Abstract
Sirtuin is a nicotinamide adenine dinucleotide–dependent deacetylase. One of its isoforms, Sirt1, is a key molecule in glucose, lipid, and energy metabolism. The renal protective effects of Sirt1 are found in various models of renal disorders with metabolic impairment, such as diabetic nephropathy. Protective effects include the maintenance of glomerular barrier function, anti–fibrosis effects, anti–oxidative stress effects, and regulation of mitochondria function and energy metabolism. Various target molecules subject to direct deacetylation or epigenetic gene regulation have been identified as effectors of the renal protective function of sirtuin. Recently, it was demonstrated that Sirt1 expression decreases in proximal tubules before albuminuria in a mouse model of diabetic nephropathy, and that albuminuria is suppressed in proximal tubule–specific mice overexpressing Sirt1. These findings suggest that decreased Sirt1 expression in proximal tubular cells causes abnormal nicotine metabolism and reduces the supply of nicotinamide mononucleotide from renal tubules to glomeruli. This further decreases expression of Sirt1 in glomerular podocytes and increases expression of a tight junction protein, claudin-1, which results in albuminuria. Activators of the sirtuin family of proteins, including resveratrol, may be important in the development of new therapeutic strategies for treating metabolic kidney diseases, including diabetic nephropathy.
Keywords: cell signaling, chronic kidney disease, diabetic nephropathy, mitochondria, podocyte, proximal tubule
THE FUNCTIONS OF SIRTUIN AND NICOTINE AMIDE DINUCLEOTIDE METABOLISM
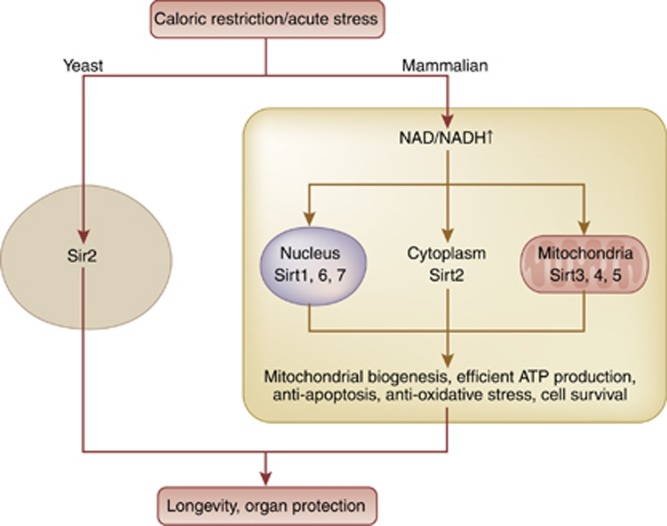
Recently, there has been an explosion of studies on sirtuin in health and diseases. The founding member of the sirtuin gene family was originally found in yeast as silent information regulator 2, Sir2.1 In 1986, the Sir2 gene was isolated and identified as a gene associated with lifespan of cells from yeast.2 In late 1990s, a study demonstrated that deletion of Sir2 shortens yeast life span and that Sir2 overexpression extends yeast life span.3 Sir2 came to be known as a longevity-related factor.4 However, a possible mechanism was not elucidated until a study showing the true enzyme activity of Sir2 as a nicotine amide dinucleotide (NAD+)–dependent histone deacetylase.5 Sir2 comprises the class II family of histone deacetylase enzymes. Unlike class I and class II, which requires only zinc as a cofactor, Sir2 depends on NAD+ for activation. In the presence of NAD+, Sir2 catalyzes the conversion of an acetylated substrate to a deacetylated substrate with O-acetyl-ADP-ribose and nicotinamide as side products.6 Sirtuins are mammalian homologs of Sir2, which are composed of seven isoforms Sirt1 to Sirt7. These seven isoforms share the same universal catalytic core region composed of 275 amino acids and show a diverse subcellular localization. Sirt1, Sirt6, and Sirt7 are mainly found in the nucleus, Sirt2 is in the cytoplasm, while Sirt3, Sirt4, and Sirt5 are localized in mitochondria.7 Among the seven isoforms, Sirt1 is the most studied, is homologous to Sir2, and is induced by calorie restriction, which has been verified as a life-extending process in mammals (Figure 1). Since substrates of Sirt1 vary from transcription factors that are involved in energy metabolism, including glucose and lipid metabolism, Sirt1 may have an important role in a number of biological processes like cell apoptosis, cell survival, longevity, and stress resistance. Human Sirt1 has been implicated to have a role in a number of age-related diseases like diabetes, neurodegenerative diseases, and kidney diseases. In particular, Sirt1 deacetylates uncoupling protein-1, peroxisome proliferator-activated receptor α, peroxisome proliferator-activated receptor gamma coactivator α, and peroxisome proliferator-activated receptor γ and increases their activities.8 Hence, the functions of Sirt1 are intimately related to pathological conditions in diabetes and insulin resistance. Systemic and liver-specific Sirt1 knockout leads to insulin resistance in mice,9, 10 and adipose tissue–specific Sirt1 knockout increases obesity in high-fat diet-fed mice.11 In parallel with those observations, systemic Sirt1 transgenic mice show reduced insulin resistance,12 and adipose tissue–specific Sirt1 overexpression leads to reduced obesity in high-fat diet-fed mice.11 Thus, Sirt1 enables effective use of biogenic energy and increases insulin sensitivity.
Figure 1.
Sirtuins and their functions. Sirtuin, a mammalian homolog of the Sir2 gene in yeasts, comprises seven isoforms. Sirt1, 6, and 7 are located predominantly in the nucleus, Sirt2 in cytoplasm, and Sirt3, 4, and 5 in mitochondria. These genes are activated or induced by calorie restriction or by acute cellular stresses mainly through increased levels of NAD+, thereby having a function in cellular survival. Changes contribute to longevity and organ protection. NAD, nicotine amide dinucleotide; Sirt, Sirtuin.
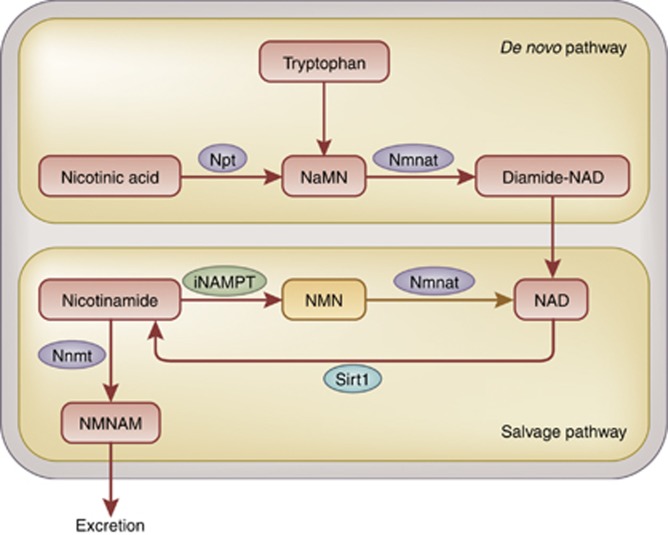
As mentioned, Sirt1 catalyzes deacetylation with the aid of the coenzyme NAD+, the cellular level of which is important for its enzymatic activity. NAD+ is also important for oxidizing and reducing reactions as a coenzyme. NAD+ is synthesized through two biological pathways, these being de novo synthesis using an essential amino-acid tryptophan supplied by dietary intake, and a salvage pathway in which NAD+ is resynthesized from nicotinamide (NAM). The rate-limiting factor in the salvage pathway is nicotinamide phosphoribosyltransferase (NAMPT), which catalyzes the synthesis of nicotinamide mononucleotide (NMN) from NAM and 5'-phosphoribosyl-1-pyrophosphate (Figure 2).13 Two NAMPT have been identified in mammals: intracellular NAMPT (iNAMPT) and extracellular NAMPT (eNAMPT), found in the circulation. iNAMPT is known to be involved in energy metabolism.14 For instance, overexpression of iNAMPT in the liver suppresses induction of hepatic steatosis.15 It is also reported that systemic administration of iNAMPT-expression vector alleviates abnormal glucose tolerance.16 Calorie restriction increases the expression level of iNAMPT in muscles and mitochondria.17 These effects are possibly due to the activation of Sirt1, which is caused by activation of iNAMPT, followed by an increase in NAD+ supply. Activation of Sirt1 leads to activation of the salvage pathway through an increase in NAM and following activation of iNAMPT. At the same time, activation of Sirt1 leads to suppression of the clock genes, thereby reducing iNAMPT expression. This prevents excess activation of Sirt1 and iNAMPT and an excess in the positive feedback loop.18, 19 NAD, iNAMPT, and Sirt1 are currently recognized as important new factors in energy metabolism.
Figure 2.
Nicotinic acid metabolism. NAD+, an essential factor in cellular respiration and metabolism, is produced through metabolism of nicotinic acid. NAD+ is synthesized via a de novo pathway from essential amino acid. NAD+ is also recycled through a salvage pathway where iNAMPT acts as a rate-limiting enzyme. In case NAD+ salvage was insufficient, Sirt1 activity decreased, further downregulating iNAMPT activity and salvage pathway. NAD, nicotine amide dinucleotide; Npt, nicotinic acid phosphoribosyltransferase; NaMN, nicotinic acid mononucleotide; Nmnat, nicotinamide mononucleotide adenylyltransferase; NMN, nicotinamide mononucleotide; iNAMPT, intracellular nicotinamide phosphoribosyltransferase; NMNAM, n-methylnicotinamide; Nnmt, nicotinamide n-methyltransferase.
THE RENAL PROTECTIVE EFFECTS OF SIRTUIN
Sirtuin increases resistance against acute stress and also regulates energy metabolism. Increasing evidence suggests that sirtuin provides protective effects against the onset and development of various renal disorders.
Acute kidney injury
The renal protective effects of sirtuin have been demonstrated using various models of acute kidney injury. For example, using the cisplatin-induced kidney injury model, researchers found that Sirt1 exhibited renal protective effects by deacetylation of p53 and by inducing catalase activity, which lead to anti–apoptotic and anti–oxidant effects, respectively.20 Deacetylation and activation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha by a specific Sirt1 activator, SRT1720, increases biogenesis of mitochondria, mitochondrial proteins as well as mitochondrial respiration. As a result, a differentiated, polarized tubule epithelium is restored, leading to a decrease in ischemia-reperfusion injury by SRT1720.21 These functions are related to the protection against acute tissue or cell insults by effective energy utilization. It was recently reported that Sirt2 also exhibits a renal protective effect through deacetylation of mitogen-activated protein kinase phosphatase-1 and the p65 subunit of nuclear factor-κB (NF-κB) in the lipopolysaccharide-induced acute kidney injury model. SIRT2 regulated p65 binding to the promoters of chemokines, CXCL2 and CCL2 and expressions of these chemokines leading to anti–inflammatory effects.22 Stanniocalcin (STC)-1 and -2 are secreted glycoproteins, which are known to regulate serum calcium and phosphate homeostasis. STC1 is important for activation of AMP kinase in the kidney, which mediates STC1-induced expression of uncoupling protein-2 and Sirt3 leading to protection from ischemia-reperfusion injury. The suppression of ischemia-reperfusion injury by STC-1 is via anti–oxidant effect.23 All of these effects are related to the anti–inflammatory and anti–oxidative effects of sirtuin.
Renal fibrogenesis
Sirtuin prevents renal fibrogenesis in a model of unilateral ureteral obstruction. Using an activator and inhibitor of sirtuin, it was demonstrated that Sirt1 and Sirt2 suppressed renal fibrogenesis by suppressing phosphorylation of STAT3 (signal transducer and activator of transcription 3), epidermal growth factor receptor, and platelet-derived growth factor receptor.24 Using Sirt1 heterozygous knockout mice, it was also shown that Sirt1 suppressed oxidative stress and inflammation in the interstitium through suppression of the expression of cyclooxygenase-2 and inhibited renal oxidative stress and fibrogenesis.25 Additionally, endothelial cell-specific Sirt1 knockout mice showed significant increases of folic acid–induced fibrogenesis. In the kidneys of endothelial cell–specific Sirt1 knockout mice, impaired angiogenesis, reduced matrilytic activity, and retention of the profibrotic cleavage substrates tissue transglutaminase and endoglin accompanied the suppressed expression of matrix metalloproteinase-14. The restoration of matrix metalloproteinase-14 expression in these mice improved angiogenic and matrilytic functions of the endothelium, prevented renal fibrosis, and ameliorated nephrosclerosis.26 Sirt1 was also shown to inhibit the tissue fibrosis by deacteylating Smad4 and repressing the effect of transforming-growth factor β signaling on a Smad4 target gene, matrix metalloproteinase-7. Consequently, less E-cadherin is cleaved from the cell surface and β-catenin remains bound to E-cadherin at the cell–cell junctions leading to a reduced fibrosis.27
Cyst formation
The role of Sirt1 in renal cyst formation was recently reported. Mice lacking both Pkd1, the causative gene for polycystic kidney disease, and Sirt1 showed decreased cyst formation. Administration of Sirt1 inhibitor to Pkd1 knockout mice decreased the cyst formation rate.28 Overexpression of Sirt2 in renal epithelial cells disrupted the cilia formation observed in the Pkd1 knockdown. The aberrant centrosome amplification and polyploidy in Pkd1 mutant or depleted cells in polycystic kidney disease was mediated through overexpression of Sirt2.29 These functions of Sirt1 and Sirt2 suggest their involvement in cystic kidney disease and ciliopathy-associated disease progression. In the former study, Sirt1 induced renal epithelial cell proliferation by inhibiting the function of both Rb and p53 through the deacetylation of these molecules.28 This effect promotes continuous epithelial cell growth and cyst formation, which enhances the disease progression. On the other hand, this effect is favorable for the recovery from the kidney damages where epithelial cells are subjected to apoptosis in ischemia-reperfusion injury or in acute tubular necrosis as already described above. Therefore, the role of Sirtuin in the initiation and progression of disease varies based on the disease background.
Renal aging
Activation of sirtuin prevents age-related impairment in renal functions. Mice lacking angiotensin II type 1a receptor show a prolonged lifespan compared with wild-type mice. These mice developed decreased cardiac and vascular injury, and multiple organs from these mice show less oxidative damage than wild-type mice. The increased number of mitochondria and upregulation of Nampt and Sirt3 in the kidney were associated with the longevity phenotype.30 Although calorie restriction is known to extend the lifespan of mice, this effect is canceled in Sirt1 heterozygous knockout mice. Sirt1 mediates deacetylation of forkhead box (Fox) O3a and induces expression of BCL2/adenovirus E1B 19-kDa interacting protein 3 (Bnip3), which is a key molecule in mitophagy, leading to longevity. This study highlights the role of the Sirt1–Foxo3 axis in cellular adaptation to hypoxia.31 The aged kidney shows increased vulnerability to acute stresses, such as ischemia-reperfusion. It has been reported that the vulnerability of the aged kidney to acute injury improves with administration of an activator of Sirt1, SRT-1720, and is further impaired in Sirt1 heterozygous knockout mice. The mechanisms underlying the protective effect of Sirt1 include the suppression of cell apoptosis.32
Diabetic nephropathy
Many studies have examined the roles of sirtuin in the onset and development of diabetic nephropathy. 3,5-diiodothyronine activates Sirt1, which deacetylates and inactivates NF-κB in mesangial cells. This results in suppression of phosphorylation of c-JUN and suppression of mesangial expansion and decreases urinary proteins.33 Sirtuin also has a role in podocyte injury in diabetic nephropathy. Podocyte-specific Sirt1 knockout mice show increased urinary proteins in diabetic nephropathy, suggesting that Sirt1 deacetylates and inactivates the p65 subunit of NF-κB and STAT3 and suppresses impairment and dysfunction of podocytes.34 It is also reported that Sirt1 deacetylates FoxO4 and decreases expression of a pro-apoptotic factor, Bcl2L11. This results in decreased apoptotic degeneration of podocytes and reduction of urinary proteins.35
The protective roles of sirtuin in kidney cells
The renal effects of sirtuin are conferred via activation of the canonical molecular targets of sirtuin, such as PCG1α and p53. Several reports have identified kidney cell–specific pathways activated by sirtuin. For example, cortactin is an F-actin binding protein involved in cell migration and cytoskeleton organization in various cells including podocytes. Sirt1 interacts with and induces deacetylation and activation of cortactin. A recent study has revealed that Sirt1 is required for the maintenance of cytoskeletal integrity by regulating cortactin function in podocytes. In podocyte-specific Sirt1-deficient mice, increases in urinary albumin excretion and severity of glomerular injury were significantly greater compared with wild-type mice, and also exhibited enhanced podocyte foot process effacement.36 By using Sirt3-deficient embryonic fibroblasts, another study reported that mitochondrial sirtuin, Sirt3 interacts with the 39-kDa protein NDUFA9, a subunit of complex I in the mitochondrial electron transfer system, thereby activating complex I and increasing ATP production. In the kidney of Sirt3-deficient mice, the basal ATP content decreased.37 In mouse mesangial cells, Sirt1 interacts with and induces deacetylation of Smad7, a mediator of transforming-growth factor β-induced cellular apoptosis. Through this process, Sirt1 inhibits transforming-growth factor β-induced mesangial cell apoptosis.38
Sirtuin activator
Sirtuin activator has gained increased attention as a potential renoprotective agent based on the recently reported renal protective effects of sirtuin. The most recognized activator of Sirt1 is resveratrol, a type of polyphenol. The renal protective effect of resveratrol was demonstrated in models of diabetic nephropathy: the streptozotocin (STZ)-administered type 1 diabetes model39 and the type 2 diabetes db/db mice model.40 It is reported that resveratrol suppresses renal lesions in obesity-related nephropathy, which is caused by a high-fat intake.41 Resveratrol also shows a renal protective effect in models of acute kidney injury such as the cisplatin-induced model, the ischemia-reperfusion injury model, and the sepsis-associated model.42, 43, 44
The current mechanisms proposed for how resveratrol exhibits its renal protective effects include various pathways besides activation of Sirt1; for example, activation of AMPK or superoxide dismutase 2, and anti–oxidant and anti–inflammatory effects. A synthetic activator of Sirt1, SRT1720, has also been reported to have a renal protective effect in a model of ischemia-reperfusion injury.45 However, these studies were conducted using rodent models, and it is necessary to examine the effects of sirtuin activators in humans, with particular attention being paid to the dosage.
Human studies
Despite its apparent pivotal role in the pathogenesis of kidney disease, clinical evidence showing the relationship between Sirt1 expression and human disease progression is scarce. One study examined the relationship between the biological properties of kidney tumors and Sirt1 expression and found that mutated alleles of Sirt1 in tumor samples of hereditary type 2 papillary renal cell carcinoma caused activation of the tumor-related gene, nuclear factor-like 2.46 Another study examined the expression of Sirt1 in peripheral mononuclear cells of patients with stage 2 and 3 chronic kidney disease and found that treatment with the phosphate-lowering reagent, sevelamer carbonate, lowered serum inflammatory markers and increased mRNA expression of Sirt1 in peripheral mononuclear cells. These findings suggest novel actions of sevelamer carbonate on metabolic and inflammatory abnormalities in early diabetic chronic kidney disease.47 Using human kidney biopsy samples, a novel pathogenic mechanism was confirmed in diabetic nephropathy where the lack of glomerular Sirt1 epigenetically increased the expression of the tight junction protein claudin-1, thereby aggravating proteinuria.48 This study is discussed below.
SIRT1 EXPRESSION IN PROXIMAL TUBULES AND ABNORMAL NICOTINE METABOLISM IN DIABETES
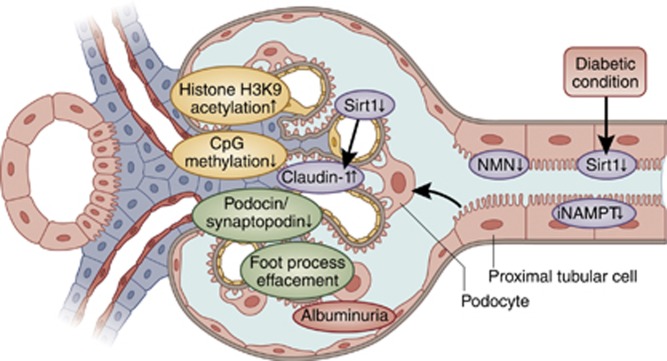
Recently, the disease-initiating role of metabolic disruption in proximal tubular cells is reported in diabetic nephropathy, which is triggered by downregulation of Sirt1 (Figure 3).48
Figure 3.
Tubule–glomeruli communication hypothesis. Primary downregulation of Sirtuin (Sirt1) or iNAMPT reflects metabolic disruption of proximal tubular cells in the diabetic condition and initiates albuminuria. This downregulation decreases NMN excretion from proximal tubules, which is taken up by podocytes upstream and close to proximal tubules. Reduced uptake levels of NMN decrease Sirt1 expression in podocytes, which epigenetically upregulate claudin-1 expression through increased acetylation of histone H3K9 and through the hypermethylation of the claudin-1 gene CpG island. Ectopic overexpression of claudin-1 in podocytes disrupts the slit membrane structure via reduced expression of synaptopodin and podocin. This molecular change leads to changes in the podocyte structure and foot process effacement. Finally, the molecular events propagating proximal tubular cells to podocytes initiate albuminuria in diabetic nephropathy. NMN, nicotinamide mononucleotide; iNAMPT, intracellular nicotinamide phosphoribosyltransferase.
Proximal tubule–specific Sirt1 genetically modified mice
Proximal tubules, which actively mediate reabsorption and require vast quantities of energy, suggesting the potential importance of Sirt1 in this tissue. Sirt1 is also thought to have a critical role in nephropathy and renal injury in diabetes, an energy metabolism dysfunction disease. The biological significance of Sirt1 was examined using proximal tubule–specific Sirt1 genetically modified mice. In STZ-induced diabetic mice models, expression of Sirt1 in proximal tubules of wild-type mice was significantly reduced at 8 weeks after STZ administration, and further reduced at 24 weeks. Reduction in glomeruli was first observed at 24 weeks after administration. At 8 weeks, serum glucose increased, whereas albuminuria, signifying glomerular failure, was not yet observed, indicating that proximal tubule integrity was disrupted at the molecular level before glomerular failure. Overexpression of Sirt1 in proximal tubules of transgenic mice improved the downregulation of Sirt1 not only in proximal tubules but also in podocytes at 24 weeks after STZ administration. Although Sirt1 was overexpressed only in proximal tubules and serum glucose levels did not fall in transgenic mice, albuminuria at 24 weeks was scarcely detected and foot process effacement of podocytes was mitigated. The amelioration of albuminuria in transgenic mice was not complete and it is deduced that Sirt1 overexpression in proximal tubules followed by Sirt1 recovery in podocytes overcame, to a certain extent, a harmful influence of high glucose on podocytes.
Gene profiling showed a significant difference in expression levels of claudin-1, a tight junction component, between STZ-treated wild-type and transgenic mice. In wild-type mice, claudin-1 expression was only detected in parietal epithelial cells (PEC) of the Bowman' capsule, and was strongly expressed in PEC and glomerular podocytes in the diabetic model. The increased expression was suppressed in transgenic mice. Using laser microdissection, it was observed that STZ administration in wild-type mice resulted in decreased Sirt1 expression in both proximal tubules and PEC and increased claudin-1 expression in PEC. In transgenic mice, although the mice overexpressed Sirt1 only in proximal tubules, the Sirt1 expression level increased also in PEC, and claudin-1 expression decreased in PEC.
Introduction of claudin-1 expression vector into cultured podocytes led to suppression of podocin and synaptopodin expression, which regulate the albumin permeability of podocytes. Furthermore, the injection of claudin-1 gene-integrated Sendai virus vector successfully led to overexpression of claudin-1 protein in the kidneys of wild-type mice and resulted in increased albuminuria and aggravation of podocyte foot process effacement.48
Sirt1-mediated epigenetic gene regulation
The molecular mechanism of claudin-1 expression was examined using human renal epithelial cells. High concentration of glucose upregulated and overexpression of Sirt1 downregulated claudin-1 expression, respectively. Focused on the epigenetic regulatory mechanism by Sirt1 as a histone deacetylase, it was found that both mouse and human claudin-1 promoter contained CpG regions that are consensus gene sequences for methylation modification. Methylation-specific PCR analysis revealed that high glucose increased, and Sirt1 overexpression decreased, methylation of claudin-1 gene. Silencing DNA methyltransferase 1 (Dnmt1) abrogated Sirt1-mediated claudin-1 gene methylation. In addition, a chromatin immunoprecipitation assay showed that Sirt1 induced histone H3K9 deacetylation. Thus, Sirt1 activation epigenetically suppressed claudin-1 expression via histone H3K9 deacetylation and Dnmt1-mediated CpG methylation in podocytes and PEC. The diabetic condition resulted in a downregulation of Sirt1 in podocytes, which reduced claudin-1 gene methylation and increased claudin-1 gene expression.
In renal biopsy samples from patients with diabetic nephropathy, intrarenal expression of Sirt1 in both proximal tubular and glomerular regions decreased, and claudin-1 expression in the glomerular region increased, in the kidneys of patients with heavy proteinuria compared with those with moderate proteinuria. Claudin-1 expression negatively correlated with Sirt1 expression in both proximal tubules and glomerular regions. Among several clinical parameters, only proteinuria correlated with both proximal tubular and glomerular Sirt1 immunostaining and also with glomerular claudin-1 immunostaining. Methylation levels of claudin-1 gene negatively correlated with claudin-1 expression levels, confirming epigenetic gene regulation of claudin-1 expression in human samples.48
Renal tubule–glomeruli communication hypothesis and its mediator
These data implied that decreased Sirt1 expression in proximal tubules leads to a decrease in glomerular Sirt1 and subsequent upregulation of glomerular claudin-1. It was thought that the release of an unknown mediator from proximal tubules affected Sirt1 expression in podocytes and glomeruli. The authors48 named such a potential retrograde functional association between proximal tubules and glomeruli as ‘renal tubule–glomeruli communication' (Figure 3). In in vitro experiment, conditioned medium obtained from proximal tubule cells cultured with high glucose medium led to significant suppression of Sirt1 and upregulation of claudin-1 in cultured podocytes. This result suggested that there was a humoral factor secreted from proximal tubule cells that regulated Sirt1 and claudin-1 expressions in podocytes. NMN is an intermediary metabolite of nicotinic acid metabolism (Figure 1), and its generation decreases when Sirt1 activity decreases. In the previous report, NMN is secreted from perivascular adipose tissue and alters vascular smooth muscle cell morphology, suggesting its function as a mediator for cell-to-cell interaction.49 In proximal tubule cells, NMN decreased when cells were cultured with high glucose. Addition of NMN to the conditioned medium obtained from proximal tubule cells cultured with high glucose medium restored Sirt1 expression and reduced claudin-1 expression in podocytes. Fluorescence staining for NMN showed that the intracellular NMN level decreased in proximal tubule cells cultured with high glucose. The NMN level also decreased in the kidney of STZ-administered diabetic mice, and did not change in transgenic mice. Intrarenal administration of fluorescent-labeled NMN showed NMN first accumulated mainly in the renal tubule 1 h after administration, and was subsequently transferred to glomeruli 2–4 h after administration.
These sets of experiments provide the evidence for NMN as a mediator of renal tubule–glomeruli communication. Similar events are not detected in the 5/6 renal ablation model, suggesting that hyperglycemic stimulation is important in the pathological process. Consistently, in this study, essential parts of the results in STZ-induced diabetic mice were reproduced not only in obese-type diabetic db/db mice but also in the db/db mice crossed with proximal tubule–specific Sirt1 transgenic mice. Moreover, the phenotypes observed in diabetic mice were reproduced in tissue culture systems using proximal tubular cell line, HK-2 cell, and primary podocytes exposed to high glucose. The phenotypes in STZ-treated mice in this study are primarily due to diabetic condition, but not to STZ-induced nephrotoxicity.
NAMPT and sirtuin
Since NAMPT is a key NAD biosynthetic enzyme, it is suggested that iNMPT in proximal tubules played a crucial role in regulating Sirt1 activity in the kidney. The expression of iNAMPT is predominantly in tubular cells.48 Consistently, the concentration of NMN in the tubular area was greater than that in the glomerular area in normal mice, although these levels decreased in diabetic mice. The treatment with an iNAMPT inhibitor, FK866, provoked proteinuria in normal mice, suggesting that Sirt1 downregulation in podocytes with the onset of diabetic nephropathy was caused by the preceding downregulation of iNAMPT in proximal tubules.48
PERSPECTIVE
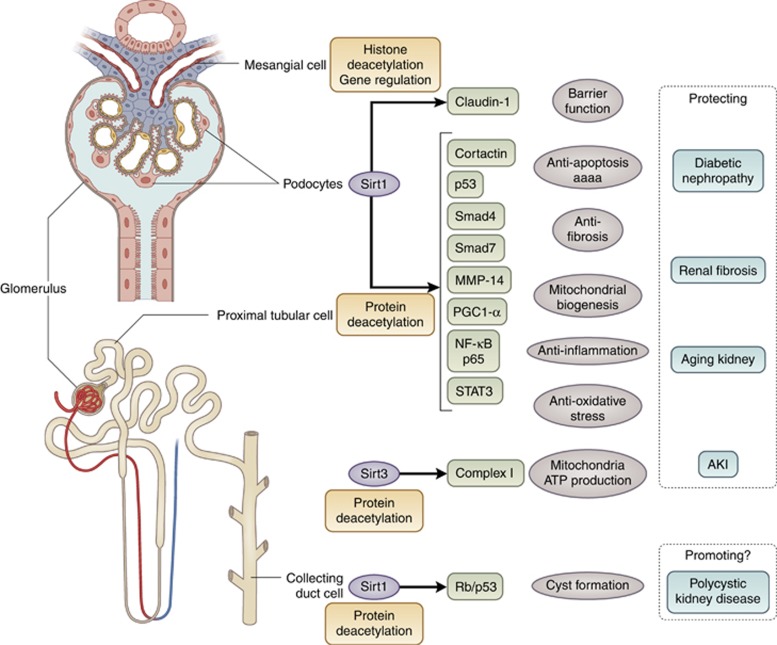
Sirt1 is a key molecule in cellular energy metabolism and exhibits renal protective effects through deacetylation and regulation of various factors such as p53, NF-κB p65 subunit, STAT, FoxO1, and FoxO3, which are transcriptional factors related to apoptosis, cellular aging, and inflammation (Figure 4). Sirtuin also exhibits histone deacetylation activity and renal protective effects through epigenetic regulation of gene expression. Recent study identified claudin-1 gene as a new target of epigenetic regulation by Sirt1. There have been few studies examining the epigenetic function–mediated renal protective effect of Sirt1, which remains to be elucidated. In addition to Sirt1, Sirt2 and Sirt3 also function in the kidney. It is therefore important to examine their roles in kidney diseases. In addition, the development of a kidney-specific sirtuin activator may be useful as a therapeutic agent for treatment of renal disorders.
Figure 4.
Scheme depicting the role of sirtuin in kidney disease. Sirt1 and Sirt3 have significant roles in the pathogenesis of various kidney diseases and in renal damage. These effects were targeted at various sites or cells of the kidney and at various downstream molecules that were subject to deacetylation or epigenetic gene regulation by sirtuin. AKI, acute kidney injury; MMP14, matrix metalloproteinase 14; NF-κB, nuclear factor-κB; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Sirt, Sirtuin.
Also of interest is elucidating the significance of NAMPT in kidney diseases, as NAMPT is a key molecule in nicotinic acid metabolism through which NAD+, the most important factor for regulation of sirtuin activity in vivo, is supplied. The role of iNAMPT as an activator of Sirt1 was reported in a recent study.19 Intriguingly, iNAMPT is expressed only in renal tubules and not in glomeruli. It is suggested that organs with low iNAMPT activity such as pancreatic β cells, neural tissues, and adipose tissues could be metabolic frailty points, and metabolic energy failure may be propagated from there throughout the entire body.13 The activity of iNAMPT is low in glomerulus-forming cells such as podocytes, mesangial cells, and endothelial cells, and thus NAD+ is easily depleted in glomeruli. Therefore, the NAD+ supply from renal tubules is necessary and reduction of iNAMPT expression in proximal tubules could be intimately related to the pathological development of diseases. Future studies using proximal tubule–specific iNAMPT gene–modified mice would solve these issues. These studies also will reveal the significance of iNAMPT in renal diseases.
All the authors declared no competing interests.
References
- 1Kim S, Benguria A, Lai CY et al. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol Biol Cell 1999; 10: 3125–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Ivy JM, Klar AJ, Hicks JB. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol 1986; 6: 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 1999; 13: 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 2005; 6: 298–305. [DOI] [PubMed] [Google Scholar]
- 5Imai S, Armstrong CM, Kaeberlein M et al. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000; 403: 795–800. [DOI] [PubMed] [Google Scholar]
- 6Guarente L., Franklin H. Epstein lecture: sirtuins, aging, and medicine. N Engl J Med 2011; 364: 2235–2244. [DOI] [PubMed] [Google Scholar]
- 7Mouchiroud L, Houtkooper RH, Auwerx J. NAD+ metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 2013; 48: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Imai S. Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett 2011; 585: 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Cheng HL, Mostoslavsky R, Saito S et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 2003; 100: 10794–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Purushotham A, Schug TT, Xu Q et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 2009; 9: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Gillum MP, Kotas ME, Erion DM et al. SirT1 regulates adipose tissue inflammation. Diabetes 2011; 60: 3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Bordone L, Cohen D, Robinson A et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007; 6: 759–767. [DOI] [PubMed] [Google Scholar]
- 13Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab 2013; 15: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Revollo JR, Körner A, Mills KF et al. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab 2007; 6: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Tao R, Wei D, Gao H et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem 2011; 286: 14681–14690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Sun Q, Li L, Li R et al. Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats. Ann Med 2009; 41: 311–320. [DOI] [PubMed] [Google Scholar]
- 17Fulco M, Cen Y, Zhao P et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 2008; 14: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Imai S. ‘Clocks' in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim Biophys Acta 2010; 1804: 1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Ramsey KM, Yoshino J, Brace CS et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009; 324: 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20Hasegawa K, Wakino S, Yoshioka K et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 2010; 285: 13045–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Funk JA, Schnellmann RG. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol 2013; 273: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Jung YJ, Lee AS, Nguyen-Thanh T et al. SIRT2 regulates LPS-induced renal tubular CXCL2 and CCL2 expression. J Am Soc Nephrol 2014. (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- 23Pan JS, Huang L, Belousova T et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol 2014; 26: 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Ponnusamy M, Zhou X, Yan Y et al. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J Pharmacol Exp Ther 2014; 350: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25He W, Wang Y, Zhang M et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 2010; 120: 1056–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Vasko R, Xavier S, Chen J et al. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol 2014; 25: 276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Simic P, Williams EO, Bell EL et al. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep 2013; 3: 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Zhou X, Fan LX, Sweeney WE Jr et al. Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J Clin Invest 2013; 123: 3084–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Zhou X, Fan LX, Li K et al. SIRT2 regulates ciliogenesis and contributes to abnormal centrosome amplification caused by loss of polycystin-1. Hum Mol Genet 2014; 23: 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Benigni A, Corna D, Zoja C et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest 2009; 119: 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Kume S, Uzu T, Horiike K et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 2010; 120: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Fan H, Yang HC, You L et al. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int 2013; 83: 404–413. [DOI] [PubMed] [Google Scholar]
- 33Shang G, Gao P, Zhao Z et al. 3,5-Diiodo-l-thyronine ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. Biochim Biophys Acta 2013; 1832: 674–684. [DOI] [PubMed] [Google Scholar]
- 34Liu R, Zhong Y, Li X et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes 2014; 63: 2440–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Chuang PY, Dai Y, Liu R et al. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One 2011; 6: e23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Motonishi S, Nangaku M, Wada T et al. Sirtuin1 maintains actin cytoskeleton by deacetylation of cortactin in injured podocytes. J Am Soc Nephrol 2014. (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- 37Ahn BH, Kim HS, Song S et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 2008; 105: 14447–14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Kume S, Haneda M, Kanasaki K et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem 2007; 282: 151–158. [DOI] [PubMed] [Google Scholar]
- 39Tikoo K, Singh K, Kabra D et al. Change in histone H3 phosphorylation, MAP kinase p38, SIR2 and p53 expression by resveratrol in preventing streptozotocin induced type I diabetic nephropathy. Free Radic Res 2008; 42: 397–404. [DOI] [PubMed] [Google Scholar]
- 40Kitada M, Kume S, Imaizumi N et al. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes 2011; 60: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Pearson KJ, Baur JA, Lewis KN et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 2008; 8: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Do Amaral CL, Francescato HD, Coimbra TM et al. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch Toxicol 2008; 82: 363–370. [DOI] [PubMed] [Google Scholar]
- 43Bertelli AA, Migliori M, Panichi V et al. Resveratrol, a component of wine and grapes, in the prevention of kidney disease. Ann NY Acad Sci 2002; 957: 230–238. [DOI] [PubMed] [Google Scholar]
- 44Holthoff JH, Wang Z, Seely KA et al. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int 2012; 81: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Khader A, Yang WL, Kuncewitch M et al. Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation 2014; 98: 148–156. [DOI] [PubMed] [Google Scholar]
- 46Ooi A, Dykema K, Ansari A et al. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res 2013; 73: 2044–2051. [DOI] [PubMed] [Google Scholar]
- 47Vlassara H, Uribarri J, Cai W et al. Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol 2012; 7: 934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Hasegawa K, Wakino S, Simic P et al. Renal tubular Sirt1 attenuates diabetic albuminuria by epigenetically suppressing Claudin-1 overexpression in podocytes. Nat Med 2013; 19: 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Wang P, Xu TY, Guan YF et al. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 2009; 81: 370–380. [DOI] [PubMed] [Google Scholar]