Abstract
Six new polycyclic polyprenylated acylphloroglucinols (PPAPs), named hyperisampsins H–M (1–6), were isolated from the aerial parts of Hypericum sampsonii, together with five known analogs (7–11). The structures of 1–6 were established by extensive spectroscopic analyses, including HRESIMS and NMR. In addition, the absolute configurations of these new compounds were determined by electronic circular dichroism (ECD) calculations. Compounds 1 and 2 represent the first examples of PPAPs possessing a unique γ-lactone ring at C-23, while 3–6 differed from normal PPAPs with an unprecedented 1,2-dioxane ring. Compounds 1–7 were evaluated for their cytotoxic activities against a panel of human cancer cell lines in vitro, of which 3, 4, and 6 exhibited significant cytotoxic activities with IC50 values ranging from 0.56 to 3.00 μM. Moreover, compound 3 induces leukemia cell apoptotic death, evidenced by activation of caspase-3, degradation of PARP, up-regulation of Bax, and down-regulation of Bcl-2 and Bcl-xl.
Polycyclic polyprenylated acylphloroglucinols (PPAPs) are a class of secondary metabolites that usually possess bicyclo[3.3.1]nonane-2,4,9-trione, adamantyl, or homoadamantyl-like core structures and associated with various bioactivities, such as anti-tumors, anti-bacterial, and anti-HIV1. Recently, studies on naturally occurring PPAPs and on the total synthesis of complex PPAPs have attracted growing interest due to their challenging structures and potential biological activities1,2,3,4,5,6. Plants of the genus Hypericum are well known as rich sources of PPAPs, and a large number of PPAPs have been discovered and reported as the main components of this genus1. Hypericum sampsonii is a traditional Chinese medicine that is used to treat backache, burns, diarrhea, and swelling. Over the past two decades, phytochemical investigations conducted by several groups have resulted in the isolation and characterization of more than 50 PPAPs with various carbon skeletons and bioactivities from this plant7,8,9,10,11,12,13,14,15,16,17,18.
As part of our continuing efforts to discover bioactive PPAPs in the genus Hypericum, a series of PPAPs and other components were previously identified19,20,21,22,23. In our current study, further chemical investigation of H. sampsonii has led to the isolation of six new PPAPs, termed hyperisampsins H–M (1–6), together with five known analogs (7–11). Compounds 1 and 2 represent the first examples of PPAPs possessing a rare γ-lactone ring at C-23, while 3–6 differed from normal PPAPs with an unprecedented 1,2-dioxane ring. Here, we report the isolation, structural elucidation, and absolute configuration determination of these new PPAPs. In addition, the cytotoxic activities of compounds 1–7 against five human cancer cell lines were evaluated, of which 3, 4, and 6 exhibited significant cytotoxic activities, with IC50 values ranging from 0.56 to 3.00 μM. The apoptosis-inducing potential of compound 3 and the mechanism of action were further studied by FACS and western blotting, and the results showed that it induced apoptosis in leukemia cells, mediated by activation of caspase-3, degradation of PARP, upregulation of Bax, and downregulation of Bcl-2 and Bcl-xl.
Results and Discussion
Isolation and Structure Elucidation
A 95% EtOH extract of the aerial parts of H. sampsonii was suspended in water and successively partitioned with petroleum ether and CHCl3. The petroleum ether and CHCl3 extracts were then subjected to a series of chromatographic separations, including silica gel, RP-C18 (reverse-phase), Sephadex LH-20, and semipreparative HPLC to yield six new (1–6) and five known (7–11) PPAPs (Fig. 1). The structures of the known compounds were elucidated by comparing their NMR data with those reported in the literature, and they were identified as attenuatumione C (7)24, sampsonione K (8)10, otogirinin D (9)25, and sampsoniones M (10)10 and N (11)11.
Figure 1. Structures of isolated compounds.
Hyperisampsin H (1), a colorless oil, had a molecular formula of C35H42O6 based on 13C NMR data and on a quasi-molecular ion peak at m/z 581.2799 [M + Na]+ in HRESIMS that required 15 indices of hydrogen deficiency. The 1H NMR (Table 1) spectrum of 1 showed resonances for a monosubstituted phenyl group (δH 7.43, 2H, d, J = 8.2 Hz; 7.38, 1H, t, J = 7.3 Hz; and 7.27, 2H, t, J = 7.6 Hz), two olefinic protons (δH 4.95, 1H, t, J = 7.2 Hz and 4.88, 1H, t, J = 7.1 Hz), an oxygenated methine (δH 4.72, 1H, dd, J = 10.3, 6.1 Hz), and seven methyl singlets (δH 1.40, 1.45, 1.48, 1.54, 1.56, 1.58, and 1.69). The 13C NMR and DEPT (Table 2) of 1 revealed 35 carbons, which resolved into three carbonyls, one ester carbonyl, a monosubstituted benzene ring, six olefinic carbons, four quaternary carbons (one oxygenated), two methines (one oxygenated), six methylenes, and seven methyls. These characteristic NMR data suggested that 1 was a PPAP derivative related to sampsonione K (8)10.
Table 1. 1H NMR Data for Compounds 1–6 in CDCl3 (400 MHz, J in Hz).
| no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 6α | 2.29 d (14.1) | 2.34 d (14.1) | 2.30 d (14.1) | 2.30 d (14.2) | 2.36 d (14.1) | 2.31 d (14.1) |
| 6β | 2.19 dd (14.1, 7.3) | 2.18 dd (14.1, 7.5) | 2.16 m | 2.16 m | 2.22 m | 2.18 m |
| 7 | 1.50 m | 1.51 m | 1.50 m | 1.48 m | 1.56 m | 1.51 m |
| 12 | 7.43 d (8.2) | 7.44 d (8.2) | 7.45 d (7.4) | 7.45 d (8.0) | 7.51 d (7.3) | 7.46 d (8.5) |
| 13 | 7.27 t (7.6) | 7.23 t (7.8) | 7.21 t (7.8) | 7.22 t (7.8) | 7.27 t (7.8) | 7.21 t (7.8) |
| 14 | 7.38 t (7.3) | 7.39 t (7.4) | 7.38 t (7.4) | 7.38 t (7.4) | 7.44 t (7.4) | 7.38 t (7.4) |
| 15 | 7.27 t (7.6) | 7.23 t (7.8) | 7.21 t (7.8) | 7.22 t (7.8) | 7.27 t (7.8) | 7.21 t (7.8) |
| 16 | 7.43 d (8.2) | 7.44 d (8.2) | 7.45 d (7.4) | 7.45 d (8.0) | 7.51 d (7.3) | 7.46 d (8.5) |
| 17a | 3.09 dd (14.2, 7.9) | 3.07 dd (14.2, 7.0) | 3.08 dd (13.9, 7.4) | 3.08 dd (13.9, 7.4) | 3.13 dd (14.2, 7.5) | 3.08 dd (13.9, 7.7) |
| 17b | 2.91 m | 2.97 dd (14.2, 7.6) | 3.00 dd (13.9, 7.8) | 3.00 dd (13.9, 7.9) | 3.05 dd (14.2, 7.6) | 3.01 dd (13.9, 7.7) |
| 18 | 4.95 t (7.2) | 4.94 t (7.0) | 5.07 t (7.6) | 5.07 t (7.5) | 5.11 t (7.5) | 5.05 t (7.7) |
| 20 | 1.58 s | 1.60 s | 1.61 s | 1.61 s | 1.65 s | 1.60 s |
| 21 | 1.56 s | 1.60 s | 1.63 s | 1.63 s | 1.66 s | 1.61 s |
| 22a | 2.88 m | 2.46 dd (13.3, 11.1) | 2.63 dd (13.3, 11.0) | 2.64 dd (13.3, 10.8) | 2.65 dd (13.3, 10.9) | 2.58 dd (13.3, 11.0) |
| 22b | 1.91 dd (13.3, 6.1) | 1.92 dd (13.3, 5.8) | 1.86 dd (13.3, 5.8) | 1.86 m | 1.90 m | 1.86 dd (13.3, 6.1) |
| 23 | 4.72 dd (10.3, 6.1) | 4.88 dd (11.1, 5.8) | 4.67 dd (11.0, 5.9) | 4.65 dd (10.8, 5.8) | 4.78 m | 4.75 m |
| 25a | 2.54 dd (13.1, 2.2) | 2.14 m | 1.88 m | 1.88 m | 1.88 m | 1.81 m |
| 25b | 2.16 m | 2.01 dt (13.2, 8.4) | 1.81 m | 1.78 m | 1.72 m | 1.66 m |
| 26 | 1.45 s | 1.47 s | 1.35 s | 1.37 s | 1.37 s | 1.31 s |
| 27a | 2.79 m | 2.69 t (8.4) | 1.97 m | 1.87 m | 1.90 m | 1.96 m |
| 27b | 2.66 m | 1.82 m | 1.62 m | 1.81 m | ||
| 28 | 3.91 dd (10.6, 3.2) | 4.27 dd (9.7, 3.2) | 4.29 dd (10.8, 3.2) | 3.88 dd (11.0, 3.5) | ||
| 30 | 1.23 s | 1.27 s | 1.32 s | 1.23 s | ||
| 31 | 1.27 s | 1.28 s | 1.38 s | 1.28 s | ||
| 32 | 2.16 m | 2.14 m | 2.16 m | 2.16 m | 2.22 m | 2.18 m |
| 33 | 4.88 t (7.1) | 4.87 t (7.5) | 4.87 t (7.2) | 4.87 t (7.2) | 4.93 t (7.2) | 4.87 t (7.1) |
| 35 | 1.69 s | 1.69 s | 1.69 s | 1.69 s | 1.66 s | 1.69 s |
| 36 | 1.54 s | 1.53 s | 1.54 s | 1.53 s | 1.75 s | 1.54 s |
| 37 | 1.48 s | 1.48 s | 1.48 s | 1.47 s | 1.59 s | 1.49 s |
| 38 | 1.40 s | 1.41 s | 1.40 s | 1.40 s | 1.45 s | 1.40 s |
Table 2. 13C NMR Data for Compounds 1–6 in CDCl3 (100 MHz).
| no. | 1 | 2 | 3 | 4 | 5 | 6 | no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76.9 | 77.2 | 77.2 | 77.2 | 77.7 | 77.1 | 20 | 25.6 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 |
| 2 | 193.8 | 193.7 | 193.8 | 193.9 | 193.8 | 193.7 | 21 | 17.7 | 17.7 | 17.8 | 17.8 | 17.8 | 17.8 |
| 3 | 116.0 | 116.2 | 115.8 | 115.8 | 115.8 | 115.8 | 22 | 31.1 | 31.8 | 31.0 | 30.9 | 31.0 | 31.1 |
| 4 | 171.4 | 171.5 | 172.2 | 172.2 | 172.6 | 172.2 | 23 | 88.3 | 87.0 | 87.1 | 87.1 | 86.8 | 86.7 |
| 5 | 58.4 | 58.4 | 58.2 | 58.2 | 58.2 | 58.2 | 24 | 84.1 | 85.7 | 80.9 | 80.9 | 81.5 | 81.7 |
| 6 | 36.9 | 36.4 | 36.5 | 36.5 | 36.7 | 36.6 | 25 | 31.3 | 27.7 | 27.5 | 27.5 | 27.5 | 28.1 |
| 7 | 47.6 | 47.6 | 47.7 | 47.7 | 47.7 | 47.7 | 26 | 23.1 | 23.1 | 17.3 | 17.4 | 15.4 | 15.3 |
| 8 | 49.2 | 49.4 | 49.3 | 49.3 | 49.2 | 49.2 | 27 | 28.9 | 28.6 | 19.2 | 19.4 | 19.4 | 18.9 |
| 9 | 204.9 | 205.1 | 205.3 | 205.8 | 205.4 | 205.5 | 28 | 175.5 | 175.3 | 87.0 | 84.4 | 84.3 | 86.9 |
| 10 | 193.3 | 193.2 | 193.4 | 193.4 | 193.4 | 193.4 | 29 | 72.0 | 83.1 | 83.0 | 72.1 | ||
| 11 | 136.5 | 136.9 | 136.9 | 136.9 | 136.9 | 136.8 | 30 | 24.9 | 20.6 | 20.6 | 24.7 | ||
| 12 | 128.1 | 128.0 | 128.1 | 128.1 | 128.1 | 128.1 | 31 | 26.2 | 21.1 | 21.1 | 25.8 | ||
| 13 | 128.1 | 127.9 | 127.9 | 127.9 | 127.9 | 127.9 | 32 | 29.0 | 28.9 | 29.0 | 28.9 | 28.9 | 28.9 |
| 14 | 132.1 | 132.1 | 131.9 | 132.0 | 132.0 | 132.0 | 33 | 124.4 | 124.0 | 124.3 | 124.3 | 124.3 | 124.2 |
| 15 | 128.1 | 127.9 | 127.9 | 127.9 | 127.9 | 127.9 | 34 | 132.8 | 133.2 | 132.9 | 133.0 | 133.0 | 133.0 |
| 16 | 128.1 | 128.0 | 128.1 | 128.1 | 128.1 | 128.1 | 35 | 25.8 | 25.8 | 25.8 | 25.8 | 25.8 | 26.1 |
| 17 | 22.4 | 22.3 | 22.4 | 22.4 | 22.3 | 22.3 | 36 | 17.7 | 17.8 | 17.7 | 17.7 | 17.8 | 17.8 |
| 18 | 119.0 | 119.2 | 119.6 | 119.5 | 119.4 | 119.4 | 37 | 22.2 | 22.2 | 22.3 | 22.2 | 22.3 | 22.3 |
| 19 | 133.4 | 133.2 | 132.6 | 132.6 | 132.9 | 132.8 | 38 | 26.9 | 26.9 | 27.0 | 26.9 | 26.9 | 26.9 |
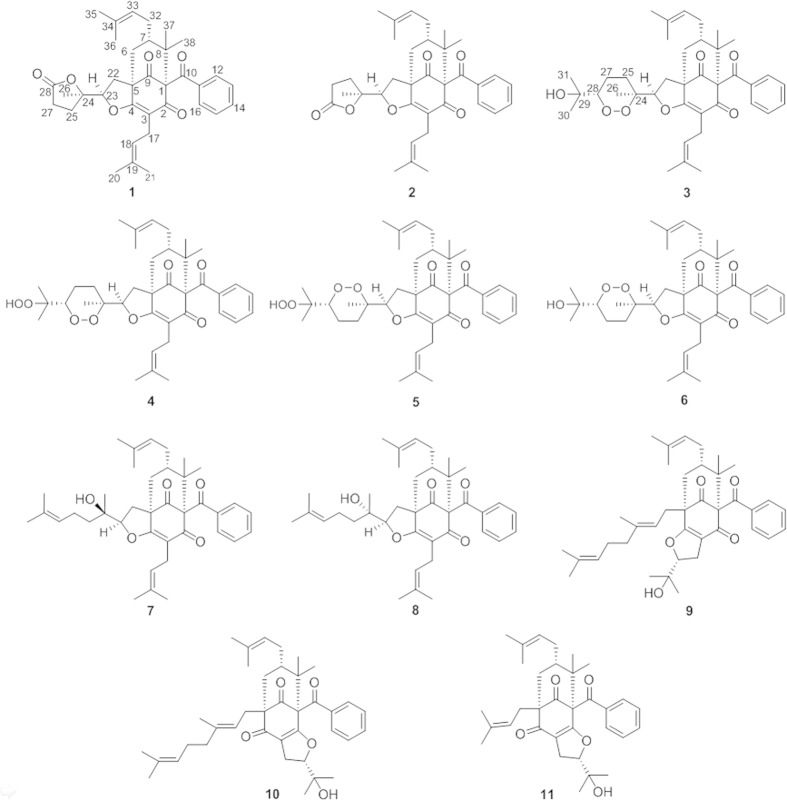
A side-by-side comparison of the 1H and 13C NMR data of 1 with those of 8 along with a tracing of the connectivities observed in the HMBC and 1H–1H COSY spectra (Fig. 2) revealed that both 1 and 8 have the same bicyclo[3.3.1]nonane core structure. However, compound 1 differs from 8 by the absence of two olefinic carbons and two methyl groups, which, together with the presence of an unexpected carboxyl group at δC 175.5 on the side chain at C-23, imply that 1 is an oxidative degradation product of 810. This conclusion was supported by the 1H–1H COSY correlations of H-25/H-27 and H-22/H-23 and by the long-range HMBC interactions from H-23 to C-4, H-26 to C-23, C-24, and C-25, and from H-25 to C-23, C-27, and C-28. In addition, with 14 indices of hydrogen deficiency occupied by the tricyclic core, a phenyl group, three carbonyls, and three double bonds, the remaining index indicated the presence of an unprecedented γ-lactone ring between C-28 (δC 175.5) and C-24 (δC 85.7).
Figure 2.
(a) Selected 2D NMR correlations for the core structures of 1–6; (b) Newman projections for C-24/C-23 of 1–6; (c) key NOESY correlations for the 1,2-dioxane ring of 3.
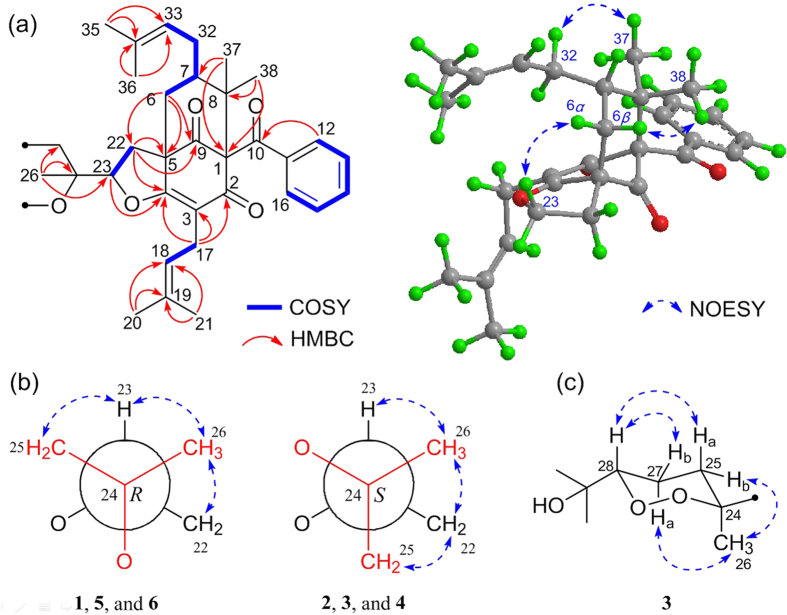
The relative configurations at C-1, C-5, and C-7 of the core skeleton of 1 were established through a NOESY experiment (Fig. 2) and was further confirmed by comparing its 1H and 13C NMR data (Tables 1 and 2) with those of sampsonione K (8)10. The NOESY correlation between Me-38 and H-6β revealed their cofacial and axial relationship. Therefore, the large coupling constant of H-6β/H-7 (J6β/7 = 7.3 Hz) and the absence of coupling between H-6α and H-7 indicated that H-7 was equatorial and β oriented. These analyses were also supported by the literature1, which indicated that when the isoprenyl at C-7 is in an axial position, the deviation of the chemical shifts of the two protons attached to C-6 should be in the range of 0.0–0.2 ppm, while the chemical shift of C-7 should be in the range of 45–49 ppm1. With compound 1, the deviation of the chemical shifts of H-6α and H-6β (ΔδH = 0.1 ppm), along with that of C-7 (δC 47.6), corresponded well with the above conditions, confirming that the isoprenyl at C-7 was in the axial orientation. Furthermore, the α-configuration of H-23 was determined by the NOESY correlation of H-23/H-6α, which was identical to that of 810. To determine the configuration of C-24, the rotation energy barrier of the single bond C-23/C-24 was calculated (Fig. 3a)26. As the literature reported, rotational energy barrier of 20 kcal mol–1 was a threshold to distinguish between atropisomers and non-atropisomers26, therefore, theoretically, the carbon-carbon bond between C-23 and C-24 could rotate to certain extent. However, the calculated Boltzmann distribution of the most stable conformation of 1 (conformation 1a) could reach up to 96.09% (Fig. 3), which together with the observed NOESY correlations (Fig. 2b) of Me-26 with H-23 and H-22 and of H-23/H-25 indicated that this rotation was restricted27, and suggested a R* configuration for C-24. Thus, the structure of 1 was elucidated as shown, and it represents the first example of a PPAP with a unique γ-lactone ring.
Figure 3.
(a) Energy profiles displayed for the torsion angle of C-23/C-24 of compound 1; (b) Three stable conformations of compound 1 showing the rotation of C-23/C-24; (c) Abbreviated Newman drawing of C-23/C-24 of 1.
The molecular formula of hyperisampsin I (2) was identical to that of 1 as revealed by the HRESIMS spectrum (m/z 581.2798, [M + Na]+). The structural elucidation of 2 was straightforward, as a comparison of its 1H and 13C NMR data (Tables 1 and 2) with those of 1, suggested that 1 and 2 shared great structural similarity. Careful analysis of the 2D NMR (1H–1H COSY and HMBC) spectra of 2 suggested that the planar structure of 2 was identical to that of 1. The relative configurtions at C-1, C-5, and C-7 were established to be identical to those of 1 in the same mannar as described for 1. In additon, the NOESY correlation of H-23/H-6α suggested that the H-23 of 2 was also α-oriented. Therefore, the only difference between 2 and 1 was the relative configuration of C-24, which was revealed by the NOESY cross-peaks (Fig. 2b) of Me-26 with H-22 and H-23 and of H-22 with H-25. The relative configuration of C-24 of 2 was thereby elucidated as S*, and compound 2 was revealed as the C-24 epimer of 1.
Hyperisampsin J (3) was isolated as a colorless oil. A molecular formula of C38H50O7 was elucidated by the HRESIMS spectrum, indicating 14 indices of hydrogen deficiency. Extensive comparison of the 1H and 13C NMR (Tables 1 and 2) data of 3 with those of 8 suggested that the structure of 3 resembled that of 810 except for the absence of the double bond at C-28 and C-29 in 8 and the presence of two oxygenated carbons (δC 87.0 and 72.0) in 3. Careful analysis of the HMBC and 1H–1H COSY spectra of 3 revealed that it possessed the same carbon connectivities as 8, albeit with significantly higher degrees of oxidation. Considering the chemical shifts of C-24 (δC 80.9) and C-28 (δC 87.0) in 3, along with the 14 indices of hydrogen deficiency required by its HRESIMS, it was reasonable to locate a 1,2-dioxane ring at C-23 via a peroxide linkage between C-24 and C-2813, which was also supported by the TLC detection colorated with KI-starch (Figure S1). This deduction was further confirmed by a comparison of the 13C NMR chemical shift values of this fragment (sequence from C-24 to C-28) in 3 with those of the 1,2-dioxane ring reported in the literature28. Thus, the planar structure of 3 was elucidated.
The relative configurations of C-1, C-5, C-7, and C-23 in 3 were established to be the same as those of 1 by careful analyses of the 1H and 13C NMR spectra and the NOESY spectra (Fig. 2). Considering the distributions of 1 based on the former conformation analyses, the S* configuration of C-24 in 3 was determined by NOESY correlations from Me-26 to H-23 and H-22 and from H-22 to H-25. Molecular modeling depicted the 1,2-dioxane ring to be in a chair conformation containing an axially oriented methyl group (Me-26) and an equatorially orientated carbon-carbon single bond at C-24/C-23. Additionally, the NOESY (in C5D5N, Fig. 2c) correlations from H-27a to H-25b and Me-26 and from H-28 to H-25a and H-27b, together with the large coupling constant of H-28/H-27a (J = 10.6 Hz), revealed that H-28 was axial and β-oriented. Therefore, the relative configuration of 3 was established, and it appears to be the first example of PPAPs possessing an unexpected 1,2-dioxane ring.
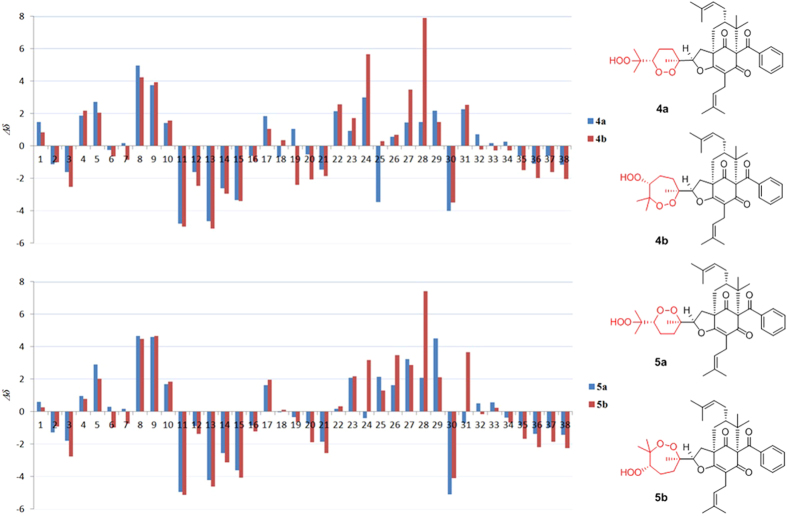
Detailed inspection of the 1H and 13C NMR spectroscopic data (Tables 1 and 2) of compounds 4–6 indicated that their structures closely resembled that of 3. Comprehensive analyses of the HMBC, 1H–1H COSY, and HRESIMS spectra of 4–6 suggested that compound 6 has the same planar structure as that of 3, while 4 and 5 might have structures with 1,2-dioxane rings or 1,2-dioxepane rings (4a/4b and 5a/5b, Fig. 4). In the end, the complete structures of 4 and 5 were determined to be 4a and 5a by comparing the experimental 13C NMR data with those calculated for 4a, 4b, 5a, and 5b (Fig. 4). The relative configurtions at C-1, C-5, C-7, and C-23 of 4–6 were identical to those of 3 as revealed by their NOESY experiments and coupling constants. Consequently, the NOESY correlations from Me-26 to H-23 and H-22 and from H-23 to H-25 in 5 suggested an R* configuration of C-24. As with 5, the relative configurations of C-24 in compounds 4 and 6 were also established by NOESY experiments. Similar to 3, the orientations of H-28 in 4–6 were all set in axial orientations, owing to the large coupling constants of H-28 (9.7–11.0 Hz). Thus, the structures of 4–6 were established and named as hyperisampsins K–M.
Figure 4. Calculated 13C NMR data for 4 and 5.
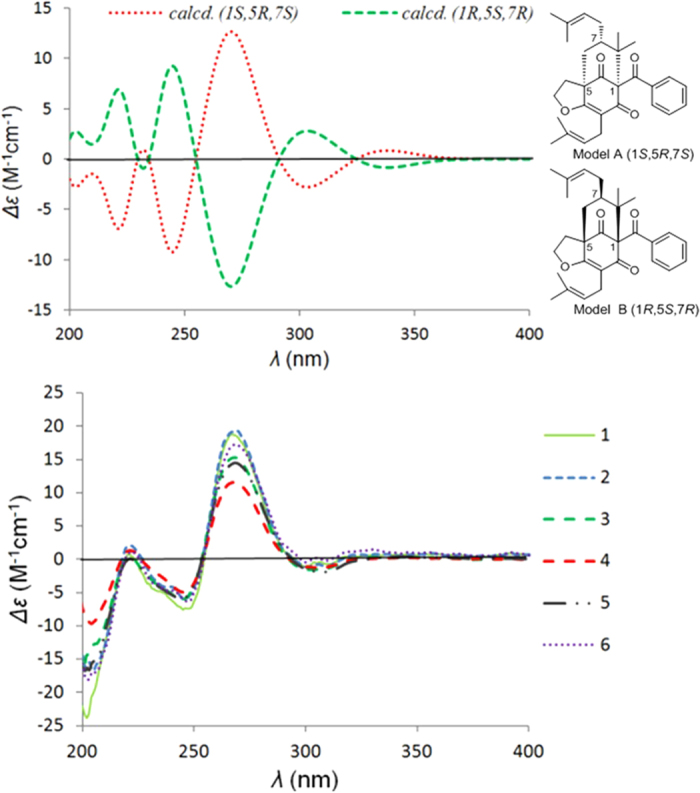
Comparison of the electronic circular dichroism (ECD) spectra (Fig. 5) of the closely related compounds 1–6 suggested that all of them had a strong positive Cotton effect (CE) at λmax 270 nm and two negative CEs at λmax 246 and 305 nm. To determine the absolute configurations of 1–6, the ECD spectra of two simplified models A and B (Fig. 5) were calculated using time-dependent density functional theory (TDDFT) with Gaussian 09. The ECD spectra of 1–6 were subsequently compared with the calculated ECD curves of models A (1S,5R,7S) and B (1R,5S,7R) (Fig. 5), which revealed a good agreement between the experimental curves and the calculated curve of model A (1S,5R,7S). In addition, the experimental ECD curves of 1–6 were closely similar to those of hyperattenins A and B21, suggesting the same absolute configurations of the core structures. Thus, the absolute configurations of 1–6 were determined as shown.
Figure 5. Calculated ECD for models (A,B) and the experimental ECD spectra of 1–6.
Cytotoxic Activities Evaluation
Compounds 1–7 were tested for their cytotoxic activities against five human tumor cell lines, including a myeloid leukemia line (HL-60 cells), a hepatocellular carcinoma line (SMMC-7721), a lung carcinoma line (A-549), a breast cancer line (MCF-7), and a colon cancer line (SW480). Cis-platin (DDP) was used as positive control for antitumor activity (Table 3)19. Among the compounds tested, compounds 3, 4, and 6 exhibited the most potent cytotoxicity, with IC50 values ranging from 0.56 to 3.00 μM. Compounds 5 and 7 showed moderate cytotoxic activities, with IC50 values over a range of 3.03–25.92 μM.
Table 3. Cytotoxic Activities of 1–7 (IC50 in μM).
| Compounds | HL-60 | SMMC-7721 | A-549 | MCF-7 | SW480 | BEAS-2B |
|---|---|---|---|---|---|---|
| 1 | >40.00 | >40.00 | >40.00 | >40.00 | >40.00 | – |
| 2 | >40.00 | >40.00 | >40.00 | >40.00 | >40.00 | – |
| 3a | 0.56 | 0.58 | 0.53 | 0.88 | 2.49 | 1.50 |
| 4 | 1.67 | 2.15 | 2.13 | 2.73 | 3.00 | 2.71 |
| 5 | 3.03 | 11.30 | 11.13 | 11.54 | 13.59 | 15.77 |
| 6 | 1.42 | 2.28 | 1.89 | 1.66 | 2.90 | 3.04 |
| 7 | 15.52 | 18.36 | 15.19 | 5.72 | 20.10 | 17.08 |
| DDPb | 1.17 | 6.43 | 9.24 | 15.86 | 13.42 | 11.11 |
aThe IC50 value of compound 3 against NB4 cell was 0.63 μM.
bDDP (cis-platin) was used as a positive control; “–” not tested.
Flow cytometry analysis of cell apoptosis
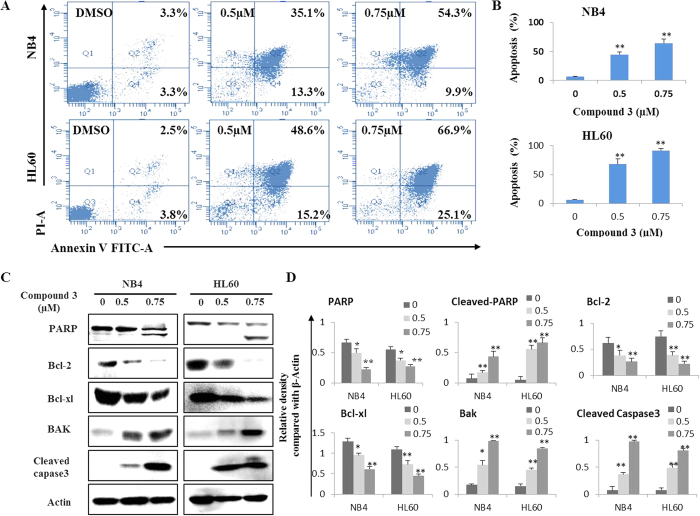
To analyze the potential cell apoptosis induced by these cytotoxic PPAPs, two acute myeloid leukemia cell lines (HL60 and NB4) were treated with compound 3 for 48 hours. As show in Fig. 6(A,B), treated HL60 and NB4 cells underwent dramatic cellular apoptosis in a concentration-dependent manner. Compared with the control group, the 0.75 μM compound 3 resulted in about 64.2% and 92.0% apoptosis incidence in NB4 and HL60 cells, respectively.
Figure 6. Compound 3 induces apoptosis in leukemia cells.
(A) After 48 h treatment, cell apoptosis was determined by Annexin V-FITC and PI staining using flow cytometric analysis. Cells in the lower right quadrant indicate early apoptotic cells, and cells in the upper right quadrant indicate late apoptotic cells. (B) Columns, means of three different experiments; bars, SD, *P < 0.05, **P < 0.01 vs control group. (C) Western blot analysis for PARP, Bcl-2, Bcl-xl, Bak and caspase-3 levels, β-Actin was used as a loading control. (D) The relative density compared with β-Actin of each protein was detected by Image J, data are presented as the means of three experiments, bars, SD, *P < 0.05, **P < 0.01 vs control group.
Western blot analysis of apoptosis related proteins
Caspase 3 activation is responsible for the proteolytic degradation of PARP, a hallmark of cells undergoing apoptosis. Bcl-2 family also plays a central regulatory role in the mitochondrial pathway of apoptosis. The balance between Bak verses Bcl-2 and Bcl-xl is important for apoptotic induction29. As shown in Fig. 6(C,D), compound 3 treatments activatedthe expression levels of Caspase 3 and PARP.It also up-regulated Bak, but down-regulated Bcl-2 and Bcl-xl. In conclusion, these data suggest that compound 3-induced apoptosis was mediated by the activation of caspase-3, upregulation of Bax, downregulation of Bcl-2/Bcl-xl, and degradation of PARP.
PPAPs are a special class of phloroglucinol derivatives, which have attracted great interest from both chemistry and pharmacology communities, since the report of the first natural occurring adamantyl derivative (plukenetione A) in 199630. Recently, many bioactive PPAPs with complex and intriguing skeletons were reported, such as hyperuralones A and B31, hypersubones A and B32, and hyperisampsins A–D19. In this study, six new PPAPs (1–6), possessing novel unique γ-lactone or 1,2-dioxane rings, were isolated from the aerial parts of Hypericum sampsonii. Compounds 3, 4, and 6 exhibited significant cytotoxic activities with IC50 values ranging from 0.56 to 3.00 μM. Moreover, we have demonstrated that compound 3 has the capacity to induce cell apoptotic death in leukemia cells, revealing, for the first time, the mechanism of PPAP-mediated cytotoxicity, which may attract more attentions from synthesis chemistry and pharmacology communities. In conclusion, the novel structure of 3 combined with its significant cytotoxic activities reported in this study may greatly promote the anti-tumor studies of PPAPs, and further investigations on the mechanism and structure-function relationship for developing more excellent agent are necessary.
Methods
General experimental procedures
Optical rotations were determined with a Perkin-Elmer 341 polarimeter. UV, ECD, and FT-IR spectra were measured using a Varian Cary 50, a JASCO-810 spectrometer, and a Bruker Vertex 70, respectively. NMR spectra were recorded on a Bruker AM-400 spectrometer, and the 1H and 13C NMR chemical shifts were referenced to the solvent or solvent impurity peaks for CDCl3 (δH 7.26 and δC 77.0) and pyridine-d5 (δH 7.19 and δC 123.5). High-resolution electrospray ionization mass spectra (HRESIMS) were obtained in the positive ion mode with a Thermo Fisher LC-LTQ-Orbitrap XL spectrometer. Semi-preparative HPLC was performed on an Agilent 1200 quaternary system with a UV detector or on a Dionex HPLC system equipped with an Ultimate 3000 pump, an Ultimate 3000 autosampler injector, and an Ultimate 3000 diode array detector (DAD) controlled by Chromeleon software (version 6.80) using a reverse-phase C18 column (5 μm, 10 × 250 mm, Welch Ultimate XB-C18). Column chromatography was performed using silica gel (100–200 and 200–300 mesh; Qingdao Marine Chemical Inc., China), ODS (50 μm, Merck, Germany), Sephadex LH-20 (Merck, Germany), or MCI gel (75–150 μm, Merck, Germany). Thin-layer chromatography (TLC) was performed with silica gel 60 F254 (Yantai Chemical Industry Research Institute) and RP-C18 F254 plates (Merck, Germany).
Plant material
The aerial parts of H. sampsonii were collected from the Da-bie Mountain areas of Hubei Province, P. R. China, in October 2011, and were identified by Professor Jianping Wang. A voucher specimen (ID 20111008) has been deposited with the Herbarium of Materia Medica, Faculty of Pharmacy, Tongji Medical College of Huazhong University of Science and Technology, China.
Extraction and isolation
The air-dried aerial parts of H. sampsonii (50 kg) were extracted with 95% EtOH, and the extract was partitioned with petroleum ether and CHCl3 against water. The petroleum ether soluble extract (800 g) was separated by chromatography on a silica gel column (5 kg, 20 × 120 cm; petroleum ether to acetone, 100:0 → 0:100) to furnish eight fractions (Fr. 1–Fr. 8). Fr. 3 was further purified by column chromatography (silica gel CC, 1 kg, 10 × 100 cm), eluting with a gradient of petroleum ether in isopropyl alcohol to yield three subfractions (Fr. 3.1–Fr. 3.3). Fr. 3.2 was separated over silica gel (petroleum ether to acetone, 20:1 → 5:1) to obtain six parts (Fr. 3.2.1–Fr. 3.2.6). These parts were subjected to Sephadex LH-20 (MeOH) and ODS (MeOH-H2O) and were then purified by semi-preparative HPLC. Compounds 7 (8 mg) and 10 (10 mg) were obtained from Fr. 3.2.2; Compounds 8 (10 mg) and 11 (5 mg) were purified from Fr. 3.2.3; Compound 9 (17 mg) was isolated from Fr. 3.2.3. Fr. 6 was applied to ODS (MeOH to H2O, 50% → 100%) to obtain six subfractions (Fr. 6.1–Fr. 6.6). Fr. 6.3 was separated with Sephadex LH-20 (MeOH) and ODS (MeOH to H2O, 60% → 100%) to obtain three mixtures (III–V). Mixture III was purified by semi-preparative HPLC (CH3CN-H2O, 88%) to obtain 3 (15 mg). Fr. 6.4 was further separated by Sephadex LH-20 (MeOH) and ODS (MeOH to H2O, 70% → 100%) and was then purified by semi-preparative HPLC (CH3CN-H2O, 75%) to obtain 1 (8 mg), 2 (9 mg), and 6 (5 mg).
The CHCl3 soluble extract (approximately 1 kg) was also separated by chromatography on silica gel (6 kg, 20 × 120 cm; petroleum ether to acetone, 100:0 → 0:100) to furnish six parts (A–F). Fraction B was subjected to silica gel CC and was eluted with petroleum ether to acetone (50:1 → 1:1) to obtain seven subfractions (B.1–B.7). Subfraction B.2 was further subjected to Sephadex LH-20 (MeOH) and then MPLC (ODS, MeOH to H2O, from 40% to 100%) to obtain six additional subfractions (B.2.1–B.2.6). B.2.3 was further purified by Sephadex LH-20 (MeOH), silica gel CC, and finally by semi-preparative HPLC (CH3OH-H2O, 90%) to obtain compounds 4 (15 mg) and 5 (9 mg).
Hyperisampsin H (1)
Colorless oil, 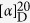 + 4 (c = 0.2, CHCl3); UV (CH3OH) λmax (log ε) = 204 (4.29), 248 (4.01), and 274 (3.90) nm; ECD (MeOH) λmax (Δε) 221 (+0.7), 247 (−7.4), 267 (+18.8), 300 (−1.1) nm; IR νmax = 1780, 1729, 1695, and 1627 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 581.2799 [M + Na]+ (calculated for C35H42O6Na, 581.2879).
+ 4 (c = 0.2, CHCl3); UV (CH3OH) λmax (log ε) = 204 (4.29), 248 (4.01), and 274 (3.90) nm; ECD (MeOH) λmax (Δε) 221 (+0.7), 247 (−7.4), 267 (+18.8), 300 (−1.1) nm; IR νmax = 1780, 1729, 1695, and 1627 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 581.2799 [M + Na]+ (calculated for C35H42O6Na, 581.2879).
Hyperisampsin I (2)
Colorless oil, 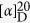 + 42 (c = 0.2, CHCl3); UV (CH3OH) λmax (log ε) = 204 (4.35), 248 (4.08), and 275 (3.98) nm; ECD (MeOH) λmax (Δε) 221 (+2.0), 245 (−6.0), 269 (+19.3), 300 (−1.2) nm; IR νmax = 1780, 1728, 1696, and 1627 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 581.2798 [M + Na]+ (calculated for C35H42O6Na, 581.2879).
+ 42 (c = 0.2, CHCl3); UV (CH3OH) λmax (log ε) = 204 (4.35), 248 (4.08), and 275 (3.98) nm; ECD (MeOH) λmax (Δε) 221 (+2.0), 245 (−6.0), 269 (+19.3), 300 (−1.2) nm; IR νmax = 1780, 1728, 1696, and 1627 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 581.2798 [M + Na]+ (calculated for C35H42O6Na, 581.2879).
Hyperisampsin J (3)
Colorless oil, 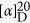 + 21 (c = 0.3, CH3OH); UV (CH3OH) λmax (log ε) = 205 (4.46), 247 (4.17), and 277 (4.15) nm; ECD (MeOH) λmax (Δε) 221 (+0.3), 245 (−6.0), 268 (+15.3), 302 (−1.6) nm; IR νmax = 3518, 1728, 1698, and 1627 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 641.3429 [M + Na]+ (calculated for C38H50O7Na, 641.3454).
+ 21 (c = 0.3, CH3OH); UV (CH3OH) λmax (log ε) = 205 (4.46), 247 (4.17), and 277 (4.15) nm; ECD (MeOH) λmax (Δε) 221 (+0.3), 245 (−6.0), 268 (+15.3), 302 (−1.6) nm; IR νmax = 3518, 1728, 1698, and 1627 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 641.3429 [M + Na]+ (calculated for C38H50O7Na, 641.3454).
Hyperisampsin K (4)
Colorless oil, 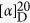 + 12 (c = 0.4, CHCl3); UV (CH3OH) λmax (log ε) = 203 (4.25), 247 (4.00), and 274 (3.88) nm; ECD (MeOH) λmax (Δε) 221 (+1.3), 246 (−5.0), 268 (+11.6), 307 (−1.2) nm; IR νmax = 3419, 1734, and 1700 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 657.3380 [M + Na]+ (calculated for C38H50O8Na, 657.3403).
+ 12 (c = 0.4, CHCl3); UV (CH3OH) λmax (log ε) = 203 (4.25), 247 (4.00), and 274 (3.88) nm; ECD (MeOH) λmax (Δε) 221 (+1.3), 246 (−5.0), 268 (+11.6), 307 (−1.2) nm; IR νmax = 3419, 1734, and 1700 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 657.3380 [M + Na]+ (calculated for C38H50O8Na, 657.3403).
Hyperisampsin L (5)
Colorless oil, 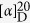 + 9 (c = 0.1, CH3OH); UV (CH3OH) λmax (log ε) = 203 (4.39), 248 (4.04), and 277 (3.95) nm; ECD (MeOH) λmax (Δε) 221 (+0.1), 244 (−5.9), 268 (+14.5), 307 (−1.9) nm; IR νmax = 3423, 1728, 1697, and 1626 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 657.3383 [M + Na]+ (calculated for C38H50O8Na, 657.3403).
+ 9 (c = 0.1, CH3OH); UV (CH3OH) λmax (log ε) = 203 (4.39), 248 (4.04), and 277 (3.95) nm; ECD (MeOH) λmax (Δε) 221 (+0.1), 244 (−5.9), 268 (+14.5), 307 (−1.9) nm; IR νmax = 3423, 1728, 1697, and 1626 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 657.3383 [M + Na]+ (calculated for C38H50O8Na, 657.3403).
Hyperisampsin M (6)
Colorless oil, 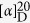 + 47 (c = 0.1, CHCl3); UV (CH3OH) λmax (log ε) = 204 (4.22), 248 (3.95), and 275 (3.88) nm; ECD (MeOH) λmax (Δε) 221 (+1.0), 247 (–6.3), 267 (+17.3), 300 (−0.4) nm; IR νmax = 3421, 1722, 1696, and 1626 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 641.3374 [M + Na]+ (calculated for C38H50O7Na, 641.3454).
+ 47 (c = 0.1, CHCl3); UV (CH3OH) λmax (log ε) = 204 (4.22), 248 (3.95), and 275 (3.88) nm; ECD (MeOH) λmax (Δε) 221 (+1.0), 247 (–6.3), 267 (+17.3), 300 (−0.4) nm; IR νmax = 3421, 1722, 1696, and 1626 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables 1 and 2, respectively; HRESIMS m/z 641.3374 [M + Na]+ (calculated for C38H50O7Na, 641.3454).
Computational details
The theoretical calculations of compound 1 and the simplified models (A and B) of compounds 1–6 were performed using Gaussian 09. Conformational analysis was initially performed using Maestro in Schrödinger 2010 conformational searching together with the OPLS_2005 molecular mechanics methods. The optimized conformation geometries and thermodynamic parameters of all conformations were provided. The OPLS_2005 conformers were optimized at the B3LYP/6–31G(d, p) level. The theoretical calculation of ECD was performed using time-dependent density functional theory (TDDFT) at the B3LYP/6–31G(d, p) level in methanol with a PCM model. The calculated ECD curve was generated using SpecDis 1.5133. Rvel was used in this work. The 3D structures of 4a/4b and 5a/5b, generated by Chem3D, were optimized in chloroform by using Gaussian 09 at the B3LYP/6–31G* level. Both optimized structures were then further used as the input structures for NMR calculations. For each conformation, the NMR calculation was performed using Gaussian 09 at the B3LYP/6–31G* level. Finally, the relative errors between the computed and recorded 13C NMR spectra were calculated34.
Cytotoxic assay
Five human cancer cell lines (HL-60, SMMC-7721, A-549, MCF-7, and SW-480), together with one noncancerous cell line, the Beas-2B human bronchial epithelial cell line, were used in the cytotoxic activity assay. Cytotoxic activity was measured as described in our previous report19.
Flow cytometry analysis of cell apoptosis
Apoptosis analysis was carried out using an apoptosis detection kit (Keygen, Nanjing, China) according to the manufacturer’s instructions. Briefly, HL60 and NB4 cells were exposed to vehicle (DMSO < 0.01%) and compound 3 (0.5 and 0.75 μM) for 48 h, then cells were collected and washed with cold PBS, and then resuspended in 500 μL binding buffer. After that, 5 μL of AnnexinV-FITC and 10 μL of PI were added. After supravital staining, cell apoptosis was analyzed by flow cytometry (Becton Dickinson, San Jose, CA, USA).
Western blot analysis
Western blot analysis was conducted as described previously (citation). Briefly, cells were treated with DMSO and compound 3 (0.5 and 0.75 μM) for 48 h, and then lysed in a radio immune-precipitation assay buffer. Protein concentrations were determined using a BCA protein assay kit (Byontime, Beijing, China). Samples were subjected to electrophoresis in 10% SDS-PAGE gels followed by transfer to PVDF membrane and probed with specific antibodies, including PARP, Bcl-2, BCL-XL, Bak, Cleaved Caspase 3, and β-Actin (Cell Signaling Technology, Inc.). Blots bands were visualized using the horseradish peroxidase conjugated secondary antibodies and chemiluminescent substrate.
Additional Information
How to cite this article: Zhu, H. et al. Hyperisampsins H–M, Cytotoxic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum sampsonii. Sci. Rep. 5, 14772; doi: 10.1038/srep14772 (2015).
Supplementary Material
Acknowledgments
The authors would like to thank the Analytical and Testing Center at Huazhong University of Science and Technology for assistance in conducting ECD and IR analyses. This work was financially supported by the Program for New Century Excellent Talents in University, State Education Ministry of China (NCET-2008-0224), the National Natural Science Foundation of China (Nos. 21502057, 31370372, 31200258, and 81202423), and the National Science and Technology Project of China (Nos. 2011ZX09102-004 and 2013ZX09103001-020).
Footnotes
Author Contributions H.Z. and C.C. contributed equally to this work. They conducted the main experiments, analyzed the data, and wrote the manuscript; Q.T. and X.C. performed the biological assay; J.Y. did the ECD calculations; J.L. and B.S. did the 13C NMR calculations of 4 and 5 as well as energy barriers calculations and conformations optimization of compound 1; J.W. authenticated the plant material; G.Y. and Z.L. edited and polished this manuscript; Y.X. and Y.Z. designed the experiments and commented the manuscript. All authors reviewed the manuscript.
References
- Ciochina R. & Grossman R. B. Polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 106, 3963–3986 (2006). [DOI] [PubMed] [Google Scholar]
- Richard J. A., Pouwer R. H. & Chen D. Y. K. The chemistry of the polycyclic polyprenylated acylphloroglucinols. Angew. Chem. Int. Ed. 51, 4536–4561 (2012). [DOI] [PubMed] [Google Scholar]
- Singh I. P., Sidana J., Bharate S. B. & Foley W. J. Phloroglucinol compounds of natural origin: Synthetic aspects. Nat. Prod. Rep. 27, 393–416 (2010). [DOI] [PubMed] [Google Scholar]
- Boyce J. H. & Porco J. A. Jr. Asymmetric, stereodivergent synthesis of (–)-clusianone utilizing a biomimetic cationic cyclization. Angew. Chem. Int. Ed. 53, 7832–7837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance G. & Barriault L. Total syntheses of hyperforin and papuaforins A–C, and formal synthesis of nemorosone through a gold(I)-catalyzed carbocyclization. Angew. Chem. Int. Ed. 53, 6701–6704 (2014). [DOI] [PubMed] [Google Scholar]
- Grenning A. J., Boyce J. H. & Porco J. Jr. Rapid synthesis of polyprenylated acylphloroglucinol analogs via dearomative conjunctive allylic annulation. A. J. Am. Chem. Soc. 136, 11799–11804 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L. H & Sim K. Y. Complex caged polyisoprenylated benzophenone derivatives, sampsoniones A and B, from Hypericum sampsonii. Tetrahedron Lett. 39, 7999–8002 (1998). [Google Scholar]
- Hu L. H. & Sim K. Y. Cytotoxic polyprenylated benzoylphloroglucinol derivatives with an unusual adamantyl skeleton from Hypericum sampsonii (Guttiferae). Org. Lett. 1, 879–882 (1999). [DOI] [PubMed] [Google Scholar]
- Hu L. H. & Sim K. Y. Sampsoniones C–H, a unique family of polyprenylated benzophenone derivatives with the novel tetracyclo[7.3.1.13,11.03,7]tetradecane-2,12,14-trione skeleton, from Hypericum sampsonii (Guttiferae). Tetrahedron Lett. 40, 759–762 (1999). [Google Scholar]
- Hu L. H. & Sim K. Y. Sampsoniones A–M, a unique family of caged polyprenylated benzoylphloroglucinol derivatives, from Hypericum sampsonii. Tetrahedron 56, 1379–1386 (2000). [Google Scholar]
- Xiao Z. Y., Mu Q., Shiu W. K. P., Zeng Y. H. & Gibbons S. Polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. J. Nat. Prod. 70, 1779–1782 (2007). [DOI] [PubMed] [Google Scholar]
- Zeng Y. H. et al. Geranyl bearing polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. Chem. Lett. 38, 440–441 (2009). [DOI] [PubMed] [Google Scholar]
- Xiao Z. Y., Zeng Y. H., Mu Q., Shiu W. K. P. & Gibbons S. Prenylated benzophenone peroxide derivatives from Hypericum sampsonii. Chem. Biodiversity 7, 953–958 (2010). [DOI] [PubMed] [Google Scholar]
- Zeng Y. H., Osman K., Xiao Z. Y., Gibbons S. & Mu Q. Four geranyl-bearing polyisoprenylated benzoylphloroglucinol derivatives from Hypericum sampsonii. Phytochemistry Lett. 5, 200–205 (2012). [Google Scholar]
- Tian W. J. et al. Norsampsones A–D, four new decarbonyl polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Org. Lett. 16, 3448–3451 (2014). [DOI] [PubMed] [Google Scholar]
- Dai Y. et al. Novel polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii. Tetrahedron 80, 822–822 (2014). [Google Scholar]
- Lin Y. L. & Wu Y. S. Polyprenylated phloroglucinol derivatives from Hypericum sampsonii. Helv. Chim. Acta 86, 2156–2163 (2003). [Google Scholar]
- Xin W. B. et al. Prenylated phloroglucinol derivatives from Hypericum sampsonii. Fitoterapia 83, 1540–1547 (2012). [DOI] [PubMed] [Google Scholar]
- Zhu H. et al. Bioactive acylphloroglucinols with adamantyl skeleton from Hypericum sampsonii. Org. Lett. 16, 6322–6325 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu H. et al. Hyperascyrones A–H, polyprenylated spirocyclic acylphloroglucinol derivatives from Hypericum ascyron Linn. Phytochemistry 115, 222–230 (2015). [DOI] [PubMed] [Google Scholar]
- Li D. et al. Hyperattenins A–I, bioactive polyprenylated acylphloroglucinols from Hypericum attenuatum Choisy. RSC Adv. 5, 5277–5287 (2015). [Google Scholar]
- Li D. et al. Two new adamantyl-like polyprenylated acylphloroglucinols from Hypericum attenuatum choisy. Tetrahedron Lett. 56, 1953–1955 (2015). [Google Scholar]
- Chen C. et al. A new 3, 4-seco-oleanane-type triterpenoid with an unusual enedione moiety from Hypericum ascyron. Fitoterapia 103, 227–230 (2015). [DOI] [PubMed] [Google Scholar]
- Zhou Z. B., Zhang Y. M., Pan K., Luo J. G. & Kong L.Y. Cytotoxic polycyclic polyprenylated acylphloroglucinols from Hypericum attenuatum. Fitoterapia 95, 1–7 (2014). [DOI] [PubMed] [Google Scholar]
- Ishida Y. et al. Polyprenylated benzoylphloroglucinol-type derivatives including novel cage compounds from Hypericum erectum. Chem. Pharm. Bull. 58, 336–343 (2010). [DOI] [PubMed] [Google Scholar]
- LaPlante S. R., Edwards P. J., Fader L. D., Jakalian A. & Hucke O. Revealing atropisomer axial chirality in drug discovery. Chem. Med. Chem. 6, 505–513 (2011). [DOI] [PubMed] [Google Scholar]
- Luo Q. et al. Applanatumin A, a new dimeric meroterpenoid from Ganoderma applanatum that displays potent antifibrotic activity. Org. Lett. 17, 1110–1113 (2015). [DOI] [PubMed] [Google Scholar]
- Boukouvalas J., Pouliot R. & Fréchette Y. Concise synthesis of yingzhaosu C and epi-yingzhaosu C by peroxyi radical cyclization. Assignment of relative configuration. Tetrahedron Lett. 36, 4167–4170 (1995). [Google Scholar]
- Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 35, 495–516 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. E., Jacobs H., Carrington C. M. S., McLean S. & Reynolds W. F. Plukenetione A. An unusual adamantyl ketone from Clusia plukenetii (guttiferae). Tetrahedron Lett. 37, 8663–8666 (1996). [Google Scholar]
- Zhang J. J. et al. Hyperuralones A and B, new acylphloroglucinol derivatives with intricately caged cores from Hypericum uralum. Org. Lett. 16, 4912–4915 (2014). [DOI] [PubMed] [Google Scholar]
- Liao Y. et al. Hypersubones A and B, new polycyclic acylphloroglucinols with intriguing adamantane type cores from Hypericum subsessile. Org. Lett. 17, 1172–1175 (2015). [DOI] [PubMed] [Google Scholar]
- Bruhn T. H., Schaumlöffel Y., Bringmann A., G. Spec Dis, version 1.51, University of Würzburg, Germany (2010).
- Rychnovsky S. D. Predicting NMR spectra by computational methods: Structure revision of hexacyclinol. Org. Lett. 8, 2895–2898 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.