Abstract
Mild traumatic brain injury (mTBI) can produce long lasting cognitive dysfunction. There is typically no cell death and only diffuse structural injury after mTBI. Thus, functional changes in intact neurons may contribute to symptoms. We have previously shown altered intrinsic properties of axotomized and intact neurons within 2 d after a central fluid percussion injury in mice expressing yellow fluorescent protein (YFP) that allow identification of axonal state prior to recording. Here, whole–cell patch clamp recordings were used to examine synaptic properties of YFP+ layer V pyramidal neurons. An increased frequency of spontaneous and miniature excitatory postsynaptic currents (EPSCs) was recorded from axotomized neurons at 1 d and intact neurons at 2 d after injury, likely reflecting an increased number of afferents. This also was reflected in the increased amplitude of the EPSC evoked by local extracellular stimulation for all neurons from injured cortex and increased likelihood of producing an action potential for intact cells. Field potentials recorded in superficial layers after online deep layer stimulation contained a single negative peak in controls but multiple negative peaks in injured tissue. The amplitude of this evoked negativity was significantly larger than controls over a series of stimulus intensities at both the 1 d and 2 d survival times. Interictal-like spikes never occurred in the field potential recordings from controls but were observed in 20–80% of stimulus presentations in injured cortex. Together, these results suggest an overall increase in network excitability and the production of particularly powerful (intact) neurons that have both increased intrinsic and synaptic excitability.
Key words: : axotomy, field potential, neocortex, patch clamp, synaptic excitation
Introduction
Traumatic brain injury (TBI) is recognized by the Centers for Disease Control and Prevention as a major health problem costing billions of dollars a year within the United States.1,2 In recent years, the high incidence of TBI has included as much as 23% of soldiers returning to the U.S. from overseas wars.3 Given the high incidence, commonly chronic nature of the symptoms, and few effective treatments, there is a clear need for a greater understanding of the basic mechanisms that underlie cognitive difficulties associated with TBI. The most commonly occurring and survivable form is mild,4–7 but even mild TBI (mTBI) can result in significant and chronic attention, learning and executive-function difficulties.8–12
The cause of this morbidity associated with mTBI is not fully appreciated; however, the use of modern imaging has shown structural and functional changes in this patient population.13–15 The use of diffusion tensor imaging (DTI) and susceptibility weighted imaging has shown regional changes in fractional anisotropy and microbleeds respectively that have been linked to more diffuse/global axonal damage and disconnection.15 These imaging signatures and their implications for axonal damage also are entirely consistent with limited pathological studies performed in patients who have sustained mTBI, as well as numerous animal studies that have confirmed diffuse axonal injury (DAI) following various forms of mild injury.16–18 These structural findings also have been confirmed by functional magnetic resonance imaging (MRI) and resting state MRI and magnetoencephalography that implicates DAI with subsequent circuit disruption and disorders of brain connectivity following mTBI.19–22
In addition to these structural changes in axonal connectivity, axonal stretch and ionic imbalances after injury likely induce additional changes not immediately obvious in structural studies.15 In fact, recent studies have begun to posit that mTBIs can involve primary electrophysiological change related to an imbalance of excitatory and inhibitory neurotransmission capable of disrupting the normal function of synaptic circuits.23,24 Our group has previously used an animal model of mTBI, which allows reliable differentiation between neurons sustaining DAI and intact neurons. This enabled us to convincingly demonstrate that both groups of neurons have altered intrinsic properties following mTBI. These results are suggestive of widespread electrophysiological dysfunction.25 Accordingly, in this same model system, we now revisit mTBI to determine if these same neuronal groups also demonstrate abnormalities in their synaptic function.
Methods
Mild TBI or sham-injury was induced in 6–8 weeks old male C57bl6 mice using the previously characterized model of central fluid percussion injury (cFPI).26,27 The mice were genetically modified to express yellow fluorescent protein (YFP) under control of the Thy1 promoter (Thy1-YFP-H mice) that induces labeling of a subset of neurons and allows identification of axotomized and structurally intact neurons. Brain slices were harvested following a 1 d or 2 d post-injury or post-sham injury survival period or from naïve mice and used for either whole–cell patch clamp or field potential recordings. All animal procedures were approved by the institutional animal care and use committee of Virginia Commonwealth University.
cFPI
The cFPI model of mild TBI26 was used as previously described.28 Briefly, mice were anaesthetized using 2.0% isoflurane in oxygen. Blood oxygenation and the heart and respiratory rates were monitored using a pulse oximeter placed on the right thigh (MouseOx; Starr Life Sciences, Oakmont, PA) and core body temperature maintained at 37°C using a feed-back controlled heating pad (Harvard Apparatus, Holliston, MA). A 3 mm craniotomy centered on the midline and halfway between bregma and lambda was made using a trephine and a plastic hub made from a 20 gauge needle was placed over the craniotomy and secured using cyanoacrylate and dental cement. Following a recovery period to allow isoflurane clearance, the animals were re-anesthetized and brain injury was induced with a 1.7±0.04 atmospheres pressure pulse delivered using a fluid percussion device (Custom Design & Fabrication; Virginia Commonwealth University; Richmond, VA). Sham-injured animals received identical surgical procedures and were attached to the fluid percussion device but without pressure pulse delivery.
Patch clamp recordings
One day or 2 d post-injury, sham-injury, and age-matched naive male mice were anesthetized with isoflurane and perfused with a cold sucrose slicing solution (in mM: 2.5 KCl, 1.25 NaH2PO4, 10 MgCl2, 0.5 CaCl2, 26 NaHCO3, 11 glucose, and 234 sucrose) prior to coronal sectioning through somatosensory cortex at 300 μm. All in vitro and whole–cell patch clamp methods were performed as previously described.29 Whole–cell patch clamp recordings were made from YFP+layer V pyramidal neurons of somatosensory cortex whose axon could be seen either to end in an axonal swelling (axotomized) or descend into the white matter (intact). Recordings were made in normal artificial cerebrospinal fluid (aCSF; in mM: 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgCl2, 2 CaCl2, 26 NaHCO3, and 10 glucose) maintained at 32°C and in some cases containing 1 μM tetrodotoxin (TTX). The intracellular solution contained (in mM): 130 K-gluconate, 10 HEPES (N-2-hydroxyethylpiperazine-N-2 ethane sulphonic acid), 11 EGTA, 2.0 MgCl2, 2.0 CaCl2, 4 Na-ATP, and 0.2 Na-GTP, and in some cases also contained biocytin or Alexa-Fluor 594. Only cells in which the access resistance was <20 MΩ, and the input resistance was >40 MΩ were accepted for data collection.
Spontaneous (s-), evoked (e-) and miniature (m-) EPSCs were recorded in voltage clamp at a holding potential of −70 mV with a Multiclamp 700A (Molecular Devices, Sunnyvale, CA), and digitized at 10 KHz with a Digidata 1440 (Molecular Devices). In order to apply a range of stimulus intensities, the threshold level of stimulation was first determined by varying the current applied during a 40 msec square pulse until the smallest possible response was observed on approximately 50% of stimulus trials. The current was then maintained while the duration of the pulse was increased to 2×, 4×, 8×, and 16×, and then repeated for a total of three series. The mEPSCs were recorded with 1 μM TTX in the bathing medium. Mean peak (averaged over 0.3 msec) and area of the response were calculated with Clampfit (Molecular Devices, Sunnyvale, CA) and Excel (Microsoft, Redmond, WA). Both sEPSCs and mEPSCs were evaluated with MiniAnalysis (Synaptosoft, Ft. Lee, NJ).
Field potential recordings
Field potentials were recorded with tungsten microelectrodes (1–3 MΩ) using a Cygnus ER-1 amplifier (Cygnus Technology, Inc., Delaware Water Gap, PA), and digitized with a Digidata 1440 (Molecular Devices, Sunnyvale, CA) at 5 KHz. The recording electrode was placed in layer II/III of somatosensory cortex, and a concentric bipolar tungsten stimulating electrode was placed in layer VI online directly beneath the recording site. Threshold current level was defined as that required to produce a 0.2 mV amplitude field negativity with a 0.02 msec pulse. After this was identified, this stimulus level was applied 10–20 times at 0.1 Hz to look for epileptiform activity. Next, a series of increasing stimulus intensities was applied by increasing the duration of the pulse to 2, 4, 8 and 16 times (×) threshold. This was repeated for a total of three stimulus presentations that were averaged prior to calculating peak and time to peak.
Statistical analysis
We have previously demonstrated the absence of significant differences in patch clamp recordings between naïve and sham-injured animals, and these were accordingly combined into a single group of control animals.25 Animals exposed to cFPI were divided into a 1 d and a 2 d survival group, and cells used for patch clamp recordings from injured animals were further sub-divided into a group for axotomized neurons and one for structurally intact neurons. Individual cells were used as the experimental unit for analysis of patch clamp data and individual slices for field potential recordings. Group comparisons of continuous variables were made using a one-way analysis of variance (ANOVA) followed by a Tukey post hoc test; distributions were compared using the Kolmogorov-Smirnov (KS) test and proportions using a z-test. Results are reported as mean±standard error of the mean (SEM) in all figures.
Results
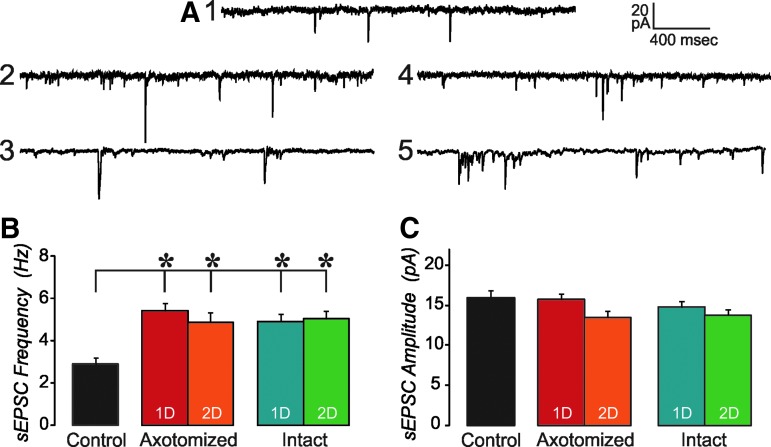
As previously described,30 this injury produced no evidence of contusion or intraparenchymal hemorrhage, and therefore was consistent with mTBI. In addition, axotomized neurons were readily identified in living brain slices utilizing the YFP expression that clearly demonstrated both axonal swellings and the associated disconnection.25 In most cases, this occurred within 100 μM of the soma; however, some neurons showed this pathology several hundred microns distally. In contrast, the axons of non-axotomized or intact neurons could be followed all the way to the underlying subcortical white matter. Spontaneous EPSCs recorded in YFP+layer V cells within 2 d of injury were not qualitatively different from those recorded from control tissue (Fig. 1A). However, the frequency of sEPSCs, which was similar in both axotomized and intact neurons at both 1 d and 2 d after injury, was significantly increased in neurons from brain injured animals to almost twice the level of the controls (Fig. 1B; one-way ANOVA, Tukey post hoc, p<0.05). The amplitude of sEPSCs was not different from control for any of the injured groups (Fig. 1C).
FIG. 1.
Spontaneous excitatory postsynaptic currents (sEPSCs) are increased after mild traumatic brain injury (mTBI). (A) Examples of sEPSCs recorded in a control (1), 1 d injured axotomized (2), 2 d injured axotomized (3), 1 d injured intact (4), and 2 d injured intact (5) layer V, yellow fluorescent protein (YFP)+pyramidal neurons. No qualitative difference is observed in sEPSCs recorded from cells of these different groups. (B) Mean sEPSC frequency is increased in all injured groups, compared with control cells (analysis of variance,*=p<0.05, 1D=1 d after injury, 2D=2 d after injury). (C) Mean sEPSC amplitude is unaltered in these experimental groups, compared with controls. n=48 control, 27 one day axotomized, 15 two day axotomized, 16 one day intact, and 15 two day intact.
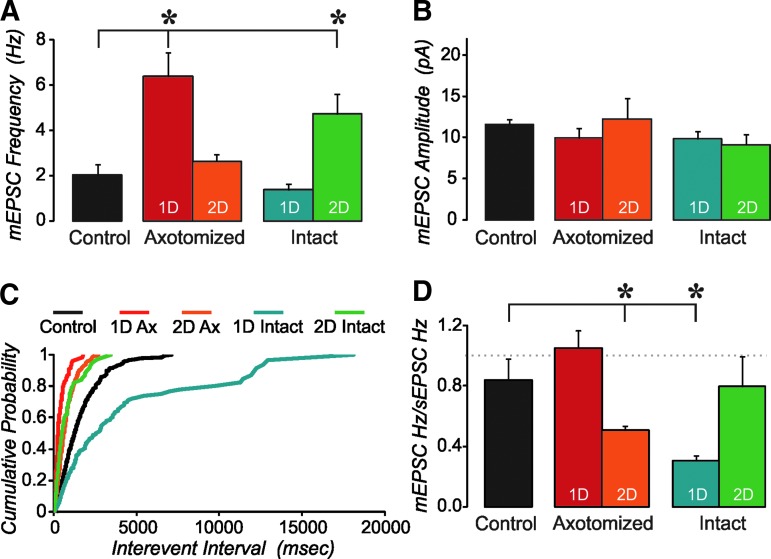
We have previously shown increased intrinsic excitability in some neurons of the injured cortex.25 Since the increase in sEPSC frequency could be due to a combination of changes in presynaptic inputs to these cells, intrinsic properties that may cause increased activity in those presynaptic inputs, and overall activity levels, we also examined mEPSCs. In axotomized neurons, the mEPSC frequency was significantly higher at 1 d post-injury but returned to near control levels by 2 d post-injury (Fig. 2A; one-way ANOVA, Tukey post hoc, p<0.05). In contrast, for intact neurons, the mEPSC frequency was not different from controls at 1 d post-injury but was significantly higher than controls at 2 d post-injury. The mEPSC amplitude was not different from control for any of the injured groups (Fig. 2B), suggesting that the changes in excitatory synapses onto these layer V pyramidal neurons are predominantly presynaptic and not postsynaptic. Cumulative probability plots for the interevent interval are shown in Fig. 2C. For this plot, the mEPSCs analyzed were restricted to the first 200 events in order to have equal influence between cells. These data show that only 25% of control cells had an interevent interval less than 700 msec, while this percentage was 93%, 53%, and 60% for 1 d axotomized, 2 d axotomized, and 2 d intact, respectively. This distribution was significantly different from control for axotomized neurons at both 1 d and 2 d survival ages (KS test, p<0.05). While these groups had a greater number of short intervals than controls, the 1 d intact group, on the other hand, had fewer short intervals than controls. For the 1 d intact group, the majority (81%) of events were greater than 700 msec apart. The cumulative probability distribution for this group was significantly different from control (KS test; p<0.05).
FIG. 2.
Axotomized and intact neurons of injured cortex are differentially altered. (A) Mean frequency of miniature excitatory postsynaptic currents (mEPSCs) was increased at 1 d, but returned to control levels at 2 d after injury in axotomized cells. In contrast, intact neurons showed a significant increase only at 2 d post injury. (analysis of variance [ANOVA]; *=p<0.05). (B) Mean amplitude of mEPSCs was not affected. (C) Cumulative probability of interevent interval shows that most events occur at longer intervals in 1 d intact neurons relative to control cells, but at shorter intervals than controls in all three other groups. (D) Ratio of mEPSC frequency to spontaneous EPSC (sEPSC) frequency. In control cells ∼80% of events come from miniature events. This ratio is not significantly different for 1 d axotomized and 2 d intact neurons, but is significantly lower for 2 d axotomized and 1 d intact cells (ANOVA; *=p<0.05). n=15 control, five 1 d axotomized, 10 two day axotomized, five 1 d intact, and four 2 d intact layer V yellow fluorescent protein (YFP)+pyramidal neurons.
In control cells, the ratio of mEPSC to sEPSC frequency was near 80% (Fig. 2D), suggesting that the majority of events under conditions of normal aCSF are non–action-potential driven. This also was true for the two injury groups that showed an increased mEPSC frequency (1 d axotomized, and 2 d intact). In contrast, the mEPSC to sEPSC ratio was significantly lower than controls for the 2 d axotomized and 1 d intact groups. This suggests that there is increased network activity driving the increased sEPSC frequency while maintaining the same number of afferents onto these cells.
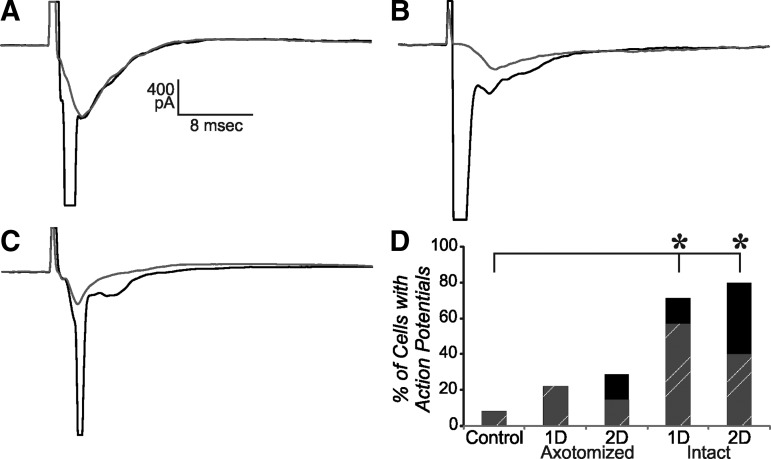
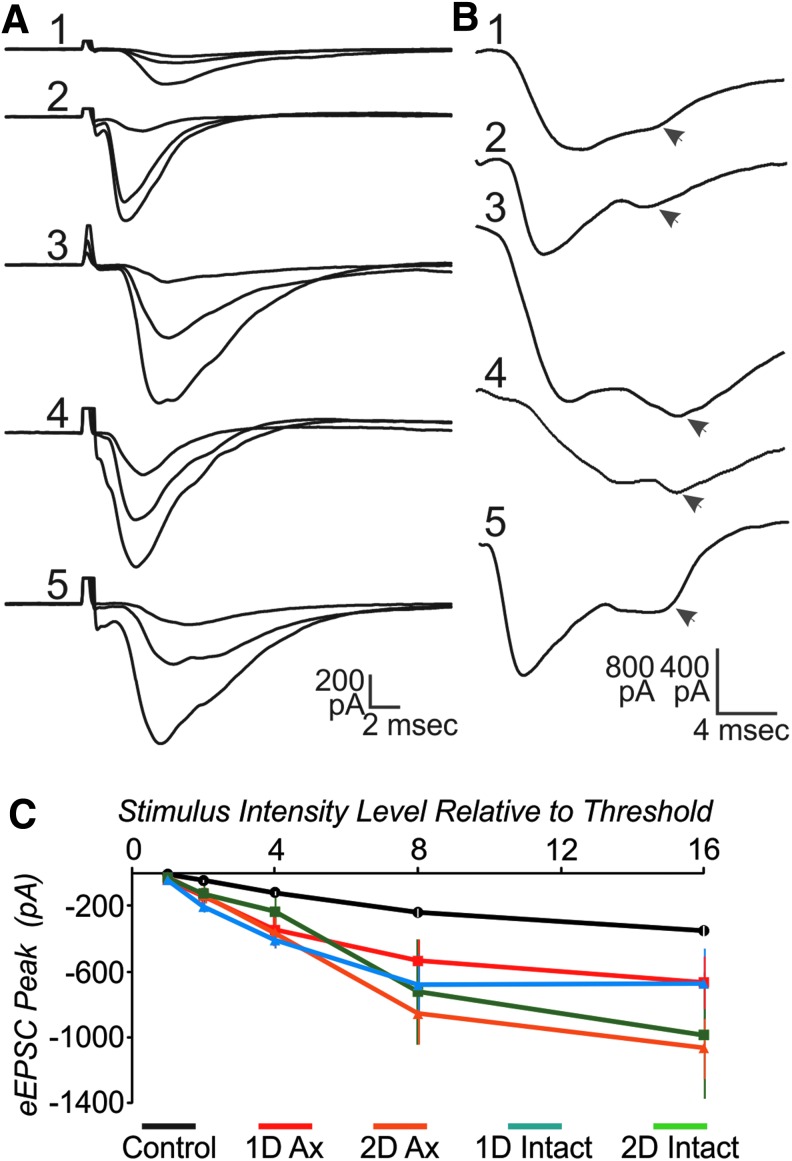
To further assess network changes, we also examined the evoked EPSC resulting from nearby local stimulation. In the majority of control cells, only a single peaked EPSC was evoked, even at high stimulus intensities. In contrast, in the majority of intact cells, an action potential was evoked in addition to the EPSC (Fig. 3D). In some cases the action potential occurred prior to the peak of the EPSC, possibly reflecting an antidromic spike (Fig. 3A, 3B), while in other cells the action potential arose from the peak of the EPSC (Fig. 3C). The proportion of cells with action potentials was significantly increased for both the 1 d and 2 d intact groups, compared with controls (z-test; p<0.05). The increase in both orth- and anti-dromic action potentials may reflect local sprouting by the recorded and adjacent neurons. For the peak amplitude of the EPSC, a two-way ANOVA showed that there were significant effects for group, stimulus intensity, and the interaction of these two. Post hoc statistical analysis showed that control was significantly lower than all experimental groups, except for the 2 d intact group. (Fig. 4; two-way ANOVA, Tukey post hoc, p<0.05). The 2 d intact group had larger SEM and was closer to controls at low stimulus intensities. Although the time to peak varied between cells (Fig. 4A), the mean time to peak was not significantly different between groups, being around 5 msec for intensities higher than threshold (two-way ANOVA, p<0.05). In control cells, a “notch” in the response was commonly observed within a few msec of the peak, possibly reflecting activation of a slower conducting axonal group or more multi-synaptic response. This late response was more clearly evident, often as a second peak in the cells in injured cortex, for both 1 d and 2 d and both axotomized and intact groups (Fig. 4B).
FIG. 3.
Action potentials were sometimes produced after extracellular stimulation 250–300 um lateral to cell in layer V. (A, B) Examples of action potentials evoked prior to the peak of the synaptic current (black trace) overlaid on response to next lowest stimulus intensity (gray). (C) Same as in A and B, except that action potential is evoked at peak of synaptic current. All examples shown are from intact cells of injured cortex. (D) Percent of cells in which action potentials were evoked (total bar height). Also shown by striped region is the percent of cells with action potentials that occur prior to the peak of the evoked excitatory postsynaptic currents. *=z-test; p<0.05. n=12 control, nine 2 d axotomized, seven 2 d axotomized, seven 1 d intact, and five 2 d intact.
FIG. 4.
The evoked excitatory postsynaptic current (eEPSC) is significantly different across stimulus intensities, experimental groups and the interaction of these two (two-way analysis of variance; p<0.05). (A) Examples of responses to extracellular stimulation (averages of three sweeps, for 2×, 4×, and 8×) for control (1), 1 d axotomized (2), 2 d axotomized (3), 1 d intact (4), and 2 d intact (5). (B) Control cells often had a late “notch” (arrowhead) in the evoked response, while in some cells of injured cortex this second peak (arrowhead) was larger than the earlier peak. Shown are single sweep examples from control (1); 1 d axotomized (2); 2 d axotomized (3); 1 d intact (4); and 2 d intact (5). Scalebar in B is 400 pA for 1–3, 5 and 800 pA for 4. (C) Mean peak of the eEPSC versus stimulus intensity level. Note control error bars are present in white, but smaller than mean symbol. N=10 control, eight 1 d axotomized, five 2 d axotomized, four 1 d intact, and four 2 d intact cells.
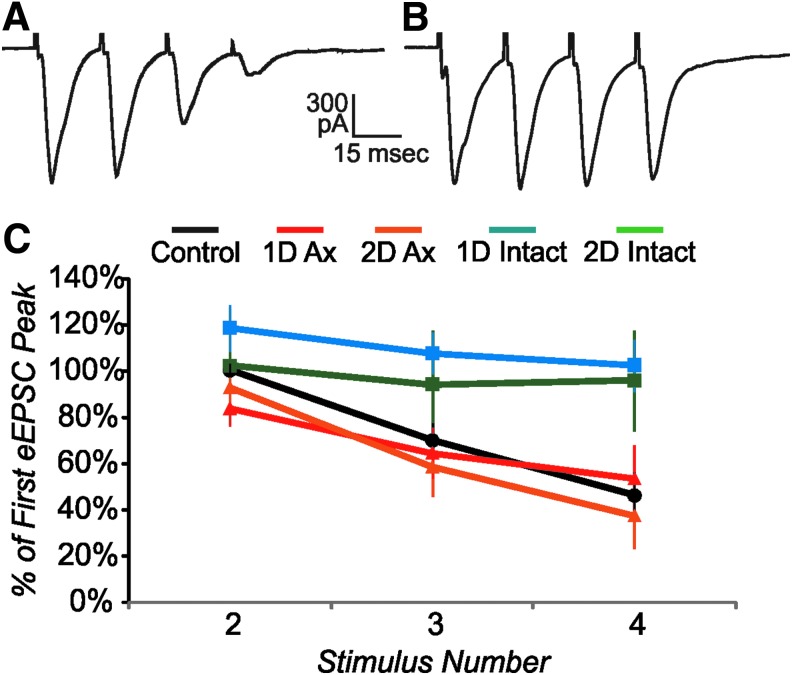
The increase in mEPSC frequency described above for specific injury groups could be due to either an increased number of excitatory afferents onto the recorded cell or to an increase in probability of release, or a combination of the two. We examined whether there are changes in probability of release by applying multiple extracellular stimuli at a short interval (20 msec). In control cells, we observed a relatively high level of probability of release for the excitatory afferents, as the eEPSC showed a depressing response (diminishing in amplitude with successive pulses; Fig. 5A). Both 1 d and 2 d axotomized groups showed a similar depressing response (Fig. 5C). In contrast, the afferents to intact cells typically showed a relatively low probability of release, since there was little change in the eEPSC amplitude with repeated stimuli (Fig. 5B, 5C). Since the intact cells have a lower probability of release than controls, the increased mEPSC frequency cannot be accounted for by an increased probability of release. The 1 d axotomized group shows a depressing level similar to controls and thus, a change in probability of release also is unlikely to account for the large increase in mEPSC frequency in this group, compared with control. Thus, in the case of both 1 d axotomized and 2 d intact cells, the increased mEPSC frequency likely reflects an increase in the number of presynaptic excitatory afferents. Note that this differs from the 1 d intact and 2 d axotomized cells, which likely have unaltered numbers of presynaptic excitatory afferents (see above).
FIG. 5.
Effect of repetitive stimulation (20 msec interstimulus interval) on excitatory postsynaptic currents (eEPSC) peak. (A) Example of a depressing EPSC, average of four stimulus presentations, from a control cell. (B) Example of a response that depresses little with successive stimuli, average of four stimulus presentations from a 2 d intact cell. (C) Mean eEPSC peak relative to first eEPSC peak for stimuli 2–4. Control and axotomized cells show depressing pattern, while intact cells depress little. Only cells with either no action potential or in which the eEPSC peak could be clearly distinguished from an earlier action potential were used for this analysis. n=9 control, nine 1 d axotomized, six 2 d axotomized, four 1 d intact, and three 2 d intact cells.
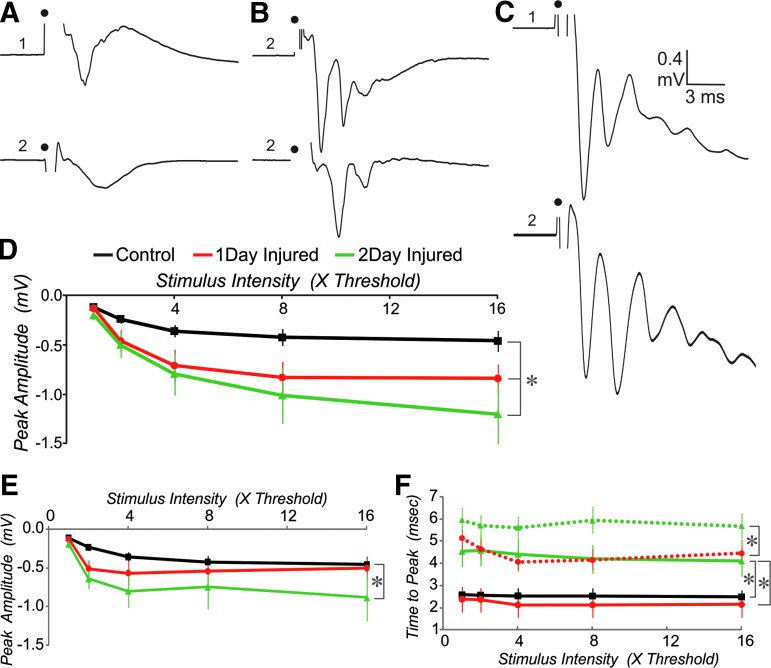
We further examined the changes in the excitability of the network by recording field potentials from superficial layers after online stimulation of the deep layers below the recording site. We normalized the stimulus between slices by setting threshold equal to a 0.2 mV short latency field negativity. A series of stimuli of increasing intensity were then tested. As expected in control cortex, this stimulation produced a single peaked short latency response that was graded with stimulus intensity (Fig. 6A, 6D). In most slices from injured cortex, two distinct peaks were observed at high intensity stimulation (Fig. 6B, 6C). The first peak in responses from injured cortex was significantly larger than the single peak of sham-injured controls at both 1 d and 2 d post-injury (Fig. 6D, two-way ANOVA, Tukey post-hoc, p<0.05). The second peak tended to be smaller than the first peak in injured cortex. The ratio for peak two relative to peak one was 67±19% and 89±12% at the maximum stimulus intensity (16×) for 1 d and 2 d injured, respectively. There was no significant difference between these two groups on this measure (t-test, p>0.05). The second peak, however, was significantly larger than the single peak of controls only for the 2 d injured group (Fig. 6E, two-way ANOVA, Tukey post hoc, p<0.05). A time to peak of approximately 2.5 msec was typical for controls and did not vary much with stimulus intensity (Fig. 6F). At 1 d post injury, the first peak occurred with a similar latency to that of controls, while the second peak typically arrived 1–2 msec later. In contrast, for the 2 d injured group, the time to peak for both the first and second peaks was significantly longer, compared with that for the single peak of controls. The 1 d and 2 d injured also were significantly different on this measure for both the first and second peaks.
FIG. 6.
Field potentials have a larger amplitude and are multi-peaked in injured compared with control cortex. (A-C) Two examples from different slices for control (A), 1 d injured (B), and 2 d injured (C) at 16× threshold stimulation. (D) Mean peak amplitude versus stimulus intensity for early peaks. (E) Comparison of single peak amplitude for controls and second peak amplitude for cells from injured cortex. (F) Time to peak for single peak in control, early peak in injured (solid lines) and second peak in injured (dashed lines). n=12 control, eight 1 d injured and eight 2 d injured slices from four, three, and three animals, respectively. *=Significantly different, two-way analysis of variance, Tukey post hoc, p<0.05.
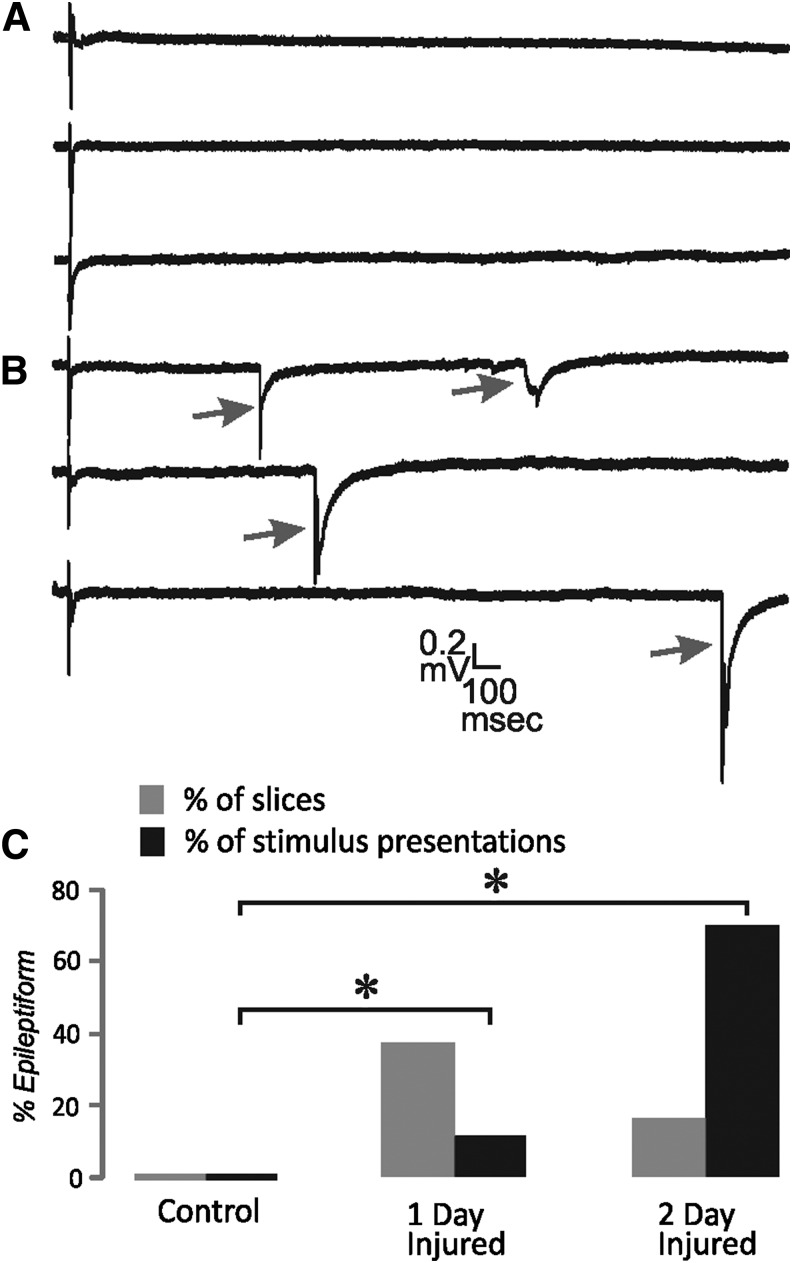
Increases in network excitability also can produce long latency all-or-none field events that are similar to interictal-like activity.31 We looked for such events by recording successive trials at threshold stimulation (prior to the recording of the stimulus intensity series). In control tissue, late events were never observed in the field potential recordings. Some slices from injured cortex did in fact have these interictal-like responses following the short latency field potential (Fig. 7). They were observed in a minority of slices for both 1 d and 2 d injured groups. Particularly for the 2 d injured group, in slices with epileptiform activity, these events were present in most of the stimulus presentations. The proportion of total stimulus presentations with epileptiform events was significantly greater than control for both 1 d and 2 d injured groups (z-test; p<0.05).
FIG. 7.
Interictal-like epileptiform field potentials. (A) Examples of the field potential evoked in superficial layers at threshold stimulus intensity shows only short latency response. (B) Examples of interictal-like late “spikes” (arrows) evoked at threshold stimulus intensity in injured cortex. (C) Percent of slices (gray bars) and percent of stimulus presentations (black bars) that showed these late interictal-like spikes. In each slice 10–20 stimulus presentations at threshold were tested. n=12 and 120 for control, 11 and 130 for 1 d injured and 9 and 90 for 2 d injured for slices and stimulus presentations, respectively. *=z-test; p<0.05.
Discussion
Here, we have demonstrated synaptic alterations in both intact and axotomized neurons after cFPI inducing mTBI with DAI. This work has important implications for clinical mTBI, where efforts are often focused on identifying locations of structural injury. Our study shows that the number of excitatory connections onto axotomized and intact layer V pyramidal neurons is likely increased 1 d and 2 d after injury, respectively. This is reflected in the increased mEPSC frequency and lack of increased release probability, detected with repeated extracellular activation of EPSCs. Overall network activity is increased, as reflected in the increased peak of the short latency field negativity and the occurrence of interictal-like epileptiform events. In addition this is supported by the increased propensity to activate both ortho- and anti-dromic action potentials in recorded neurons after nearby stimulation. Even spatially and temporally focal occurrences of increased excitation may alter the functional network. Such clinically diagnosed functional network changes have been demonstrated and suggested to account for specific cognitive deficits in patients with TBI.32–34
We previously showed that both axotomized and intact layer V pyramidal neurons have altered intrinsic properties after a mild cFPI.25 For intact neurons 2 d after injury, regular spiking neurons fire more action potentials in response to intracellular depolarizing current pulses. Here, we have shown that the same neurons, those with intact axons at 2 d post-injury, also has increased synaptic excitability. These neurons have a higher mEPSC frequency, compared with controls (Fig. 2A) and are more likely to fire an action potential in response to extracellular stimulation (Fig. 3). The higher mEPSC frequency is unlikely to be due to an altered presynaptic calcium or increases in the number of presynaptic vesicles released since the mEPSC amplitude is unchanged. In theory, the higher mEPSC frequency could be produced by either increased excitatory synaptic afferents onto the recorded cell or an increased release probability from these afferents, or a combination of both of these mechanisms. It is unlikely, however, that an increase in release probability occurs in the afferents to the intact neurons recorded here, since such a change would be expected to produce an increase in the depressing response profile during repeated stimulation of the afferents. We instead observed a loss of the depression in response to stimuli given at 50 Hz (Fig. 5), suggesting that the increased mEPSC frequency is due instead to increased afferents onto these intact pyramidal neurons. Axonal sprouting has previously been observed in response to TBI30,35,36 and axotomy.37–40 It is unclear why a delay to sprouting onto these cells occurs. Based on the cumulative probability plot of mEPSC frequency (Fig. 2C), these cells may have a slightly depressed level of synaptic excitation 1 d after injury that is converted to double the level of control synaptic excitation on Day 2 after injury. The combination of increased intrinsic excitability and increased synaptic excitation at 2 d after injury is likely to make these cells particularly powerful within the network. This may contribute to the creation of neuronal hubs that have been shown to provide a mechanism for hyperexcitability in the epileptic brain.41
The increased mEPSC frequency in axotomized neurons 1 d after injury also likely reflects an increased excitatory synaptic input onto these cells, since for this group the synaptic depression after 50 Hz extracellular stimulation is not increased relative to controls (Fig. 5C). It is not clear why the sprouting occurs exclusively to axotomized neurons at 1 d nor why for these neurons mEPSC frequency returns to control level at 2 d after injury. The initial sprouting may be a network form of homeostasis42,43 where increased excitatory input is provided selectively to cells that have a reduced output. The fact that the timing of altered synaptic function is different for axotomized and intact neurons suggests that the feedback to the network from these cells is involved in determining levels of synaptic input. There also may be an interaction between intrinsic and synaptic properties. The slope of action potential frequency versus injected current was significantly decreased in axotomized cells, compared with intact cells, 1 d after injury.25 In addition, the amount of injected depolarizing current needed to produce an action potential (rheobase) is significantly higher in axotomized cells, compared with both intact cells and controls, 1 d after injury. The depressed intrinsic properties combined with the lack of output may promote increased synaptic excitation onto the axotomized cells.42,43 As a whole, network homeostasis is not achieved because excitability is increased above network control levels as shown from field potential recordings (Fig. 6). Cells in other layers and changes in inhibition also may contribute to this overall increase in network excitability. This network hyperexcitability may then contribute to the return to control levels of excitatory input in axotomized cells 1 d later. In fact, the reduction in the mEPSC to sEPSC frequency ratio (Fig. 2D) in axotomized neurons at 2 d suggests that the increased sEPSC frequency in these cells is due primarily to network hyperactivity since the number of afferents onto these cells is likely similar to that of control cells based on mEPSC frequency.
Increased synaptic excitation after TBI is consistent with the undercut model of post-traumatic epilepsy, where neocortical axons are severed at the subcortical white matter, as well as partially isolated from adjacent tissue.44,45 In this model that develops epileptiform activity at two weeks, layer V pyramidal neurons have an increased mEPSC frequency and activation of caged glutamate has shown that local excitatory synaptic input to these cells is increased.46, 47 These changes also are similar to those found in other TBI models that develop post-traumatic epilepsy, such as CCI.48,49 Although initial suppression of activity has been proposed to prime the brain for hyperexcitability,24 here we expect that few of these animals would be likely to develop spontaneous seizures, as others have shown that more severe FPI resulting in more severe TBI is required to consistently evoke seizures 6–10 weeks later.50,51
Our findings of increased synaptic and network excitation are in opposition to findings obtained using models of severe injury that cause cell death or in other models, such as the stretch/strain model of cultured cells and the weight drop impact acceleration (WDIA) model.52,53 In these models, a depression of synaptic and network activity has been observed. One reason for the disparate findings may be that different brain structures respond differently to the trauma. Within the hippocampus, CA1 cells are typically depressed, while dentate gyrus cells show increased excitation after TBI.23 For the WDIA model, only superficial neocortical layers showed depressed responses to whisker stimulation.52 In addition, the level of spontaneous activity was significantly increased within layer V in this report. In the stretch model, increased responses to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors have been observed.54 Here, AMPA receptor responses were likely unaffected since the amplitude of the mEPSC was not different for either axotomized or intact neurons relative to controls. Although the stretch model is performed on cortical neurons, it is possible that discrepancies with our findings reflect the developmental state, which is likely to be younger in these cultures, compared with our 6- to 8-week-old animals. In contrast to these studies and similar to the changes in excitability demonstrated with the current study, others have shown an immediate depression followed by a rapid increase in excitability by 2 h after focal compression TBI within somatosensory cortex.24
It is well known that TBI lesion volume is correlated with injury severity; however, using conventional imaging techniques, it has sometimes been challenging to find abnormalities that could account for chronic cognitive dysfunction in mild cases of TBI.13 The ability to correlate functional abnormalities, in addition to detailing structural anomalies with DTI irregularities, has been a major clinical advancement. While numerous studies have shown correlations between measures of fractional anisotropy and cognitive function following mTBI,13,14,55–57 a recent report with a relatively large healthy cohort of patients failed to identify any DTI-related changes associated with post-concussion symptoms.58 These differing results, perhaps related to the timing of imaging as Ilvesmäki and colleagues obtained images acutely within 14 d of injury, at a minimum question the extent to which various imaging modalities can detect the most subtle of head injuries. Critical to this discussion, the presented data suggest that post-concussional symptoms may not reflect structural white matter changes detectable by advanced imaging but rather may be due to altered function and synaptic connectivity of neurons with intact axons. Our work supports the idea that altered functional connectivity at the synaptic level may play a critical role in overall network health and cognitive operations.
Acknowledgments
This work was supported by National Institutes of Health Grant NS077675.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.(2013). CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb. Mortal. Wkly. Rep. 62, 549–552 [PMC free article] [PubMed] [Google Scholar]
- 2.Gerberding J.L. and Binder S. (2003). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. U.S. Centers for Disease Control: Atlanta, GA, pps. 1–45 [Google Scholar]
- 3.O'Neil M.E., Carlson K., Storzbach D., Brenner L., Freeman M., Quinones A., Motu'apuaka M., Ensley M., and Kansagara D. (2013). VA evidence-based synthesis program reports. In: Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review. Department of Veterans Affairs: Washington, DC: [PubMed] [Google Scholar]
- 4.Asemota A.O., George B.P., Bowman S.M., Haider A.H., and Schneider E.B. (2013). Causes and trends in traumatic brain injury for United States adolescents. J. Neurotrauma 30, 67–75 [DOI] [PubMed] [Google Scholar]
- 5.Hall E.C., Lund E., Brown D., Murdock K.R., Gettings L., Scalea T.M., and Stein D.M. (2014). How are you really feeling? A prospective evaluation of cognitive function following trauma. J. Trauma Acute Care Surg. 76, 859–865 [DOI] [PubMed] [Google Scholar]
- 6.Carlson K.F., Kehle S.M., Meis L.A., Greer N., Macdonald R., Rutks I., Sayer N.A., Dobscha S.K., and Wilt T.J. (2011). Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J. Head Trauma Rehabil. 26, 103–115 [DOI] [PubMed] [Google Scholar]
- 7.Kerr Z.Y., Harmon K.J., Marshall S.W., Proescholdbell S.K., and Waller A.E. (2014). The epidemiology of traumatic brain injuries treated in emergency departments in North Carolina, 2010–2011. N. C. Med. J. 75, 8–14 [DOI] [PubMed] [Google Scholar]
- 8.Rabinowitz A.R. and Levin H.S. (2014). Cognitive sequelae of traumatic brain injury. The Psychiatr. Clin. North Am. 37, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geary E.K., Kraus M.F., Pliskin N.H., and Little D.M. (2010). Verbal learning differences in chronic mild traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 506–516 [DOI] [PubMed] [Google Scholar]
- 10.Catroppa C. and Anderson V. (2005). A prospective study of the recovery of attention from acute to 2 years following pediatric traumatic brain injury. J Int. Neuropsychol. Soc. 11, 84–98 [DOI] [PubMed] [Google Scholar]
- 11.Catroppa C., Anderson V., Godfrey C., and Rosenfeld J.V. (2011). Attentional skills 10 years post-paediatric traumatic brain injury (TBI). Brain Inj. 25, 858–869 [DOI] [PubMed] [Google Scholar]
- 12.Mangels J.A., Craik F.I., Levine B., Schwartz M.L., and Stuss D.T. (2002). Effects of divided attention on episodic memory in chronic traumatic brain injury: a function of severity and strategy. Neuropsychologia 40, 2369–2385 [DOI] [PubMed] [Google Scholar]
- 13.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd A.B., Epstein K., Ling J., and Mayer A. (2014). Diffusion tensor imaging findings in semi-acute mild traumatic brain injury. J. Neurotrauma. 31, 1235–1248 [DOI] [PubMed] [Google Scholar]
- 15.Bigler E.D. and Maxwell W.L. (2012). Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 6, 108–136 [DOI] [PubMed] [Google Scholar]
- 16.Farkas O. and Povlishock J.T. (2007). Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog. Brain Res. 161, 43–59 [DOI] [PubMed] [Google Scholar]
- 17.Buki A. and Povlishock J.T. (2006). All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir.(Wien.) 148, 181–193 [DOI] [PubMed] [Google Scholar]
- 18.Bigler E.D. and Maxwell W.L. (2011). Neuroimaging and neuropathology of TBI. NeuroRehabilitation 28, 63–74 [DOI] [PubMed] [Google Scholar]
- 19.Mishra A.M., Bai X., Sanganahalli B.G., Waxman S.G., Shatillo O., Grohn O., Hyder F., Pitkanen A., and Blumenfeld H. (2014). Decreased resting functional connectivity after traumatic brain injury in the rat. PloS One 9, e95280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palacios E.M., Sala-Llonch R., Junque C., Roig T., Tormos J.M., Bargallo N., and Vendrell P. (2013). Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol. 70, 845–851 [DOI] [PubMed] [Google Scholar]
- 21.Tarapore P.E., Findlay A.M., Lahue S.C., Lee H., Honma S.M., Mizuiri D., Luks T.L., Manley G.T., Nagarajan S.S., and Mukherjee P. (2013). Resting state magnetoencephalography functional connectivity in traumatic brain injury. J. Neurosurg. 118, 1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo Q., Xu D., Roskos T., Stout J., Kull L., Cheng X., Whitson D., Boomgarden E., Gfeller J., and Bucholz R.D. (2013). Complexity analysis of resting state magnetoencephalography activity in traumatic brain injury patients. J. Neurotrauma 30, 1702–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen A.S., Pfister B.J., Schwarzbach E., Grady M.S., Goforth P.B., and Satin L.S. (2007). Injury-induced alterations in CNS electrophysiology. Prog. Brain Res. 161, 143–169 [DOI] [PubMed] [Google Scholar]
- 24.Ding M.C., Wang Q., Lo E.H., and Stanley G.B. (2011). Cortical excitation and inhibition following focal traumatic brain injury. J. Neurosci. 31, 14085–14094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greer J.E., Povlishock J.T., and Jacobs K.M. (2012). Electrophysiological abnormalities in both axotomized and nonaxotomized pyramidal neurons following mild traumatic brain injury. J. Neurosci. 32, 6682–6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon C.E., Lyeth B.G., Povlishock J.T., Findling R.L., Hamm R.J., Marmarou A., Young H.F., and Hayes R.L. (1987). A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 67, 110–119 [DOI] [PubMed] [Google Scholar]
- 27.Dixon C.E., Lighthall J.W., and Anderson T.E. (1988). Physiologic, histopathologic, and cineradiographic characterization of a new fluid-percussion model of experimental brain injury in the rat. J. Neurotrauma 5, 91–104 [DOI] [PubMed] [Google Scholar]
- 28.Greer J.E., Hanell A., McGinn M.J., and Povlishock J.T. (2013). Mild traumatic brain injury in the mouse induces axotomy primarily within the axon initial segment. Acta Neuropathol. 126, 59–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George A.L. and Jacobs K.M. (2011). Altered intrinsic properties of neuronal subtypes in malformed epileptogenic cortex. Brain Res. 1374, 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer J.E., McGinn M.J., and Povlishock J.T. (2011). Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J. Neurosci. 31, 5089–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connors B.W. (1984). Initiation of synchronized neuronal bursting in neocortex. Nature 310, 685–687 [DOI] [PubMed] [Google Scholar]
- 32.Sharp D.J., Scott G., and Leech R. (2014). Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 10, 156–166 [DOI] [PubMed] [Google Scholar]
- 33.Mayer A.R., Mannell M.V., Ling J., Gasparovic C., and Yeo R.A. (2011). Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 32, 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarapore P.E., Findlay A.M., Lahue S.C., Lee H., Honma S.M., Mizuiri D., Luks T.L., Manley G.T., Nagarajan S.S., and Mukherjee P. (2013). Resting state magnetoencephalography functional connectivity in traumatic brain injury. J. Neurosurg. 118, 1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa H., Ueno M., Itokazu T., and Yamashita T. (2013). Bilateral movement training promotes axonal remodeling of the corticospinal tract and recovery of motor function following traumatic brain injury in mice. Cell Death Dis. 4, e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falo M.C., Reeves T.M., and Phillips L.L. (2008). Agrin expression during synaptogenesis induced by traumatic brain injury. J. Neurotrauma 25, 769–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allegra Mascaro A.L., Cesare P., Sacconi L., Grasselli G., Mandolesi G., Maco B., Knott G.W., Huang L., De Paola V., Strata P., and Pavone F.S. (2013). In vivo single branch axotomy induces GAP-43-dependent sprouting and synaptic remodeling in cerebellar cortex. Proc. Natl. Acad. Sci. U. S. A. 110, 10824–10829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makwana M., Werner A., Acosta-Saltos A., Gonitel R., Pararajasingham A., Ruff C., Rumajogee P., Cuthill D., Galiano M., Bohatschek M., Wallace A.S., Anderson P.N., Mayer U., Behrens A., and Raivich G. (2010). Peripheral facial nerve axotomy in mice causes sprouting of motor axons into perineuronal central white matter: time course and molecular characterization. J. Comp. Neurol. 518, 699–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blizzard C.A., Haas M.A., Vickers J.C., and Dickson T.C. (2007). Cellular dynamics underlying regeneration of damaged axons differs from initial axon development. Eur. J. Neurosci. 26, 1100–1108 [DOI] [PubMed] [Google Scholar]
- 40.Dickson T.C., Chung R.S., McCormack G.H., Staal J.A., and Vickers J.C. (2007). Acute reactive and regenerative changes in mature cortical axons following injury. Neuroreport 18, 283–288 [DOI] [PubMed] [Google Scholar]
- 41.Morgan R.J. and Soltesz I. (2008). Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc. Natl Acad. Sci. U. S. A. 105, 6179–6184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakayama K., Kiyosue K., and Taguchi T. (2005). Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J. Neurosci. 25, 4040–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turrigiano G. (2007). Homeostatic signaling: the positive side of negative feedback. Curr. Opin. Neurobiol. 17, 318–324 [DOI] [PubMed] [Google Scholar]
- 44.Prince D.A. and Tseng G.F. (1993). Epileptogenesis in chronically injured cortex: in vitro studies. J. Neurophysiol. 69, 1276–1291 [DOI] [PubMed] [Google Scholar]
- 45.Hoffman S.N., Salin P.A. and Prince D.A. (1994). Chronic neocortical epileptogenesis in vitro. J. Neurophysiol. 71, 1762–1773 [DOI] [PubMed] [Google Scholar]
- 46.Li H. and Prince D.A. (2002). Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol. 88, 2–12 [DOI] [PubMed] [Google Scholar]
- 47.Jin X., Prince D.A., and Huguenard J.R. (2006). Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J. Neurosci. 26, 4891–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt R.F., Scheff S.W., and Smith B.N. (2009). Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp. Neurol. 215, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt R.F., Scheff S.W., and Smith B.N. (2010). Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J. Neurophysiol. 103, 1490–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharatishvili I., Nissinen J.P., McIntosh T.K., and Pitkanen A. (2006). A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–697 [DOI] [PubMed] [Google Scholar]
- 51.Bolkvadze T. and Pitkanen A. (2012). Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J. Neurotrauma 29, 789–812 [DOI] [PubMed] [Google Scholar]
- 52.Johnstone V.P., Yan E.B., Alwis D.S., and Rajan R. (2013). Cortical hypoexcitation defines neuronal responses in the immediate aftermath of traumatic brain injury. PloS One 8, e63454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goforth P.B., Ren J., Schwartz B.S., and Satin L.S. (2011). Excitatory synaptic transmission and network activity are depressed following mechanical injury in cortical neurons. J. Neurophysiol. 105, 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goforth P.B., Ellis E.F., and Satin L.S. (1999). Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. J. Neurosci. 19, 7367–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 29, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hulkower M.B., Poliak D.B., Rosenbaum S.B., Zimmerman M.E., and Lipton M.L. (2013). A decade of DTI in traumatic brain injury: 10 years and 100 articles later. A.J.N.R. Am. J. Neuroradiol. 34, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toth A., Kovacs N., Perlaki G., Orsi G., Aradi M., Komaromy H., Ezer E., Bukovics P., Farkas O., Janszky J., Doczi T., Buki A., and Schwarcz A. (2013). Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: can we see the difference? J. Neurotrauma 30, 2–10 [DOI] [PubMed] [Google Scholar]
- 58.Ilvesmaki T., Luoto T.M., Hakulinen U., Brander A., Ryymin P., Eskola H., Iverson G.L., and Ohman J. (2014). Acute mild traumatic brain injury is not associated with white matter change on diffusion tensor imaging. Brain 137, 1876–1882 [DOI] [PubMed] [Google Scholar]