Abstract
Background: Bacterial biofilms are involved in a large proportion of clinical infections, including device-related infections. Unfortunately, biofilm-associated bacteria are typically less susceptible to antibiotics, and infected devices must often be removed. On the basis of a recent observation that lipid-rich biofilm matrix material is present in early biofilm formation and may protect a population of bacteria from interacting with ordinarily diffusible small molecules, we hypothesized that surfactants may be useful in preventing biofilm development.
Methods: Experimental Staphylococcus aureus or Enterococcus faecalis biofilms were cultivated on surgical suture suspended in a growth medium supplemented with the natural surfactant glycerol monolaurate (GML) or with a component molecule, lauric acid. After 16 h incubation, the numbers of viable biofilm-associated bacteria were measured by standard microbiologic techniques and biofilm biomass was measured using the colorimetric crystal violet assay.
Results: Both GML and lauric acid were effective in inhibiting biofilm development as measured by decreased numbers of viable biofilm-associated bacteria as well as decreased biofilm biomass. Compared with lauric acid on a molar basis, GML represented a more effective inhibitor of biofilms formed by either S. aureus or E. faecalis.
Conclusions: Because the natural surfactant GML inhibited biofilm development, resulting data were consistent with the hypothesis that lipids may play an important role in biofilm growth, implying that interfering with lipid formation may help control development of clinically relevant biofilms.
Microbial biofilms develop in a variety of clinical situations and it is now recognized that biofilms are involved in more than 60% of infections [1]. Biofilms can be defined as surface-associated microbial communities that develop in liquid environments. Microbes within biofilms are often embedded in a hydrated matrix composed of an extracellular polymeric substance containing proteins, glycoproteins, glycolipids, polysaccharides, and extracellular DNA [2–5]. Biofilm-related infections encompass a variety of clinical processes and include periodontitis, otitis media, ventilator- and cystic fibrosis-related pneumonias, endocarditis, biliary tract infections, prostatitis, osteomyelitis, burn wound infections, other surgical site infections, and device-related infections such as those associated with catheters, sutures, and stents [1,6]. Device-related infections complicate treatment and may require removal of the infected device.
Biofilm-associated bacteria are generally less susceptible to antibiotic therapy compared with free-living planktonic bacteria [1,2,6] and the mechanisms responsible for this resistance are unclear. One explanation for the decreased antibiotic susceptibility of biofilm bacteria may be that antibiotic molecules are unable to interact directly with bacteria because of the proximity of impermeable matrix substance, or that charge characteristics of the matrix may interfere with binding between the antibiotic and its target microbe. For example, positively charged aminoglycosides are inhibited by negatively charged matrix material [6]. Although a variety of studies have reported unrestricted antibiotic diffusion through the biofilm [1,7,8], none of these studies had the resolution required to observe whether antibiotic was able to diffuse to each cell within the biofilm, i.e., none of these studies was able to verify that antibiotics were uniformly accessible to individual cells within a biofilm. We have used cytochemistry and fluorescent microscopy to observe and characterize the biofilm matrix material of in vitro and in vivo biofilms [9]. Our studies revealed the presence of occasional areas of lipid-containing matrix encasing some bacteria within the biofilm. This lipid matrix prevented comparatively small, ordinarily diffusible molecules from coming into contact with the encased bacterial cells. In light of these findings, it is conceivable that antibiotics may be able to diffuse through the biofilm but not come in contact with all bacterial cells throughout the biofilm. There may be areas in the biofilm that are shielded by a lipid hydrophobic barrier that prevents diffusion of antibiotics into these areas. Because we observed that lipid material may be identified early in biofilm development and appears to prevent penetration of small molecules into a portion of the bacterial cells [9], we now hypothesize that surfactants (surface-acting agents capable of disrupting lipid-containing structures) may interfere with biofilm development.
As an initial challenge to this hypothesis, the present study was designed to determine whether a natural surfactant, namely glycerol monolaurate (GML), could prevent development of experimental Staphylococcus aureus or Enterococcus faecalis biofilms. GML is a monoester composed of glycerol and lauric acid and is used as a surfactant in cosmetics and as an emulsifier in foods. In human beings, lauric acid is converted into GML and can be found in human breast milk. Although the clinical usefulness of GML has not been established firmly, GML has potent antimicrobial activity against enveloped viruses [10] as well as a variety of planktonic (free-living) bacteria including some gram-negative bacteria and some gram-positive bacteria such as S. aureus and Streptococcus species [11]. Resulting data from our study indicated that both GML and lauric acid interfered with development of experimental S. aureus or E. faecalis biofilms cultivated on surgical suture.
Materials and Methods
Bacterial strains and surfactants
Staphylococcus aureus RN6390 and ATCC 25923 are wild-type strains known to produce biofilms [12–15]. Enterococcus faecalis OG1RF is a plasmid-free strain, often used as a parent strain for genetic manipulations of this species [16] and E. faecalis VA1128 is a clinical isolate; both E. faecalis strains are also known to produce biofilms [15]. Bacterial inocula were washed cells from overnight cultures incubated at 35°C in tryptic soy broth, with bacterial concentrations confirmed by standard microbiologic methods. Surfactants (Sigma-Aldrich, St. Louis, MO) included GML (also known as glyceryl laurate or 1-Lauroyl-glycerol) and lauric acid, and the original powders were stored at −20°C. A 182 mM stock solution of GML was diluted in chloroform and stored at room temperature in the dark, and two stock solutions of 500 and 50 mM lauric acid were diluted in 100% ethanol and stored at −20°C. For experiments, working dilutions of GML and lauric acid were further diluted in biofilm growth medium (described below). Preliminary experiments showed that the residual chloroform in working dilutions of GML (<2.5 mcL/mL) and the residual ethanol in working dilutions of lauric acid (<25 mcL/mL) did not affect bacterial viability.
Development and analysis of suture-associated biofilms
Suture-associated biofilms were cultivated as described [12,13,15] with minor modifications. Briefly, each well of a 24-well microtiter plate contained a 1-cm segment of black braided 3-0 silk suture (Ethicon, Inc., Somerville, NJ) suspended in 1 mL of biofilm growth medium, namely 66% tryptic soy broth supplemented with 0.2% glucose [14], and the medium was supplemented additionally with varying concentrations of GML or lauric acid. Control wells contained no surfactant. Each well was inoculated with 107 S. aureus or E. faecalis and incubated 16 h at 37°C with gentle rotation (50 rpm). Suture-associated biofilms were photographed under phase contrast microscopy with an Olympus IMT-2 inverted microscope (Lake Success, NY). Suture-associated biofilms were analyzed for numbers of viable bacteria and for biofilm biomass as described below.
To assess the numbers of viable suture-associated biofilm bacteria, each suture was gently rinsed, transferred to 3 mL of sterile Hank's balanced salt solution, sonicated at approximately 50 joules at 100% amplitude for 5 sec using a sonicator at 20 kHz (Sonics and Materials, Newtown, CT). Sonication had no noticeable effect on bacterial viability [17], and microscopy confirmed that sonicated bacteria were single-cell suspensions. Bacterial concentrations in sonicates were determined by standard microbiologic methods, and the lower detection limit was 1.7 log10 colony forming units per suture. Biofilm biomass was measured with the basic dye crystal violet as described [18] with minor modifications. Crystal violet binds negatively charged surface molecules, including those on live and dead bacteria, as well as on polysaccharides in the biofilm extracellular matrix. Biofilm-laden sutures were gently rinsed with Hank's balanced salt solution, fixed in 99% methanol for 15 min, air-dried, incubated for 20 min with 0.5% crystal violet (Fisher Chemical, Pittsburgh, PA), washed, then incubated 20 to 30 min in 33% acetic acid to release the crystal violet, with absorbance read at 590 nm.
Statistical analysis
Comparisons of two treatment groups were analyzed by unpaired Student t-test and more than two groups were analyzed by one-way analysis of variance with Fisher post hoc. Significance was set at p<0.05.
Results
Effect of GML and lauric acid on S. aureus biofilm development
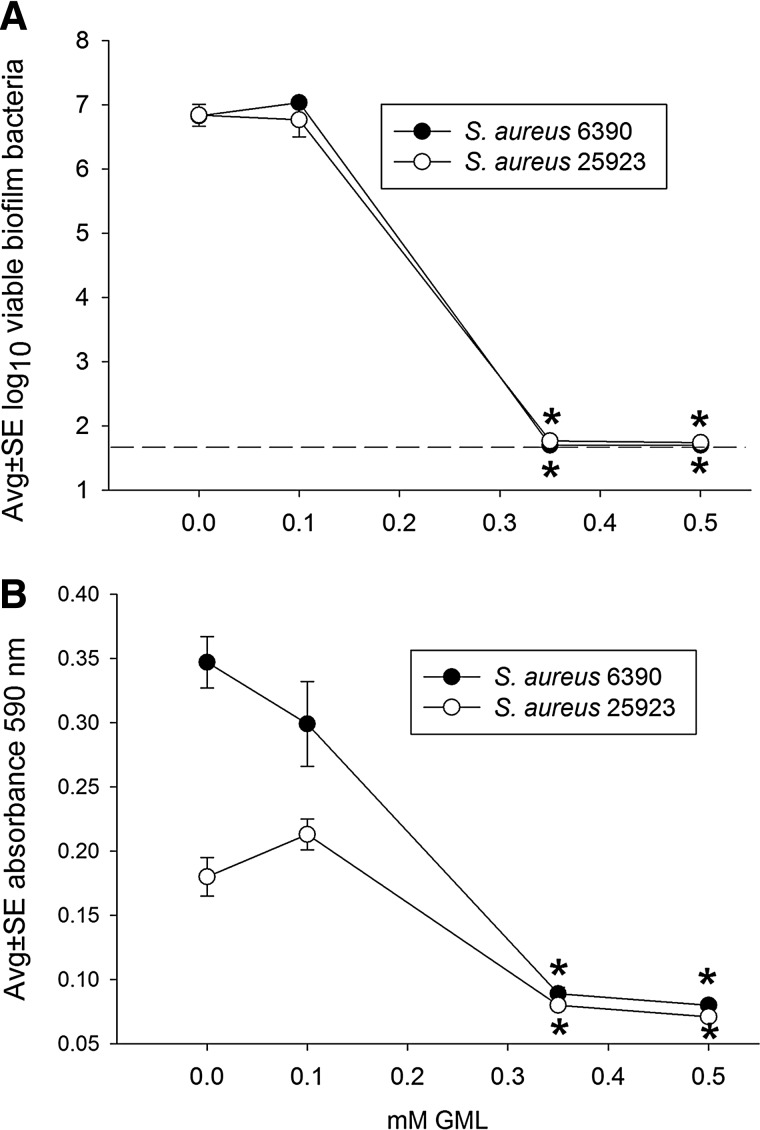
Figure 1 shows that GML inhibited biofilm development with both strains of S. aureus used in this study. In the absence of GML, each suture held a biofilm containing approximately 107 S. aureus. As little as 0.35 mM GML inhibited biofilm development by at least 100,000-fold, reflected in the decreased numbers of viable bacteria from 107 bacteria per control suture-associated biofilm to <101.7 bacteria per suture, the lower limit of assay detection (Fig. 1A). Similarly, the biomass associated with both S. aureus strains was effectively inhibited at a concentration of 0.35 mM GML (Fig. 1B), and this is presented in Figure 2. This inhibition of biomass was noted with both S. aureus strains, although the biomass of samples treated at the lower concentrations of 0 and 0.1 mM GML were greater with the 6390 strain compared with the 25923 strain.
FIG. 1.
Effect of glycerol monolaurate (GML) on development of Staphylococcus aureus RN 6390 and ATCC 25923 biofilms incubated 16 h on silk suture, as measured by the numbers of viable biofilm bacteria (A) and biofilm biomass (B). Each data point represents 12 biofilms. Dashed line represents the lower limit of assay detection. *, decreased at p<0.01 compared with corresponding 0 mM GML.
FIG. 2.
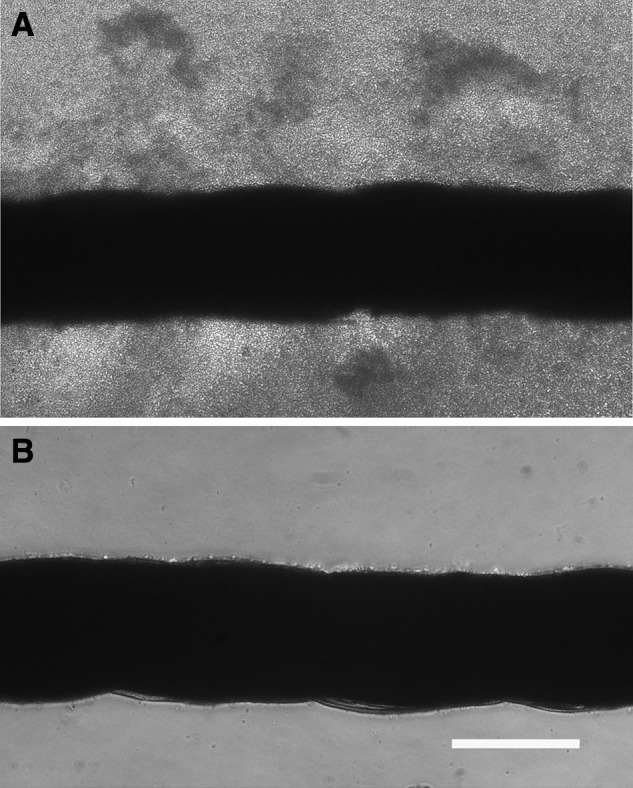
Phase contrast micrographs of silk suture incubated 16 h with Staphlococcus aureus RN 6390 cultivated in growth medium alone (A) or in growth medium supplemented with 0.35 mM GML (B). Scale bar is 200 μm.
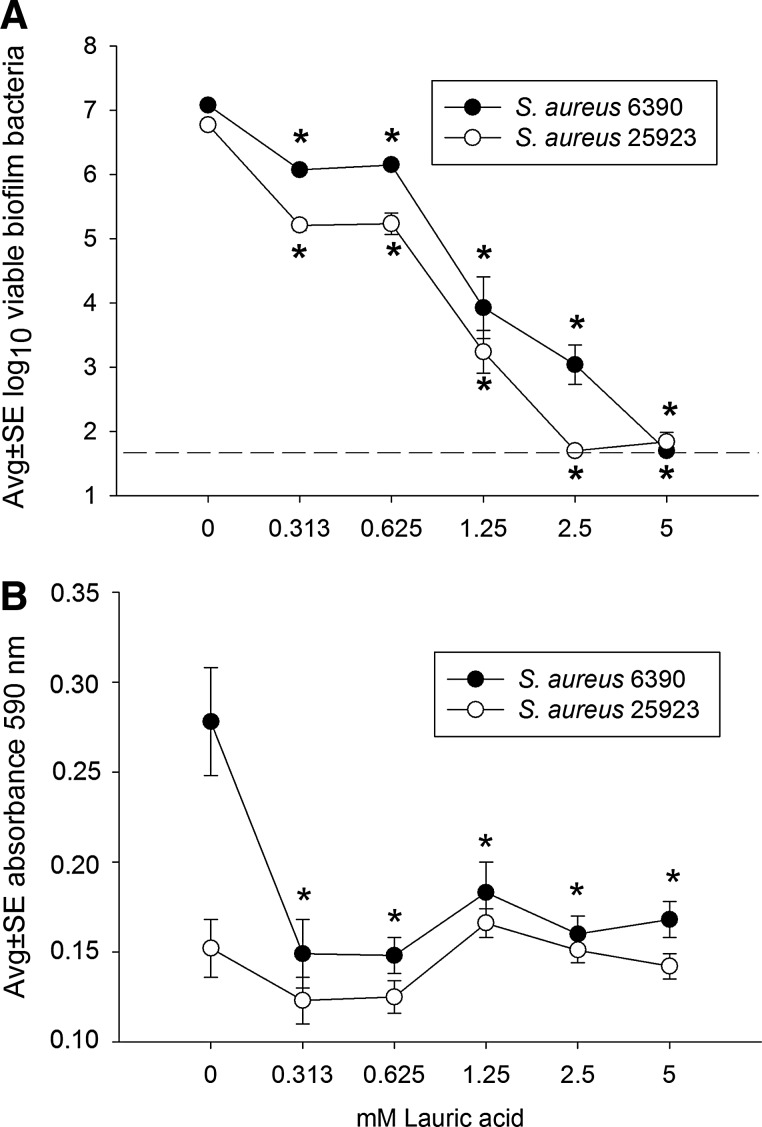
Figure 3 presents evidence that lauric acid alone also inhibited biofilm development of both strains of S. aureus used in this study. As expected, in the absence of lauric acid, each suture again held a biofilm containing approximately 107 S. aureus (Fig. 3A). In contrast to the results with GML in which only 0.35 mM of GML (Fig. 1A) was needed to inhibit bacterial viability down to the lower detection limit (<101.7 bacteria per suture-associated biofilm), 2.5 to 5 mM lauric acid was needed to achieve a similar result (Fig. 3A). However, similar to the results with GML (Fig. 1B), only 0.35 mM lauric acid was required to decrease the biomass of S. aureus RN6390 biofilms (Fig. 3B). In contrast to GML, lauric acid was not associated with a decrease in the biomass of the 25923 strain at all concentrations of lauric acid tested, a result likely because of the comparatively low biomass in control samples treated with 0 mM lauric acid (Fig. 3B).
FIG. 3.
Effect of lauric acid on development of Staphylococcus aureus RN 6390 and ATCC 25923 biofilms incubated 16 h on silk suture, as measured by the numbers of viable biofilm bacteria (A) and biofilm biomass (B). Each data point represents 12 biofilms. Dashed line represents the lower limit of assay detection. *, decreased at p<0.01 compared with corresponding 0 mM lauric acid.
Effect of GML and lauric acid on E. faecalis biofilm development
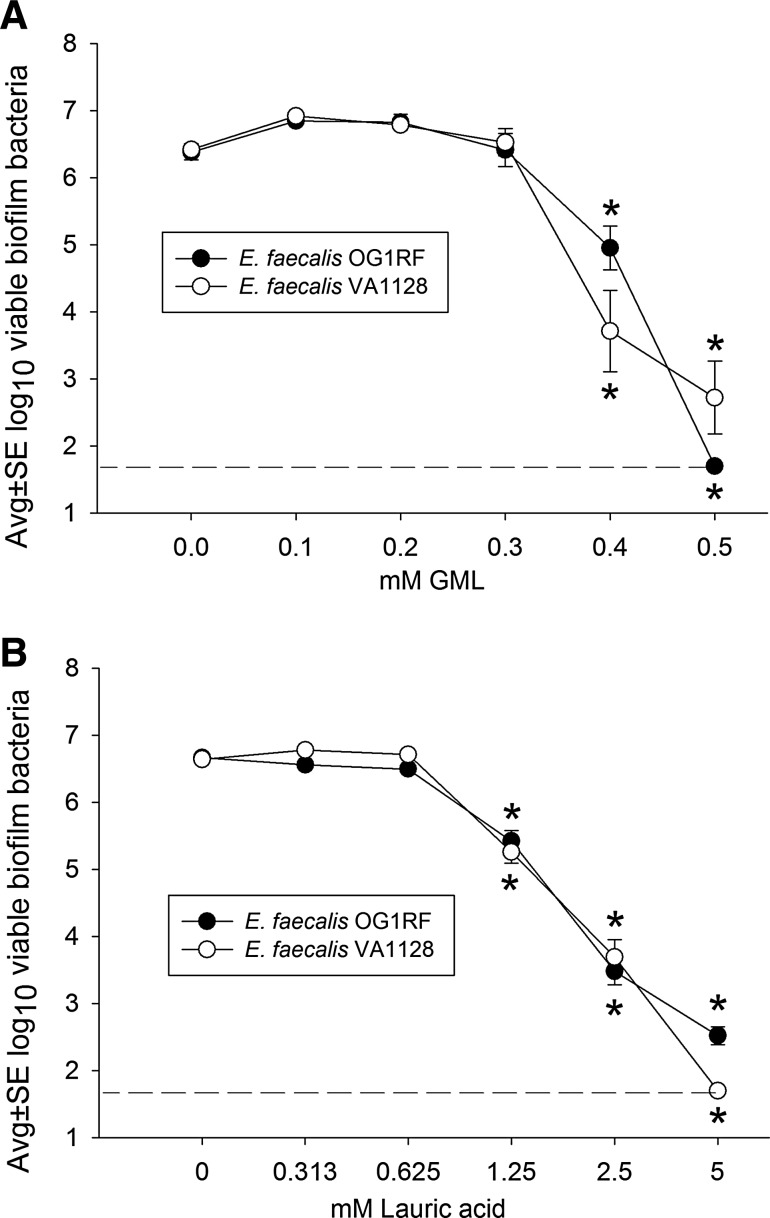
Figure 4A shows that GML also inhibited biofilm development with both strains of E. faecalis used in this study. In the absence of GML, each suture held a biofilm containing approximately 106.4 E. faecalis. At a concentration of 0.4 mM, GML was bactericidal for E. faecalis biofilms, resulting in a reduction in the numbers of viable enterococci, i.e., approximately a 100- to 1,000-fold decrease from control values. Thus, similar concentrations of GML (0.3 to 0.4 mM) were bactericidal for both S. aureus and E. faecalis, and approximately 0.5 mM inhibited enterococcal viability to values near the lower limit of assay detection (1.7 log10 or 50 bacteria). Interestingly, similar to experiments with S. aureus, lauric acid alone effectively limited development of E. faecalis biofilms (Fig. 4B), where 1.25 mM lauric acid resulted in a decrease in the numbers of viable bacteria and 5 mM lauric acid decreased these numbers down to values near the lower limit of assay detection. In addition, similar to the results with S. aureus, developing E. faecalis biofilms were approximately 10-fold more susceptible to GML compared with lauric acid, where 0.5 mM GML and 5 mM lauric acid were associated with bacterial numbers that were at the lower limit of assay detection. (The effects of GML and lauric acid on E. faecalis biomass were not determined because preliminary experiments revealed a comparatively low biomass associated with enterococcal suture biofilms, making it difficult to observe a biomass decrease with this assay [data not shown]).
FIG. 4.
Effect of glycerol monolaurate (GML) (A) and lauric acid (B) on development of Enterococcus faecalis OG1RF and VA1128 biofilms incubated 16 h on silk suture, as measured by the numbers of viable biofilm bacteria. Each data point represents 8 biofilms. Dashed line represents the lower limit of assay detection. *, decreased at p<0.01 compared with corresponding 0 mM GML or lauric acid.
Discussion
The goal of this study was to assess the effectiveness of GML in preventing development of S. aureus and E. faecalis biofilms cultivated on surgical suture. This investigation was based on substantial evidence that GML has an antibacterial effect on a wide variety of clinically relevant microbes cultivated as planktonic cultures. For example, Schlievert et al. [11] reported that GML was bactericidal for a wide variety of aerobic and anaerobic gram-positive bacteria, including S. aureus and Streptococcus species, but gram-negative bacteria in the family Enterobacteriaceae (such as Escherichia coli) and Pseudomonas aeruginosa were resistant. Preuss et al. [19] also noted that GML was bactericidal for S. aureus, but not E. coli or Klebsiella pneumoniae, another member of Enterobacteriaceae. There is evidence that GML inhibited production of S. aureus virulence factors, such as β-lactamase, α-hemolysin, and toxic shock syndrome toxin-1, presumably by inhibiting signal transduction [20,21]. Glycerol monolaurate also inhibited induction of vancomycin resistance in E. faecalis, and this mechanism also appeared to involve signal transduction [22]. Strandberg et al. [23] reported that GML inhibited effectively Candida species and Gardnerella vaginalis, two potential vaginal pathogens. In addition, GML inhibited biomass formation in S. aureus biofilms cultivated in plastic dishes, and GML also inhibited production of toxic shock syndrome toxin-1 in a model of biofilms cultivated on tampon fibers [11]. Thus, there is substantial evidence that GML is bactericidal for a wide variety of microbes but the effect of GML on biofilm development has received relatively little attention.
Data from the present study indicated that GML inhibited the development of detectable viable S. aureus and E. faecalis biofilms, two gram-positive bacteria. Because others have shown that many gram-negative bacteria may not be susceptible to GML, it will be interesting to test the effect of GML on development of biofilms initiated with gram-negative bacteria such as P. aeruginosa or E. coli. With the gram-positive species in this study, GML-associated inhibition of biofilm development was noted with 0.35 mM GML (Figs. 1 and 4A), which corresponds to approximately 100 mcg/mL GML. On a molar basis, compared with lauric acid, GML was approximately 10-fold more effective in inhibiting development of viable S. aureus or E. faecalis biofilms because 2.5 to 5.0 mM lauric acid was needed to prevent recovery of viable bacteria in this model (Figs. 3 and 4B). Thus, although lauric acid was antibacterial on its own, its ability to inhibit biofilm development appeared to be enhanced by the presence of the glycerol molecule in GML. This may be important clinically because, in liquid culture, S. aureus can rapidly hydrolyze GML to glycerol and lauric acid with a half-life of approximately 5 min, but lauric acid persists for at least 2 h [20].
Using S. aureus planktonic cells, others have similarly noted that GML has greater bactericidal activity than lauric acid, and that GML is more effective in inhibiting production of toxic shock syndrome toxin 1 [11]. However, Ruzin and Novick [20] reported that both lauric acid and GML have similar effects on production of staphylococcal exoproteins, including beta-lactamase and toxic shock syndrome toxin 1. Because GML inhibited suture biofilm formation more effectively than lauric acid and because the antimicrobial effects of GML and lauric acid on planktonic cultures vary between studies, our data indicated that the mechanisms responsible for the antimicrobial action of GML and lauric acid may be different in the case of biofilm-associated versus planktonic bacteria.
Our original hypothesis was that if lipids are important in development of S. aureus biofilms, then the addition of a surfactant should interfere with biofilm formation. Because the surfactant GML inhibited biofilm development, the resulting data were consistent with that hypothesis, and data from the present study provided additional evidence that the natural surfactant GML might be a viable candidate for controlling clinically relevant biofilms.
Acknowledgments
This work was supported in part by U.S. National Institutes of Health Grant R01 GM095553 and in part by funds from the Department of Surgery, University of Minnesota, Minneapolis, Minnesota.
Author Disclosure Statement
None of the authors has any commercial associations that might create a conflict of interest in connection with this article.
References
- 1.Fux CA, Costerton JW, Stewart PS, et al. Survival strategies of infectious biofilms. Trends Microbiol 2005;13:34–40 [DOI] [PubMed] [Google Scholar]
- 2.Cos P, Tote K, Horemans T, Maes L. Biofilms: An extra hurdle for effective antimicrobial therapy. Curr Pharm Des 2010;16:2279–2295 [DOI] [PubMed] [Google Scholar]
- 3.Flemming H-C, Wingender J. The biofilm matrix. Nat Rev 2010;8:623–633 [DOI] [PubMed] [Google Scholar]
- 4.Flemming H-C, Neu TR, Wozniak DJ. The EPS matrix: The house of biofilm cells. J Bacteriol 2007;198:7945–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol 2008;6:199–210 [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol 2009;11:1034–1043 [DOI] [PubMed] [Google Scholar]
- 7.Anderl JN, Zahller J, Roe F, et al. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother 2003;47:1251–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daddi Oubekka S, Briandet R, Fontaine-Aupart MP, et al. Correlative time-resolved fluorescence microscopy to assess antibiotic diffusion-reaction in biofilms. Antimicrob Agents Chemother 2012;56:3349–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess DJ, Henry-Stanley MJ, Barnes MT, et al. Ultrastructure of a novel bacterial form located in Staphylococcus aureus in vitro and in vivo catheter-associated biofilms. J Histochem Cytochem 2012;60:770–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thormar H, Isaacs CE, Brown HR, et al. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother 1987;31:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlievert PM, Peterson ML. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 2012;7:e40350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells CL, Henry-Stanley M, Barnes AMT, et al. Relationship between antibiotic susceptibility and ultrastructure of Staphylococcus aureus biofilms on surgical suture. Surg Infect 2011;12:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hess DJ, Henry-Stanley MJ, Wells CL. Interplay of antibiotics and bacterial inoculum on suture-associated biofilms. J Surg Res 2012;177:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanks RM, Donegan NP, Graber ML, et al. Heparin stimulates Staphylococcus aureus biofilm formation. Infect Immun 2005;73:4596–4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry-Stanley MJ, Hess DJ, Barnes AMT, et al. Bacterial contamination of surgical suture resembles a biofilm. Surg Infect 2010;11:433–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunny GM, Craig RA, Carron RL, et al. Plasmid transfer in Streptococcus faecalis: Production of multiple pheromones by recipients. Plasmid 1979;2:454–4651 [DOI] [PubMed] [Google Scholar]
- 17.Henry-Stanley MJ, Hess DJ, Wells CL. Aminoglycoside inhibition of Staphylococcus aureus biofilm formation is nutrient dependent. J Med Microbiol 2014;63:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Meth 2008;72:157–165 [DOI] [PubMed] [Google Scholar]
- 19.Preuss HG, Echard B, Enig M, et al. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol Cell Biochem 2005. 272:29–34 [DOI] [PubMed] [Google Scholar]
- 20.Ruzin A, Novick RP. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J Bacteriol 2000;82:2668–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Projan SJ, Brown-Skrobot S, Schlievert PM, et al. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J Bacteriol 1994;176:4204–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruzin A, Novick RP. Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis. J Bacteriol 1998;180:182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strandberg KL, Peterson ML, Lin YC, et al. Glycerol monolaurate inhibits Candida and Gardnerella vaginalis in vitro and in vivo but not Lactobacillus. Antimicrob Agents Chemother 2010;54:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]