Abstract
Colorectal surgery is one of the most common procedures performed around the world with more than 600,000 operations each year in the United States, and more than a million worldwide. In the past two decades, there has been a clear trend toward minimal access and surgeons have embraced this evolution. Widespread adoption of advanced minimally invasive procedures is often limited by procedural complexity and the need for specific technical skills. Furthermore, the loss of 3D vision, limited overview of the surgical field, and diminished tactile sensation make major colorectal procedures more challenging and have an impact on the surgeons' learning curves. New technologies are emerging that can compensate for some of the sensory losses associated with laparoscopy. High-definition picture acquisition, 3D camera systems, and the use of biomarkers will allow improved identification of the target structures and help differentiate them from surrounding tissues. In this article, we describe some of the new technologies available and, in particular, focus on the possible implications of biomarkers and fluorescent laparoscopic imaging.
Keywords: laparoscopic colorectal cancer resection, colorectal surgery, biomarkers, indocyanine green, enhanced reality, imaging
Enhancing Reality in Colorectal Surgery
Restoring 3D Vision
3D vision is important for judgement of depth, spatial localization, and perception of different reflective surfaces. All these elements are fundamental to any surgical procedure involving tissue dissection and suturing. The loss of 3D vision occurs in standard laparoscopy which induces a distortion of reality and requires adaptation by the surgeon. However, this can be partially overcome by the use of new laparoscopes that provide 3D vision. While there are few publications that specifically investigate the benefits of 3D vision in laparoscopic surgery,1 it is clear that the restoration of 3D vision is a key advantage of using the robotic platform.
Perioperative perception of human anatomy is still very crude based on white light reflectance and limited tactile feedback, and yet there is a potential wealth of knowledge provided by preoperative radiological images that is now part of the routine diagnostic assessment of any patient with a diagnosis of colorectal cancer.
Few surgeons are able to use all the information they get from these images during the operation. The use of an open source DICOM compatible available freeware (OSIRIX, Pixmeo, Geneva, Switzerland)2 allows surgeons to improve their reality perception by vascular reconstructions or tagging the location of the tumor. Once this has been done, this can be imported in theater as an image overlay to help navigation during the operation. Preoperative computed tomographic (CT) mesenteric angiograms can be useful to identify anatomical variants.3 For example, a recent randomized controlled trial demonstrated that surgeons who had preoperative access to 3D CT reconstructions of mesenteric vessels had shorter operative times and better identification of aberrant vascular anatomy.4
Smaller tumors, if not marked by endoluminal tattoo, could also be mapped out to allow better localization. However, even the most advanced systems currently available are not yet ready to do this in real-time because of limitations in the ability to integrate preoperative imaging with surgical views, including dealing with tissue deformity and motion artifacts.
Use of Biomarkers and Fluorescence Imaging
Surgeons have always depended on white light to illuminate their field of vision. Historically, operating theaters have been located at the top of buildings, exemplified by the Old Operating Theatre situated in the garret of St Thomas's Church, London, and the Ether Dome at Massachusetts General Hospital, Boston. Their location allowed the maximum amount of light into the operating room, and emphasized the need for optimal visual conditions required by surgeons. Unfortunately, anatomical structures are not as distinct and differentially colored as presented in standard anatomy textbooks. In reality, under white light visualization, body structures vary in limited shades of red, yellow, white, and gray making it difficult to identify which structures are important and which need to be removed.
There has been much interest in recent years in the use of fluorescence to help guide surgery. Biomarkers which are either inherently fluorescent or which are conjugated to a fluorophore may be used in surgery in the following ways:
To identify normal anatomical structures.
To identify abnormal tissue, such as dysplasia or cancer.
To assess the perfusion and microvasculature of tissue for wound healing or assessment of an anastomosis.
To assess the lymphatic drainage and identify sentinel lymph nodes.
Biomarkers may be either specific or nonspecific. Specific biomarkers bind to their targets only, are not normally fluorescent, and, therefore, require a conjugated fluorophore. Nonspecific biomarkers are inherently fluorescent and although they do not bind specifically to any targets, they help identify the natural channels within the body, for example, lymph node drainage, blood vessels.
The principle of fluorescence imaging is the use of a special endoscope that releases light at a certain wavelength (excitation wavelength), which then stimulates a signal to be released by the biomarker (emission wavelength). The fluorescence signal emitted from the biomarker is detected by the endoscope and the image is mapped digitally onto a screen. The fluorescent image may then be mapped over a white light image.
Most fluorescent-guided surgeries have used near infrared (NIR) technology as the use of this wavelength results in a greater depth of tissue penetration and a reduction of autofluorescence from tissue. NIR endoscopes are most commonly used together with indocyanine green (ICG) as a nonspecific biomarker. ICG is clinically approved to be used in patients, has few side effects (allergic reaction 1/300,000) and a very large safety margin, the maximal dose being of 2 mg/kg of body weight. There are currently two endoscopes available for NIR imaging in laparoscopy (Pinpoint, Novadaq, Canada and D-Light, Storz, Germany) and one in robotic surgery (Firefly, Da Vinci, Intuitive Surgical, Inc., Sunnyvale, CA).5 Fluorescent imaging in open abdominal surgery is also possible (Spy Scope, Novadaq, Richmond, British Columbia, Canada).
Identifying Normal Structures
During surgery, it is often difficult to identify important structures that are located within the operative field, which need to be preserved to conserve normal physiological function. Although this application has not been used before in colorectal surgery, fluorescent biomarkers have been used to identify and preserve nerves and the biliary tree in animal models and patients, respectively.
It is important to identify nerves during surgery as accidental injury can lead to significant impairment in neurological and physiological function. However, peripheral nerves are often difficult to identify and may be involved in the diseased tissue. Whitney et al demonstrated in a mouse model that the fluorescent NP41 peptide, when injected intravenously, localized to the connective tissue surrounding peripheral nerves, and highlighted these nerves without any obvious toxicity.6 The same probe also labeled nerves in human tissue samples.
ICG is metabolized by the liver and excreted into the biliary tree. This has been exploited in fluorescent cholangiography, where the biliary tree can be highlighted in laparoscopic surgery following an intravenous injection of ICG. In a study of 52 patients,7 Ishizawa et al demonstrated that ICG enabled real-time identification of the cystic duct-common hepatic duct junction, as well as accessory bile ducts in 8 patients. Similarly, ICG has been demonstrated to help delineate the biliary tree during robotic cholecystectomy, with the cystic duct and the common bile duct being visualized in 97.8 and 96.1% of the cases.8
Identifying Dysplasia and Cancer
Fluorescent biomarkers have been used to help identify dysplasia and cancer during surgery.
One of the most commonly used biomarkers is 5-aminolevulinic acid (5-ALA). 5-ALA is metabolized by tumors into protoporphyrin IX, which is fluorescent in blue light. To date, the largest clinical study involved 322 patients undergoing surgery for malignant gliomas who were randomized to either conventional white light microsurgery or 5-ALA fluorescence-guided surgery. This study demonstrated that 5-ALA fluorescence could guide surgeons to perform more complete resections of the tumor, resulting in improved progression-free survival.9 In colorectal surgery, most work on 5-ALA has been performed on animal models.10 11 However, Kondo et al demonstrated that 5-ALA was able to identify peritoneal metastasis in patients with colorectal cancer that were not seen in white light vision.12
Specific molecular markers have also been developed that identify specific molecular changes that occur on the cell surface membrane during carcinogenesis. In epithelial ovarian cancer, folate receptor-α (FR-α) expression is increased in 90 to 95% of the patients. van Dam et al demonstrated that fluorescein-labeled folate, which is the ligand for FR-α, was able to detect FR-α expressing tumors intraoperatively, as well as additional tumor deposits compared with white light visualization alone, suggesting that this tool could be useful in intraoperative staging and radical cytoreductive surgery.13
Lectins are specific carbohydrate recognition proteins that may be used to help identify dysplasia and cancer, as glycosylation changes occur early during carcinogenesis. Bird–Lieberman et al have shown that fluorescein labeled wheat germ agglutinin (WGA) can act as a negative marker of dysplasia in Barrett esophagus in ex vivo resection specimens in conditions simulating endoscopy, which would have been missed using white light examination alone.14 This may have applications in colorectal neoplasia. Although the identification of dysplasia using a negative marker does not appear intuitive, positive markers are not without their limitations. With negative markers, there is a risk of a false-positive result, whereas with positive markers, there is a risk of a false-negative result, which is more devastating to the patient.
Assessment of Bowel Perfusion
One of the major complications in colorectal surgery is an anastomotic leak. This is a devastating complication of colorectal surgery for the patient, surgeon, and health-care provider. Average leak rates range between 1 and 3% for ileo–colic anastomosis but can go up to 20% for a low colorectal anastomosis.15 The process of anastomotic healing can be divided into three steps: inflammation phase (0–6 days), proliferation phase (3–10 days), and healing phase (6–15 days). During the initial inflammatory process, there is an intense inflammatory cell infiltration with secretion of a new extracellular matrix. The collagen I to collagen III ratio will change during healing, and there will be less than 10% of collagen III at the end of the process. Neovascularization starts around the 4th postoperative day and will allow a complete healing of the anastomosis. While administration of vascular endothelial growth factors (VEGF) decreases the anastomosis leak rate,16 it is fundamental to have a good perfusion of the anastomosis to allow a proper healing.17 Animal studies show that a stapled anastomosis will heal in the same way as a simple enterotomy if the microvascularization is well preserved.18
Multiple factors have been shown to be associated with anastomotic leak, including patient comorbidities such as age, gender (male), smoking habits, diabetes, nutritional status, and use of steroids. Intraoperative technical problems account for the other factors involved, such as blood loss (or transfusion), type of resection, use of a drain, number of stapler lines fired, peri-anastomotic hematoma, an abscess, and anastomotic tension at the time of construction of the anastomosis.19 Although the cause of a leak is multifactorial, few of the factors can be easily modified to prevent a leak. The assessment of perfusion of the colon during the surgery is, however, one of them.
A large number of tools have been developed to try to address this question, but none has yet been adopted into daily clinical practice. The ideal tool for laparoscopic surgery should be easy to use, accurate with a minimum of false-negative results and, more importantly, with a few or no false positives. It would be as objective and reproducible as possible and cost effective. While the traditional clinical criteria of good perfusion of tissue include the identification of a bleeding marginal vessel and pink vascularized mucosa, newer techniques have tried to make this step more objective (Table 1).
Table 1. Techniques to assess blood supply of an anastomosis.
| Technique | Laparoscopic surgery | Easy to use | Accurate | Objective | Reproducible | Cost effective |
|---|---|---|---|---|---|---|
| Color of the bowel | + | + | − | − | ± | $ |
| Marginal blood vessels | + | + | − | − | ± | $ |
| On table angiography28 | + | − | + | + | + | $$$ |
| Pulse oxymetry29 | − | + | − | + | + | $ |
| Polarographic oxygen tension30 31 | − | − | + | − | + | $$ |
| Doppler Ultrasound32 | − | + | ± | ± | ± | $ |
| Intravital microscopy | − | − | ± | ± | ± | $$$ no human use |
| Spectrophotometry | − | − | + | + | + | $$ |
| Bowel wall contractility | − | − | ± | + | ± | $$$ |
| pH measurement | − | − | ± | + | + | $$ |
| Microdialysis | − | − | ± | ± | ± | $$ |
| Fluorescein fluorescence33 |
± | − | ± | + | − | $$ |
| Laser Doppler flowmetry15 | ± | − | ± | ± | ± | $$ |
| Near infrared24 | + | + | + | + | + | $ |
The microcirculation at the anastomosis plays a crucial role in any anastomotic dehiscence. Dividing the colonic blood supply leads to a loss of small vessel collaterals as shown in studies using intravenous angiography in open surgery, but this is not practical in everyday practice.20 However, NIR technology with the use of ICG seems to have all the characteristics needed. It is possible to use it in laparoscopic surgery, it is easy to use, is reproducible, and accurate.
Once we can assess the microvascularization of an anastomosis in real time, it will be feasible to alter the course of surgery to prevent the development of a leak. During the operation, the conventional scope is replaced by an NIR scope, an intravenous injection of ICG is given and the prepared colon can be assessed before and after anastomosis. The anastomosis can be inspected both extraluminally and intraluminally using a modified rectoscope passed per anally. Fig. 1 gives an example of NIR imaging to assess the microvascularization in real time, showing a clear cut off at the level of the loss of the signal after vessels division. Fig. 2 shows the NIR fluorescence after the anastomosis has been completed.
Fig. 1.
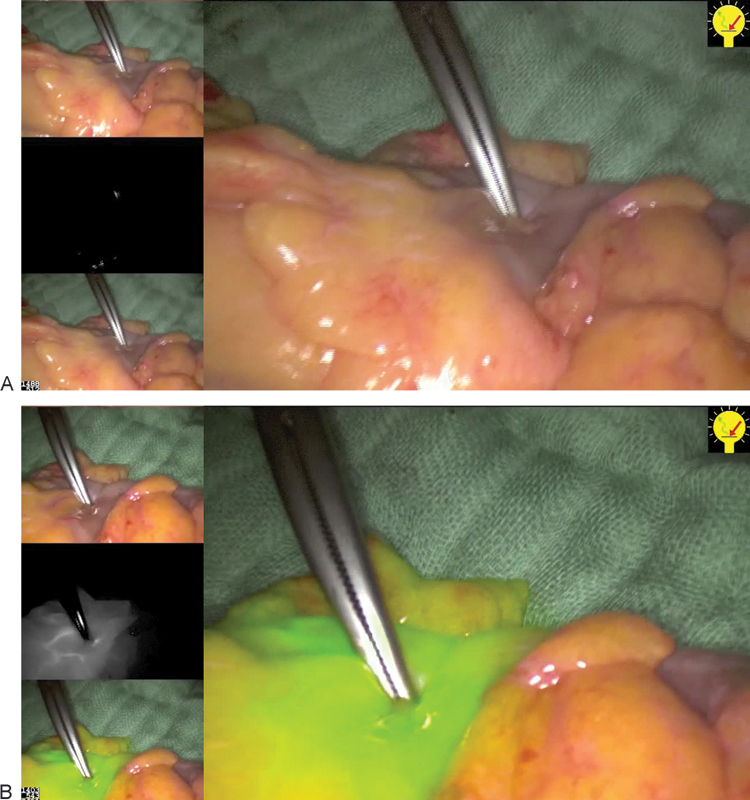
Near infrared (NIR) imaging after vessel division in a low anterior resection (Left up: normal view, middle left: NIR mode, and low left: composite view mixed). (A) Before injection of indocyanine green (ICG), the clamp shows the planned level of transection. (B) After apparition of the signal with the main screen with the composite view, showing a clear cut off of the signal.
Fig. 2.
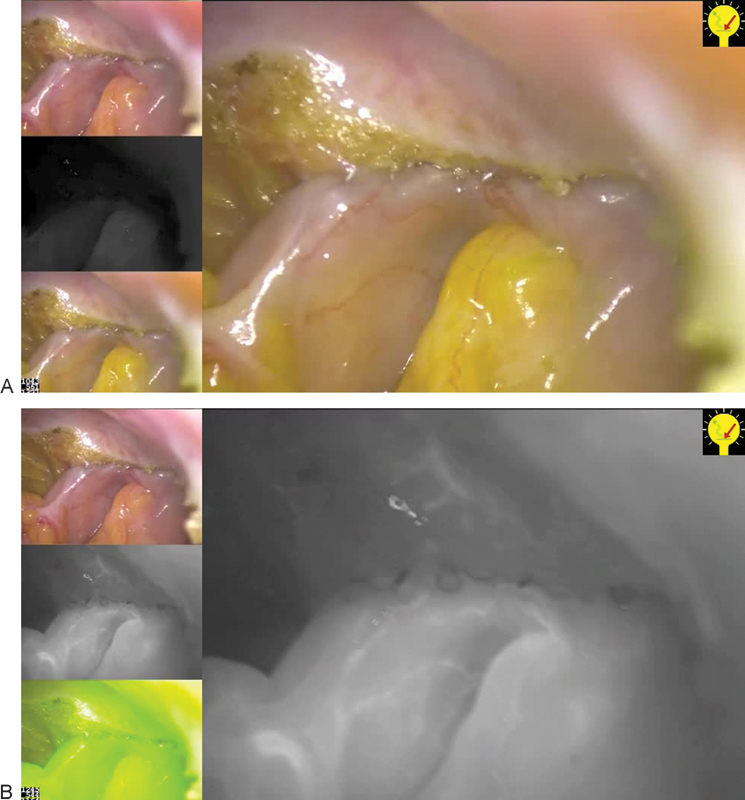
Near infrared (NIR) imaging after side to end anastomosis in a low anterior resection (Left up: normal view, middle left: NIR mode, and low left: composite view mixed). (A) Before injection of indocyanine green (ICG). (B) After ICG injection, main screen with the NIR black and white image, showing a good microperfusion of the anastomosis.
As this is such a novel area of research, there are only a few prospective feasibility studies that have been published. Sherwinter et al21 visualized 20 patients who underwent a low anterior resection using transanal NIR imaging and discovered that 50% of the patients with poor perfusion around the anastomosis developed a leak. Jafari et al22 showed in a small prospective robotic series of patients undergoing low anterior resections that the use of NIR imaging led to a decrease in the anastomotic leak rate from 18 to 6%. This has been confirmed in a recent study in robotic surgery where imaging led to a change in the proximal transection area in 40% of the patients, and resulted in a 5% anastomotic leak rate in those patients.23 Finally, in our small prospective study of laparoscopic colorectal resections, we had no leaks in a selected group of low anterior and high anterior resections or right hemicolectomies.24 A larger prospective study, the PILLAR study, is about to be reported with similarly low leakage rates.
There is much to be learned about this technology in the prevention of anastomotic leak particularly after a low anterior resection. While these early studies are promising, there will a need for further refinement of the technique. Outstanding questions remain on whether intraluminal imaging is better than extraluminal, and whether the perfusion signal can be quantified. Clearly, randomized controlled trials will be needed before the benefits of this new technology can be established.
Lymph Node Mapping and the Potential for Tailored Surgery for Colorectal Cancer
Currently, the same surgical operation (excision of the primary lesion and all draining lymph nodes) is proposed for all patients with colorectal cancer irrespective of stage and tumor size. With the introduction of bowel cancer screening programs, more patients are being detected with early stage disease without involvement of any lymph nodes. As there is no treatment benefit for these patients from having such normal lymph nodes excised, they are currently receiving a much more extensive operation than what is actually required for cure. This exposes them to additional risks of injury during their operation and necessitates a longer hospital stay than would otherwise be the case. Indeed, a small group of patients (around 10% of the total) is currently undergoing a radical operation when their cancer is potentially removable (and curable) by an endoscopic procedure that would not entail removal of any length of bowel and that could be performed potentially as a much simpler procedure.
Radical surgery, the classical oncological operation, involving resection of as many nodes as possible may still have a place in advanced cancer but this has an increased morbidity and mortality and a low level of evidence.25 As 97.5% of all the significant lymph nodes are found within 5 cm of the tumor,26 extended radical surgery will be unnecessary especially with aberrant lymphatic drainage in up to 22% of the cases.27 In this situation, a tailored limited resection could be sufficient if we can correctly identify involved nodes at the time of resection. This is particularly important in rectal cancer surgery, where the patient risks either a long-term stoma or major functional disturbance.
The concept of sentinel lymph node mapping is currently being developed for patients with breast cancer, melanoma, and, more recently, early stage stomach cancer. In colorectal cancer, blue dye has been used to look at lymph node drainage patterns in resected specimens and at open surgery. Lymphatic drainage in colorectal cancer is less predictable. Although first draining lymph nodes in the primary lymph node basin have been demonstrated, we prefer to use the term significant lymph node.
We have been using the NIR laparoscope and ICG injection intraluminally around the tumor to identify significant lymph nodes intraoperatively at laparoscopic colorectal cancer resections. We have selected patients with small tumors in the descending colon, sigmoid colon, and upper rectum. At the beginning of the procedure, a colonoscope is passed into the rectum. Insufflation with Co 2 is essential to prevent over distension of the colon and subsequent difficulty with the resection. A submucosal injection of ICG is given around the tumor and after 30 minutes the draining lymph nodes will be made visible. It is possible to inject the ICG a day before the surgery but this can be difficult for the patient and hospital to arrange.
The conventional laparoscope is changed for the NIR scope and the nodes can be identified as a white on black image, or a superimposed image on the conventional image, as seen in Fig. 3. The significant node can then be marked with a clip for subsequent histopathological analysis.
Fig. 3.
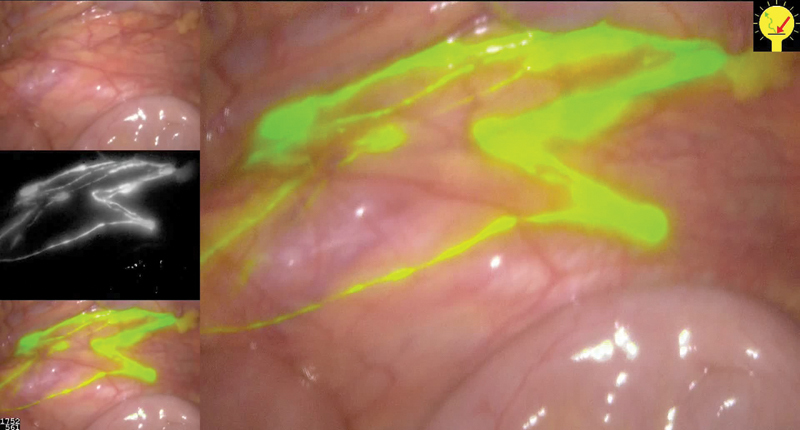
Lymph node mapping after tumoral indocyanine green injection (Left up: normal view, middle left: NIR mode, and low left: composite view mixed).
Now that the significant nodes can be reliably identified, there remain two important issues. We do not as yet have enough evidence for the predictive value and accuracy of the node sampling for it to be used in routine clinical practice. In addition, assessment of nodes for cancer needs to be in real time during the procedure. We have been using one-step nucleic acid amplification on resected specimen significant nodes to test the feasibility of immediate histopathological assessment in the operating room and this work is in progress.
The use of lymphatic mapping can also improve the visualization of draining lymph nodes lying outside the field of standard operative resection (aberrant drainage). This occurs in approximately 10 to 20% of the patients and explains why some low-risk cancer could recur.
Conclusion and Perspective for Colorectal Surgery
We are entering a new era of surgery where the surgeon's eyesight can be augmented using new technologies to provide 3D endoscopic images, fusion of operative field, cross-sectional imaging, identification of important structures using fluorescent biomarkers, the provision of real-time information on blood flow and colonic microcirculation, and the intraoperative identification of lymph nodes previously only seen by the histopathologist. The challenge is to translate these exciting concepts into improvements in cancer outcomes, with minimal surgical resection, less morbidity, and better functional results.
Footnotes
Both the authors contributed equally to this work.
References
- 1.Buchs N C, Morel P. Three-dimensional laparoscopy: a new tool in the surgeon's armamentarium. Surg Technol Int. 2013;23(9):19–22. [PubMed] [Google Scholar]
- 2.Volonté F, Pugin F, Buchs N C. et al. Console-integrated stereoscopic OsiriX 3D volume-rendered images for da Vinci colorectal robotic surgery. Surg Innov. 2013;20(2):158–163. doi: 10.1177/1553350612446353. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi M, Morishita S, Okabayashi T. et al. Preoperative assessment of vascular anatomy of inferior mesenteric artery by volume-rendered 3D-CT for laparoscopic lymph node dissection with left colic artery preservation in lower sigmoid and rectal cancer. World J Gastroenterol. 2006;12(4):553–555. doi: 10.3748/wjg.v12.i4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mari F S, Nigri G, Pancaldi A. et al. Role of CT angiography with three-dimensional reconstruction of mesenteric vessels in laparoscopic colorectal resections: a randomized controlled trial. Surg Endosc. 2013;27(6):2058–2067. doi: 10.1007/s00464-012-2710-9. [DOI] [PubMed] [Google Scholar]
- 5.Cahill R A, Ris F, Mortensen N J. Near-infrared laparoscopy for real-time intra-operative arterial and lymphatic perfusion imaging. Colorectal Dis. 2011;13(7) 07:12–17. doi: 10.1111/j.1463-1318.2011.02772.x. [DOI] [PubMed] [Google Scholar]
- 6.Whitney M A, Crisp J L, Nguyen L T. et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol. 2011;29(4):352–356. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97(9):1369–1377. doi: 10.1002/bjs.7125. [DOI] [PubMed] [Google Scholar]
- 8.Daskalaki D, Fernandes E, Wang X. et al. Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov. 2014;21(6):615–621. doi: 10.1177/1553350614524839. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W Pichlmeier U Meinel T Wiestler O D Zanella F Reulen H J; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial Lancet Oncol 200675392–401. [DOI] [PubMed] [Google Scholar]
- 10.Kato S, Kawamura J, Kawada K, Hasegawa S, Sakai Y. Fluorescence diagnosis of metastatic lymph nodes using 5-aminolevulinic acid (5-ALA) in a mouse model of colon cancer. J Surg Res. 2012;176(2):430–436. doi: 10.1016/j.jss.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Murayama Y, Harada Y, Imaizumi K. et al. Precise detection of lymph node metastases in mouse rectal cancer by using 5-aminolevulinic acid. Int J Cancer. 2009;125(10):2256–2263. doi: 10.1002/ijc.24707. [DOI] [PubMed] [Google Scholar]
- 12.Kondo Y, Murayama Y, Konishi H. et al. Fluorescent detection of peritoneal metastasis in human colorectal cancer using 5-aminolevulinic acid. Int J Oncol. 2014;45(1):41–46. doi: 10.3892/ijo.2014.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam G M, Themelis G, Crane L M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17(10):1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 14.Bird-Lieberman E L, Neves A A, Lao-Sirieix P. et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med. 2012;18(2):315–321. doi: 10.1038/nm.2616. [DOI] [PubMed] [Google Scholar]
- 15.Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43(1):76–82. doi: 10.1007/BF02237248. [DOI] [PubMed] [Google Scholar]
- 16.Ishii M, Tanaka E, Imaizumi T. et al. Local VEGF administration enhances healing of colonic anastomoses in a rabbit model. Eur Surg Res. 2009;42(4):249–257. doi: 10.1159/000210671. [DOI] [PubMed] [Google Scholar]
- 17.Attard J A, Raval M J, Martin G R. et al. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48(7):1460–1470. doi: 10.1007/s10350-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 18.Grommes J, Binnebösel M, Klink C D. et al. Comparison of intestinal microcirculation and wound healing in a rat model. J Invest Surg. 2013;26(1):46–52. doi: 10.3109/08941939.2012.692759. [DOI] [PubMed] [Google Scholar]
- 19.Bertelsen C A Andreasen A H Jørgensen T Harling H; Danish Colorectal Cancer Group. Anastomotic leakage after anterior resection for rectal cancer: risk factors Colorectal Dis 201012137–43. [DOI] [PubMed] [Google Scholar]
- 20.Allison A S, Bloor C, Faux W. et al. The angiographic anatomy of the small arteries and their collaterals in colorectal resections: some insights into anastomotic perfusion. Ann Surg. 2010;251(6):1092–1097. doi: 10.1097/SLA.0b013e3181deb649. [DOI] [PubMed] [Google Scholar]
- 21.Sherwinter D A, Gallagher J, Donkar T. Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis. 2013;15(1):91–96. doi: 10.1111/j.1463-1318.2012.03101.x. [DOI] [PubMed] [Google Scholar]
- 22.Jafari M D, Lee K H, Halabi W J. et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc. 2013;27(8):3003–3008. doi: 10.1007/s00464-013-2832-8. [DOI] [PubMed] [Google Scholar]
- 23.Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia J A. The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc. 2014;28(5):1695–1702. doi: 10.1007/s00464-013-3377-6. [DOI] [PubMed] [Google Scholar]
- 24.Ris F, Hompes R, Lindsey I, Cunningham C, Mortensen N J, Cahill R A. Near Infra-Red (NIR) Laparoscopic Assessment of the Adequacy of Blood Perfusion of Intestinal Anastomosis. Colorect Dis. 2014;16(8):646–647. doi: 10.1111/codi.12593. [DOI] [PubMed] [Google Scholar]
- 25.Killeen S, Mannion M, Devaney A, Winter D C. Complete Mesocolic Resection and Extended Lymphadenectomy for Colon Cancer: A Systematic Review. Colorect Dis. 2014;16(8):577–594. doi: 10.1111/codi.12616. [DOI] [PubMed] [Google Scholar]
- 26.Hashiguchi Y, Hase K, Ueno H, Mochizuki H, Shinto E, Yamamoto J. Optimal margins and lymphadenectomy in colonic cancer surgery. Br J Surg. 2011;98(8):1171–1178. doi: 10.1002/bjs.7518. [DOI] [PubMed] [Google Scholar]
- 27.Cahill R A, Anderson M, Wang L M, Lindsey I, Cunningham C, Mortensen N J. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg Endosc. 2012;26(1):197–204. doi: 10.1007/s00464-011-1854-3. [DOI] [PubMed] [Google Scholar]
- 28.Leaper D J. Angiography as an index of healing in experimental laparotomy wounds and colonic anastomoses. Ann R Coll Surg Engl. 1983;65(1):20–23. [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips J P, Kyriacou P A, Jones D P, Shelley K H, Langford R M. Pulse oximetry and photoplethysmographic waveform analysis of the esophagus and bowel. Curr Opin Anaesthesiol. 2008;21(6):779–783. doi: 10.1097/ACO.0b013e328317794d. [DOI] [PubMed] [Google Scholar]
- 30.Sheridan W G, Lowndes R H, Williams G T, Young H L. Determination of a critical level of tissue oxygenation in acute intestinal ischaemia. Gut. 1992;33(6):762–766. doi: 10.1136/gut.33.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan W G, Lowndes R H, Young H L. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;30(11):867–871. doi: 10.1007/BF02555426. [DOI] [PubMed] [Google Scholar]
- 32.Boerma E C, Mathura K R, van der Voort P H, Spronk P E, Ince C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care. 2005;9(6):R601–R606. doi: 10.1186/cc3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter M S, Fantini G A, Sammartano R J, Mitsudo S, Silverman D G, Boley S J. Qualitative and quantitative fluorescein fluorescence in determining intestinal viability. Am J Surg. 1984;147(1):117–123. doi: 10.1016/0002-9610(84)90044-8. [DOI] [PubMed] [Google Scholar]


