Abstract
Since the advent of laparoscopy, minimally invasive techniques such as single port laparoscopy, robotics, endoscopically assisted laparoscopy, and transanal endoscopic surgery continue to revolutionize the field of colorectal surgery. Transanal natural orifice transluminal endoscopic surgery (NOTES) represents a further paradigm shift by combining the advantages of these earlier techniques to reduce the size and number of abdominal incisions and potentially optimize rectal dissection, especially with respect to performance of an oncologically adequate total mesorectal excision (TME) for rectal cancer. Since the first experimental report of transanal rectosigmoid resection in 2007, the potential impact of transanal NOTES in colorectal surgery has been extensively investigated in experimental models and recently transitioned to clinical application. There have been 14 clinical trials of transanal TME (taTME) for rectal cancer that have demonstrated the feasibility and preliminary oncologic safety of this approach in carefully selected patients, with results comparable to outcomes after laparoscopic and open TME, including cumulative intraoperative and postoperative complication rates of 5.5 and 35.5%, respectively, 97.3% rate of complete or near-complete specimens, and 93.6% rate of negative margins. Transanal NOTES has also been safely applied to proctectomy and colectomy for benign indications. The consensus among published series suggests that taTME is most safely performed with transabdominal assistance by surgeons experienced with laparoscopic TME, transanal endoscopic surgery, and sphincter-preserving techniques including intersphincteric resection. Future applications of transanal NOTES may include evolution to a pure endoscopic transanal approach for TME, colectomy, and sentinel lymph node biopsy for rectal cancer, with a potential role for robotic assistance.
Keywords: transanal, NOTES, TME, mesorectal excision, proctectomy
Though adoption of the surgical principles of total mesorectal excision (TME) in combination with chemoradiation for locally advanced disease has continued to improve local control and survival rates of resectable rectal cancer, radical resection continues to be associated with high morbidity and mortality.1 2 Standard multiport and robotic-assisted laparoscopy, although oncologically safe and associated with shorter length of hospital stay (LOS) than open resections, have not had a measurable impact on the incidence of postoperative complications, sexual and urinary dysfunction, or quality of life. In addition, widespread adoption of laparoscopic techniques in colorectal surgery has been severely limited by the technical complexity and steep learning curve required to gain expertise in minimally invasive pelvic resections. In an effort to harness the advantages of a minimally invasive approach to benefit patients with colorectal pathology, transanal natural orifice transluminal endoscopic surgery (NOTES) has been explored, with promising preliminary results, particularly when used for rectal cancer. Here, we review the history of NOTES, evolution of minimally invasive and transanal approaches applied to colorectal diseases, results of transanal NOTES proctectomy clinical trials for both malignant and benign indications, current indications for NOTES proctectomy, and future applications of NOTES.
History of Natural Orifice Transluminal Endoscopic Surgery
The first clinical report of transgastric appendectomy performed using a flexible endoscope by Reddy and Rao in 2004 ignited worldwide interest in NOTES as a method of extending flexible endoscopy into the realm of minimally invasive intra-abdominal surgery.3 Over the subsequent few years, NOTES was intensely explored in animal models with the hope of significantly reducing access trauma by operating through a natural orifice instead of the abdominal or thoracic wall.4 Theoretical advantages of this approach included decreased postoperative pain, faster postoperative recovery, decreased postoperative complications, including wound infections and incisional hernias, as well as improved cosmesis.3 5
After experimental evidence in animal models demonstrated the feasibility and safety of peritoneal access via transoral, transanal, transurethral, and transvaginal routes, NOTES cautiously entered clinical practice.4 6 7 While transgastric approaches were initially most popular, the risks of creating a gastrotomy solely for access purposes were deemed prohibitively high.3 Transvaginal NOTES procedures quickly surpassed all other transluminal access routes with thousands of transvaginal cholecystectomies performed to date using either rigid or flexible instrumentation with excellent safety profile and patient satisfaction scores.3 8 9 10 Transanal and transcolonic NOTES were adopted more slowly because of initial concerns about risk of infection and reliable colorectal incisional closure. Extensive evidence in swine survival models, however, demonstrated the safety of transcolonic NOTES peritoneoscopy and other intra-abdominal procedures, as long as adequate closure of the colotomy was achieved.11 12 13 14 15
Colorectal resection ensued as the most logical application of transanal NOTES. Beyond the theoretical benefits common to all NOTES procedures, transanal NOTES had the distinct advantage of creating the viscerotomy within the diseased target organ and incorporating it into the colorectal anastomosis. Several endoscopic prototypes designed to achieve safe transluminal access, dissection, and endoscopic closure were explored without successful transition to clinical trial, highlighting the obstacles faced by NOTES with performing complex endoscopic procedures with maladapted instrumentation.16 17 18 In this setting, the report by Whiteford et al in 2007, describing rectosigmoid resection in three human cadavers using a purely transanal approach with transanal endoscopic microsurgery (TEM), was a turning point in the evolution of transanal NOTES.19 It highlighted the potential of performing complex colorectal dissection using existing transanal endoscopic platforms, thus enabling experimental and then clinical trials using this innovative approach.
Evolution of Minimally Invasive Approaches to Rectal Surgery
The development of transanal NOTES approach for rectal resection comes in the context of efforts to minimize the morbidity and mortality of open rectal surgery. Three decades following its original description by Heald,20 TME has become the gold standard for curative rectal cancer resection. However, it continues to be associated with a 1 to 6% mortality rate as well as high perioperative morbidity, including anastomotic leak (0–21%), wound complications (0–47%), and hernia formation (0–11%), as well as functional disorders, including defecatory (0.5–37%), urinary (5–14%), and sexual dysfunction (33–36%).1 2 21 22 23 24 25 26 27 28 29 30 31 32 33 34
Relative to open TME, laparoscopic TME is associated with decreased postoperative pain, decreased LOS, and faster return of bowel function, with similar oncologic outcomes based on large randomized controlled trials, including the CLASSIC and COLOR II trials.35 36 37 However, laparoscopic TME has not been widely adopted because of the difficulty of the pelvic dissection, long operative time, steep learning curve, and minimal impact of the laparoscopic approach on functional outcomes.1 38 39 40 41 42 Regardless of the specific reasons for the lack of wider adoption, it is estimated that well under 30% of the rectal cancer resections in the United States are performed using laparoscopy, without significant improvement over the past decade, and persistently high conversion rates (15–30%).35 36 37 Single-port laparoscopy for colon resections has not been shown to provide advantages over traditional laparoscopy and has been minimally investigated for rectal resections because of technical difficulty.43 44 45 46 Preliminary data from large series on robotic TME suggest equivalent perioperative and oncologic outcomes relative to laparoscopic TME, but with decreased conversion rates to open surgery.47 48 However, robotic surgery is associated with significantly longer operative times and higher procedural costs, potentially problematic in this environment of health-care cost reduction.49 Furthermore, laparoscopic and robotic TME still require a sizeable abdominal incision for specimen removal, minimizing their wound-related benefits over open TME.
In addition to NOTES, natural orifice specimen extraction (NOSE) has gained increasing popularity, with many groups reporting successful transvaginal and transanal removal of colorectal resection specimens.50 51 52 53 54 55 56 57 Many of these procedures have been performed for benign indications, such as rectal prolapse and diverticulitis;58 59 however, there have also been several studies employing this technique for colorectal cancer resections. Park et al compared laparoscopic right colectomy with transvaginal versus transabdominal extraction (n = 68) and found decreased postoperative pain and LOS associated with transvaginal removal, though wound infections and intra-abdominal abscesses were not significantly decreased.60 Leung et al (n = 70) conducted a randomized trial of laparoscopic left colectomy with transanal versus transabdominal extraction, finding decreased postoperative pain (p = 0.017) and wound infections (p = 0.005) with transanal NOSE.61 A large retrospective comparison (n = 432) by Liang et al of laparoscopic TME for rectal cancer with transanal or transabdominal specimen extraction found no difference in intraoperative complications (p = 0.69), postoperative complications (p = 0.59), LOS (p = 0.83), or 2-year local recurrence rates (p = 0.15).62 63 Thus, preliminary results show NOSE to be feasible for colorectal cancer resection, with possible advantages including reduction in postoperative pain, LOS, and wound infection rates.
Evolution of Transanal Approaches to Rectal Surgery
Alongside these minimally invasive transabdominal approaches to colorectal surgery, transanal techniques for the removal of rectal lesions have been developed. Over the last three decades, TEM has provided excellent endoscopic local control for proximal rectal lesions through improved visualization and full thickness resection, resulting in decreased fragmentation of specimens relative to conventional transanal excision.64 65 66 In addition, TEM has been associated with exceedingly low mortality and morbidity relative to radical proctectomy.67 68 More recently, transanal minimally invasive surgery (TAMIS), a newer technique that utilizes flexible and disposable transanal endoscopic platforms and accommodates traditional laparoscopic instruments, has accelerated the popularity of transanal endoscopic surgery worldwide.69 70 71 72 Inherent limitations of TEM and TAMIS apply to all local excision techniques, namely the lack of mesorectal clearance with prohibitively high recurrence rates when used to resect T2 and more advanced rectal tumors. As a result, TEM and TAMIS are only indicated for resection of benign rectal lesions and carefully selected T1 rectal tumors with low-risk features, as local recurrence rates for those lesions after TEM are similar to rates after TME.73 74 75 76 77 78 79 80 81
Transanal NOTES expands the conventional indications of TEM and TAMIS by taking advantage of the exposure and access provided by transanal endoscopic platforms. Transanal rectal distention with Co 2 combined with magnified TEM and TAMIS optics permits excellent visualization of tissue planes and precise tissue manipulation.82 Full thickness rectal dissection can be extended beyond the rectal wall to encompass the mesorectum, and pneumodissection facilitates identification of the presacral space and rectovaginal or rectoprostatic plane (Fig. 1). This transanal endoscopic “down-to-up” approach may be particularly helpful in patients with a narrow pelvis and significant visceral obesity, in whom laparoscopic pelvic dissection would be challenging and where the risk for conversion to open surgery is the highest.43 83 84
Fig. 1.
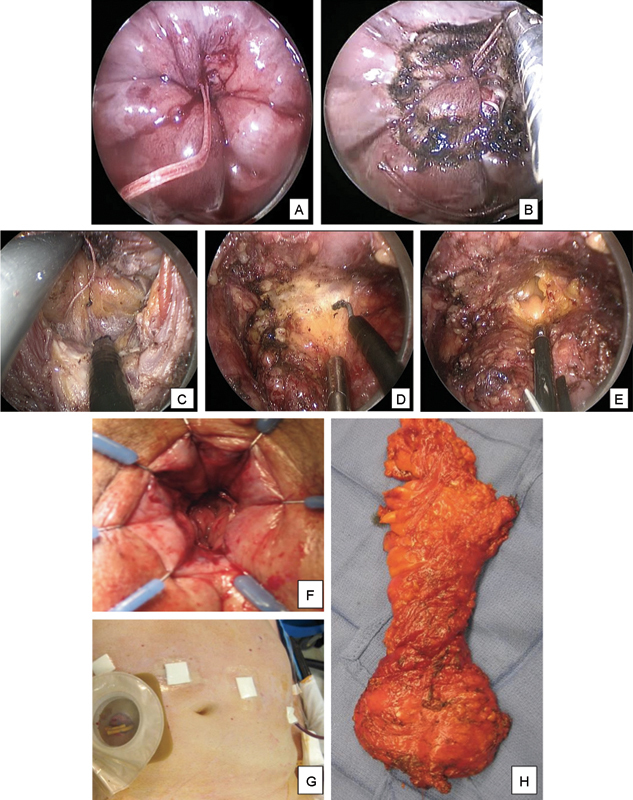
Laparoscopic-assisted taTME in an obese male patient with a T2N0 low rectal cancer. The rectum is sutured closed below the tumor with a purse string located just above the dentate line (A) and the transanal endoscopic platform is inserted. After Co 2 insufflation of the rectum, the rectal mucosa is scored circumferentially with monopolar cautery (B). Full-thickness rectal and mesorectal dissection is completed posteriorly along the presacral space (C), laterally, and anteriorly along the rectoprostatic plane (D) until the peritoneal reflection is reached and the abdominal cavity entered anteriorly (E). Rectal and mesorectal dissection is completed using a combined abdominal and transanal approach, and the specimen is transected transanally if feasible. Stapled coloanal anastomosis is performed (F) followed by a protective diverting loop ileostomy (G). A complete TME is achieved with negative margins and 25 negative lymph nodes (H). taTME, transanal total mesorectal excision; TME, total mesorectal excision.
With regard to low rectal cancers abutting the anorectal ring, transanal NOTES access is also expanding the application of intersphincteric resection (ISR). ISR has been used since the early 1990s as a sphincter-preserving approach for very low rectal tumors, and involves extending the rectal dissection into the intersphincteric plane and removing part or all the internal sphincter to achieve negative distal margins.85 ISR is combined with open, laparoscopic, or robotic transabdominal TME (TATA or transanal abdominotransanal resection) with equivalent oncologic outcomes as abdominoperineal resection (APR) for tumors lower than 4 cm from the anal verge (AV).86 87 Transanal NOTES is particularly well suited for intersphincteric completion proctectomy for inflammatory bowel disease (IBD) and for low rectal tumors when ISR would otherwise be indicated to achieve negative distal margins. Following intersphincteric dissection of the internal anal sphincter, the rectal stump is sutured and a transanal platform is inserted to complete the transanal NOTES proctectomy or TME.84 88 Unlike TATA, where more proximal mesorectal dissection is limited by poor exposure with conventional anal retractors, the transanal NOTES approach allows transanal completion of the rectal and mesorectal excision all the way toward the sacral promontory where the peritoneal reflection is opened and the abdominal cavity entered. Finally, size of rectum and width of pelvic inlet permitting transanal specimen extraction can be combined with transanal NOTES resection followed by transanal handsewn or stapled anastomosis, without the absolute need for an abdominal extraction site (Fig. 1).
Thus, transanal NOTES combines the advantages of transanal endoscopic surgery (TEM and TAMIS), sphincter-preserving techniques including ISR, and NOSE. By facilitating identification of the distal resection margin and improving visualization, exposure and dissection of the perirectal and mesorectal planes, transanal NOTES may optimize the quality of rectal resections while minimizing the morbidity of these procedures.
Clinical Trials of Transanal Natural Orifice Transluminal Endoscopic Surgery
Numerous porcine survival and human cadaver trials have confirmed the safety and feasibility of a transanal NOTES approach to colorectal resection.19 89 90 91 92 93 94 95 96 97 98 In the largest cadaver study (n = 32), adequate TME was achieved in every cadaver and, when comparing different transanal approaches, the laparoscopic-assisted technique was found to be particularly important in minimizing organ injury and maximizing specimen length,97 leading to the subsequent use of laparoscopic assistance in many clinical trials.
Currently, transanal NOTES has been most thoroughly investigated for TME for rectal cancer. However, several reports have described both pure and laparoscopic-assisted transanal NOTES approaches to perform colorectal resections for benign indications.
Transanal Natural Orifice Transluminal Endoscopic Surgery Proctectomy and Colectomy for Benign Disease
Lacy et al were the first to report a successful laparoscopic-assisted transanal NOTES total colectomy in a 36-year-old man with medically refractory ulcerative colitis.99 Total operative time was 240 minutes and there were no surgical complications.
Fuchs et al performed laparoscopic-assisted transanal colon resections in 15 patients with additional rectopexy in 11 of the 15 patients for benign indications (full-thickness rectal prolapse, internal rectal intussusception with pelvic obstruction, recurrent sigmoid diverticulitis, and severe slow-transit constipation).59 There were no intraoperative complications except for one conversion to full laparoscopy with a minilaparotomy to remove the bulky specimen in a patient with diverticulitis. Mean operative time was 131 minutes. There were two postoperative complications (postoperative ileus that resolved with conservative management and intra-abdominal bleeding that required a transfusion but no other intervention). At 6-month follow-up, the median Gastrointestinal Quality of Life Index increased significantly (p < 0.05) and no patient reported any functional complaints.
Liyanage et al report a series of 12 patients who underwent transanal completion proctectomy for inflammatory bowel disease, rectal adenomas, or radiation proctitis.88 All procedures were performed purely transanally except in two patients. Mean rectal stump length was 17.8 cm. Median LOS was 5.5 days. There were seven postoperative complications (five delayed wound healing, one temporary incarceration of a parastomal hernia, and one colocutaneous fistula to the perineum in the patient with radiation proctitis, which required operative intervention).
Wolthuis et al describe a series of 14 transanal rectal excisions, laparoscopically assisted in 11 of the cases, for benign and malignant indications.100 Among the nine patients with benign disease (inflammatory bowel disease, fistula, adenoma, fecal incontinence, and anastomotic complications), ISR with coloanal anastomosis was performed in six patients and proctectomy with end colostomy was performed in the remaining three patients. Mean operating time was 146 minutes. Two patients required conversion to open surgery because of extensive adhesions. Mean LOS was 7.6 days and minor complications occurred in 4 of the 14 patients.
Thus, these early trials suggest that transanal NOTES is feasible for proctectomy and colectomy in carefully selected patients with benign disease, and is most commonly and safely performed with laparoscopic assistance.
Transanal Natural Orifice Transluminal Endoscopic Surgery Total Mesorectal Excision for Rectal Cancer
With regard to rectal cancer resection, there have been an increasing number of transanal NOTES TME (taTME) cases performed since the first clinical report of laparoscopic-assisted taTME in a 76-year-old woman with rectal cancer.101 Thus far, 14 clinical series have been reported from groups in the United States, Asia, and Europe, involving a total of 110 patients (Table 1).34 43 82 84 101 102 103 104 105 106 107 108 109 110 111 112 Sixty-seven percent of the patients were male, mean patient age was 61.5 years, mean body mass index (BMI) was 25.8 kg/m2, and 80.6% of the patients received neoadjuvant therapy.
Table 1. Patient characteristics of published clinical series on transanal TME.
| Series | N | Age (y)a | Gender | BMI (kg/m2)a | Tumor location (cm)a | Neoadjuvant CRT | Operative technique | Transanal platform | Operating time (min)a | Final TNM stage (n) | Number of lymph nodes collecteda | TME quality | Margins (n) Distal margina Circumferential margina |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sylla et al 2010101 | 1 | 76 | F | 20 | 6 cm from AV | Yes | LA | Rigid metal (TEO; Karl Storz Endoscopy-America, Inc., El Segundo, CA) | 270 | ypT1N0 | 23 | Complete | Negative |
| Chen et al 2010103 | 1 | 47 | M | 22 | 5 cm from AV | Yes | LA | Custom-made | 290 | pT3N2 | 25 | Complete | Negative |
| Tuech et al 2011109 | 1 | 45 | F | 20 | 3 cm from DL | No | LA | Rigid single port (Endorec; Apside Medical; La Talaudiere, France) | 300 | pT1sm3N0 | 15 | Complete | Negative |
| Zorron et al 2012112 | 2 | 54, 73 | M (1), F (1) |
NR | 6, 8 cm from AV | No | LA | Colonoscope, single port (Triport; Olympus America, Southborough, MA) | 390, 410 | pT3N1M0 (2) | 11, 12 | Complete | Negative |
| Dumont et al 201243 | 4 | 66.8 (60–76) | M (4) | 23.4 (22.4–24.5) | 5.3 (4–7) cm from AV | Yes | LA | Single port (Gelpoint; Applied Medical, Rancho Santa Margarita, CA) | 360 (270–460) | NR | 16 (8–22) | Complete | Negative 22.5 mm (10–40) 7.4 mm (1.5–15) |
| Lacy et al 2013106 | 3 | 73 (71–75) | M (1), F (2) |
21.7 (16–25) | 9.7 (9–10) cm from AV | Yes (2), No (1) |
LA | Single port (Gelpoint; Applied Medical) | 143 (125–155) | pT1N0M0 (1), pT3N0M0 (2) | NR | Complete | Negative |
| Velthuis et al 2013110 | 5 | 69.4 (63–79) | M (3), F (2) |
NR | 6 (5–8) cm from AV | Yes | LA | Single port (SILS, Covidien, Mansfield, MA) | 178.2 (160–194) | ypT0N0 (1), ypT2N0 (2), ypT3N0 (1), ypT3N1 (1) | 13.4 (11–17) | Complete | Negative |
| Lacy et al 2013104 | 20 | 65 (44–77) | M (11), F (9) | 25.3 (19–33) | 6.5 (2–15) cm from AV | Yes (14), No (6) | LA | Single port (Gelpoint, Applied Medical) | 234.7 (150–325) | high-grade dysplasia (2), stage I (4), stage II (7), stage III (6), stage IV (1) | 15.9 ± 4.3b | Complete | Negative 2.6 cm (0.7–5) 1.8 cm (0.5–3) |
| Rouanet et al 2013108 | 30 | 65 (43–82) | M (30) | 26.0 (21.0–32.4) | < 5 cm from AV (n = 20), 5 to 10 cm from AV (n = 10) | Yes (29), No (1) | LA | Rigid metal (TEO; Karl Storz Endoscopy-America, Inc.) | 304 (120–432) | pT1 (1), pT2 (8), pT3 (18), pT4 (3); pN0 (14), pN1 (13), pN2 (3) |
13 (8–32) | Complete | Negative (26), positive (4). 9 mm (3–40) 7 mm (0–17) |
| Sylla et al 201384 | 5 | 48.6 (36–63) | M (3), F (2) |
25.7 (22–28) | 5.7 (4–10) cm from AV | Yes (2), No (3) | LA | Rigid metal (TEO; Karl Storz Endoscopy-America, Inc.) | 274.6 (214–423) | pT0N0 (1), pT1N0 (1), pT2N1 (1), ypT2N0 (2) | 33 (16–53) | Complete | Negative 3.6 cm (0.8–10) 0.73 cm (0.2–1.1) |
| Leroy et al 2013107 | 1 | 56 | F | NR | Mid-third of rectum | No | P | Rigid metal (TEO; Karl Storz Endoscopy-America, Inc.) | 190 | Adenoma with low-grade dysplasia | 16 | Complete | NR |
| Zhang et al 2013111 | 1 | 48 | F | 20 | 7 cm from AV | NR | P | Single port (PPH; Ethicon Endo-surgery, Inc., Cincinnati, OH) | 300 | pT3N1M0 | 12 | Complete | Negative |
| Atallah et al 2013102 | 20 | 57 (36–73) |
M (14), F (6) | 24 (18–41) |
5 (1–9) cm from AV | Yes (17), No (3) | LA (11), RA (6), OA (3) | Single port (Gelpoint, Applied Medical) and Single port (SILS, Covidien USA) | 243 (140–495) |
ypT0N0 (5), ypT0N2 (1), ypT2N0 (3), ypT3N0 (4), ypT3N1 (2), ypT3N2 (3), ypT4N0 (2) | 22.5 (9–51) | Complete (11), near complete (6), incomplete (2) | Negative (18), positive (2) |
| Chouillard et al 201482 | 16 | 57.7 (34–81) | M (6), F (10) |
27.9 (21–38) | Mid- or low-rectal tumors | NR | LA (6), P (10) |
Single port (SILS, Covidien USA) | 265 (155–440) |
pTyN0 (1), pT1N0 (3), pT2N0 (3), pT2N1 (1), pT3N0 (3), pT3N1 (3), pT3N2 (1), pT4N0 (1) |
17 (12–81) | Complete | Negative |
| Overall | 110 | 61.5 (34–82) | M (74), F (36) | 25.8 (16–41) | < 5 cm from AV (n = 41), 5 to 10 cm from AV (n = 49), > 10 cm from AV (n = 3) | Yes (75), No (18) | LA (89), RA (6), OA (3), P (12) |
— | 265.6 (120–495) | pT0 (10), pT1 (8), pT2 (20), pT3 (42), pT4 (6), N0 (66), N1–2 (40) | 17.9 (8–81) |
Complete (101), near complete (6), incomplete (2) | Negative (103), positive (6) |
Abbreviations: AV, anal verge; CRT, chemoradiation therapy; DL, dentate line; F, female; LA, laparoscopic assisted; M, male; NR, not reported; OA, open-assisted; P, pure NOTES; RA, robotic assisted; TME, total mesorectal excision; TNM, tumor-node-metastasis.
Given as mean (range) for series with n ≥ 3.
Data reported as mean ± standard error of the mean.
In general, most series selected patients based on tumor-specific criteria including low- and mid-rectal resectable tumors (staged by pelvic magnetic resonance imaging [MRI] and/or endorectal ultrasound), with no evidence of metastases on computed tomographic (CT) scan, and excluded patients with BMI > 35 kg/m2 and previous extensive abdominal or pelvic surgery. By contrast, two series intentionally selected patients with anatomical features predictive of difficult rectal dissection (narrow pelvis, BMI > 30 kg/m2, and large prostate).43 108 Rouanet et al also selected patients with T4 and recurrent tumors, including 25 patients (75%) with predicted circumferential resection margins ≤ 1 mm based on MRI and 3 patients (10%) with known metastases before surgery.108 Overall, in all 14 series, 44.1% of the tumors were located in the lower rectum (< 5 cm from the AV), 52.7% were in the middle (5–10 cm from the AV), and 3.2% were high (> 10 cm from the AV). Of the 43 patients for whom preoperative nodal status was reported, 41.9% had positive nodes. On final pathology, 37.7% of the patients had positive nodes.
Mean operative time was 265.6 minutes and mean estimated blood loss was 138.2 mL. Most of the series utilized a hybrid NOTES technique; 89 cases were laparoscopic assisted, 6 were robotic assisted, 3 were open assisted, and 12 were unassisted, purely transanal NOTES. Despite the majority of these trials being transabdominally assisted, most of the rectal dissection was performed transanally, hence their classification as transanal NOTES, as opposed to NOSE. The rectum was occluded transanally with a purse-string suture before making the enterotomy in 92.7% of the patients. In 69.1% of the patients, the splenic flexure was partially or fully mobilized. Seventy-seven percent of the patients received a diverting ileostomy. All groups extracted the specimen transanally, except Dumont et al who utilized the future ileostomy site and Atallah et al who used a Pfannenstiel incision.43 83 102
The overall intraoperative complication rate across all 14 clinical series of taTME was 5.5%, with 2 conversions to open surgery, 2 urethral injuries, 1 possible air embolism, and 1 episode of pneumatosis of the small bowel mesentery (Table 2). Rouanet et al, who published the series of high-risk patients, reported five of the six intraoperative events, explaining that the complications occurred early in the surgeons' learning curve and in patients with large, fixed tumors.108 The rate of conversion to open surgery was 1.8%, which compares favorably to published conversion rates after laparoscopic TME of 0 to 34%.1 36 37 38 42 113 114
Table 2. Postoperative outcomes of published clinical series on transanal TME.
| Series | Length of stay (d)a | Intraoperative complications (n) | Postoperative complications (n) | Oncologic outcomes (n) | Functional outcomes |
|---|---|---|---|---|---|
| Sylla et al 2010101 | 5 | None | None | NR | NR |
| Chen et al 2010103 | NR | None | None | NR | NR |
| Tuech et al 2011109 | NR | NR | NR | NR | NR |
| Zorron et al 2012112 | 6 | None | Feet paresthesia related to intraoperative positioning (1) | NR | NR |
| Dumont et al 201243 | 13 (10–21) | None | Anastomotic fistula (treated with antibiotics, drainage) (1) | No recurrence at 4.3-month follow-up | No severe incontinence (median Wexner score 5) at 3-month follow-up |
| Lacy et al 2013106 | 4.7 (4–5) | None | Dehydration requiring readmission (1) | NR | NR |
| Velthuis et al 2013110 | NR | Pneumatosis of small bowel mesentery (1) | Pneumonia and ileus (1), and presacral abscess (1) | NR | NR |
| Lacy et al 2013104 | 6.5 ± 3.1b | None | Urinary retention (2), ileus (1), and dehydration (1) | No recurrence at 30-day follow-up | NR |
| Rouanet et al 2013108 | 14 (9–25) | Conversion to open (2), urethral injury (2), and air embolism (1) | Peritonitis (2), septic shock (1), bowel obstruction (2), anastomotic leakage (1), and urinary dysfunction (2) | No recurrence (13), treated for recurrence (12), cancer-related deaths (4) at 21-month follow-up | 40% continent, 15% incontinent to liquids, 35% to gas, 25% with stool fragmentation (median Wexner score 11) at 12-month follow-up (n = 12) |
| Sylla et al 201384 | 5.2 (4–10) | None | Urinary dysfunction (2), and ileus (1) | No recurrence at 5.4-month follow-up | NR |
| Leroy et al 2013107 | NR | None | Small pelvic hematoma (treated with CT-guided drainage) (1) | NR | NR |
| Zhang et al 2013111 | NR | None | None | NR | NR |
| Atallah et al 2013102 | 4.5 (3–24) | None | Wound infection (2), pelvic abscess (4), prolonged ileus (4), pneumonia (1), acute renal failure (1), anastomotic leak requiring reoperation (1), and peri-anastomotic fluid collection (2) | No local recurrence at 6-month follow-up. One patient with distant metastases found 9 months after surgery. | “Most” patients with mild fecal incontinence (< 1 accident/d) 8 weeks after ileostomy closure. One patient with lifestyle-limiting incontinence (Wexner score 16)c |
| Chouillard et al 201482 | 10 (4–29) | None | Small bowel obstruction requiring reoperation (2), and pelvic abscess requiring reoperation (1) | No recurrence at 9-month follow-up | NR |
| Overall | 9.5 (3–29) | 6 intraoperative complications | 39 postoperative complications | – | – |
Abbreviations: CT, computed tomography; NR, not reported; TME, total mesorectal excision.
Given as mean (range) for series with n ≥ 3.
Data reported as mean ± standard error of the mean.
Functional outcomes were reported in the 14 patients who underwent diverting ileostomy closure.
The cumulative postoperative complication rate across all series of taTME was 35.5%, comparable to published morbidity rates of 21 to 44% and 24 to 51% after laparoscopic and open TME, respectively.1 36 37 42 113 115 Morbidity included 12 infectious (10.9%) and 5 anastomotic (4.5%) complications. Six patients experienced urinary dysfunction (5.5%), likely because of pelvic nerve injury and comparable to reported rates after laparoscopic (up to 20%) and open (5–14%) TME.116 117 Mean LOS after taTME was 9.5 days, no patient died within 30 days of surgery, and no patient developed incisional hernias during follow-up.
Oncologic results are similarly promising, with R0 resections achieved in 93.6% of the patients. Six patients had positive margins: four were high-risk patients with aggressive tumors reported by Rouanet et al and two had margins of 1 mm reported by Atallah et al.102 108 This rate of positive margins (5.5%) is consistent with historical rates published in the literature for laparoscopic (1.2–16%) and open (1.3–16%) TME.1 36 37 38 40 42 113 Mean lymph node harvest was 17.9 nodes, which compares favorably to published rates of laparoscopic and open TME.1 37 40 42 113 Ninety-seven percent of the patients had complete (n = 101) or near-complete (n = 6) specimens, comparable to rates of oncologically satisfactory specimens after laparoscopic (72–88%) and open (75–92%) TME.1 37 42 113 Only two specimens were deemed incomplete; Atallah et al explained that these two specimens contained defects of ≥ 5 mm in the mesorectal envelopes but margins were negative and the patients did not recur during follow-up.102
Lacy et al, Dumont et al, Sylla et al, and Chouillard et al reported no tumor recurrence after follow-up periods of 30 days, 4.3 months, 5.4 months, and 9 months, respectively.43 82 84 104 Atallah et al found no local recurrence after median follow-up of 6 months; however, one patient developed distant metastases.102 Though that patient's final pathology was ypT3N0 with complete TME specimen and negative margins and negative nodes, liver metastases were found 9 months after surgery and the patient required curative liver resection. In the high-risk patient cohort of Rouanet et al, 13 had no recurrence (43%), 12 were treated for recurrence (40%), and 4 died of cancer-related causes (13%) during their 21-month follow-up period.108 Disease-free survival rates were 93.3% and 88.9% and overall survival rates were 96.6% and 80.5% at 12- and 24-month follow-up, respectively. Even in this patient population selected for high-risk tumors, these overall survival rates are similar to those after laparoscopic (85.8–90.7% and 77.1–80.4% at 12 and 24 months, respectively) and open (80.5–92.7% and 69.5–83.3%) TME.35 42
Preliminary results of functional outcomes after taTME demonstrate similar postoperative sphincter function when compared with laparoscopic or open TME. Rouanet et al, who performed partial ISR and used a rigid transanal platform, demonstrated a median Wexner score of 11 at 12-month follow-up, at which time 40% of the patients who underwent ileostomy closure were fully continent, 15% were incontinent to liquids, 35% were incontinent to gas, and 25% had stool fragmentation.108 This is comparable to published results of TME with ISR, which report median Wexner scores of 10.8 to 11.118 119 120 Dumont et al and Atallah et al both used flexible transanal platforms and externalized the specimen transabdominally; Dumont et al's cohort had a median Wexner score of 5 at 3 months after ileostomy reversal, while “most” of Atallah et al's cohort had mild fecal incontinence (< 1 accident per day) at 8 weeks after ileostomy takedown.43 102 One patient reported by Atallah et al had lifestyle-limiting incontinence, with a Wexner score of 16. These results are similar to functional outcomes after TME with low coloanal anastomoses (median Wexner score of 6.9).118
Thus, these clinical series demonstrate taTME to be feasible and oncologically safe. However, these preliminary results must be interpreted carefully, as they are based on aggregating data from a few studies with small cohorts, variable patient characteristics, different surgical techniques and equipment, and short follow-up periods. The morbidity, oncologic outcomes, and functional outcomes of transanal TME must be clarified by large trials with long-term follow-up before widespread adoption of transanal NOTES. Future trials may also elucidate any differences in postoperative pain, wound complications, and recovery time after NOTES versus conventional surgical approaches.
Current Indications for Transanal Natural Orifice Transluminal Endoscopic Surgery Proctectomy and Total Mesorectal Excision
Based on the current published experience with transanal NOTES proctectomy and TME, these complex procedures should be performed by surgeons with significant experience in laparoscopic or robotic TME, TEM or TAMIS, and ISR.121 Like with many minimally invasive techniques, a steep learning curve must be overcome to master transanal NOTES. In the largest cadaver series of transanal TME, Telem et al demonstrated decreased operative time (p = 0.13) and increased specimen length (p = 0.001) after the first five cadavers.97 This data suggests that procedural training with fresh human cadavers should be considered as the optimal training model for this approach. Alternatively, Buscaglia et al recently found that transanal NOTES sigmoidectomy training using an endoscopy-simulation model decreased operative time by 42% and may also be effective in gaining proficiency.122
Because of the limitations of currently available transanal instruments, NOTES colorectal procedures should be performed using a hybrid fashion, with transabdominal assistance. The same cadaver study by Telem et al compared three different approaches: transanal alone, transanal with transgastric assistance, and transanal with laparoscopic assistance.97 They found decreased operative time with the laparoscopic-assisted technique, as well as decreased complications, though the latter was not statistically significant. Laparoscopic assistance is helpful for mobilization of the splenic flexure, retraction of the colon during transanal rectal dissection, and identification of the ureters and pelvic nerves. While there have been 12 cases of unassisted transanal TME,107 111 platforms and instruments tailored to complex transanal surgery must be developed before pure transanal NOTES procedures can become commonplace.
The initial clinical experiences of transanal TME also demonstrate the importance of careful patient selection, particularly while many surgeons are early in the learning curve, and while larger studies on this technique continue to investigate long-term functional and oncologic outcomes of this approach. Currently, transanal NOTES procedures should only be performed in patients with benign disease or premalignant or resectable malignant tumors, preferably located in the low- or mid-rectum.17 These patients should have no history of extensive abdominal or pelvic surgery.
Ideally, with increased surgeon expertise, taTME may be safely and consistently performed in patients likely to benefit most from a transanal approach. These are patients with characteristics that predict difficult transabdominal rectal dissections, including a narrow or deep pelvis, male gender, obesity, large prostate, low rectal tumor (< 5 cm from AV), and previous neoadjuvant radiation.102 Transanal TME may be particularly appropriate for these patients because of enhanced visualization and technically easier rectal and mesorectal dissection, which has the potential to improve the quality of the specimen and reduce the incidence of positive margins.123 124 Some groups, notably Rouanet et al and Dumont et al, have already begun investigating taTME in this subset of patients, with early results demonstrating equivalent morbidity and oncologic outcomes to previously published data from laparoscopic and open TME.43 108 Nevertheless, taTME should not be performed on these high-risk patients until the surgical team has gained clinical expertise with NOTES techniques and until the long-term oncologic outcomes of these procedures are reported.
Future Applications of Transanal Natural Orifice Transluminal Endoscopic Surgery
Improvements in transanal platforms and specialized instruments have the potential to revolutionize applications of transanal NOTES. Pure, unassisted NOTES procedures will likely become easier to perform and may maximize the theoretical benefits of natural orifice surgery, namely decreased postoperative pain, wound complications, and recovery time. There have been 12 reported cases of pure transanal NOTES TME so far, 2 case reports and 10 patients who were part of a larger series.82 107 111 In all cases, the specimen was intact and ≥ 11 nodes were collected. There were no intraoperative complications and three postoperative complications (a small pelvic hematoma treated with CT-guided drainage, a small bowel obstruction requiring reoperation, and a pelvic abscess requiring reoperation). Pure transanal NOTES has also been successfully reported for pull-through colectomy in six pediatric patients with long-segment intestinal aganglionosis, suggesting that pure NOTES may be used for indications beyond simply the removal of isolated lesions.125 126
Development of longer, more flexible scopes and instruments may also enable transanal NOTES procedures for more proximal colorectal lesions. Very few groups have pursued transanal colon resections because of the difficulty of dissection using currently available tools, though laparoscopic-assisted transanal colectomy for benign disease has been shown to be safe and feasible by Lacy et al and Fuchs et al59 99 In addition, Hall et al reported an excision of a rectal scar and successful transanal intraperitoneal creation of the colorectal anastomosis using a TEM platform, TEM instruments, and PDS suture, with no laparoscopic assistance.127 Though the resection margins were already closely approximated because of the small size of the scar and were thus under minimal tension, this case demonstrates the feasibility of suturing a colorectal anastomosis entirely intraperitoneally using a transanal platform, suggesting that more proximal transanal colorectal resections may be possible without the need to externalize the margins to perform a handsewn anastomosis. Nevertheless, the EURO-NOTES working group's 2012 recommendations state that transanal NOTES should currently only be utilized if the colotomy can be incorporated into the specimen or anastomosis, essentially restricting it to left-sided colon and rectal procedures.128
Another potential future application of NOTES is to biopsy sentinel lymph nodes (SLN) for rectal cancer. Currently, there is much debate over how to determine which patients with rectal cancer require radical resection and which will have similar oncologic outcomes after local excision. Radiologic staging has poor sensitivity for nodal spread, and local excision with transanal endoscopic surgery does not enable assessment of nodal status.129 Transanal NOTES offers the possibility of obtaining more accurate staging by removing the tumor and then sampling SLN through the colotomy site, particularly appropriate given that 98% of the positive nodes are located in the laterodorsal mesorectum within 5 cm of the tumor.130 131 Transanal NOTES segmental sigmoid resection and SLN extraction was demonstrated to be feasible in a porcine model that used submucosal injection of methylene blue dye to locate the SLN.132 Arezzo reported a small human series (n = 3) in which indocyanine green was instilled in the submucosa around the rectal tumor, the tumor was removed, the fat dissected, and the SLN successfully removed, all via transanal NOTES.133 There were no complications specific to nodal dissection and removal, and the mesorectal fascia was preserved, maintaining oncologic integrity. Nevertheless, the utility of SLN biopsy in colorectal cancer is still under debate, as results are widely variable, with sensitivity rates ranging from 25 to 100% and false negative rates ranging from 0 to 75%.134
Another area of innovation with transanal NOTES is the incorporation of robotic technology. Robotics has been reported to improve visualization using 3-dimensional optical technology, enable ambidextrous movements, decrease tremor, and improve dexterity, particularly in confined spaces.135 Cadaver models have proven the feasibility of robotic TAMIS,136 137 and an initial clinical series (n = 16) by Hompes et al of robotic TAMIS for rectal lesions has demonstrated promising results.135 There has also been a cadaver series of laparoscopic-assisted robotic transanal TME, in which complete TME specimens were obtained in all four cadavers.138 Atallah et al reported the only clinical case thus far of robotic-assisted robotic transanal TME in a 51-year-old woman with a preoperatively staged T3N1 rectal tumor located 4 cm from the AV.83 The operative time was 381 minutes, there were no complications, negative margins were achieved, and the specimen quality was near-complete. These preliminary results demonstrate the safety and feasibility of robotic transanal NOTES; however, more trials are needed to elucidate the benefits and costs of adding robotic technology to the standard transanal endoscopic approach.
Conclusion
Transanal NOTES represents a paradigm shift in minimally invasive colorectal surgery, combining the benefits of TEM and TAMIS, ISR, and NOSE to minimize access trauma and optimize the quality and ease of rectal dissection. The transanal NOTES approach to TME for rectal cancer and proctocolectomy for benign disease has shown encouraging preliminary results; however, there is a need for larger trials to better characterize outcomes, advantages, disadvantages, and cost before widespread adoption. Currently, transanal NOTES procedures should only be performed in carefully selected patients with transabdominal assistance by surgeons experienced with laparoscopic TME, TEM or TAMIS, and ISR.
References
- 1.Kang S B, Park J W, Jeong S Y. et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11(7):637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 2.Ohtani H, Tamamori Y, Azuma T. et al. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and conventional open surgery for rectal cancer. J Gastrointest Surg. 2011;15(8):1375–1385. doi: 10.1007/s11605-011-1547-1. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs K H, Meining A, von Renteln D. et al. Euro-NOTES Status Paper: from the concept to clinical practice. Surg Endosc. 2013;27(5):1456–1467. doi: 10.1007/s00464-013-2870-2. [DOI] [PubMed] [Google Scholar]
- 4.Mutter D, Dallemagne B, Perretta S. et al. Innovations in minimally invasive surgery: lessons learned from translational animal models. Langenbecks Arch Surg. 2013;398(7):919–923. doi: 10.1007/s00423-013-1115-0. [DOI] [PubMed] [Google Scholar]
- 5.Telem D A, Berger D L, Bordeianou L G, Rattner D W, Sylla P. Update on transanal NOTES for rectal cancer: transitioning to human trials. Minim Invasive Surg. 2012;2012:287613. doi: 10.1155/2012/287613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flora E D, Wilson T G, Martin I J, O'Rourke N A, Maddern G J. A review of natural orifice translumenal endoscopic surgery (NOTES) for intra-abdominal surgery: experimental models, techniques, and applicability to the clinical setting. Ann Surg. 2008;247(4):583–602. doi: 10.1097/SLA.0b013e3181656ce9. [DOI] [PubMed] [Google Scholar]
- 7.Hochberger J, Lamadé W. Transgastric surgery in the abdomen: the dawn of a new era? Gastrointest Endosc. 2005;62(2):293–296. doi: 10.1016/j.gie.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann K S, Ritz J P, Wibmer A. et al. The German registry for natural orifice translumenal endoscopic surgery: report of the first 551 patients. Ann Surg. 2010;252(2):263–270. doi: 10.1097/SLA.0b013e3181e6240f. [DOI] [PubMed] [Google Scholar]
- 9.Salinas G, Saavedra L, Agurto H. et al. Early experience in human hybrid transgastric and transvaginal endoscopic cholecystectomy. Surg Endosc. 2010;24(5):1092–1098. doi: 10.1007/s00464-009-0733-7. [DOI] [PubMed] [Google Scholar]
- 10.Zorron R, Palanivelu C, Galvão Neto M P. et al. International multicenter trial on clinical natural orifice surgery—NOTES IMTN study: preliminary results of 362 patients. Surg Innov. 2010;17(2):142–158. doi: 10.1177/1553350610370968. [DOI] [PubMed] [Google Scholar]
- 11.Fong D G, Pai R D, Thompson C C. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65(2):312–318. doi: 10.1016/j.gie.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Fong D G, Ryou M, Pai R D, Tavakkolizadeh A, Rattner D W, Thompson C C. Transcolonic ventral wall hernia mesh fixation in a porcine model. Endoscopy. 2007;39(10):865–869. doi: 10.1055/s-2007-966916. [DOI] [PubMed] [Google Scholar]
- 13.Pai R D, Fong D G, Bundga M E, Odze R D, Rattner D W, Thompson C C. Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model (with video) Gastrointest Endosc. 2006;64(3):428–434. doi: 10.1016/j.gie.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 14.Ryou M, Fong D G, Pai R D, Tavakkolizadeh A, Rattner D W, Thompson C C. Dual-port distal pancreatectomy using a prototype endoscope and endoscopic stapler: a natural orifice transluminal endoscopic surgery (NOTES) survival study in a porcine model. Endoscopy. 2007;39(10):881–887. doi: 10.1055/s-2007-966908. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm D, Meining A, von Delius S. et al. An innovative, safe and sterile sigmoid access (ISSA) for NOTES. Endoscopy. 2007;39(5):401–406. doi: 10.1055/s-2007-966438. [DOI] [PubMed] [Google Scholar]
- 16.Wagh M S, Thompson C C. Surgery insight: natural orifice transluminal endoscopic surgery—an analysis of work to date. Nat Clin Pract Gastroenterol Hepatol. 2007;4(7):386–392. doi: 10.1038/ncpgasthep0867. [DOI] [PubMed] [Google Scholar]
- 17.Sylla P. Current experience and future directions of completely NOTES colorectal resection. World J Gastrointest Surg. 2010;2(6):193–198. doi: 10.4240/wjgs.v2.i6.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrifield B F, Wagh M S, Thompson C C. Peroral transgastric organ resection: a feasibility study in pigs. Gastrointest Endosc. 2006;63(4):693–697. doi: 10.1016/j.gie.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Whiteford M H, Denk P M, Swanström L L. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc. 2007;21(10):1870–1874. doi: 10.1007/s00464-007-9552-x. [DOI] [PubMed] [Google Scholar]
- 20.Heald R J. A new approach to rectal cancer. Br J Hosp Med. 1979;22(3):277–281. [PubMed] [Google Scholar]
- 21.Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc. 2013;27(5):1485–1502. doi: 10.1007/s00464-012-2649-x. [DOI] [PubMed] [Google Scholar]
- 22.Asoglu O, Matlim T, Karanlik H. et al. Impact of laparoscopic surgery on bladder and sexual function after total mesorectal excision for rectal cancer. Surg Endosc. 2009;23(2):296–303. doi: 10.1007/s00464-008-9870-7. [DOI] [PubMed] [Google Scholar]
- 23.Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2006;18(4):CD005200. doi: 10.1002/14651858.CD005200.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Dahlberg M Glimelius B Graf W Påhlman L Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study Dis Colon Rectum 1998415543–549., discussion 549–551 [DOI] [PubMed] [Google Scholar]
- 25.Hida J, Yoshifuji T, Tokoro T. et al. Comparison of long-term functional results of colonic J-pouch and straight anastomosis after low anterior resection for rectal cancer: a five-year follow-up. Dis Colon Rectum. 2004;47(10):1578–1585. doi: 10.1007/s10350-004-0654-4. [DOI] [PubMed] [Google Scholar]
- 26.Köhler A, Athanasiadis S, Ommer A, Psarakis E. Long-term results of low anterior resection with intersphincteric anastomosis in carcinoma of the lower one-third of the rectum: analysis of 31 patients. Dis Colon Rectum. 2000;43(6):843–850. doi: 10.1007/BF02238025. [DOI] [PubMed] [Google Scholar]
- 27.Machado M, Nygren J, Goldman S, Ljungqvist O. Similar outcome after colnic pouch and side-to-end anastomosis in low anterior resection for rectal cancer: a prospective randomized trial. Ann Surg. 2003;238(2):214–220. doi: 10.1097/01.sla.0000080824.10891.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maggiori L, Panis Y. Is it time for a paradigm shift: “laparoscopy is now the best approach for rectal cancer”? Transl Gastrointest Cancer. 2014;3(1):1–3. [Google Scholar]
- 29.Schlachta C M, Mamazza J, Gregoire R, Burpee S E, Poulin E C. Could laparoscopic colon and rectal surgery become the standard of care? A review and experience with 750 procedures. Can J Surg. 2003;46(6):432–440. [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor G W, Jayne D G, Brown S R. et al. Adhesions and incisional hernias following laparoscopic versus open surgery for colorectal cancer in the CLASICC trial. Br J Surg. 2010;97(1):70–78. doi: 10.1002/bjs.6742. [DOI] [PubMed] [Google Scholar]
- 31.Temple L K, Bacik J, Savatta S G. et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48(7):1353–1365. doi: 10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 32.Vignali A, Fazio V W, Lavery I C. et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 1997;185(2):105–113. doi: 10.1016/s1072-7515(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 33.Williamson M E, Lewis W G, Finan P J, Miller A S, Holdsworth P J, Johnston D. Recovery of physiologic and clinical function after low anterior resection of the rectum for carcinoma: myth or reality? Dis Colon Rectum. 1995;38(4):411–418. doi: 10.1007/BF02054232. [DOI] [PubMed] [Google Scholar]
- 34.Emhoff I, Lee G, Sylla P. Future directions in surgery for colorectal cancer: the evolving role of transanal endoscopic surgery. Colorectal Cancer. 2014;3(2):195–213. [Google Scholar]
- 35.Green B L, Marshall H C, Collinson F. et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100(1):75–82. doi: 10.1002/bjs.8945. [DOI] [PubMed] [Google Scholar]
- 36.Guillou P J, Quirke P, Thorpe H. et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 37.van der Pas M H, Haglind E, Cuesta M A. et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 38.Aziz O, Constantinides V, Tekkis P P. et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13(3):413–424. doi: 10.1245/ASO.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Surg Endosc. 2009;23(2):403–408. doi: 10.1007/s00464-008-9912-1. [DOI] [PubMed] [Google Scholar]
- 40.Krane M K, Fichera A. Laparoscopic rectal cancer surgery: where do we stand? World J Gastroenterol. 2012;18(46):6747–6755. doi: 10.3748/wjg.v18.i46.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Künzli B M, Friess H, Shrikhande S V. Is laparoscopic colorectal cancer surgery equal to open surgery? An evidence based perspective. World J Gastrointest Surg. 2010;2(4):101–108. doi: 10.4240/wjgs.v2.i4.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos M D, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96(9):982–989. doi: 10.1002/bjs.6662. [DOI] [PubMed] [Google Scholar]
- 43.Dumont F, Goéré D, Honoré C, Elias D. Transanal endoscopic total mesorectal excision combined with single-port laparoscopy. Dis Colon Rectum. 2012;55(9):996–1001. doi: 10.1097/DCR.0b013e318260d3a0. [DOI] [PubMed] [Google Scholar]
- 44.Kim S J, Ryu G O, Choi B J. et al. The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg. 2011;254(6):933–940. doi: 10.1097/SLA.0b013e318237826b. [DOI] [PubMed] [Google Scholar]
- 45.Pedraza R, Aminian A, Nieto J, Faraj C, Pickron T B, Haas E M. Single-incision laparoscopic colectomy for cancer: short-term outcomes and comparative analysis. Minim Invasive Surg. 2013;2013:283438. doi: 10.1155/2013/283438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sirikurnpiboon S, Jivapaisarnpong P. Single-access laparoscopic rectal surgery is technically feasible. Minim Invasive Surg. 2013;2013:687134. doi: 10.1155/2013/687134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akmal Y, Baek J H, McKenzie S, Garcia-Aguilar J, Pigazzi A. Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc. 2012;26(9):2471–2476. doi: 10.1007/s00464-012-2216-5. [DOI] [PubMed] [Google Scholar]
- 48.Trastulli S, Farinella E, Cirocchi R. et al. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14(4):e134–e156. doi: 10.1111/j.1463-1318.2011.02907.x. [DOI] [PubMed] [Google Scholar]
- 49.Fung A K, Aly E H. Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum. 2013;56(6):786–796. doi: 10.1097/DCR.0b013e318285b810. [DOI] [PubMed] [Google Scholar]
- 50.Awad Z T, Qureshi I, Seibel B, Sharma S, Dobbertien M A. Laparoscopic right hemicolectomy with transvaginal colon extraction using a laparoscopic posterior colpotomy: a 2-year series from a single institution. Surg Laparosc Endosc Percutan Tech. 2011;21(6):403–408. doi: 10.1097/SLE.0b013e31823945ac. [DOI] [PubMed] [Google Scholar]
- 51.Choi G S, Park I J, Kang B M, Lim K H, Jun S H. A novel approach of robotic-assisted anterior resection with transanal or transvaginal retrieval of the specimen for colorectal cancer. Surg Endosc. 2009;23(12):2831–2835. doi: 10.1007/s00464-009-0484-5. [DOI] [PubMed] [Google Scholar]
- 52.D'Hoore A, Wolthuis A M. Laparoscopic low anterior resection and transanal pull-through for low rectal cancer: a Natural Orifice Specimen Extraction (NOSE) technique. Colorectal Dis. 2011;13 07:28–31. doi: 10.1111/j.1463-1318.2011.02773.x. [DOI] [PubMed] [Google Scholar]
- 53.Franklin M E Jr, Liang S, Russek K. Natural orifice specimen extraction in laparoscopic colorectal surgery: transanal and transvaginal approaches. Tech Coloproctol. 2013;17 01:S63–S67. doi: 10.1007/s10151-012-0938-y. [DOI] [PubMed] [Google Scholar]
- 54.Lacy A M, Delgado S, Rojas O A, Almenara R, Blasi A, Llach J. MA-NOS radical sigmoidectomy: report of a transvaginal resection in the human. Surg Endosc. 2008;22(7):1717–1723. doi: 10.1007/s00464-008-9956-2. [DOI] [PubMed] [Google Scholar]
- 55.Ooi B S, Quah H M, Fu C W, Eu K W. Laparoscopic high anterior resection with natural orifice specimen extraction (NOSE) for early rectal cancer. Tech Coloproctol. 2009;13(1):61–64. doi: 10.1007/s10151-009-0460-z. [DOI] [PubMed] [Google Scholar]
- 56.Torres R A, Orban R D, Tocaimaza L, Vallejos Pereira G, Arévalo J R. Transvaginal specimen extraction after laparoscopic colectomy. World J Surg. 2012;36(7):1699–1702. doi: 10.1007/s00268-012-1528-x. [DOI] [PubMed] [Google Scholar]
- 57.Wang Q, Wang C, Sun D H, Kharbuja P, Cao X Y. Laparoscopic total mesorectal excision with natural orifice specimen extraction. World J Gastroenterol. 2013;19(5):750–754. doi: 10.3748/wjg.v19.i5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arezzo A, Zornig C, Mofid H. et al. The EURO-NOTES clinical registry for natural orifice transluminal endoscopic surgery: a 2-year activity report. Surg Endosc. 2013;27(9):3073–3084. doi: 10.1007/s00464-013-2908-5. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs K-H, Breithaupt W, Varga G, Schulz T, Reinisch A, Josipovic N. Transanal hybrid colon resection: from laparoscopy to NOTES. Surg Endosc. 2013;27(3):746–752. doi: 10.1007/s00464-012-2534-7. [DOI] [PubMed] [Google Scholar]
- 60.Park J S, Choi G S, Kim H J, Park S Y, Jun S H. Natural orifice specimen extraction versus conventional laparoscopically assisted right hemicolectomy. Br J Surg. 2011;98(5):710–715. doi: 10.1002/bjs.7419. [DOI] [PubMed] [Google Scholar]
- 61.Leung A L, Cheung H Y, Fok B K, Chung C C, Li M K, Tang C N. Prospective randomized trial of hybrid NOTES colectomy versus conventional laparoscopic colectomy for left-sided colonic tumors. World J Surg. 2013;37(11):2678–2682. doi: 10.1007/s00268-013-2163-x. [DOI] [PubMed] [Google Scholar]
- 62.Franklin M E Jr, Liang S, Russek K. Integration of transanal specimen extraction into laparoscopic anterior resection with total mesorectal excision for rectal cancer: a consecutive series of 179 patients. Surg Endosc. 2013;27(1):127–132. doi: 10.1007/s00464-012-2440-z. [DOI] [PubMed] [Google Scholar]
- 63.Liang S, Franklin M. , Jr. Baltimore, MD: Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) 2013; 2013. Transanal versus Transabdominal Specimen Extraction with Laparoscopic Low Anterior Resection: A Comparative Analysis on 432 Patients with Rectal Cancer. [Google Scholar]
- 64.Buess G, Theiss R, Günther M, Hutterer F, Pichlmaier H. Endoscopic surgery in the rectum. Endoscopy. 1985;17(1):31–35. doi: 10.1055/s-2007-1018451. [DOI] [PubMed] [Google Scholar]
- 65.Buess G, Theiss R, Hutterer F. et al. Transanal endoscopic surgery of the rectum - testing a new method in animal experiments [in German] Leber Magen Darm. 1983;13(2):73–77. [PubMed] [Google Scholar]
- 66.Moore J S Cataldo P A Osler T Hyman N H Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses Dis Colon Rectum 20085171026–1030., discussion 1030–1031 [DOI] [PubMed] [Google Scholar]
- 67.Langer C, Liersch T, Süss M. et al. Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis. 2003;18(3):222–229. doi: 10.1007/s00384-002-0441-4. [DOI] [PubMed] [Google Scholar]
- 68.Winde G, Nottberg H, Keller R, Schmid K W, Bünte H. Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum. 1996;39(9):969–976. doi: 10.1007/BF02054683. [DOI] [PubMed] [Google Scholar]
- 69.Albert M R, Atallah S B, deBeche-Adams T C, Izfar S, Larach S W. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56(3):301–307. doi: 10.1097/DCR.0b013e31827ca313. [DOI] [PubMed] [Google Scholar]
- 70.Alves F E, Costa P, Guerra J. Transanal minimally invasive surgery with single-port (TAMIS) for the management of rectal neoplasms: a pilot study. J Coloproctol. 2012;32(4):402–406. [Google Scholar]
- 71.Atallah S B, Albert M R. Transanal minimally invasive surgery (TAMIS) versus transanal endoscopic microsurgery (TEM): is one better than the other? Surg Endosc. 2013;27(12):4750–4751. doi: 10.1007/s00464-013-3111-4. [DOI] [PubMed] [Google Scholar]
- 72.Rega D, Cardone E, Montesarchio L. et al. Transanal minimally invasive surgery with single-port laparoscopy for rectal tumors. Eur J Surg Oncol. 2012;38(10):982. [Google Scholar]
- 73.National Comprehensive Cancer Network . Fort Washington, PA: National Comprehensive Cancer Network; 2013. Clinical Practice Guidelines in Oncology. Rectal Cancer. [Google Scholar]
- 74.Bach S P, Hill J, Monson J R. et al. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg. 2009;96(3):280–290. doi: 10.1002/bjs.6456. [DOI] [PubMed] [Google Scholar]
- 75.Bentrem D J Okabe S Wong W D et al. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg 20052424472–477., discussion 477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greenberg J A, Shibata D, Herndon J E II, Steele G D Jr, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum. 2008;51(8):1185–1191, discussion 1191–1194. doi: 10.1007/s10350-008-9231-6. [DOI] [PubMed] [Google Scholar]
- 77.Monson J R, Weiser M R, Buie W D. et al. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2013;56(5):535–550. doi: 10.1097/DCR.0b013e31828cb66c. [DOI] [PubMed] [Google Scholar]
- 78.Nash G M, Weiser M R, Guillem J G. et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52(4):577–582. doi: 10.1007/DCR.0b013e3181a0adbd. [DOI] [PubMed] [Google Scholar]
- 79.Nesbakken A, Nygaard K, Westerheim O, Mala T, Lunde O C. Local recurrence after mesorectal excision for rectal cancer. Eur J Surg Oncol. 2002;28(2):126–134. doi: 10.1053/ejso.2001.1231. [DOI] [PubMed] [Google Scholar]
- 80.Peng J, Chen W, Sheng W. et al. Oncological outcome of T1 rectal cancer undergoing standard resection and local excision. Colorectal Dis. 2011;13(2):e14–e19. doi: 10.1111/j.1463-1318.2010.02424.x. [DOI] [PubMed] [Google Scholar]
- 81.You Y N, Baxter N N, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245(5):726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chouillard E Chahine E Khoury G et al. NOTES total mesorectal excision (TME) for patients with rectal neoplasia: a preliminary experience Surg Endosc 201428113150–3157. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 83.Atallah S, Nassif G, Polavarapu H. et al. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol. 2013;17(4):441–447. doi: 10.1007/s10151-013-1039-2. [DOI] [PubMed] [Google Scholar]
- 84.Sylla P, Bordeianou L G, Berger D. et al. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc. 2013;27(9):3396–3405. doi: 10.1007/s00464-013-2922-7. [DOI] [PubMed] [Google Scholar]
- 85.Schiessel R, Karner-Hanusch J, Herbst F, Teleky B, Wunderlich M. Intersphincteric resection for low rectal tumours. Br J Surg. 1994;81(9):1376–1378. doi: 10.1002/bjs.1800810944. [DOI] [PubMed] [Google Scholar]
- 86.Akagi Y, Shirouzu K, Ogata Y, Kinugasa T. Oncologic outcomes of intersphincteric resection without preoperative chemoradiotherapy for very low rectal cancer. Surg Oncol. 2013;22(2):144–149. doi: 10.1016/j.suronc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Marks G, Mohiuddin M, Goldstein S D. Sphincter preservation for cancer of the distal rectum using high dose preoperative radiation. Int J Radiat Oncol Biol Phys. 1988;15(5):1065–1068. doi: 10.1016/0360-3016(88)90185-x. [DOI] [PubMed] [Google Scholar]
- 88.Liyanage C, Ramwell A, Harris G J, Levy B F, Simson J N. Transanal endoscopic microsurgery: a new technique for completion proctectomy. Colorectal Dis. 2013;15(9):e542–e547. doi: 10.1111/codi.12316. [DOI] [PubMed] [Google Scholar]
- 89.Bhattacharjee H K, Buess G F, Becerra Garcia F C. et al. A novel single-port technique for transanal rectosigmoid resection and colorectal anastomosis on an ex vivo experimental model. Surg Endosc. 2011;25(6):1844–1857. doi: 10.1007/s00464-010-1476-1. [DOI] [PubMed] [Google Scholar]
- 90.Bhattacharjee H K, Kirschniak A, Storz P, Wilhelm P, Kunert W. Transanal endoscopic microsurgery-based transanal access for colorectal surgery: experience on human cadavers. J Laparoendosc Adv Surg Tech A. 2011;21(9):835–840. doi: 10.1089/lap.2011.0045. [DOI] [PubMed] [Google Scholar]
- 91.Fajardo A D, Hunt S R, Fleshman J W, Mutch M G. Video. Transanal single-port low anterior resection in a cadaver model. Surg Endosc. 2010;24(7):1765. doi: 10.1007/s00464-009-0838-z. [DOI] [PubMed] [Google Scholar]
- 92.Leroy J, Cahill R A, Perretta S, Forgione A, Dallemagne B, Marescaux J. Natural orifice translumenal endoscopic surgery (NOTES) applied totally to sigmoidectomy: an original technique with survival in a porcine model. Surg Endosc. 2009;23(1):24–30. doi: 10.1007/s00464-008-0102-y. [DOI] [PubMed] [Google Scholar]
- 93.Rieder E, Spaun G O, Khajanchee Y S. et al. A natural orifice transrectal approach for oncologic resection of the rectosigmoid: an experimental study and comparison with conventional laparoscopy. Surg Endosc. 2011;25(10):3357–3363. doi: 10.1007/s00464-011-1726-x. [DOI] [PubMed] [Google Scholar]
- 94.Sohn D K, Jeong S Y, Park J W. et al. Comparative study of NOTES rectosigmoidectomy in a swine model: E-NOTES vs. P-NOTES. Endoscopy. 2011;43(6):526–532. doi: 10.1055/s-0030-1256239. [DOI] [PubMed] [Google Scholar]
- 95.Sylla P, Sohn D K, Cizginer S. et al. Survival study of natural orifice translumenal endoscopic surgery for rectosigmoid resection using transanal endoscopic microsurgery with or without transgastric endoscopic assistance in a swine model. Surg Endosc. 2010;24(8):2022–2030. doi: 10.1007/s00464-010-0898-0. [DOI] [PubMed] [Google Scholar]
- 96.Sylla P, Willingham F F, Sohn D K, Gee D, Brugge W R, Rattner D W. NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg. 2008;12(10):1717–1723. doi: 10.1007/s11605-008-0637-1. [DOI] [PubMed] [Google Scholar]
- 97.Telem D A, Han K S, Kim M-C. et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013;27(1):74–80. doi: 10.1007/s00464-012-2409-y. [DOI] [PubMed] [Google Scholar]
- 98.Trunzo J A, Delaney C P. Natural orifice proctectomy using a transanal endoscopic microsurgical technique in a porcine model. Surg Innov. 2010;17(1):48–52. doi: 10.1177/1553350609359516. [DOI] [PubMed] [Google Scholar]
- 99.Lacy A M, Saavedra-Perez D, Bravo R, Adelsdorfer C, Aceituno M, Balust J. Minilaparoscopy-assisted natural orifice total colectomy: technical report of a minilaparoscopy-assisted transrectal resection. Surg Endosc. 2012;26(7):2080–2085. doi: 10.1007/s00464-011-2117-z. [DOI] [PubMed] [Google Scholar]
- 100.Wolthuis A M, de Buck van Overstraeten A, D'Hoore A. Dynamic article: transanal rectal excision: a pilot study. Dis Colon Rectum. 2014;57(1):105–109. doi: 10.1097/DCR.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 101.Sylla P, Rattner D W, Delgado S, Lacy A M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24(5):1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 102.Atallah S Martin-Perez B Albert M et al. Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution Tech Coloproctol 2013; Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Hu M, Lei J, Chen J, Li J. NOTES transanal endoscopic total mesorectal excision for rectal cancer. China Journal of Endoscopy. 2010;16(12):1261–1265. [Google Scholar]
- 104.de Lacy A M, Rattner D W, Adelsdorfer C. et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)—short-term outcomes in the first 20 cases. Surg Endosc. 2013;27(9):3165–3172. doi: 10.1007/s00464-013-2872-0. [DOI] [PubMed] [Google Scholar]
- 105.Emhoff I A, Lee G C, Sylla P. Transanal colorectal resection using natural orifice translumenal endoscopic surgery (NOTES) Dig Endosc. 2014;26 01:29–42. doi: 10.1111/den.12157. [DOI] [PubMed] [Google Scholar]
- 106.Lacy A M, Adelsdorfer C, Delgado S, Sylla P, Rattner D W. Minilaparoscopy-assisted transrectal low anterior resection (LAR): a preliminary study. Surg Endosc. 2013;27(1):339–346. doi: 10.1007/s00464-012-2443-9. [DOI] [PubMed] [Google Scholar]
- 107.Leroy J, Barry B D, Melani A, Mutter D, Marescaux J. No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery. JAMA Surg. 2013;148(3):226–230, discussion 231. doi: 10.1001/jamasurg.2013.685. [DOI] [PubMed] [Google Scholar]
- 108.Rouanet P, Mourregot A, Azar C C. et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56(4):408–415. doi: 10.1097/DCR.0b013e3182756fa0. [DOI] [PubMed] [Google Scholar]
- 109.Tuech J J, Bridoux V, Kianifard B. et al. Natural orifice total mesorectal excision using transanal port and laparoscopic assistance. Eur J Surg Oncol. 2011;37(4):334–335. doi: 10.1016/j.ejso.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 110.Velthuis S van den Boezem P B van der Peet D L Cuesta M A Sietses C Feasibility study of transanal total mesorectal excision Br J Surg 20131006828–831., discussion 831 [DOI] [PubMed] [Google Scholar]
- 111.Zhang H, Zhang Y S, Jin X W, Li M Z, Fan J S, Yang Z H. Transanal single-port laparoscopic total mesorectal excision in the treatment of rectal cancer. Tech Coloproctol. 2013;17(1):117–123. doi: 10.1007/s10151-012-0882-x. [DOI] [PubMed] [Google Scholar]
- 112.Zorron R, Phillips H N, Coelho D, Flach L, Lemos F B, Vassallo R C. Perirectal NOTES access: “down-to-up” total mesorectal excision for rectal cancer. Surg Innov. 2012;19(1):11–19. doi: 10.1177/1553350611409956. [DOI] [PubMed] [Google Scholar]
- 113.Lujan J, Valero G, Biondo S, Espin E, Parrilla P, Ortiz H. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc. 2013;27(1):295–302. doi: 10.1007/s00464-012-2444-8. [DOI] [PubMed] [Google Scholar]
- 114.Targarona E M, Balague C, Pernas J C. et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg. 2008;247(4):642–649. doi: 10.1097/SLA.0b013e3181612c6a. [DOI] [PubMed] [Google Scholar]
- 115.Leroy J, Jamali F, Forbes L. et al. Laparoscopic total mesorectal excision (TME) for rectal cancer surgery: long-term outcomes. Surg Endosc. 2004;18(2):281–289. doi: 10.1007/s00464-002-8877-8. [DOI] [PubMed] [Google Scholar]
- 116.Kim N K, Aahn T W, Park J K. et al. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum. 2002;45(9):1178–1185. doi: 10.1007/s10350-004-6388-5. [DOI] [PubMed] [Google Scholar]
- 117.Maurer C A. Urinary and sexual function after total mesorectal excision. Recent Results Cancer Res. 2005;165:196–204. doi: 10.1007/3-540-27449-9_21. [DOI] [PubMed] [Google Scholar]
- 118.Bretagnol F, Rullier E, Laurent C, Zerbib F, Gontier R, Saric J. Comparison of functional results and quality of life between intersphincteric resection and conventional coloanal anastomosis for low rectal cancer. Dis Colon Rectum. 2004;47(6):832–838. doi: 10.1007/s10350-004-0523-1. [DOI] [PubMed] [Google Scholar]
- 119.Dumont F, Ayadi M, Goéré D, Honoré C, Elias D. Comparison of fecal continence and quality of life between intersphincteric resection and abdominoperineal resection plus perineal colostomy for ultra-low rectal cancer. J Surg Oncol. 2013;108(4):225–229. doi: 10.1002/jso.23379. [DOI] [PubMed] [Google Scholar]
- 120.Rouanet P Saint-Aubert B Lemanski C et al. Restorative and nonrestorative surgery for low rectal cancer after high-dose radiation: long-term oncologic and functional results Dis Colon Rectum 2002453305–313., discussion 313–315 [DOI] [PubMed] [Google Scholar]
- 121.Lacy A M, Adelsdorfer C. Totally transrectal endoscopic total mesorectal excision (TME) Colorectal Dis. 2011;13 07:43–46. doi: 10.1111/j.1463-1318.2011.02781.x. [DOI] [PubMed] [Google Scholar]
- 122.Buscaglia J M Karas J Palladino N et al. Simulated transanal NOTES sigmoidectomy training improves the responsiveness of surgical endoscopists Gastrointest Endosc 2014801126–132. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 123.Nahar Al-Furaji H H, Sehgal R, Mansoor T, Burke J, Cahill R A. Transanal and transrectal operations for excisional surgery of the low and mid rectum (with video) Surg Technol Int. 2014;24:124–132. [PubMed] [Google Scholar]
- 124.Sehgal R, Cahill R A. Advanced laparoscopic surgery for colorectal disease: NOTES/NOSE or single port? Best Pract Res Clin Gastroenterol. 2014;28(1):81–96. doi: 10.1016/j.bpg.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 125.Li N, Zhang W, Yu D. et al. NOTES for surgical treatment of long-segment Hirschsprung's disease: report of three cases. J Laparoendosc Adv Surg Tech A. 2013;23(12):1020–1023. doi: 10.1089/lap.2013.0180. [DOI] [PubMed] [Google Scholar]
- 126.Vahdad M R, Foroutan A, Najafi S M. et al. Totally transanal LESS pull-through colectomy: a novel approach for avoiding abdominal wall incision in children with long-segment intestinal aganglionosis. J Laparoendosc Adv Surg Tech A. 2013;23(3):276–280. doi: 10.1089/lap.2012.0058. [DOI] [PubMed] [Google Scholar]
- 127.Hall D J, Farmer K C, Roth H S, Warrier S K. Transanal endoscopic microsurgery colorectal anastomosis: a critical step to natural orifice colorectal surgery in humans. Dis Colon Rectum. 2014;57(4):549–552. doi: 10.1097/DCR.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 128.Meining A, Spaun G, Fernández-Esparrach G. et al. NOTES in Europe: summary of the working group reports of the 2012 EURO-NOTES meeting. Endoscopy. 2013;45(3):214–217. doi: 10.1055/s-0032-1326205. [DOI] [PubMed] [Google Scholar]
- 129.Kim H, Lim J S, Choi J Y. et al. Rectal cancer: comparison of accuracy of local-regional staging with two- and three-dimensional preoperative 3-T MR imaging. Radiology. 2010;254(2):485–492. doi: 10.1148/radiol.09090587. [DOI] [PubMed] [Google Scholar]
- 130.Engelen S M, Beets-Tan R G, Lahaye M J, Kessels A G, Beets G L. Location of involved mesorectal and extramesorectal lymph nodes in patients with primary rectal cancer: preoperative assessment with MR imaging. Eur J Surg Oncol. 2008;34(7):776–781. doi: 10.1016/j.ejso.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 131.Koh D M, Brown G, Temple L. et al. Distribution of mesorectal lymph nodes in rectal cancer: in vivo MR imaging compared with histopathological examination. Initial observations. Eur Radiol. 2005;15(8):1650–1657. doi: 10.1007/s00330-005-2751-8. [DOI] [PubMed] [Google Scholar]
- 132.Cahill R A, Perretta S, Forgione A, Leroy J, Dallemagne B, Marescaux J. Multimedia article. Combined sentinel node biopsy and localized sigmoid resection entirely by natural orifice transluminal endoscopic surgery: a new challenge to the old paradigm. Dis Colon Rectum. 2009;52(4):725. doi: 10.1007/DCR.0b013e31819a69af0. [DOI] [PubMed] [Google Scholar]
- 133.Arezzo A. Amsterdam: United European Gastroenterology Week; 2012. Fluorescence-guided mesorectal lymph node sampling during transanal endoscopic microsurgery for early rectal tumours. [Google Scholar]
- 134.Cahill R A, Leroy J, Marescaux J. Could lymphatic mapping and sentinel node biopsy provide oncological providence for local resectional techniques for colon cancer? A review of the literature. BMC Surg. 2008;8:17. doi: 10.1186/1471-2482-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hompes R, Rauh S M, Ris F, Tuynman J B, Mortensen N J. Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br J Surg. 2014;101(5):578–581. doi: 10.1002/bjs.9454. [DOI] [PubMed] [Google Scholar]
- 136.Atallah S B, Albert M R, deBeche-Adams T H, Larach S W. Robotic TransAnal Minimally Invasive Surgery in a cadaveric model. Tech Coloproctol. 2011;15(4):461–464. doi: 10.1007/s10151-011-0762-9. [DOI] [PubMed] [Google Scholar]
- 137.Hompes R, Rauh S M, Hagen M E, Mortensen N J. Preclinical cadaveric study of transanal endoscopic da Vinci® surgery. Br J Surg. 2012;99(8):1144–1148. doi: 10.1002/bjs.8794. [DOI] [PubMed] [Google Scholar]
- 138.Gomez Ruiz M Martin Parra I Calleja Iglesias A et al. Preclinical cadaveric study of transanal robotic proctectomy with total mesorectal excision combined with laparoscopic assistance Int J Med Robot 2014; Epub ahead of print [DOI] [PubMed] [Google Scholar]


