Abstract
Decursin, a bioactive phytochemical isolated from Angelica gigas Nakai (danggwi), has shown preclinical anticancer efficacy in various cancer models. However, the antitumor effect of decursin in melanoma models remains undefined. The antitumor activities of decursin were investigated in B16F10 cells in vitro and in vivo. In this study, we show that treatment with decursin inhibited cell proliferation in a dose-dependent manner in B16F10 cells, but not in normal cells. Decursin also induced apoptosis in B16F10 cells, as determined by annexin V-staining assay and transferase-mediated nick-end labeling (TUNEL) staining assay. Decursin increased the phosphorylation of p38 as well as the expression of Bax while decreasing the phosphorylation of extracellular signaling-regulated kinase (ERK) and the expression of Bcl-2 in B16F10 cells. Moreover, decursin activated caspase-3 in B16F10 cells and xenograft tumor tissue. Together, these findings support further investigations into the potential use of decursin in the treatment of melanoma cells.
Key Words: : antiproliferation, antitumor, apoptosis, Bax, Bcl-2
Introduction
Melanoma is less common, but much more dangerous than other skin cancers if not detected in the early stages. It is the cause of majority (75%) of deaths related to skin cancer.1 Globally, in 2012, melanoma occurred in 232,000 people and resulted in 55,000 deaths.2
Although chemotherapy is one of the most frequently used therapeutic modalities for the treatment of this cancer, chemotherapy alone does not achieve satisfactory therapeutic results.3 Moreover, cytotoxic chemotherapy and radiotherapy do not show any significant improvement in patient condition due to the high recurrence of apoptosis-resistant hormone-refractory melanoma.4 However, many nutritive and nonnutritive phytochemicals with diverse pharmacologic properties have shown promising results in the prevention and/or intervention of various cancers, including melanoma.5,6
Herbal medicines, including conventional and complementary medicines, are still prevalent and serve the medicinal needs of a large population around the world.7 Angelica gigas Nakai grows in relatively moist soil and is a biennial or herbaceous perennial plant. A. gigas Nakai is indigenous to Korea and used in traditional medicine. Its major compound, decursin, exerted antitumor activity by apoptosis induction or angiogenesis inhibition in various cancer cells, including prostate, bladder, and colon cancer.8,9 Nevertheless, little is known about its apoptotic activity in melanoma.
This study aimed to investigate the apoptotic effect and the potential mechanisms of decursin in vitro and in vivo by using B16F10 melanoma cells. We found that decursin induced apoptosis through the caspase-3-dependent pathway both in vitro and in vivo.
Materials and Methods
Cell culture
B16F10 melanoma cell and NIH-3T3 cell were obtained from the Korea Cell Line Bank (Seoul, Korea). B16F10 melanoma cells were grown in Dulbecco's Modified Eagle's Medium (DMEM; Gibco BRL, Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS). NIH-3T3 cells were also grown in DMEM supplemented with 10% newborn calf serum. The cells were cultured under a humidified atmosphere with 5% CO2 at 37°C.
Cell viability assay
The cell viability was confirmed using the MTT assay. Cells were seeded at a density of 3×103 cells per well in 96-well plates and stabilized at 37°C in a CO2 incubator. Decursin (Institute for Korea Traditional Medical Industry; KOTMIN, Gyeongsan, Korea), at different concentrations, was added to each well and the cells were incubated for 24 h. Following this, the MTT solution (5 mg/mL) was added to each well and the cells were incubated for another 3 h. After removing all the liquid from the wells, chloroform treatment was done for 1 h. The optical density was measured at 595 nm using an ELISA reader.
The proliferation assay was carried out using the bromodeoxyuridine (BrdUrd) incorporation assay. To measure the BrdUrd incorporation, cells were incubated on plates in the presence or absence of decursin in DMEM for containing 2% FBS, followed by BrdUrd (Oncogene Research Product, Boston, MA, USA) for 6 h in the culture incubator. BrdUrd uptake by the cells was detected using a Cell Proliferation Assay Kit (Oncogene Research Product).
Western blot analysis
B16F10 cells were seeded at a density of 6×105 cells per well into a 60-mm dish and stabilized for 24 h. The cells were then treated with 0, 20, 40, 60, 80, and 100 μM of decursin and lysed in a lysis buffer (150 mM NaCl, 1% TritonX-100, 1% soduim deoxycholate, 0.1% SDS, 50 mM Tris-HCl) with a protease inhibitor and phosphatase inhibitor cocktails. Total protein extracts were separated on a 15% SDS-PAGE gel and transferred to membranes blocked with 5% skim milk. Primary antibodies were incubated overnight. The bound antibodies were then incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit Ig secondary antibody. The immunoreactivity was revealed with incubation with the ECL Western Blotting Detection Reagents (Amersham, Buckinghamshire, United Kingdom). Membranes were exposed by image station 4000MM Pro Imaging System (Kodak, Rochester, USA). Antibodies for Bax (1:1000), Bcl-2 (1:1000), pro-caspase-3 (1:1000), p-p38 (1:1000), and p38 (1:1000) were purchased from Cell Signaling Technology, and antibodies for p-extracellular signaling-regulated kinase (ERK) (1:1000), ERK (1:1000), β-actin (1:1000), and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Annexin V staining
After treatment with various concentrations of decursin, the cells were collected and washed with phosphate-buffered saline (PBS), resuspended in a binding buffer, and stained with annexin V for 15 min at room temperature (RT) in the dark. The samples were then analyzed using flow cytometry.
TUNEL and DAPI staining
In situ detection of DNA fragments was performed using an ApopTag® Red In Situ Apoptosis Detection Kit. After treatment with various concentrations of decursin, the cells were fixed using 4% paraformaldehyde (PFA) in PBS for 10 min at RT, followed by two washes with PBS for 5 min each. The samples were treated with precooled ethanol:acetic acid (2:1) for 5 min at –20°C, followed by two washes with PBS for 5 min each. The excess liquid was gently removed. An equilibration buffer was applied and the cells were then incubated for 10 min at RT. After removing the excess liquid, TdT enzyme was added and the cells were incubated in a humidified chamber at 37°C for 1 h. The stop buffer was added, followed by incubation for 10 min at RT and three washes with PBS for 1 min each. After removing the excess liquid, a warmed working strength anti-digoxigenin conjugate was added to the slide and then incubated in a humidified chamber for 30 min at RT. After washing four times with PBS for 2 min each at RT, a mounting medium containing 0.5–1 μg/mL of DAPI (4′,6-diamidino-2-phenylindole) was added and mounted under a glass coverslip. Using fluorescence microscopy, the average number of fluorescence dots of three images from each treatment group was calculated.
In vivo tumor and histological assay
The laboratory animals used for the experiment were Male C57BL/6J mice from Hyochang Science (Daegu, Korea). The mice were obtained 1 week before the experiment to allow sufficient time for adaptation. The mice were cared for under an environment with 50%±10% humidity and 25°C±1°C temperature and an automatic lighting system that was employed to provide sufficient light in a 12-h cycle. All animals were given free access to water and food.
Male C57BL/6J mice (5–6 weeks old) were implanted with 1×106 B16F10 cells subcutaneously into the flank, and tumor growth after systemic treatment with decursin (10 mg/kg) was monitored. The experimental group was intraperitoneally injected with decursin on alternate days for 14–20 days with a total volume of 0.1 mL. After 20 days, the mice were sacrificed and fixed with 4% PFA for 24 h, and then, 10-μm-thick sections were prepared. Apoptotic cells were assessed by terminal deoxy-nucleotidyl transferase-mediated nick-end labeling (TUNEL) assays. After washing with PBS, the sections were incubated with Alexa-594-conjugated secondary antibody (diluted 1:200 in 1% BSA) for 60 min at RT. Sections were then washed with PBS, incubated with DAPI, slide mounted, and sealed. Samples were visualized under a fluorescence microscope.
Statistical analysis
All results shown are averages of three independent experiments. Statistical significances were determined using the Student's t-test. Statistical significance was accepted for P values<.05.
Results
Decursin inhibits the viability and proliferation of B16F10 cells, not NIH-3T3 cells
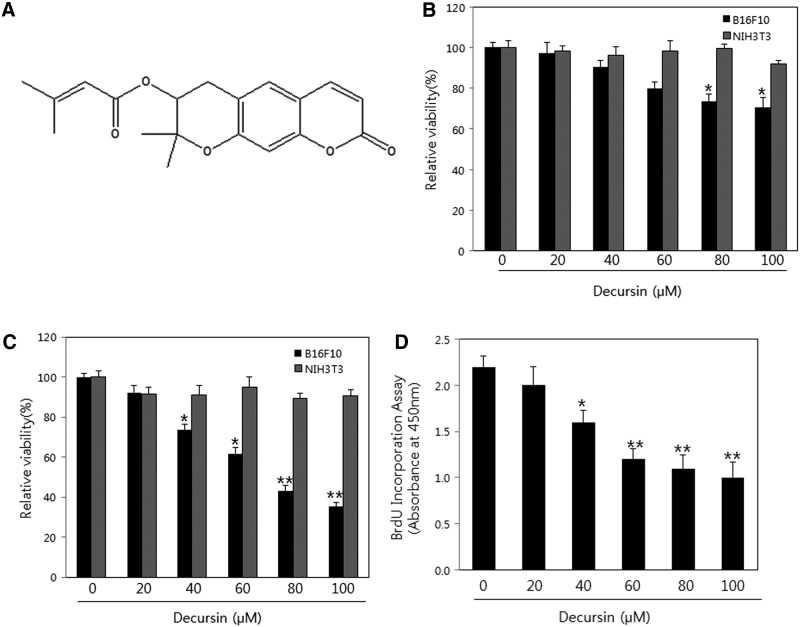
Several plant species have been studied for anticancer properties, and an estimated 40–50% of the drugs in the market today are either natural products or derived from natural products.10,11 Nonetheless, comprehensive and systematic evaluation of natural products is required to demonstrate efficacy and safety for clinical use. To determine the antiviability effect of decursin on B16F10 cells and NIH-3T3 cells, both cell lines were cultured for 24–48 h with or without various concentrations of decursin (0–100 μM). Decursin significantly inhibited viability of B16F10 cells in a dose- and time-dependent manner, but not NIH-3T3 cells (Fig. 1A, B). Similar results were obtained using the BrdUrd incorporation assay with B16F10 cells (Fig. 1C). These results show that decursin could specifically inhibit the viability and proliferation in melanoma cells, but not normal cells.
FIG. 1.
Effect of decursin on cell viability in B16F10 and NIH-3T3 cells. (A) Chemical structure of decursin isolated from roots of Angelica gigas Nakai. (B, C) B16F10 cells were seeded into 96-well plates at a density of 3×104 cells/well. Cell growth inhibition in B16F10 cells treated with decursin for 24 h (B) and 48 h (C) as measured by MTT assay. (D) Inhibited cell proliferation after decursin treatment was confirmed by the BrdUrd Incorporation Assay. *P < .05, **P < .01.
Decursin induces apoptosis in B16F10 cells
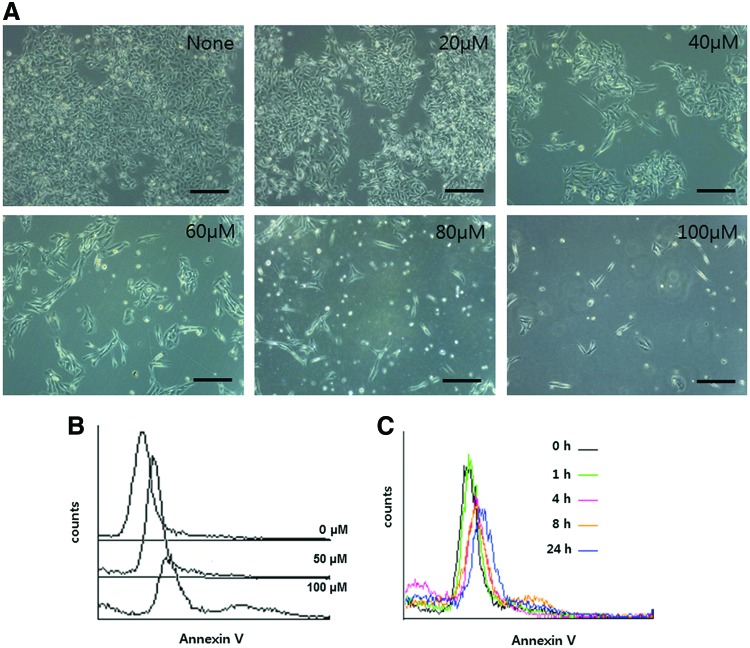
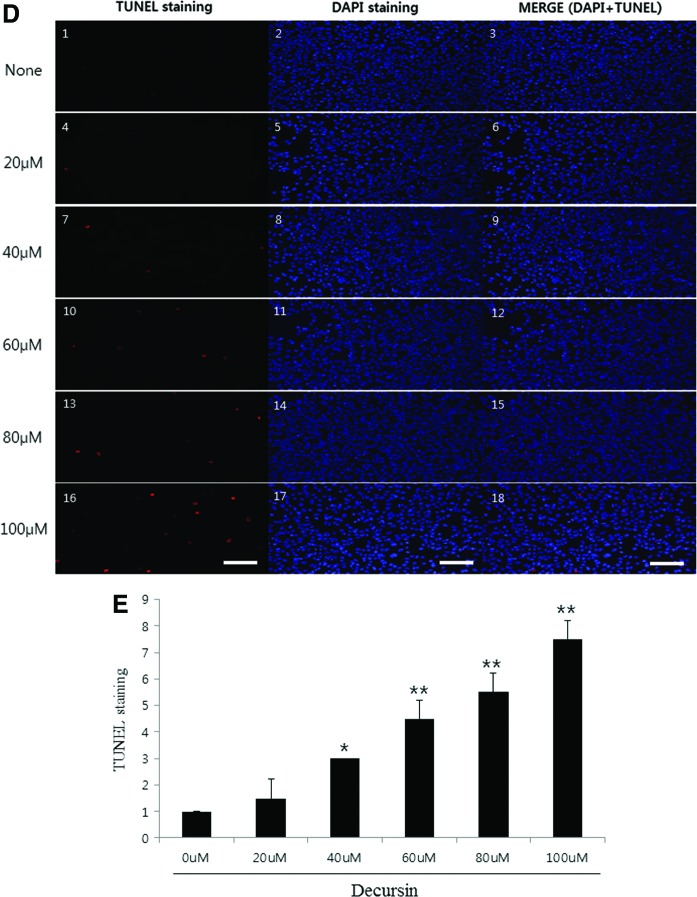
Chemotherapeutic agents induce tumor regression through inhibition and/or activation of apoptosis.12 Apoptosis, also called programmed cell death, is a morphological and biochemical change characterized by cell shrinkage, cytoplasm condensation, DNA fragmentation, and annexin V staining.13 Annexins are a family of calcium-dependent phospholipid-binding proteins, which bind to phosphatidylserine (PS) to identify apoptotic cells. In healthy cells, PS is predominantly located along the cytosolic side of the plasma membrane. Upon initiation of apoptosis, PS loses its asymmetric distribution in the phospholipid bilayer and translocates to the extracellular membrane, which is detectable using fluorescently labeled annexin V. To determine whether the inhibitory effect of decursin on cell proliferation is due to apoptotic cell death, we used phase-contrast microscopy. Direct observation showed that treatment of decursin was associated with cell shrinkage and cytoplasmic condensation in B16F10 cells (Fig. 2A). Annexin V-FITC staining method was used to confirm the presence of early apoptosis in cells. Figure 2B and C show the dose- and time-dependent increase of annexin V binding in decursin-treated B16F10 cells. To further quantify the decursin-induced apoptosis in B16F10 cells, cells were stained with TUNEL and DAPI. Representative images of TUNEL (white arrows) and DAPI staining are shown in Figure 2D. By calculating the number of fluorescent dots relative to the control group, we observed about 200% and 300% higher staining with 80 μM and 100 μM decursin treatment, respectively, compared to the control group (Fig. 2E). These results suggest that decursin induced apoptosis in a dose- and time-dependent manner.
FIG. 2.
Decursin-induced apoptosis in B16F10 cells. (A) B16F10 cells were seeded into 60-mm plates and treated by decursin at various concentrations for 48 h and examined with a microscope. (B, C) One hundred micromolars of decursin was added for an indicated time and concentration. Apoptosis was detected using the annexin V assay for flow cytometry. Decursin caused an increase in transferase-mediated nick-end labeling (TUNEL) positive cell area with an increasing time of treatment. After 48 h of treatment, cells were stained using TUNEL or DAPI. Representative image of TUNEL and DAPI staining at 400×magnification from the phosphate-buffered saline (PBS)-treated and 10 mg/kg decursin-treated groups are shown in (D). (E) The graph showed the average number of fluorescence dots in images from each treatment group. Original microscope magnification=200×. *P < .05, **P < .01. Color images available online at www.liebertpub.com/jmf
Decursin induces apoptosis of B16F10 cells
To gain insights into the mechanisms by which decursin induces apoptosis in B16F10 cells, we first investigated whether decursin treatment resulted in the activation of conventional mitogen-activated protein kinases (MAPKs), including ERK1/2, c-Jun N-terminal kinase (JNK), and p38 MAP kinase. Treatment with decursin inhibited ERK activation and induced p38 MAP kinase activation (Fig. 3). However, decursin treatment did not affect JNK activation (data not shown). These results suggest that ERK and p38 MAP kinase are important in decursin-mediated inhibition of B16F10 cell proliferation.
FIG. 3.

Effects of decursin on the apoptosis-related signal pathway. Cells were treated with decursin (0, 20, 40, 60, 80, 100 μM) for 48 h and the lysates were analyzed by Western blot analysis using the indicated protein (p, antibody specific for phosphorylated protein). Beta-actin was used as control.
In general, a balance between proapoptotic (Bax/Bad) and antiapoptotic (Bcl-2/Bcl-xl) members of Bcl-2 family proteins and their upregulation and downregulation determine whether cells undergo apoptosis or survival.14,15 Apoptosis is executed through two major caspase pathways, the mitochondria-dependent intrinsic caspase-9 pathway and the death receptor-mediated extrinsic caspase-8 pathway.16,17 Both pathways involve the proteolysis of various cellular components by activated caspase-3.18
Next, we investigated whether decursin treatment regulated the Bcl-2 family proteins and caspase-3 pathway. As shown in Figure 3, decursin downregulated the antiapoptotic protein, Bcl-2, and upregulated proapoptotic proteins, Bax and procaspase-3. Collectively, these results suggest that decursin induced cell death in B16F10 cells through the Bcl-2/Bax-mediated apoptosis pathway.
Decursin inhibits tumor growth
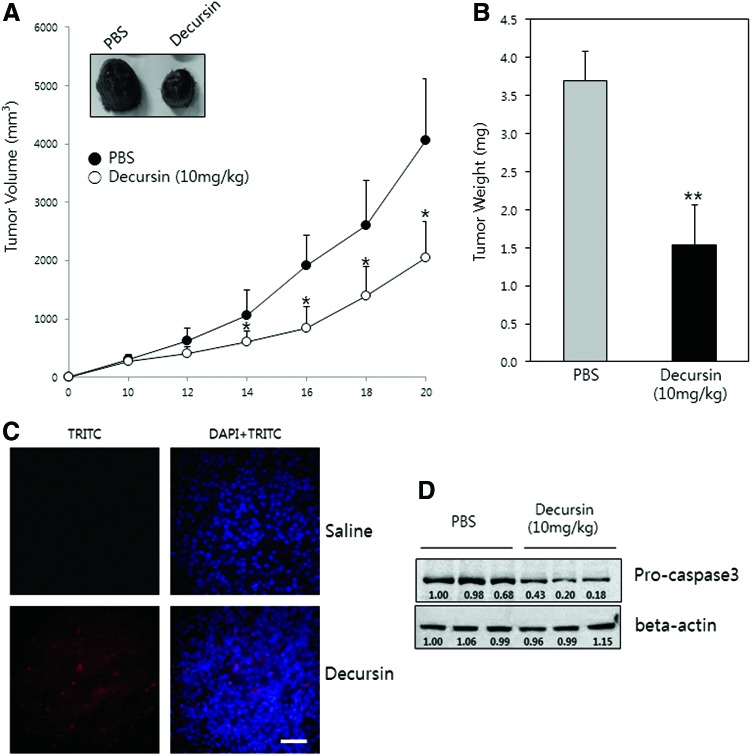
To examine the inhibitory effect of decursin on tumor growth in vivo, we implanted B16F10 cells into the flanks of C57BL6 mice and monitored tumor growth and body weight after systemic treatment with decursin. Decursin (10 mg/kg) was intraperitoneally injected on alternate days for 14–20 days after tumor cell implantation. After 20 days, mice were sacrificed and the tumor weights were measured. As shown in Figure 4A and B, decursin significantly inhibited tumor growth and tumor weight, compared to PBS treatment. The reduced sizes of tumor were consistent with increases in TUNEL-positive cells in the decursin-treated group (Fig. 4C). No significant differences in body weight were observed between the groups (data not shown). Western blot analysis of homogenized tumor tissue showed that the procaspase-3 protein expression was decreased in decursin-treated groups, compared to PBS-treated groups (Fig. 4D). Taken together, these results show that decursin can inhibit B16F10 tumor growth in vivo through apoptosis.
FIG. 4.
Decursin inhibits tumor growth in a B16F10 tumor model. (A) C57BL/6 mice with B16F10 melanomas were injected with 10 mg/kg of decursin (n=6), or PBS (n=6) for 10 alternate days. The tumor size was measured every other day and the tumor volume was calculated. (A) Whole tumor tissues at 20 days of implantation. (B) This graph indicates the tumor weights after 10 mg/kg decursin treatment. (C) Tumors after 20 days of implantation were cut into sections and immunofluoresence staining was performed. Representative image of TUNEL and DAPI staining at 400×magnification from the PBS-treated and 10 mg/kg decursin-treated groups are shown in (C). (D) Decursin induced apoptosis and modulated the protein expression of procaspase-3 and beta-actin in B16F10 tumor tissues. Representative immunoblots for phosphorylated procaspase-3 and actin in tumor tissue isolated from PBS- and decursin-treated mice. *P < .05, **P < .01. Color images available online at www.liebertpub.com/jmf
Discussion
Many studies have been undertaken to elucidate the anticancer effects of decursin on apoptosis induction and inhibition of cell growth in several cancer cell lines.19,20 However, the molecular mechanism by which decursin inhibits cell proliferation and cell apoptosis of B16F10 cells in vitro and in vivo model, is not yet understood. The present study is the first to show that decursin-induced apoptosis through caspase-3 activation is due to p38 activation in B16F10 cells and mouse tumor model.
Most of the presently available anticancer drugs have the ability to induce tumor cell apoptosis.12 Consistent with this approach, we found that decursin-induced apoptosis in B16F10 cells is associated with reduced cell viability and proliferation. This study also defined the mechanical events of apoptosis that are associated with decursin-induced inhibition of cell growth in B16F10 cells. In general, apoptosis is regulated by proapoptotic and antiapoptotic protein members of the Bcl-2 family and is executed through major caspase pathways, the mitochondrial-dependent intrinsic cytochromec/caspase-9 pathway, and the death receptor-mediated extrinsic caspase-8 pathway. Caspase-3 interacts with caspase-8 and caspase-9. Caspase-3 shares many of the typical characteristics common to all currently known caspases and initiates apoptotic cell death by both extrinsic and intrinsic pathways.21,22 In this study, we demonstrated that decursin-induced apoptotic death in B16F10 cells is mediated, at least in part, through the activation of caspase-3.
The MAPK signaling pathway is one of the critical molecular events regulating cell proliferation and migration.23–26 MAPKs include ERK, JNK, and p38 MAP kinase. MAPKs play important roles during cancer progression and have been shown to be activated during the apoptotic death of tumor cells in response to various cellular stresses.14 The RAF/MEK/ERK-MAPK pathway is activated in most melanomas.15 The activation of ERK pathway directs cell proliferation, differentiation, and survival, whereas the p38 MAPK pathway is involved in cellular stress, including chemotherapeutic agents, UV irradiation, and proinflammatory cytokines.16,17 The present study is the first to report the activation of p38 MAPK in decursin-induced B16F10 cell apoptosis induction.
Our data show that decursin results in significant suppression of tumor growth in a B16F10 mouse tumor model. From systemic treatment of tumor-bearing mice with decursin, we found that the reduced tumor sizes were correlated with increases in TUNEL-positive cells and activation of caspase-3. Our results indicated that decursin also inhibits B16F10 tumor growth through activation of caspase-3 in vivo model. To verify decursin-induced apoptosis in vivo model, we only showed procaspase-3 expression and not others, because it is well known that procaspase-3 is regulated by Bax, Bcl-2, p38, and ERK pathways. In conclusion, we provide preliminary evidence that decursin acts as a regulator of pathologic tumorigenesis, without affecting normal cells, thus supporting its development as a potential inhibitor for the control of tumorigenesis.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2064784); and by Kyungpook National University Research Fund, 2012.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ratheesh A, Ingle A, Gude RP: Pentoxifylline modulates cell surface integrin expression and integrin mediated adhesion of B16F10 cells to extracellular matrix components. Cancer Biol Ther 2007;6:1743–1752 [DOI] [PubMed] [Google Scholar]
- 2.Cummins DL, Cummins JM, Pantle H, et al. : Cutaneous malignant melanoma. Mayo Clin Proc 2006;81:500–507 [DOI] [PubMed] [Google Scholar]
- 3.Fulda S, Debatin KM: Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006;25:4798–4811 [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee AK, Basu S, Sarkar N, et al. : Advances in cancer therapy with plant based natural products. Curr Med Chem 2001;8:1467–1486 [DOI] [PubMed] [Google Scholar]
- 5.Birt DF, Bresnick E: Chemoprevention by nonnutrient components of vegetables and fruits. Cancer Nutr 1991;7:221–260 [Google Scholar]
- 6.Messina M, Barnes S: The role of soy products in reducing risk of cancer. J Natl Cancer Inst 1991;83:541–546 [DOI] [PubMed] [Google Scholar]
- 7.Tilburt JC, Kaptchuk TJ: Herbal medicine research and global health: An ethical analysis. Bull World Health Organ 2008;86:594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo MA, Song YK, Jang H, et al. : Profiling of skin anti-aging related proteins in human dermal fibroblasts by decursin in Angelica gigas Nakai. Korean J Chem Eng 2011;28:880–885 [Google Scholar]
- 9.Son SH, Park KK, Park SK, et al. : Decursin and decursinol from Angelica gigas inhibit the lung metastasis of murine colon carcinoma. Phytother Res 2010;25:959–964 [DOI] [PubMed] [Google Scholar]
- 10.Cordell GA, Beecher CW, Pezzuto JM: Can ethnopharmacology contribute to the development of new anticancer drugs? J Ethnopharmacol 1991;32:117–133 [DOI] [PubMed] [Google Scholar]
- 11.Balandrin MF, Klocke JA, Wurtele ES, et al. : Natural plant chemicals: Sources of industrial and medicinal materials. Science 1985;7:1154–1160 [DOI] [PubMed] [Google Scholar]
- 12.Ajani JA: Evolving chemotherapy for advanced gastric cancer. Oncologist 2005;10:49–58 [DOI] [PubMed] [Google Scholar]
- 13.Gerl R, Vaux DL: Apoptosis in the development and treatment of cancer. Carcinogenesis 2005;26:263–270 [DOI] [PubMed] [Google Scholar]
- 14.Afaq F, Adhami VM, Ahmad N, et al. : Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor κB by green tea polyphenol in SKH-1 hairless mice. Oncogene 2003;22:9254–9264 [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Bergami P: The role of mitogen- and stress-activated protein kinase pathways in melanoma. Pigment Cell Melanoma Res 2011;24:902–921 [DOI] [PubMed] [Google Scholar]
- 16.Seger R, Krebs EG: The MAPK signaling cascade. FASEB J 1995;9:726–735 [PubMed] [Google Scholar]
- 17.Han J, Sun P: The pathways to tumor suppression via route p38. Trends Biochem Sci 2007;32:364–371 [DOI] [PubMed] [Google Scholar]
- 18.Vaux DL, Cory S, Adams JM: Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988;335:440–442 [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Lee YS, Jung SH, et al. : Anti-tumor activities of decursinol angelatea and decursin from Angelical gigas. Arch Pharm Res 2003;26:727–730 [DOI] [PubMed] [Google Scholar]
- 20.Yim D, Singh RP, Aqarwal C, et al. : A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res 2005;65:1035–1044 [PubMed] [Google Scholar]
- 21.Ghavami S, Hashemi M, Ande SR, et al. : Apoptosis and cancer: Mutations within caspase genes. J Med Genet 2009;46:497–510 [DOI] [PubMed] [Google Scholar]
- 22.Salvesen GS: Caspase: Opening the boxes and interpreting the arrows. Cell Death Differ 2002;9:3–5 [DOI] [PubMed] [Google Scholar]
- 23.Cobb MH, Goldsmith EJ: How MAP kinases are regulated. J Biol Chem 1995;270:14843–14846 [DOI] [PubMed] [Google Scholar]
- 24.Davis RJ: The mitogen-activated protein kinase signaling transduction pathway. J Biol Chem 1993;268:14553–14556 [PubMed] [Google Scholar]
- 25.Davis RJ: MAPKs: New JNK expands the group. Trends Biochem Sci 1994;19:470–473 [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, Dickens M, Raingeaud J, et al. : Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995;270:1326–1331 [DOI] [PubMed] [Google Scholar]