Abstract
Magnetic resonance imaging (MRI) with rectal protocol modification is a reliable staging modality which is rapidly replacing transrectal ultrasound for staging. The added information delivered by MRI includes wide circumferential radial margin compromise, distant lymph node metastasis in the pelvis, and level of sphincter compromise in the low rectum. As more information becomes available through ongoing studies, MRI may be able to contribute the decision to treat rectal cancer nonoperatively.
Keywords: MRI, staging, restaging, rectal cancer
The multidisciplinary team approach to rectal cancer has been validated to improve the outcome of patients with rectal cancer.1 2 The radiologist's contribution in this arena is accurate staging and restaging local extent of disease. These are vital to improving patient survival with rectal cancer while at the same time lowering the morbidity and mortality which may be associated with local recurrence and metastatic disease. Accurate staging prevents undertreatment or overtreatment of rectal cancer. Current evidence-based guidelines support the use of either transrectal ultrasound (TRUS) or magnetic resonance imaging (MRI) assessment of local disease extent. Despite these guidelines, recent research confirms appropriate local staging remains underutilized, particularly in the hands of general surgeons.3 MRI has supplanted TRUS for staging in many institutions. MRI with rectal cancer protocol modification is now the preferred modality for rectal cancer staging and restaging in most specialty institutions. The aim of this article is (1) to highlight the utility of MRI for these purposes, emphasizing synoptic reporting, and (2) to examine barriers and solutions to appropriate MRI utilization in rectal cancer care.
Magnetic Resonance Imaging Staging
Current TNM staging in rectal cancer derives stage groupings based on (1) the depth of tumor invasion into the rectal wall and surrounding structures, including the peritoneum and surrounding viscera; (2) the presence and number of involved lymph nodes; and (3) the presence of metastatic disease to distant organs, distant lymph nodes, or distant portions of the peritoneum. The superior soft tissue contrast achieved with state-of-the-art rectal protocol MRI allows for measurement of the tumor depth of invasion (DOI) (T stage), determination of the relationship of the most invasive component of the tumor to the mesorectal fascia, and elucidation of the tumor's relationship to the sphincter complex, peritoneal reflection, and perirectal venous plexus (Fig. 1A, B).4 Furthermore, MRI is able to assess for lymph nodes and tumor deposits in the tissues beyond the mesorectal fascia, including the pelvic sidewall, which, if unaddressed, are a source of residual and/or recurrent disease (Fig. 2).5 6 7 Though not currently included in current staging guidelines, the presence of extensive extramural vascular invasion as determined by MRI places patients at high risk for metastatic disease and is associated with poor survival.8 The inception of minimally invasive treatment strategies, such as local transanal excision, underscores the need to accurately assess a tumor's relationship to the peritoneal reflection and the layers of the rectal wall muscle. Two large studies have recently confirmed the ability of MRI to determine the location of the tumor with respect to the anterior peritoneal reflection. MRI is able to identify the peritoneal reflection between 75 and 90% of cases.9 10 Given the complexity and number of parameters that need to be addressed when interpreting rectal MRI, synoptic reporting utilizing standardized templates is crucial to consistently report all facets of a tumor that have implications on management.11 12
Fig. 1.

(A) Sagittal T2-weighted image showing a large circumferential low rectal tumor (asterisk) invading the prostate (large arrow) and seminal vesicle (arrowhead). Small arrow delineates the inferior margin of the anterior peritoneal reflection. (B) Coronal T2-weighted image showing a normal left “danger triangle” containing a paucity of bright fat that separates the lower margin of the rectum from the adjacent normal T2 dark fibers of the levator ani muscle. The circumferential low rectal tumor present invades the right danger triangle (asterisk) and extends further laterally into the right levator ani (large arrow), indicating the need for an extralevator TME.
Fig. 2.
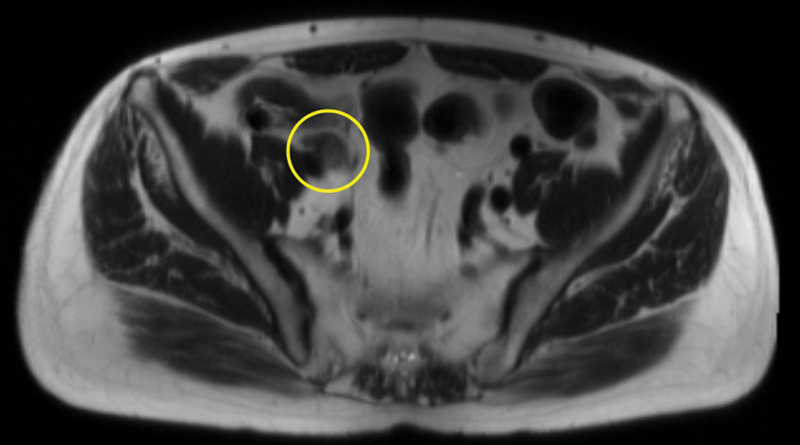
Axial large field of view image showing a metastatic right external iliac node (circle).
MRI has taken on an ever increasing role in the staging of rectal cancer since the MERCURY trial established strong concordance between radiologic and pathologic analysis of maximal depth of tumor invasion.13 The study established MRI stage can be compared with the pathological cross-section of the mesorectum and rectum as a whole mount (Fig. 3). MRI estimation of DOI utilizing axial oblique images to the long axis of the tumor was found to be within 0.5 mm of the DOI determined at pathology. Axial oblique images with respect to the tumor are critical in order to eliminate the possibility of partial volume effect on straight axial images. DOI or T stage in rectal cancer is an important predictor of local recurrence, the risk for local lymph node involvement and distant metastatic disease. The majority of patients with rectal cancer present with T3 tumors. Subclassification of T3 tumors based on DOI helps select which patients will most benefit from neoadjuvant chemoradiation therapy. The use of staging MRI limits the overutilization of neoadjuvant therapy in patients with low-risk disease to avoid the morbidity and cost of neoadjuvant therapy. Interestingly, MRI has been shown to be cost-effective when compared with TRUS for rectal cancer staging.14 TRUS is a powerful tool for evaluating superficial T1 tumors which have spread from the mucosa into the submucosa.15 MRI is best suited to evaluate T2 rectal cancers, which have invaded through the submucosa into the muscularis propria; T3 tumors, which have extended through the muscularis propria into the surrounding mesorectum; and T4 tumors, which have directly invaded adjacent pelvic viscera.
Fig. 3.

Axial gross specimen (left) and axial oblique T2-weighted MRI image showing a T3 tumor with 8-mm depth of invasion (line) and metastatic perirectal node (circle). Observe the hypointense (black) intact muscularis propria cephalad in the portions of the rectum uninvolved with tumor (small arrows).
A key strength of MRI in rectal cancer is evaluation of the tumor's relationship to the mesorectal fascia, which allows determination of the predicted circumferential tumor margin (CRM), the distance between the most invasive component of the tumor and the mesorectal fascia. It has been established that patients with tumors invading to within 1 mm of the CRM, so-called CRM positive, have increased incidence of local recurrence, shorter disease-free survival, and decreased overall survival compared with patients found to be CRM negative as assessed by MRI.16 A recent study has shown that TRUS is able to demonstrate the mesorectal fascia and estimate of the closest predicted radial margin. Agreement between TRUS and MRI has been reported at 81%.17 MRI remains the gold standard in this regard. Further studies with TRUS are warranted, particularly for the subset of patients having contraindications to MRI, for instance those with cardiac pacemakers and cochlear implants. Although there are numerous contraindications to MRI, for the unfortunate patient having an obstructing tumor which requires rectal stent for management, the stent does not preclude MRI and diagnostic images may still be obtained (Fig. 4 A, B).
Fig. 4.

(A) Sagittal T2-weighted image showing a large obstructing low rectal tumor that has undergone stenting. The stent results in posterior artifact (small arrows) but tumor invasion of the upper posterior vagina is clearly seen despite the presence of the stent (large arrow). (B) Coronal T2-weighted image showing a large obstructing low rectal tumor that has undergone stenting. The stent, which appears black, results in only minimal artifact abutting the stent (arrows).
The negative circumferential radial margin after radical resection is of paramount importance in achieving favorable oncologic outcomes. The ability of MRI to resolve a tumor's complex relationship with the surrounding pelvic visceral, sacrum, and mesorectal envelope facilitates charting a clear plane of resection. This is of particular relevance for low rectal tumors at high risk for local recurrence, as pelvic floor muscle resection can be guided by the definition of the tumor edge, allowing for determination of which patients require extralevator abdominoperineal resection for removal of invading tendrils in the deep pelvis to achieve clear circumferential radial margins (Fig. 1A, B).18 Thus, the endgame of accurate local staging is the prevention of local recurrence after abdominoperineal resection.
Magnetic Resonance Imaging Restaging
Local tumor control in patients with advanced disease may be achieved with a combination of surgical resection and chemoradiation. A minority patients will have a complete clinical response (18–20%) after neoadjuvant chemoradiotherapy (Fig. 5 A, B). Emerging data from observational studies of patients who have complete response to neoadjuvant therapy suggest that a subset of patients may be able to undergo close follow-up with deferral of extirpation. However, surgery remains a cornerstone of rectal cancer therapy. MRI may eventually be able to predict those patients who are candidates for close observation and those who should proceed with operative resection.
Fig. 5.

(A) Axial oblique T2-weighted image showing a T3 low rectal tumor from 2 o'clock to 6 o'clock (asterisk) with 5-mm depth of invasion (arrow), tumor less than 1 cm from the sphincter complex (seen on the coronal image which is not shown). (B) Axial oblique T2-weighted image showing a T3 low rectal tumor following neoadjuvant radiation therapy with favorable treatment response. Observe dense band-like fibrosis of the tumor (arrowheads) compatible with tumor regression grade 1–2.
Adequate restaging utilizing MRI relies on assessing changes of the tumor as regard to DOI, status of the CRM, reestimation of the tumor's relationship to the sphincter complex, assessing for status of extramural venous invasion, and estimation of tumor regression grade (TRG).19 20 The posttreatment status of the CRM and TRG as determined by MRI are of particular importance as to assess further treatment decisions as guided by the multidisciplinary team based on these parameters.21 The TRG can be determined by subjective assessment of the degree of tumor fibrosis following therapy. Treatment-induced fibrosis gives rise to dark T2 signal (black) replacing pretreatment intermediate T2 signal (gray) in rectal tumors. MR assessment of TRG (mrTRG) utilizes a 5-point scale with mrTRG-1 corresponding to no tumor signal (all black). mrTRG-2 corresponds to predominant fibrosis with minimal residual tumor (Fig. 5B). mrTRG-3 corresponds to mixed areas of fibrosis and residual tumor, but not a majority of residual tumor. mrTRG-4 corresponds to predominant tumor signal with little fibrosis (Fig. 6A–C). mrTRG-5 represents no fibrosis with tumor signal only.
Fig. 6.

(A) Sagittal T2-weighted image showing large polypoid low rectal tumor (asterisk). (B) Coronal T2-weighted image showing large polypoid low rectal tumor (asterisk) with less than 5 mm depth of invasion through the left rectal wall (arrowhead) appearing to abut the internal sphincter (large arrow). Observe the hypointense (black) intact muscularis propria cephalad to the tumor (small arrow). (C) Coronal T2-weighted image following neoadjuvant radiation showing decreased tumor size, but large amount of residual tumor remains (asterisk) compatible with tumor regression grade 4. The tumor has shrunk away from the sphincter complex (small arrow), affording sphincter a margin for sphincter sparing surgery (large arrow). The distal total mesorectal excision margin was negative on final pathology.
The mrTRG scale is useful for tumors which show treatment-related fibrotic change. This rating system does not characterize tumors which clearly have a size decrease following therapy yet still contain viable tumor. A tumor may be sufficiently down-staged to allow for sphincter sparing surgery despite the presence of substantial residual tumor volume (Fig. 6 A–C).
Lymph nodes which decrease in size between the index scan and restaging exams are taken to be malignant. Intermediate signal intensity nodes on the index scan which become T2 bright on the restaging exam correlate with mucinous treatment-related change. Such change infers a favorable response to therapy provided that the nodes were not T2 hyperintense on the initial staging exam as typical of mucinous metastatic nodes.
Despite optimal staging, improvements in neoadjuvant therapy, optimal restaging and advancements in surgical techniques, locally recurrent disease continues to be a challenging dilemma. MRI plays an important role for evaluation of locally recurrent tumors in the pelvis as it affords evaluation of the tumor's relationship to the pelvic side wall, all soft tissue structures in the pelvis (including the sacral plexus and iliac vessels), and any relevant bony involvement (Fig. 7). Salvage surgical planning relies accurate MRI staging of the recurrent tumor.
Fig. 7.

Sagittal T2-weighted image showing a large recurrent high rectal tumor (asterisk). Observe tumor invading the S2 sacral segment. The normal black cortical margin of the ventral S2 cortex is destroyed (arrow) with nodular tumor with the anterior S2 segment (arrowheads).
Barriers to Magnetic Resonance Imaging Utilization in Clinical Practice
There are manifold barriers to the implementation of rectal MRI in clinical practice. Although larger centers having multidisciplinary teams are able to guide radiologists about the importance of MRI in staging and restaging rectal cancer, the scope of clinical practice in many areas hampers radiologists becoming familiar with these studies. Radiologists' inexperience with the interpretation of these complex exams may lead them away from building this component of their practice. Importantly, the MRI protocols utilized for rectal cancer staging consist of standardized sequences which have been validated and published, on both 1.5 and 3.0 Tesla platforms and the hardware required to perform the exam should not be a barrier to implementation of MRI in practice.22 23
An important aspect to accurate tumor staging is clear communication from the referring clinician to the radiologist with respect to tumor location and histology. The proper performance of a staging or restaging rectal MRI exam depends on getting images which are in an axial plane oblique to the tumor. This requires knowing the precise location of the tumor, including the distance of the tumor from the anal verge, the tumor morphology, and ideally the clock-face location of the tumor with respect to the rectum. In busy clinical practices, radiologists may not be available during the acquisition of the MRI images. In such practices, MRI technicians have the sole responsibility for acquiring the images. If the tumor location is not properly communicated when the exam is ordered, the likelihood of improperly staging the tumor increases.
The current discussion strictly applies to rectal adenocarcinoma, which is well defined on noncontrast high-resolution T2 MRI images. Rectal tumors of non-adenocarcinoma types (e.g., neuroendocrine tumors and sarcomas) have different staging systems and require additional sequences, such as gadolinium postcontrast images, to be properly staged. The radiologist relies heavily on accurate and complete clinical data to avoid misdiagnosis. A policy of open communication between the radiologist and surgeon has the dual benefit of preventing patient anxiety which may arise when the patient must be recalled for additional images if the tumor cannot be localized on the images and, more importantly, preventing inappropriate treatment and associated morbidity that comes along with inaccurate staging.
It has been demonstrated that reader experience is of paramount importance for accurately staging rectal carcinoma, whether staging is achieved with MRI or TRUS.24 Accurate interpretation of rectal MRI requires considerable experience and care, particularly regarding the differentiation between T2 tumors, which involve the full thickness of the muscularis propria, and T3a tumors which extend to <1 mm into the perirectal tissue. Such challenging interpretation quandaries may discourage radiologists from acquiring the skill to interpret rectal MRI exams.
Conclusion
MRI with rectal protocol modification is a reliable staging modality which is rapidly replacing TRUS for staging. The added information delivered by MRI includes circumferential radial margin compromise, distant lymph node metastasis in the pelvis, assessment of extramural vascular invasion, and level of sphincter compromise in the low rectum. MRI provides pivotal restaging information regarding circumferential resection margin and TRG, thereby directing further therapy. As more information becomes available through ongoing studies, MRI may be able to contribute the decision to treat rectal cancer nonoperatively.
References
- 1.Taylor A, Sheridan M, McGee S, Halligan S. Preoperative staging of rectal cancer by MRI; results of a UK survey. Clin Radiol. 2005;60(5):579–586. doi: 10.1016/j.crad.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Augestad K M, Lindsetmo R O, Stulberg J. et al. International preoperative rectal cancer management: staging, neoadjuvant treatment, and impact of multidisciplinary teams. World J Surg. 2010;34(11):2689–2700. doi: 10.1007/s00268-010-0738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlton M E, Mattingly-Wells L R, Marcet J E, McMahon Waldschmidt B C, Cromwell J W. Association between surgeon characteristics and their preferences for guideline-concordant staging and treatment for rectal cancer. Am J Surg. 2014;208(5):817–823. doi: 10.1016/j.amjsurg.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson G S, Eardley N, McNicol F, Healey P, Hughes M, Rooney P S. Circumferential resection margin (CRM) positivity after MRI assessment and adjuvant treatment in 189 patients undergoing rectal cancer resection. Int J Colorectal Dis. 2014;29(5):585–590. doi: 10.1007/s00384-014-1846-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim D J, Kim J H, Ryu Y H, Jeon T J, Yu J S, Chung J J. Nodal staging of rectal cancer: high-resolution pelvic MRI versus 18F-FDGPET/CT. J Comput Assist Tomogr. 2011;35(5):531–534. doi: 10.1097/RCT.0b013e318225720f. [DOI] [PubMed] [Google Scholar]
- 6.Park J S, Jang Y J, Choi G S. et al. Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features. Dis Colon Rectum. 2014;57(1):32–38. doi: 10.1097/DCR.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 7.Tatli S, Mortele K J, Breen E L, Bleday R, Silverman S G. Local staging of rectal cancer using combined pelvic phased-array and endorectal coil MRI. J Magn Reson Imaging. 2006;23(4):534–540. doi: 10.1002/jmri.20533. [DOI] [PubMed] [Google Scholar]
- 8.Smith N J, Shihab O, Arnaout A, Swift R I, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191(5):1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 9.Gollub M J, Maas M, Weiser M. et al. Recognition of the anterior peritoneal reflection at rectal MRI. AJR Am J Roentgenol. 2013;200(1):97–101. doi: 10.2214/AJR.11.7602. [DOI] [PubMed] [Google Scholar]
- 10.Jung E J, Ryu C G, Kim G. et al. Is rectal MRI beneficial for determining the location of rectal cancer with respect to the peritoneal reflection? Radiol Oncol. 2012;46(4):296–301. doi: 10.2478/v10019-012-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor F, Mangat N, Swift I R, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging. 2010;10(1A):S142–S150. doi: 10.1102/1470-7330.2010.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nougaret S, Reinhold C, Mikhael H W, Rouanet P, Bibeau F, Brown G. The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”? Radiology. 2013;268(2):330–344. doi: 10.1148/radiol.13121361. [DOI] [PubMed] [Google Scholar]
- 13.Group M S; MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study Radiology 20072431132–139. [DOI] [PubMed] [Google Scholar]
- 14.Brown G, Davies S, Williams G T. et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91(1):23–29. doi: 10.1038/sj.bjc.6601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Esparrach G, Ayuso-Colella J R, Sendino O. et al. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74(2):347–354. doi: 10.1016/j.gie.2011.03.1257. [DOI] [PubMed] [Google Scholar]
- 16.Taylor F G, Quirke P, Heald R J. et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32(1):34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 17.Phang P T, Gollub M J, Loh B D. et al. Accuracy of endorectal ultrasound for measurement of the closest predicted radial mesorectal margin for rectal cancer. Dis Colon Rectum. 2012;55(1):59–64. doi: 10.1097/DCR.0b013e318235b885. [DOI] [PubMed] [Google Scholar]
- 18.Shihab O C, How P, West N. et al. Can a novel MRI staging system for low rectal cancer aid surgical planning? Dis Colon Rectum. 2011;54(10):1260–1264. doi: 10.1097/DCR.0b013e31822abd78. [DOI] [PubMed] [Google Scholar]
- 19.McGlone E R, Shah V, Lowdell C, Blunt D, Cohen P, Dawson P M. Circumferential resection margins of rectal tumours post-radiotherapy: how can MRI aid surgical planning? Tech Coloproctol. 2014;18(10):937–943. doi: 10.1007/s10151-014-1199-8. [DOI] [PubMed] [Google Scholar]
- 20.Patel U B, Blomqvist L K, Taylor F. et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response—the MERCURY experience. AJR Am J Roentgenol. 2012;199(4):W486-95. doi: 10.2214/AJR.11.8210. [DOI] [PubMed] [Google Scholar]
- 21.Videhult P, Smedh K, Lundin P, Kraaz W. Magnetic resonance imaging for preoperative staging of rectal cancer in clinical practice: high accuracy in predicting circumferential margin with clinical benefit. Colorectal Dis. 2007;9(5):412–419. doi: 10.1111/j.1463-1318.2006.01167.x. [DOI] [PubMed] [Google Scholar]
- 22.Gowdra Halappa V, Corona Villalobos C P, Bonekamp S. et al. Rectal imaging: part 1, High-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol. 2012;199(1):W35-42. doi: 10.2214/AJR.11.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa-Silva L, Brown G. Magnetic resonance imaging of rectal cancer. Magn Reson Imaging Clin N Am. 2013;21(2):385–408. doi: 10.1016/j.mric.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Rafaelsen S R, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43(4):440–446. doi: 10.1080/00365520701745842. [DOI] [PubMed] [Google Scholar]


