003; 16:698–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocan KM, de la Fuente J, Blouin EF, Garcia-Garcia JC. Anaplasma marginale (Rickettsiales: Anaplasmataceae): Recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 2004; 129:S285–S300 [DOI] [PubMed] [Google Scholar]
- Kubelová M, Mazancová J, Siroký P. Theileria, Babesia, and Anaplasma detected by PCR in ruminant herds at Bié Province, Angola. Parasite 2012; 19:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ma M, Wang Z, Wang J, et al. . Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl Environ Microbiol 2012; 78:464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Liu Z, Sun M, Yang J, et al. . Development and evaluation of a loop-mediated isothermal amplification method for rapid detection of Anaplasma ovis. J Clin Microbiol 2011; 49:2143–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- M'ghirbi Y, Ghorbel A, Amouri M, Nebaoui A, et al. . Clinical, serological, and molecular evidence of ehrlichiosis and anaplasmosis in dogs in Tunisia. Parasitol Res 2009; 104:767–774 [DOI] [PubMed] [Google Scholar]
- M'ghirbi Y, Yaïch H, Ghorbel A, Bouattour A. Anaplasma phagocytophilum in horses and ticks in Tunisia. Parasit Vectors 2012; 30:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N, Inayoshi M, Kitamura K, Kawamori F, et al. . Anaplasma phagocytophilum-infected ticks, Japan. Emerg Infect Dis 2005; 11:1780–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovec M, Lotric Furlan S, Zupanc TA, Strle F, et al. . Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol 1997; 35:1556–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renneker S, Abdo J, Salih DEA, Karagenc T, et al. . Can Anaplasma ovis in small ruminants be neglected any longer? Transbound Emerg Dis 2013; 60:105–112 [DOI] [PubMed] [Google Scholar]
- Richter PJ, Kimsey RB, Madigan JE, Barlough JE, et al. . Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae). J Med Entomol 1996; 33:1–5 [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 1987; 4:406–425 [DOI] [PubMed] [Google Scholar]
- Sarih M, M'ghirbi Y, Bouattour A, Gern L, et al. . Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. J Clin Microbiol 2005; 43:1127–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltsz WH. Ovine and caprine anaplasmosis. In: Infectious Diseases of Livestock, vol. 1, 2nd ed. Coetzer J, Tustin RC, eds. Cape Town: Oxford University Press, 1994:617–624 [Google Scholar]
- Stuen S. Anaplasma phagocytophilum—the most widespread tick-borne infection in animals in Europe. Vet Res Commun 2007; 31:79–84 [DOI] [PubMed] [Google Scholar]
- Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, et al. . Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA 1996; 93:6209–6214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torina A, Caracappa S. Tick-borne diseases in sheep and goats: Clinical and diagnostic aspects. Small Rum Res 2012; 106:S6–S11 [Google Scholar]
- Torina A, Alongi A, Naranjo V, Estrada-Peña A, et al. . Prevalence and genotypes of Anaplasma species and habitat suitability for ticks in a Mediterranean ecosystem. Appl Environ Microbiol 2008a; 74:7578–7584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torina A, Alongi A, Naranjo V, Scimeca S, et al. . Characterization of Anaplasma infections in Sicily, Italy. Ann NY Acad Sci 2008b; 1149:90–93 [DOI] [PubMed] [Google Scholar]
- Torina A, Galindo RC, Vicente J, Di Marco V, et al. . Characterization of Anaplasma phagocytophilum and A. ovis infection in a naturally infected sheep flock with poor health condition. Trop Anim Health Prod 2010; 42:1327–1331 [DOI] [PubMed] [Google Scholar]
- Torina A, Agnone A, Blanda V, Alongi A, et al. . Development and validation of two PCR tests for the detection of and differentiation between Anaplasma ovis and Anaplasma marginale. Ticks Tick Borne Dis 2012; 3:283–287 [DOI] [PubMed] [Google Scholar]
- Tuomi J. Experimental studies on bovine tick-borne fever .1. Clinical and haematological data, some properties of the causative agent, and homologous immunity. Acta Pathol Microbiol Scand 1967; 70:429–445 [PubMed] [Google Scholar]
- Walker AR, Bouattour A, Camicas J-L, Estrada-Peña A, et al. . Ticks of domestic animals in Africa: A guide to identification of species. Part 4: Species of ticks. Biosci Rep 2013:45–221 [Google Scholar]
- Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol 2010; 167:108–122 [DOI] [PubMed] [Google Scholar]
- Ybañez AP, Matsumoto K, Kishimoto T, Inokuma H. Molecular analyses of a potentially novel Anaplasma species closely related to Anaplasma phagocytophilum detected in sika deer (Cervus nippon yesoensis) in Japan. Vet Microbiol 2012a; 157:232–236 [DOI] [PubMed] [Google Scholar]
- Ybañez AP, Matsumoto K, Kishimoto T, Yokoyama N, et al. . Dual presence of Anaplasma phagocytophilum and its closely related Anaplasma sp. in ixodid ticks in Hokkaido, Japan, and their specific molecular detection. J Vet Med Sci 2012b; 74:1551–1560 [DOI] [PubMed] [Google Scholar]
- Ybañez AP, Tagawa M, Matsumoto K, Kishimoto T, et al. . Specific molecular detection of Anaplasma sp. closely related to Anaplasma phagocytophilum in Ixodid ticks and cattle in a pastureland in Hokkaido, Japan. Vector Borne Zoonotic Dis 2013; 13:6–11 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Matsuyama Y, Matsuda H, Sakamoto L, et al. . Detection of Anaplasma bovis and Anaplasma phagocytophilum DNA from Haemaphysalis megaspinosa in Hokkaido, Japan. Vet Parasitol 2010; 168:170–172 [DOI] [PubMed] [Google Scholar]
- Zobba R, Anfossi AG, Parpaglia MLP, Dore GM, et al. . Molecular investigation and phylogeny of Anaplasma spp. in Mediterranean ruminants reveal the presence of neutrophil-tropic strains closely related to A. platys. Appl Environ Microbiol 2014; 80:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.