Abstract
In contrast to traditional pharmacodynamic approaches to treat substance use disorders, the use of biologics (vaccines, monoclonal antibodies, genetically modified enzymes) is based on a pharmacokinetic principle: reduce the amount of (and in the ideal, eliminate) abused drug entering the central nervous system. Preclinical studies indicate biologics are effective in both facilitating abstinence and preventing relapse to abused substances ranging from nicotine to heroin. While data are still emerging, the results from multiple clinical trials can best be described as mixed. Nonetheless, these clinical studies have already provided important insights using “first generation” tools that may inform the development of effective and commercially viable biologics to treat tobacco, cocaine, and methamphetamine use disorders.
Keywords: substance use disorders, cocaine, methamphetamine, nicotine, vaccines, monoclonal antibodies
Pharmacological treatment of substance use disorders
Target based, small molecule approaches have not yielded highly effective medications to treat substance use disorders (SUDs). Thus, there are no pharmacotherapies approved by the USA FDA that address cocaine, methamphetamine, or cannabis use disorders. Moreover, based on the current listings trials in www.clinicaltrials.gov, it is unlikely that any medications to treat these SUDs will be approved in the next five to seven years. Although there are medications approved to treat other SUDs, these are far from ideal. For example, the long term (52 week) abstinence rates of approved smoking cessation medications (nicotine replacement therapies, bupropion, varenicline) are generally <20% [1], and substitution therapies (methadone and buprenorphine) remain the most widely used medications to treat opiate use disorders.
While multiple factors contribute to the dearth of pharmacotherapies to treat SUDs [2], both the inability to develop highly effective, innovative therapies using target based approaches and advances in immunology and molecular biology has rekindled [3,4] interest in treating SUDs with biologics: vaccines, monoclonal antibodies, and enzymes.
Biologic approaches are grounded on a common pharmacokinetic principle: reducing the amount of (and in the ideal, eliminating) abused substance from reaching its target organ, the central nervous system (CNS). Three different approaches have now been explored in clinical trials: i) vaccines that stimulate the production of antibodies directed against a drug of abuse; ii) monoclonal antibodies that bind a drug of abuse; iii) genetically modified enzymes that accelerate the metabolism of abused drugs (e.g. the hydrolysis of cocaine).
Mixed Signals Emerge From “First Generation” Vaccine Trials
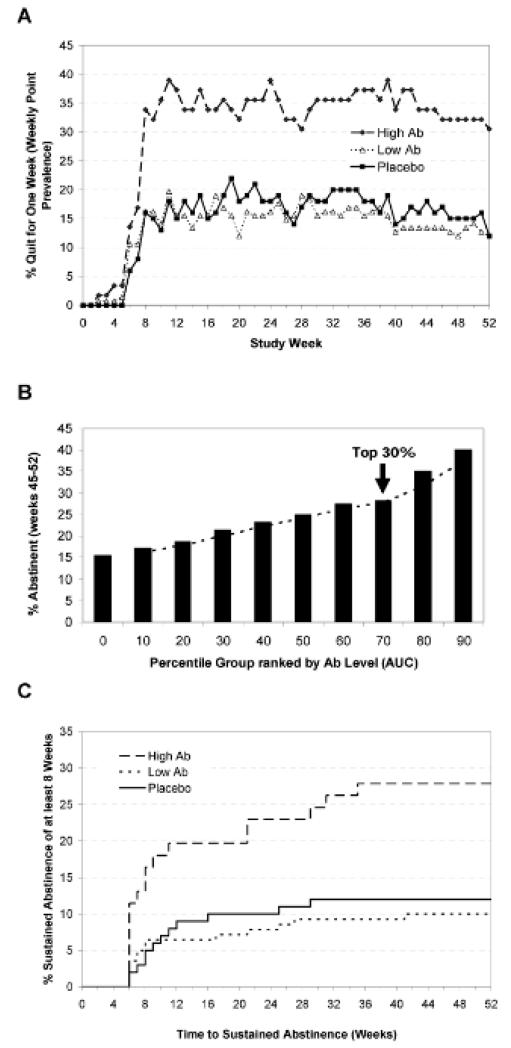
Positive signals have emerged from proof-of-principle trials with two different nicotine vaccines [5,6] and a cocaine vaccine [7,8]. However, these signals were manifested only in subpopulations of patients who developed relatively high titers of anti-drug antibodies following multiple vaccinations. For example, in a double-blind, placebo controlled trial of CYT002-NicQb® vaccine [5], subjects received five monthly injections of either active vaccine or placebo. Those subjects with the highest tertile of antibody levels exhibited significantly higher rates of abstinence (this is currently the only FDA acceptable endpoint for smoking cessation and illicit drugs [1,9]) between months 2-6 compared to placebo (56.6% versus 31.3%). However, the abstinence rate of subjects in the ‘low to mid’ antibody range did not separate from placebo. At 52 weeks, the difference in abstinence rates between subjects receiving placebo and those individuals in the highest tertile of antibody responses remained statistically significant (20.2%, p=0.012). Similar outcomes were observed in a double blind, placebo controlled Phase II trial with a second nicotine vaccine, NicVAX® [6]. In this study, subjects were injected either four or five times over the first six months of the trial with two different doses of vaccine. Subjects with the highest tertile of antibodies had a significantly higher level of sustained (8 weeks) abstinence at 6 months compared to placebo (24.6% versus 12%, p=0.024). A higher abstinence rate was maintained in this group compared to placebo at 12 months (Figure 1). Moreover, in subjects who received five injections of the high dose of vaccine, significantly higher rates of smoking cessation and long-term abstinence were observed compared with the low antibody and placebo arms. Thus, an intent-to-treat analysis (not stratified by antibody levels) of this high dose (400 μg) arm had a significantly higher abstinence rate at 6 and 12 months versus placebo (p=0.025 and p=0.038, respectively), while the abstinence rate of the group receiving the lower dose (200 μg) approached statistical significance at both 6 and 12 months compared to placebo (p=0.054 and p=0.056, respectively) [6]. Based on an apparent dose related signal, two Phase III trials used a modified vaccination regimen (six injections spaced over 26 weeks) with the high dose of NicVAX® [10]; both trials failed to separate from placebo, with identical end-of-study abstinence rates of ~11%.
Figure 1.
Effects of NicVAX® on smoking behavior: Panel A: Seven day point prevalence abstinence rates in smokers with high (top 30% based on AUC) and low (bottom 70%) antibody titers compared with placebo injections over 52 weeks. Panel B: Percentage of patients exhibiting end of study abstinence (8 weeks of continuous abstinence, weeks 45-52), grouped by antibody levels. Panel C: Time required to achieve 8 weeks of sustained abstinence before week 46 and continuous abstinence through to week 52 stratified by antibody titer (see Panel A). Ab, antibody, AUC, area under the curve. From reference [6], with permission.
Small molecules such as nicotine, cocaine, and heroin are not inherently antigenic, and must be chemically modified to enable covalent linkage to a protein. Such structural modification may interfere with the ability of the immune system to recognize (and mount an effective immune response to) the abused drug. This issue emerges in the study of Martell et al., [7], evaluating a cocaine vaccine (succinylnorcocaine linked to a recombinant cholera toxin B-subunit protein) in cocaine dependent, methadone-maintained patients. Five vaccinations were administered over a 12 week period (total trial length, 20 weeks). While robust antibody titers to cholera toxin B were detected in every patient, serum antibody levels (defined by the authors as serum IgG anticocaine antibody levels of ≥43 μg/ml) sufficient to marginally affect cocaine use (an increase in cocaine-free urine samples (45%) compared to vehicle (35%) during trial weeks 9-16) were present in far fewer than half of the patients [7,8]. A second, larger trial in cocaine dependent patients resulted in modest, albeit non-significant signals of reduced cocaine use in patients with high serum antibody levels [11].
Perhaps more problematic to the development of highly effective vaccines is the challenge created by presenting the immune system with a large quantity (generally in the range of 1-100 mg) of abused drug that must be neutralized in a timeframe of seconds to minutes in order to effectively reduce entry to the central nervous system. From both a quantitative and temporal perspective, these demands are far greater than those placed on vaccines developed against infectious agents (e.g., a measles vaccine). This is illustrated in a small study of smokers who received four monthly injections of high dose NicVAX® (400 μg). In these individuals, an intravenous bolus of nicotine (1.5 mg, ~1.5 cigarettes smoked) resulted in a modest (12.5%) reduction in SPECT ligand binding to β2*-nAChRs, corresponding to approximately a 25% reduction in brain nicotine levels [12]. These proof-of-principle trials illustrate the challenges of “first generation” vaccines that must be resolved to demonstrate the clinical efficacy necessary for both regulatory approval and successful commercialization.
Improving “First Generation” Vaccines
Clearly, it would not be feasible to develop commercially viable “anti-addiction” vaccines that require frequent, multiple boosts and raise effective antibody levels in only a subpopulation of patients. The data emerging from published clinical studies indicate that higher antibody affinity and less inter-subject variability in antibody titers will be necessary for effective vaccines. One seemingly straightforward strategy to improve antibody affinity is hapten redesign. For example, Pryde, et al., [13] recently reported significant differences in antibody affinity among nicotine vaccines using haptens derived by systematic modification of the pyridine moiety of nicotine. In this study, the size and lipophilicity of the molecules used to link a hapten to diphtheria toxoid (the carrier protein) and the ratio of hapten to carrier had profound effects on vaccine performance in vivo [13]. The haptens in both the CYT002-NicQb® and NicVAX® vaccines contain the nicotine molecule modified at the 3′ position on the pyrrolidine ring, and have similarly sized linkers, although the carrier proteins are different. The characteristics of first generation vaccines (e.g.., antibody titers and the affinity of these antibodies) may also be improved by the use of novel adjuvants such as CpG (a TLR9 agonist) and liposomes containing monophosphoryl lipid A [13,14,15]. These newer adjuvants could potentially be combined with the traditional adjuvant, alum, used in the vaccine trials described here. Thus, McCluskie, et al. [16] reported that levels of anti-nicotine antibodies were increased by >1 order of magnitude in non-human primates receiving 3′-aminomethylnicotine conjugated to diphtheria toxoid (this is the same nicotine-like hapten on NicVAX® linked to a different carrier protein) with a combination of CpG and alum compared to alum alone. These increased levels of anti-nicotine antibodies reduced the amount of free nicotine in non-human primate blood spiked with 100 ng/ml of nicotine by about 30% compared to 3% in blood from immunized animals who received alum alone as adjuvant. Moreover, a further modification of the nicotine conjugate (together with the same adjuvant combination) resulted in 100% of nicotine bound using a 10-fold higher concentration (1000 ng/ml) of nicotine [16]! Pfizer has already initiated the translation of some of these concepts. Phase I studies with two nicotine vaccines, Nic7-001 and Nic7-003 in healthy adult smokers are currently listed as active on ClinicalTrials.gov (Identifier: NCT01672645). These vaccines may be based on “hapten 7” described by Pryde et al. [13] and the protocol likely incorporates the use of multiple adjuvants.
Beyond traditional protein-carrier conjugate approaches, platforms such as nanoparticle based vaccines (incorporating immune targeting and adjuvating agents within the nanoparticle) [10; http://www.selectabio.com/company/index.cfm] and adenovirus based vaccines, produced by linking a hapten to the capsid proteins of an adenovirus [17] have been reported to produce high titers of high affinity antibodies to nicotine and cocaine, respectively, in non-human primates. A nanoparticle based nicotine vaccine (SEL-068) is in early stage clinical development, but the results have not been disclosed [10].
Using Monoclonal Antibodies to Treat Substance Use Disorders
Monoclonal antibodies (mAbs) do not require the patient to mount an immune response, which could be especially important in a population with a high proportion of injection drug users who may already be immunosuppressed. In addition, mAbs act immediately, a significant advantage over “first generation” nicotine and cocaine vaccines that require multiple immunizations and weeks to months before effective antibody titers are generated [6,7,8]. Preclinical studies have demonstrated that mAbs can modify drug seeking and intake of abused substances including nicotine, cocaine, and methamphetamine [18], but translation of these findings has been slow. Clinical studies have been initiated with a chimeric monoclonal antibody directed against methamphetamine (ch-mAb7F9), with first-in-human studies completed in 2013 [19]. This Phase I study examined both the safety and pharmacokinetics of ch-mAb7F9 in healthy volunteers. Single doses of ch-mAb7F9 (0.2 to 20 mg/kg) were administered to 32 subjects who were followed for approximately five months. Ch-mAb7F9 had a half-life of 17-19d, which is consistent with several commercially available mAbs [19]. Moreover, the volume of distribution (5-6 l) indicates it is primarily confined to the vascular compartment. Based on the high affinity (KD=7 nM) and the predicted effective plasma concentrations [19], the 20 mg dose of ch-mAb7F9 could be “protective” against methamphetamine challenge for more than a month. Of the 32 subjects receiving ch-mAb7F9, 4 (12.5%) were found to have developed a human anti-chimeric antibody by the study end; the appearance of these anti-chimeric antibodies did not appear to be dose-related. The authors indicate the FDA requested additional pre-clinical safety studies prior to initiating Phase 1b studies in non-treatment seeking methamphetamine users [19]. Despite the potential advantages of such a mAb, there is a concern that even if effective in modifying or eliminating drug use, the high cost of production would deter commercialization. However, novel technologies including mAb expression in plant systems [20] and the potential to genetically engineer mAbs with significantly longer biological half-lives [21,22] may mitigate concerns about the feasibility of developing a commercially viable mAb for SUDs.
Accelerating Cocaine Hydrolysis as a Therapeutic Strategy
Esterases, including butyrylcholinesterase, catalyze the hydrolysis of cocaine [23]. The biochemical and biophysical properties of wild-type esterases derived from bacteria and humans can be dramatically altered by genetic modification. For example, point mutations of human butyrylcholinesterase can increase the catalytic rate by more than 1000-fold compared to the wild-type enzyme; modification of bacterial cocaine esterase increases its stability in plasma [24,25,26]. Parenteral administration of a mutated butyrylcholinesterase linked to serum albumin dramatically reduces both the toxicological and behavioral effects of cocaine in rodents [23,26,27] and nonhuman primates [28], effects that are attributable to a rapid degradation of cocaine in plasma and a corresponding rise in plasma concentrations of ecgonine methyl ester, the BChE catalyzed hydrolysis product of cocaine [23,28].
Clinical studies have now established the feasibility of using mutated forms of a bacterial cocaine esterase [29] and a human butyrylcholinesterase fused to serum albumin [30,31] to treat cocaine intoxication [26] and facilitate abstinence in cocaine dependent subjects [31]; https://clinicaltrials.gov/ct2/show/NCT01887366)], respectively.
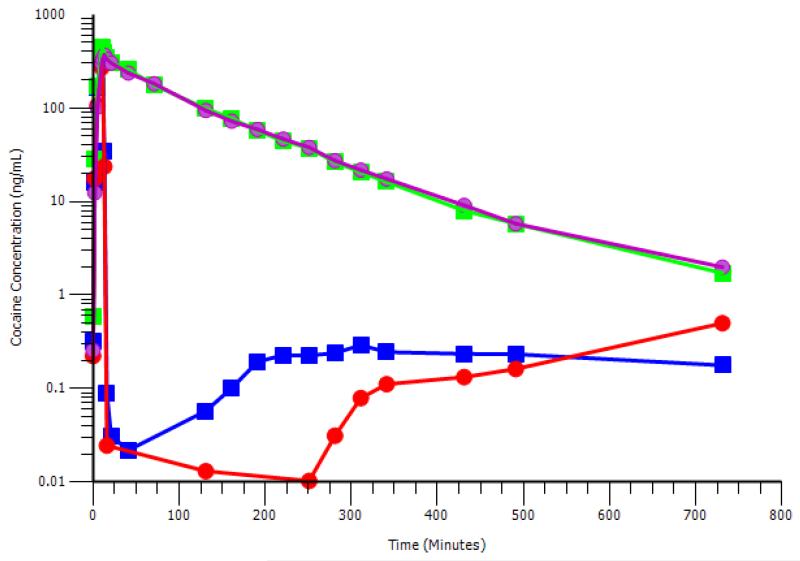
Bacterial cocaine esterase is highly efficient in hydrolyzing cocaine, but has a half-life of <2 minutes in plasma; a doubly mutated form of this enzyme (RBP-8000) significantly increases plasma half-life [25, 29] making it suitable as a potential treatment for cocaine intoxication. In studies of non-treatment seeking individuals with a diagnosis of cocaine abuse as defined by the Diagnostic and Statistical Manual of Mental Disorders, volume IV (DSM-IV), an intravenous infusion of RBP-8000 (100 mg and 200 mg) one minute after a cocaine infusion (50 mg over 10 minutes) reduced plasma cocaine levels by 90% within 2 minutes (Figure 2); corresponding dramatic rises were observed in plasma levels of ecgonine methyl ester, a pharmacologically inert hydrolysis product of cocaine. Moreover, while the data were highly variable, RBP-8000 caused a more rapid blunting of both the chronotropic and inotropic effects of cocaine compared to placebo. These doses of RBP 8000 were safe and well-tolerated, and the sponsor intends to develop this mutated enzyme as a pharmacotherapy for cocaine intoxication.
Figure 2.
RBP-8000 accelerates the hydrolysis of cocaine: Non –treatment seeking subjects with a DSM-IV diagnosis of cocaine abuse were administered an IV infusion of cocaine (50 mg) over 10 minutes. RBP-8000 (100 or 200 mg) or matched placebo was infused 1 minute after completion of cocaine infusion. Blood samples were collected between 2-720 minutes post RBP-8000 (or placebo) infusion. Symbols: Green squares, cocaine + matched placebo; magenta circles, cocaine + matched placebo; blue squares: cocaine + RBP-8000 (100 mg); red circles: cocaine + RBP (200 mg). The data used to generate this figure is taken from [26].
A mutated form of human butyrylcholinesterase, less likely to provoke an immune response than a bacterial enzyme, could be used on a chronic basis either to facilitate abstinence or to prevent relapse to cocaine. TV 1380 is a recombinant protein containing human serum albumin fused at its amino terminus to the carboxyl terminus of a human plasma butyrylcholinesterase. This enzyme contains four amino acid mutations that increase its catalytic activity more than three orders of magnitude compared to the wild-type enzyme [24]. Fusion to human serum albumin both increases plasma stability and enables expression of this protein as a single polypeptide [32]. In a Phase I study [30], normal volunteers received up to four weekly intramuscular doses of TV 1380. At doses of up to 300 mg, TV 1380 was safe and well tolerated. The plasma half-life (43-77 h) of TV 1380 is compatible with weekly dosing, and the ex vivo hydrolysis of cocaine was highly correlated with plasma concentrations in subjects receiving a single dose of enzyme. Anti-TV 1380 antibodies were detected in 3/24 subjects receiving multiple doses, but no changes in either acetyl cholinesterase or butyrylcholinesterase activities were reported. In a follow-on study in recreational cocaine users, subjects were administered single doses (50, 100, and 300 mg) of TV 1380 and challenged with an intravenous dose of cocaine (40 mg) 1, 4, and 7 days later [31]. As predicted, TV 1380 dramatically reduced the Cmax and t1/2 of cocaine in a dose dependent fashion. For example, 24 h following the 300 mg dose of TV 1380, the Cmax of a the challenge dose of cocaine was reduced from 260 to 36 ng/ml, and the t1/2 from 1.76 to 0.14 h, respectively. One week later, TV 1380 was still able to significantly reduce both the Cmax and t1/2 of the challenge dose of cocaine compared to vehicle: 257 to 96 ng/ml and 1.71 to 0.5 h, respectively. TV 1380 also produced modest reductions in the subjective ‘high’ and willingness to take cocaine again. On the strength of this effect on the pharmacokinetics of cocaine, the sponsor, in collaboration with the National Institute on Drug Abuse (NIDA), recently completed a multisite, Phase II study on facilitation of abstinence in cocaine dependent patients (https://clinicaltrials.gov/ct2/show/NCT01887366) comparing weekly administration of either 150 mg or 300 mg of TV 1380 to placebo. Top line results should be released later this year.
Concluding remarks
Biologics offer a nontraditional approach to treating SUDs with both advantages and drawbacks compared to conventional pharmacotherapies. Low medication compliance has frustrated attempts to rigorously test hypotheses in SUD trials [33,34,35]. Delivery of a long-lived treatment allows a patient to make fewer good decisions (in the ideal, one good decision to receive a vaccine and booster immunizations) compared to one (or more) good daily decision to remain on a conventional regimen. Thus, a biologic eliminates low compliance as a variable during development and minimizes the issue of patient compliance in real world practice. The specificity of a biologic (e.g., a heroin vaccine) neither precludes the patient from receiving structurally unrelated molecules as therapy (e.g., methadone, buprenorphine) nor does it prevent the abuse of these and other drugs. However, the high incidence of polydrug dependence [36] is also problematic for conventional pharmacotherapies, exemplified by a patient using cocaine while receiving depot naltrexone to prevent relapse to opiates. The “first generation” nicotine and cocaine vaccines described here require months before meaningful antibody titers are detected [6,7,8]. A delay in onset is an inherent property of all current vaccines. However, advances in vaccine technology may overcome the need for frequent, multiple immunizations and the inability to produce high titers of high affinity antibodies in the majority of patients. Resolution of these issues is critical for the development of truly effective, commercially viable vaccines.
There are unique ethical, social, and legal dimensions to treating SUDs with biologics (vaccines and gene therapy approaches, such as delivery of an engineered butyrylcholinesterase in an adenovirus vector [37]) with the potential to confer an enduring “immunity” against an abused molecule (see Outstanding Questions box). At face value, administering a biologic to an adult seeking treatment may not be substantially different from a conventional pharmacotherapy. However, the use of biologics for prevention has raised multiple concerns among bioethicists [38]. For example, given the societal harms associated with smoking, should adolescents receive a nicotine vaccine before they start experimenting with tobacco products? Should vaccination be optional or compulsory? How does prophylactic administration of such a biologic undermine an individual’s future choices? While these represent only a subset of the concerns associated with the use of biologics, to this author, many of these issues ring hollow by comparison to the anguish of a parent whose child is addicted to drugs [39], and whose addiction might be prevented by an effective biologic.
Outstanding Questions (Biologic Approaches To Treat Substance Use Disorders).
-
-
Can the efficacy of “first generation” vaccines be improved by newer adjuvants and hapten modification?
-
-
Is it commercially feasible to produce mAbs and genetically modified enzymes (that may be administered on a weekly to monthly basis) to treat SUDs?
-
-
Will the development of effective biologics create ethical issues that could limit widespread dissemination? For example: Should a nicotine vaccine be mandatory for “at risk” adolescents prior to using nicotine-based products?
-
-
Will biological approaches, used either alone or in combination with medications and/or behavioral therapies, be accepted by the treatment community?
Trends Box.
-
-
Biologics reduce the amount of abused drug entering the central nervous system.
-
-
Data emerging from clinical trials with “first generation” vaccines are “mixed”.
-
-
Novel adjuvants and hapten redesign are strategies that may improve vaccine efficacy.
-
-
A nicotine vaccine incorporating these strategies is currently in clinical trials.
-
-
An anti-methamphetamine mAb and bioengineered esterases are in clinical development.
Acknowledgement
I am grateful to Dr. Matthew Kalnik, who provided many insightful comments and suggestions. My colleagues at Indivior generously provided elements of Figure 2; these data were derived from their publication [26].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jorenby DE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 2.Acri JB, Skolnick P. Pharmacotherapy of substance use disorders. In: Charney D, et al., editors. Neurobiology of Mental Illness. 4 edn Oxford University Press; 2013. pp. 235–245. [Google Scholar]
- 3.Bonese KF, et al. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;252:708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 4.Killian A, et al. Effects of passive immunization against morphine on heroin self-administration. Pharmacol. Biochem. Behav. 1978;9:347–352. doi: 10.1016/0091-3057(78)90295-2. [DOI] [PubMed] [Google Scholar]
- 5.Cornuz J, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS. One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatsukami DK, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 2011;89:392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martell BA, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen. Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen X, Kosten TR. Immunotherapy for drug abuse. CNS. Neurol. Disord. Drug Targets. 2011;10:876–879. doi: 10.2174/187152711799219352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winchell C, et al. Reanalysis of methamphetamine dependence treatment trial. CNS. Neurosci. Ther. 2012;18:367–368. doi: 10.1111/j.1755-5949.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahim RE, et al. Therapeutic vaccines against tobacco addiction. Expert. Rev. Vaccines. 2013;12:333–342. doi: 10.1586/erv.13.13. [DOI] [PubMed] [Google Scholar]
- 11.Kosten TR, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42–47. doi: 10.1016/j.drugalcdep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esterlis I, et al. Effect of a nicotine vaccine on nicotine binding to beta2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. Am. J. Psychiatry. 2013;170:399–407. doi: 10.1176/appi.ajp.2012.12060793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pryde DC, et al. Selection of a novel anti-nicotine vaccine: influence of antigen design on antibody function in mice. PLoS. One. 2013;8:e76557. doi: 10.1371/journal.pone.0076557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matyas GR, et al. Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013;31:2804–2810. doi: 10.1016/j.vaccine.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremer PT, et al. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol. Pharm. 2014;11:1075–1080. doi: 10.1021/mp400631w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCluskie MJ, et al. Enhancing immunogenicity of a 3′aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. Int. Immunopharmacol. 2013;16:50–56. doi: 10.1016/j.intimp.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Maoz A, et al. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology. 2013;38:2170–2178. doi: 10.1038/npp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brimijoin S, et al. Prospects, promise and problems on the road to effective vaccines and related therapies for substance abuse. Expert. Rev. Vaccines. 2013;12:323–332. doi: 10.1586/erv.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens MW, et al. First human study of a chimeric anti-methamphetamine monoclonal antibody in healthy volunteers. MAbs. 2014;6:1649–1656. doi: 10.4161/19420862.2014.976431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twyman RM, et al. Optimizing the yield of recombinant pharmaceutical proteins in plants. Curr. Pharm. Des. 2013;19:5486–5494. doi: 10.2174/1381612811319310004. [DOI] [PubMed] [Google Scholar]
- 21.Hinton PR, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J. Biol. Chem. 2004;279:6213–6216. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- 22.Vaccaro C, et al. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat. Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 23.Brimijoin S. Interception of cocaine by enzyme or antibody delivered with viral gene transfer: a novel strategy for preventing relapse in recovering drug users. CNS. Neurol. Disord. Drug Targets. 2011;10:880–891. doi: 10.2174/187152711799219398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, et al. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16656–16661. doi: 10.1073/pnas.0507332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao D, et al. Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol. Pharmacol. 2009;75:318–323. doi: 10.1124/mol.108.049486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brimijoin S, et al. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology. 2008;33:2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng F, Zhan CG. Are pharmacokinetic approaches feasible for treatment of cocaine addiction and overdose? Future. Med. Chem. 2012;4:125–128. doi: 10.4155/fmc.11.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindler CW, et al. Modification of pharmacokinetic and abuse-related effects of cocaine by human-derived cocaine hydrolase in monkeys. Addict. Biol. 2013;18:30–39. doi: 10.1111/j.1369-1600.2011.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasser AF, et al. A randomized, double-blind, placebo-controlled trial of RBP-8000 in cocaine abusers: pharmacokinetic profile of rbp-8000 and cocaine and effects of RBP-8000 on cocaine-induced physiological effects. J. Addict. Dis. 2014;33:289–302. doi: 10.1080/10550887.2014.969603. [DOI] [PubMed] [Google Scholar]
- 30.Cohen-Barak O, et al. Safety, pharmacokinetics, and pharmacodynamics of TV-1380, a novel mutated butyrylcholinesterase treatment for cocaine addiction, after single and multiple intramuscular injections in healthy subjects. J. Clin. Pharmacol. 2015;55:573–583. doi: 10.1002/jcph.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loupe P, et al. Assessment of pharmacokinetic and pharmacodynamic interactions between albumin-fused mutated butyrylcholinesterase (Albu-BChE) and intravenously administered cocaine in recreational cocaine users. Journal of Clinical Psychopharmacology. 2015 doi: 10.1097/JCP.0000000000000347. in press. [DOI] [PubMed] [Google Scholar]
- 32.Gao Y, et al. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem. Biol. Interact. 2008;175:83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson AL, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120:135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015;150:170–174. doi: 10.1016/j.drugalcdep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somoza EC, et al. A multisite, double-blind, placebo-controlled clinical trial to evaluate the safety and efficacy of vigabatrin for treating cocaine dependence. JAMA Psychiatry. 2013;70:630–637. doi: 10.1001/jamapsychiatry.2013.872. [DOI] [PubMed] [Google Scholar]
- 36.Tiihonen J, et al. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am. J. Psychiatry. 2012;169:531–536. doi: 10.1176/appi.ajp.2011.11071121. [DOI] [PubMed] [Google Scholar]
- 37.Zlebnik NE, et al. Long-term reduction of cocaine self-administration in rats treated with adenoviral vector-delivered cocaine hydrolase: evidence for enzymatic activity. Neuropsychopharmacology. 2014;39:1538–1546. doi: 10.1038/npp.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young MJ, et al. Immune to addiction: the ethical dimensions of vaccines against substance abuse. Nat. Immunol. 2012;13:521–524. doi: 10.1038/ni.2321. [DOI] [PubMed] [Google Scholar]
- 39.Sheff D. Beautiful Boy: a father’s journal through his son’s addiction. Houghton-Mifflin; New York: 2008. [Google Scholar]