Abstract
Pseudomonas tolaasii is the main bacterial pathogen of several mushroom species. In this paper we report that strains of P. tolaasii produce volatile substances inducing in vitro mycelia growth inhibition of Pleurotus ostreatus and P. eryngii, and Agaricus bisporus and P. ostreatus basidiome tissue blocks brown discoloration. P. tolaasii strains produced the volatile ammonia but not hydrogen cyanide. Among the volatiles detected by GC–MS, methanethiol, dimethyl disulfide (DMDS), and 1-undecene were identified. The latter, when assayed individually as pure compounds, led to similar effects noticed when P. tolaasii volatiles natural blend was used on mushrooms mycelia and basidiome tissue blocks. Furthermore, the natural volatile mixture resulted toxic toward lettuce and broccoli seedling growth. In contrast, pure volatiles showed different activity according to their nature and/or doses applied. Indeed, methanethiol resulted toxic at all the doses used, while DMDS toxicity was assessed till a quantity of 1.25 μg, below which it caused, together with 1-undecene (≥10 μg), broccoli growth increase.
Keywords: Pseudomonas tolaasii, mushrooms, broccoli, lettuce, volatile organic compounds, methanethiol, dimethyl disulfide, 1-undecene
Introduction
Pseudomonas tolaasii (Paine, 1919) is the brown blotch disease’s causal agent in several economically important edible mushrooms such as Agaricus bisporus (Lange) Imbach (Tolaas, 1915), A. bitorquis (Quél.) Sacc. and other Agaricales (Gandy, 1979), Pleurotus ostreatus (Jacq. : Fr.) Kum (Gill, 1995), P. eryngii (D. C. : Fr.) Quél (Ferri, 1985; Rodriguez and Royse, 2007), Lentinula edodes (Berkeley) Singer (Tsuneda et al., 1995), and Flammulina velutipes (Curtis) Singer (Lee et al., 1996). Brown blotch is considered to be the cultivated mushrooms’s main disease because of the important economic losses (Fermor, 1987; Iacobellis, 2011) and the difficulty to control it. Compost and casing soil, required for mushroom cultivation were found as a primary source of P. tolaasii (Wong and Preece, 1980).
Pseudomonas tolaasii is also described as a pathogen of some plants, indeed it causes diseases on cauliflowers (Brassica oleracea L. var. botrytis) (Talame and Piccirillo, 1995), tobacco (Nicotiana tabacum L.), Solanum sp. (Rainey et al., 1991; Shirata et al., 1995) and strawberry (Fragaria × ananassa Duchesne) (Tanprasert and Reed, 1997). Moreover, it has been reported as a saprophytic bacterium associated to pear (Pyrus communis L.) phylloplane (Noval et al., 1993) and bean (Phaseolus vulgaris L.) and sugar beet (Beta vulgaris L.) rhizosphere (Zdor and Anderson, 1992).
Pseudomonas tolaasii produces tolaasins, considered the primary factors involved in the virulence and interaction between the pathogen and the hosts (Rainey et al., 1992; Soler-Rivas et al., 1999; Largeteau and Savoie, 2010; Iacobellis, 2011). The toxic lipodepsipeptides tolaasins (Nutkins et al., 1991) are the only molecules whose role in the virulence of P. tolaasii has been unequivocally ascertained (Cutri et al., 1984; Rainey et al., 1992; Grewal et al., 1995; Lo Cantore et al., 2006). In fact, when applied directly on mushrooms, tolaasins can reproduce the symptoms of the disease.
Besides tolaasins, other compounds have been reported to be produced by P. tolaasii and considered as potential factors responsible for bacterial botch symptoms. Park et al. (1994) isolated a compound from a P. tolaasii strain, characterized as an aminobenzene, containing an amylamine group, able to induce the symptoms of the disease on A. bisporus caps. Furthermore, Shirata (1996) described the ability of P. tolaasii strains to produce volatile organic compounds (VOCs) called tovsins, different from tolaasins, which are not volatiles, and able to induce the alteration of P. ostreatus and F. velutipes basidiomes.
Volatile organic compounds are small molecules (molecular masses lower 300 Da) with low polarity and high vapor pressure that are produced by both eukaryotes and prokaryotes. The biological activities and role of many bacterial VOCs, so far identified, are partly known; in fact, they have been demonstrated to act as infochemicals for inter- and intra-organism communication (Effmert et al., 2012), attraction, and defense (Ryu et al., 2004; Farag et al., 2006). They can be detected in small amounts by the organisms and diffused in the atmosphere and soil (Kai et al., 2009; Morath et al., 2012).
The bacterial volatile blends have been proved to interact with plants and fungi by inhibiting or stimulating their growth (Kai et al., 2007; Vespermann et al., 2007; Effmert et al., 2012). To date, many studies have been performed with the aim of identifying bacterial volatile substances, including those produced by Pseudomonas sp.. However, the knowledge on their biological activities is still limited. In fact, only in recent years the inhibition and/or stimulation of fungal and plant growth by carbon dioxide (CO2), hydrogen cyanide (HCN), ammonia (NH3), 2,3-butanediol, acetoin, dimethyl disulfide (DMDS), and 1-undecene, has been reported (Howell et al., 1988; Ryu et al., 2003; Haas and Défago, 2005; Afsharmanesh et al., 2006; Farag et al., 2006; Kai and Piechulla, 2009; Kai et al., 2009, 2010; Blom et al., 2011; Meldau et al., 2013; Weise et al., 2013; Hunziker et al., 2015).
Based on the above consideration, it seemed worthy of interest to confirm the production of volatiles by P. tolaasii and establish their identity and biological role on the host mushroom and plants. In this work we report a study on the bioactivity of volatile compounds, produced by three virulent strains of P. tolaasii, on P. eryngii and P. ostreatus mycelia and A. bisporus and P. ostreatus basidiome tissue blocks. Some of the pure VOCs, identified by GC–MS, were in vitro evaluated for their toxicity, respectively, on the above mycelia growth and tissue blocks. Furthermore, since P. tolaasii is also a natural rhizosphere inhabitant of some plants, in the present study is also reported the bioactivity of both natural volatile mixture and pure VOCs on lettuce and broccoli seeds germination and seedling growth.
Materials and Methods
Bacterial, Fungal Cultures, and Seeds
Bacterial type strain NCPPB2192 and the strains USB1 (ICMP13791), USB66 (ICMP13792) of P. tolaasii, and fungal strains DS256, DS270 of P. eryngii and DS226, DS284 of P. ostreatus, have been used in this study. Bacterial strains were maintained lyophilised at 4°C and subcultures were obtained on King’s B agar medium (KBA; King et al., 1954) for 48 h at 25°C. P. ostreatus and P. eryngii strains were maintained on Malt Extract Agar (MEA, Oxoid, Milan, Italy) slants at 4°C and subcultures were obtained by growing the fungi on the same medium at 25°C for 72 h.
Broccoli (Brassica oleracea L. var. italica; variety “Cima di rapa sessantina”, Blumen, Milan, Italy) and lettuce (Lactuca sativa L.; variety “Lattuga romana lentissima a montare”, Blumen, Milan, Italy) seeds were used for the evaluation of bioactivity of bacterial volatiles on plant material.
Mushroom Bioassays
Mycelia of P. eryngii and P. ostreatus strains, grown as reported above, and tissue blocks of A. bisporus and P. ostreatus, obtained from freshly harvested basidiomes, prepared according to Ercolani (1970), were used.
The assay was performed inoculating 100 μl of about 108 CFU ml-1 (OD590 = 0.2) bacterial suspension of P. tolaasii strains on KBA in each of two out of three sectors Petri dishes. In the third sector a Pleurotus sp. mycelium plug (6 mm diameter) was placed on 5 ml of MEA. In the case of basidiome tissue assays, one tissue block of A. bisporus or P. ostreatus was placed onto the plate bottom. After inoculation, Petri dishes were incubated at 25°C for 5 days and then mycelia growth was measured (colony diameter subtracted the mycelium agar plug diameter) and the effects on tissue blocks were visually evaluated.
The assays as above described were performed either sealing Petri dishes with parafilm (Pechiney Plastic Packaging Company, Chicago, IL, USA) to minimize volatile compounds exchange during the incubation period or in non-sealed Petri dishes to simulate what happens in nature in an open system such as the rhizosphere.
Controls were obtained placing mycelium plugs or tissue blocks as above, but no bacterial suspensions were inoculated on KBA in the remaining Petri dishes sectors. All mushroom bioassays were performed twice with three replicates and, in the case of mycelia growth assays, the final data were reported in percentage compared to the control (100%).
Seed Bioassays
Broccoli and lettuce seeds were surface disinfected by treating for 1 min with 0.5% chlorine, washed three times with sterile distilled water and then dried under an air flow cabinet at room temperature for 20 min. One hundred disinfected seeds were transferred on filter paper soaked with 1.5 ml of sterile distilled water in one out of three sectors of Petri dishes. The other two sectors, containing each 5 ml of KBA, were inoculated with 100 μl of about 108 CFU ml-1 bacterial suspension of P. tolaasii strains. Petri dishes containing 100 broccoli or lettuce seeds in one out three sectors, while in the other two just KBA were used as controls. The assay was performed as above both in parafilm-sealed and non-sealed dishes. After 5 days incubation at 25°C in the dark, broccoli and lettuce seeds germination was assessed and the whole seedlings, epicotyls, main rootlets were measured with a ruler. The seed bioassays were performed at least twice with three replicates and the final growth data were reported as percentage compared to the control growth (100%).
GC–MS Analysis
The three strains of P. tolaasii, plus P. eryngii strain DS256 and P. ostreatus strain DS284, were analyzed for their volatile compounds profile after a 5 days incubation period both in sealed and non-sealed conditions. The bacterial volatile analysis was carried out in Petri plates, prepared as reported in the previous paragraph, but without fungal inoculum or seeds, respectively, while the fungal volatile analysis was carried out in Petri plates prepared as reported in the mushroom bioassays, but without bacterial inoculum. Petri dishes with non-inoculated KBA and MEA were used as negative control.
Volatiles produced by bacteria and fungi were collected with SPME fiber, coated with 100 μm of polydimethylsiloxane (PDMS) phase (Supelco 57300-U, mounted on a Supelco 57330 support). The fiber was introduced in the headspace of Petri dishes through a hole previously obtained by piercing the parafilm layers, and kept inside the plate for 20 min. The fiber was then introduced into the injection port of a HP6890 plus gas-chromatograph equipped with a Phenomenex Zebron ZB-5 MS capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness). A HP5973 mass selective detector (mass range: 15–300 amu; scan rate: 1.9 scans s-1; EM voltage: 1435) with helium at 0.8 ml min-1 as carrier gas was used. The injection port, equipped with glass insert (internal diameter 0.75 mm) was split less at 250°C. The desorption time of 1.0 min was used. Detector was maintained at 230°C. Oven was maintained at 40°C for 2 min, then the temperature increased to 250°C (8°C min-1) which was maintained for 10 min. All the analysis were performed twice with three replicates. A blank run was performed after each analysis in order to check for residual compounds contaminating the fiber.
All the peaks were identified by comparison with spectra in Wiley6N and NIST98 libraries. Furthermore, the identity of some of the VOCs components was confirmed by GC–MS analysis of reference substances [acetaldehyde (Sigma–Aldrich, 402788); methanethiol (MT) (Sigma–Aldrich, 295515); DMDS (Sigma–Aldrich, W353604); p-cymene (Sigma–Aldrich, C121452); 1-undecene (Sigma–Aldrich, 242527); 2-undecanone (Sigma–Aldrich, U1303)] used as control. The volatile relative concentrations in each Petri plate were calculated based on GC–MS peak areas without using correction factors. The results were reported as percentage average of total peak area (±SE).
NH3 and HCN Production
Strains of P. tolaasii were screened for NH3 production for which freshly grown cultures were grown in 10 ml peptone water (peptone 10 g l-1, NaCl 5 g l-1, pH 7.2) tubes for 72 h at 25°C. After incubation period 0.5 ml of Nessler’s reagent [0.09 mol l-1 solution of K2(HgI4) in 2.5 mol l-1 of KOH] was added in each tube. The development of brownish color of bacterial liquid cultures was positive for NH3 production (Cappuccino and Sherman, 2010).
Hydrogen cyanide production was assessed inoculating bacteria in nutrient sucrose agar (sucrose 5 g l-1, yeast extract 4 g l-1, peptone 4 g l-1, beef extract 2 g l-1, agar 18 g l-1) amended of glycine (4.4 g l-1) Petri dishes. Before incubation a Whatman No. 1 filter paper disk, soaked with a solution of 0.5% picric acid in Na2CO3 (2%) aqueous solution was placed on the upper lid of each plate. The plates were finally incubated for 4 days at 25°C. Filter paper color switch from yellow to red-brown was positive for HCN production (Lorck, 1948).
Strains USB2106 of P. putida and USB2102 of P. brassicacearum were used as positive control for the production of NH3 and HCN, respectively. Non-inoculated media were used as negative controls. The assays were performed twice with three replicates.
VOCs Bioassays
In order to evaluate VOCs bioactivity, identified by GC-MS, belonging to P. tolaasii strains volatile mixture, three pure VOCs, DMDS, MT, and 1-undecene (Sigma–Aldrich, Milan, Italy), selected on the basis of their detection for all the P. tolaasii strains, were used in mushrooms and seeds bioassays. In these bioassays P. ostreatus mycelium plugs, A. bisporus and P. ostreatus basidiome tissue blocks and 100 broccoli seeds, were placed in two out three sectors of Petri dishes, while in the third one were dropped or injected, according to their physical state, DMDS, 1-undecene, and MT. In particular, liquid DMDS and 1-undecene were dropped in glass slide posed in one of the three sectors; then the dishes were rapidly sealed with parafilm, to minimize volatile compound exchange, able to alter the volatile quantity introduced in the system. In the bioassay with gaseous MT, at first, the plates were pierced in correspondence of the empty sector and the hole closed with a triple layer of parafilm. Then the gas were taken with a GC syringe (Supelco, Milan, Italy) from a gas Pyrex pipette device (Microglass Heim s.r.l., Naples, Italy) and injected through the hole; then the dishes were immediately re-sealed and incubated for 5 days at 25°C in the dark. Petri dishes without the VOCs, were used as controls. Then mycelia and seedlings growth was measured with a ruler as above said and the effects on tissue blocks were visually evaluated. All bioassays were performed twice with three replicates and the final data were reported in percentage compared to the control.
Statistical Analysis
All GC–MS analysis and bioassays data were statistically evaluated for the determination of standard errors and the latter subjected to analysis of variance (ANOVA) and P calculated by F test of Fisher–Snedecor. All statistical analysis was carried out using software program package SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Mushroom Bioassays
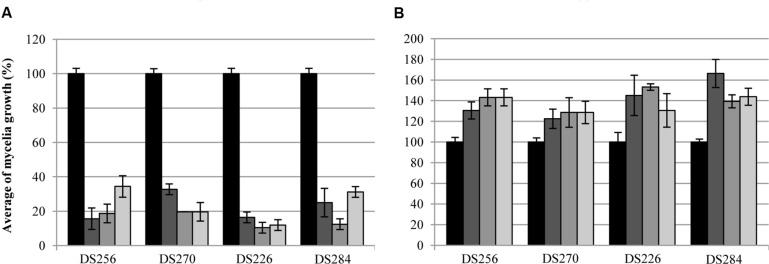
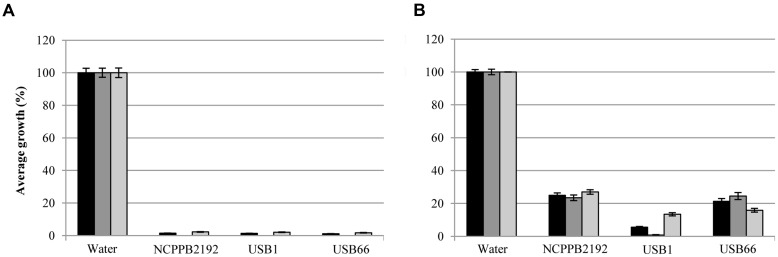
Volatile compounds produced by P. tolaasii strains caused a highly significant reduction, in non-sealed Petri dishes, (P ≤ 0.014) of P. eryngii and P. ostreatus mycelia growth (Figure 1A). In fact, the mycelia growth of the strains DS256 and DS270 of P. eryngii, in the presence of volatile compounds of the strains NCPPB2192, USB1, and USB66 of P. tolaasii was only 16 and 19% (NCPPB2192), 34 and 33% (USB1) and 20% for both fungi (USB66) compared to the control (100%), respectively (Figure 1A). In the same assay conditions, mycelia growth of the strains DS226 and DS284 of P. ostreatus was only 16 and 10% (NCPPB2192), 12 and 25% (USB1), 12 and 31% (USB66) of the control, respectively (Figure 1A).
FIGURE 1.
Average of mycelia growth (%) of strains DS256, DS270, and DS226, DS284 of Pleurotus eryngii and P. ostreatus, respectively, in presence of water ( ) and volatile compounds produced by strains NCPPB2192 (
) and volatile compounds produced by strains NCPPB2192 ( ), USB1 (
), USB1 ( ), and USB66 (
), and USB66 ( ) of Pseudomonas tolaasii in Petri dishes not sealed (A) and sealed (B) with parafilm. Bars on the columns correspond to the standard error of the mean in percentage.
) of Pseudomonas tolaasii in Petri dishes not sealed (A) and sealed (B) with parafilm. Bars on the columns correspond to the standard error of the mean in percentage.
When the bioassay was performed in sealed Petri dishes, instead, (Figure 1B) it came out that P. tolaasii strains volatiles caused a significant P. eryngii and P. ostreatus strains mycelia growth increase (P ≤ 0.038) (Figure 1B). Indeed, fungal growth of the P. eryngii strains DS256 and DS270, co-incubated with the P. tolaasii strains NCPPB2192, USB1, and USB66, was assessed to 131%, and 122% (NCPPB2192), 143 and 129% (USB1), 143 and 129% (USB66), respectively, compared to the control (Figure 1B). In a similar way, the effect of the above examined volatiles on mycelia growth of P. ostreatus strains DS226 and DS284 was 145 and 166% (NCPPB2192), 153 and 139% (USB1), 131 and 144% (USB66), respectively, compared to the control (Figure 1B).
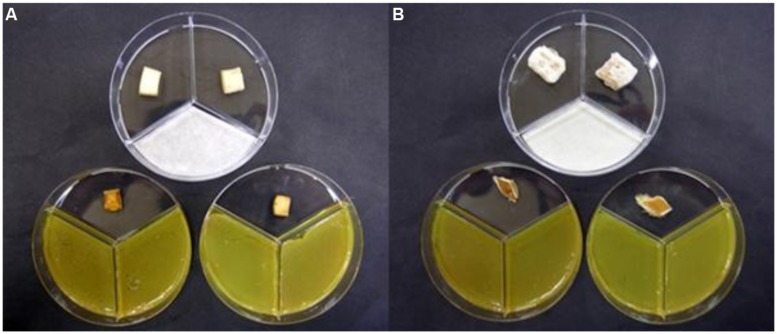
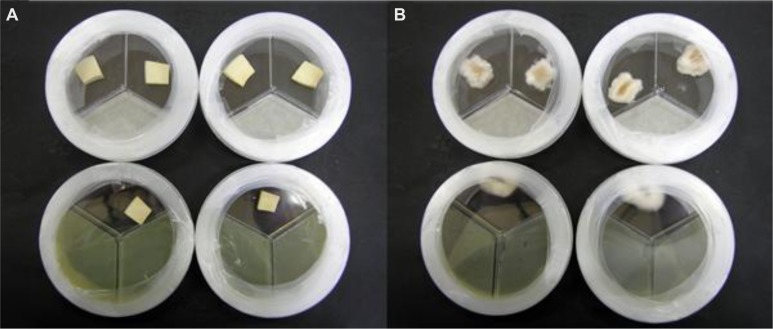
In non-sealed Petri dishes, volatiles from P. tolaasii strains caused a noticeable brown discoloration of A. bisporus and P. ostreatus tissue blocks (Figure 2); when Petri dishes were sealed, instead, no apparent alteration of the A. bisporus tissue blocks was noticed and the presence of mycelium growth surrounding P. ostreatus tissue blocks was observed (Figure 3).
FIGURE 2.
Agaricus bisporus(A) and Pleurotus ostreatus(B) basidiome tissue blocks showing marked brown discoloration caused by volatile compounds blend produced by Pseudomonas tolaasii NCPPB2192 when the Petri dishes were not sealed with parafilm. At the top the Petri dishes containing the blocks treated with water.
FIGURE 3.
Agaricus bisporus(A) and Pleurotus ostreatus(B) basidiome tissue blocks showing apparently no change (A) or a slight increase in mycelia growth (B) when they were exposed, in sealed Petri dishes, to volatile compounds blend produced by Pseudomonas tolaasii NCPPB2192. At the top the Petri dishes containing the blocks treated with water.
Seed Bioassays
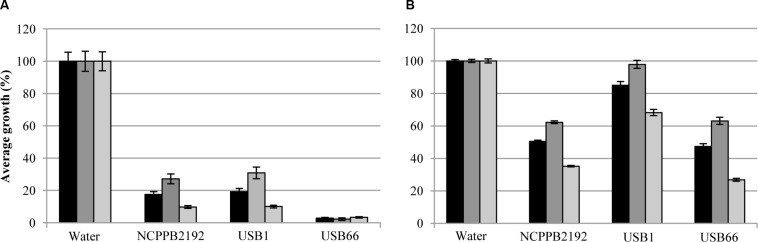
In non-sealed Petri dishes assays, volatile compounds produced by P. tolaasii strains NCPPB2192, USB1 and USB66 caused a highly significant reduction (P < 0.001) of broccoli seedlings growth, compared to the water control (Figure 4A). Indeed, the growth of whole seedlings, epicotyls and main rootlets was highly reduced, resulting in 17, 27, and 10% (NCPPB2192), 19, 31, and 10% (USB1), 3, 2, and 3% (USB66), respectively, compared to the control (100%) (Figure 4A). The above bioassays on lettuce seeds produced a similar toxic effect, though more limited. In fact, the growth of whole seedlings, epicotyls and main rootlets was highly reduced (P < 0.001) to 51, 62, 35, 47, 63, and 27 in presence of the NCPPB2192 and USB66 strains, respectively, compared to the control (Figure 4B). When the USB1 strain was used, the whole seedlings and epicotyls length of the plantlets (85 and 98%, respectively) resulted not significantly (P > 0.05) different from the controls, while the main rootlets length resulted highly reduced (P < 0.001) at 68% compared to the control (Figure 4B).
FIGURE 4.
Average growth (%) of whole seedlings  ), epicotyls (
), epicotyls ( ), and main rootlets (
), and main rootlets ( ) of broccoli (A) and lettuce (B) in presence of volatile compounds blend produced by strains NCPPB2192, USB1 and USB66 of Pseudomonas tolaasii in non-sealed Petri dishes. Bars on the columns correspond to the standard error of the mean in percentage.
) of broccoli (A) and lettuce (B) in presence of volatile compounds blend produced by strains NCPPB2192, USB1 and USB66 of Pseudomonas tolaasii in non-sealed Petri dishes. Bars on the columns correspond to the standard error of the mean in percentage.
In sealed Petri dishes assays, the reduction of broccoli and lettuce plantlets growth was more pronounced (Figure 5) and the statistical analysis confirmed that volatile compounds from the above bacterial strains led to a highly significant reduction (P < 0.001) of broccoli and lettuce seedlings growth in comparison to the control (Figure 5). Actually, the growth of the whole broccoli seedlings, epicotyls and main rootlets resulted highly reduced being only 1, 0, 2% for all P. tolaasii strains, compared to the control (Figure 5A). Similarly, the growth of the whole lettuce seedlings, epicotyls, and main rootlets was 25, 23, 27% (NCPPB2192), 6, 1, 13% (USB1), and 21, 24, 16% (USB66), respectively, compared to the control (Figure 5B).
FIGURE 5.
Average growth (%) of whole seedlings ( ), epicotyls (
), epicotyls ( ), and main rootlets (
), and main rootlets ( ) of broccoli (A) and lettuce (B) in presence of volatile compounds blend produced by strains NCPPB2192, USB1 and USB66 of Pseudomonas tolaasii in sealed Petri dishes. Bars on the columns correspond to the standard error of the mean in percentage.
) of broccoli (A) and lettuce (B) in presence of volatile compounds blend produced by strains NCPPB2192, USB1 and USB66 of Pseudomonas tolaasii in sealed Petri dishes. Bars on the columns correspond to the standard error of the mean in percentage.
Furthermore, the inspection of broccoli and lettuce seedlings made evident root browning and, in the case of lettuce, the absence of root hairs, as it was observed in the negative control (Figure 6).
FIGURE 6.
Growth of broccoli (A–D) and lettuce (E–H) seedlings in absence (A,B,E,F, respectively) and in presence (C,D,G,H, respectively) of volatile compounds produced by strain NCPPB2192 of Pseudomonas tolaasii in Petri dishes not sealed (A,C,E,G) and sealed (B,D,F,H) with parafilm.
GC–MS Analysis and NH3 and HCN Production
Pseudomonas tolaasii strains, in sealed Petri dishes, produced MT, DMDS, p-cymene, 1,4-undecadiene, 1-undecene, 2-undecanone, and 4,7-dimethylundecane, even though not all the volatile compounds identified have been systematically produced by the three P. tolaasii strains (Table 1; Figure 7). In addition, CO2 was also present in the headspace of these samples. The strains DS256 and DS284, respectively, of P. eryngii and P. ostreatus, in the same experimental conditions, produced CO2 and nitrous oxide (NO2) and CO2, ethylene, and acetaldehyde, respectively (Table 1). In the headspace of plates containing non-inoculated KBA or MEA media, CO2, acetaldehyde, and propane, and CO2 and ethylene oxide, respectively, were detected (Table 1).
Table 1.
Percentage average of total peak area (±SE) of volatile compounds in the overhead space of sealed Petri dishes, as determined by SPME–GC analysis, produced by pure cultures of Pseudomonas tolaasii, Pleurotus eryngii, and P. ostreatus strains and by non-inoculated KBA and MEA media.
| Volatile compoundsa |
P. tolaasii strainsb |
P. eryngii DS256b | P. ostreatus DS284b | Media |
|||
|---|---|---|---|---|---|---|---|
| NCPPB 2192 | USB1 | USB66 | KBc | MEAd | |||
| Carbon dioxide (CO2) | 63.66 ± 2.20 | 44.71 ± 5.22 | 42.73 ± 4.34 | 69.86 ± 1.26 | 6.75 ± 0.88 | 5.04 ± 0.22 | 5.31 ± 0.59 |
| Nitrous oxide (NO2) | – | – | – | 1.10 ± 0.11 | – | – | – |
| Ethylene oxide | – | – | – | – | 5.41 ± 0.32 | – | 45.25 ± 1.94 |
| Acetaldehyde | – | – | – | – | 69.42 ± 2.44 | 52.55 ± 1.98 | – |
| Propane | – | – | – | – | – | 2.68 ± 0.32 | – |
| Methanethiol (MT) | 2.12 ± 0.19 | 1.89 ± 0.19 | 2.03 ± 0.27 | – | – | – | – |
| Dimethyl disulfide (DMDS) | 0.25 ± 0.04 | 0.22 ± 0.04 | 0.26 ± 0.04 | – | – | – | – |
| p-cymene | Traces | 0.11 ± 0.04 | 0.10 ± 0.03 | – | – | – | – |
| 1,4-undecadiene | – | 0.49 ± 0.18 | 0.20 ± 0.04 | – | – | – | – |
| 1-undecene | 11.86 ± 2.34 | 17.04 ± 2.73 | 14.45 ± 2.08 | – | – | – | – |
| 2-undecanone | 1.98 ± 0.71 | – | 0.40 ± 0.04 | – | – | – | – |
| 4,7-dimethylundecane | Traces | 0.13 ± 0.04 | 0.15 ± 0.03 | – | – | – | – |
aVolatile compounds are reported according to their molecular weight. bPseudomonas tolaasii, Pleurotus eryngii, and P. ostreatus strains were grown on KB and MEA, respectively; NCPPB, National Collection of Plant Pathogenic Bacteria; USB, Microorganism collection of Università (U) degli Studi (S) della Basilicata (B). cKB, medium B of King et al. (1954). dMEA, Malt Extract Agar. –, no volatile compounds detected.
FIGURE 7.
Volatile compounds produced by Pseudomonas tolaasii strains (NCPPB2192, USB1, and USB66). 1- carbon dioxide (CO2); 2- methanethiol (MT); 3- dimethyl disulfide (DMDS); 4- p-cymene; 5, 1,4-undecadiene; 6- 1-undecene; 7- 2-undecanone; 8- 4,7-dimethylundecane.
In non-sealed conditions no volatiles were detected.
The three P. tolaasii strains resulted to produce NH3 but not HCN.
VOCs Bioactivity
Mushroom Bioassays
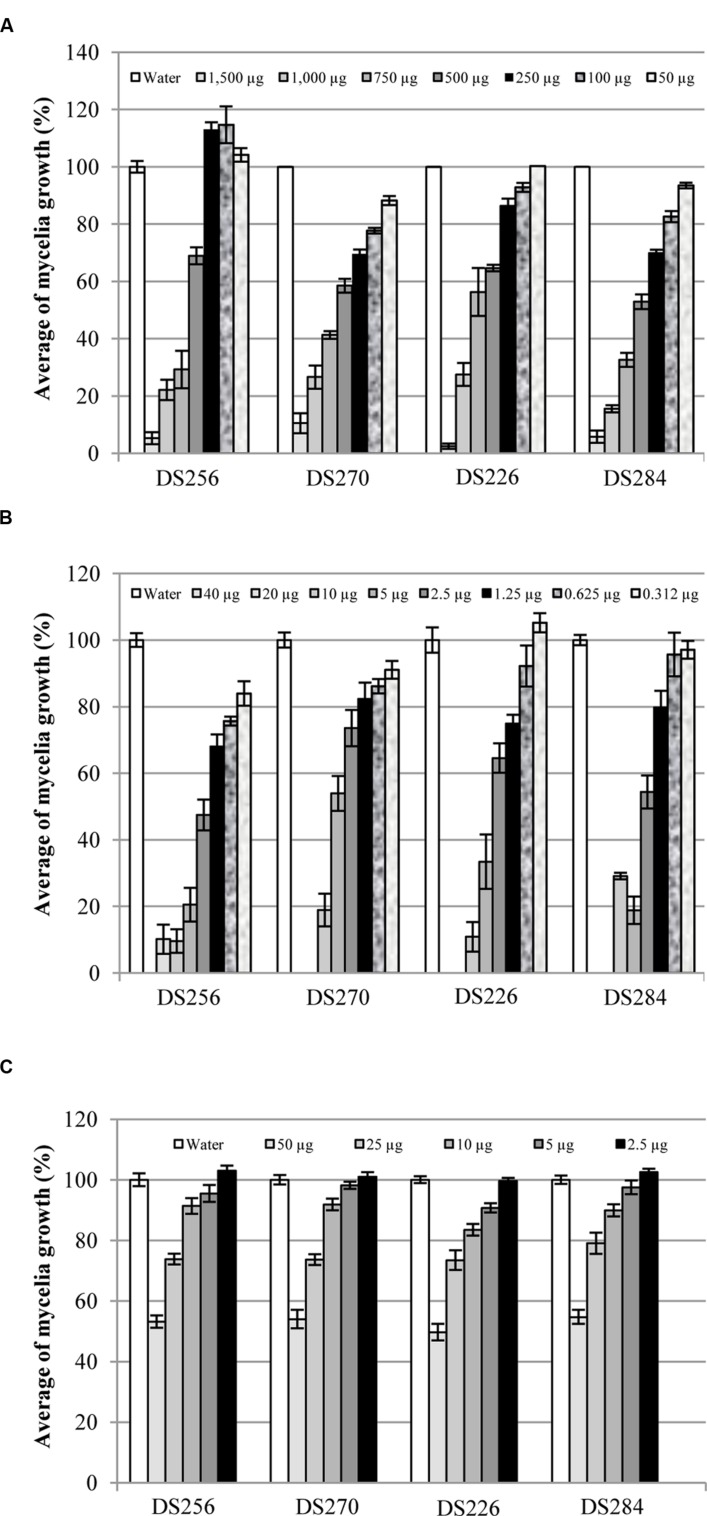
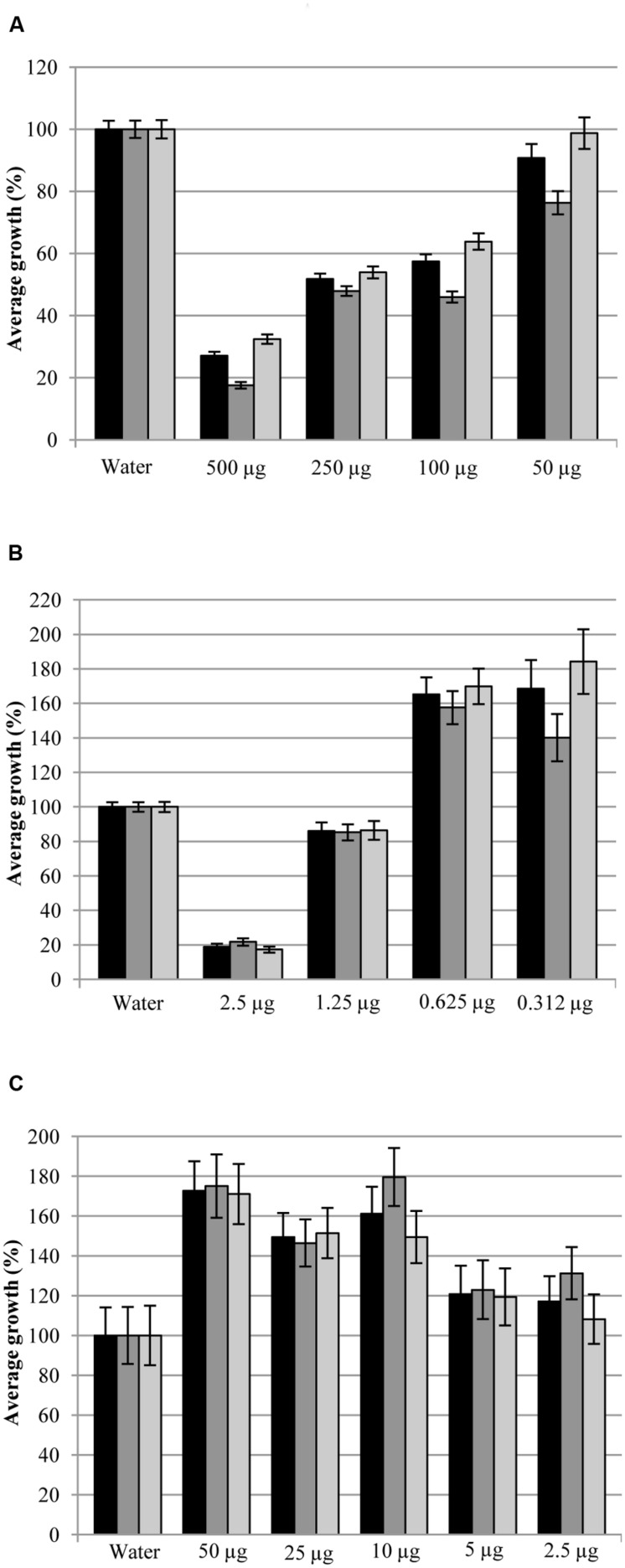
Treatments with 1,500 μg of MT per Petri dish caused a noticeable inhibition of P. eryngii and P. ostreatus strains mycelia growth (Figure 8A). In particular, P. eryngii strain DS256 resulted less sensitive than DS270 strain to MT effects. Indeed, DS256 strain growth was significantly reduced (P ≤ 0.001) by doses ≥500 μg [mycelia growth ≤69% when compared to the control (100%)], below which no significant growth reduction values were observed. P. eryngii strain DS270 resulted more affected by MT inhibiting action; using doses ≥50 μg mycelia growth was ≤88% (P = 0.01). The diverse sensitivity between two strains of the same mushroom was noticed also in the case of P. ostreatus strains DS226 and DS284, where quantities ≥500 μg of MT were necessary to cause significant growth inhibition of DS226 (mycelia growth ≤65%, P ≤ 0.001), and ≥100 μg for DS284 strain (mycelia growth ≤87%, P = 0.001) (Figure 8A).
FIGURE 8.
Average of mycelia growth (%) of strains DS256, DS270, and DS226, DS284 of Pleurotus eryngii and P. ostreatus, respectively, in presence of pure MT (A), DMDS (B), and 1-undecene (C) aliquots. Bars on the columns correspond to the standard error of the mean in percentage.
The total inhibition of P. eryngii and P. ostreatus strains mycelia growth was observed when they were treated with doses ≥20 μg per Petri dish of DMDS (Figure 8B). At lower doses of DMDS, the effect on mycelia growth was reduced but still highly significant. In particular, at doses ≥0.625 μg per Petri dish mycelia growth of the strains DS256 and DS270 of P. eryngii was ≤76% (P = 0.013) and ≤86% (P = 0.002), respectively, when compared to the control. A similar effect on P. ostreatus DS226 and DS284 strains growth was observed applying doses ≥1.25 μg of DMDS per Petri dish. Indeed, mycelia growth of the latter strains was ≤75% (P = 0.001) and ≤80% (P ≤ 0.002), respectively, compared to the control (Figure 8B).
1-undecene treatments on P. eryngii strains did not affect mycelia growth at doses ≤10 μg while, above the mentioned quantity, it caused a significant reduction (P ≤ 0.001) (Figure 8C). In particular, P. eryngii mycelia growth at doses ≥25 μg was ≤74% for both P. eryngii strains compared to the control (Figure 8C). The same treatments on P. ostreatus strains DS226 and DS284 were ineffective in mycelia growth inhibition at doses ≤2.5 and ≤5 μg, respectively; on the contrary, using quantity ≥5 and ≥10 μg, respectively, 1-undecene caused a significant reduction of mycelia growth (≤91%, P = 0.002 and ≤90%, P = 0.038) (Figure 8C).
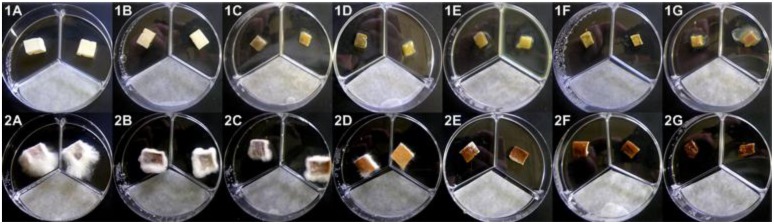
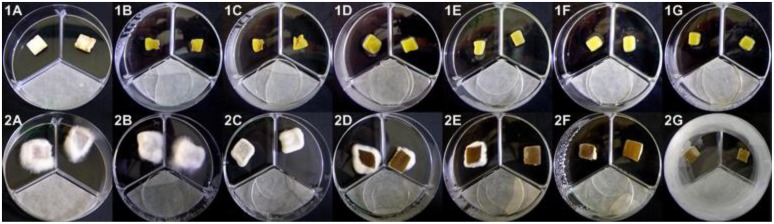
On A. bisporus and P. ostreatus tissue blocks MT aliquots determined aerial mycelia growth reduction, brown discoloration and deliquescence, with P. ostreatus being the less sensitive (Figure 9). Brown discoloration (Figure 9-1C) and tissue deliquescence of A. bisporus tissue blocks were determined with treatments of 100 and ≥250 μg of MT per Petri dish, respectively (Figure 9-1D). When P. ostreatus tissue blocks were treated with 50 or 100 μg of MT, the reduction of aerial mycelia growth was solely observed (Figure 9-2B,C); doses ≥250 μg caused a marked reduction of the mycelia growth and depressed brown discoloration (Figure 9-2D,E). The deliquescence of P. ostreatus tissue blocks was observed with treatments ≥1500 μg of MT (Figure 9-2G).
FIGURE 9.
Agaricus bisporus(1) and Pleurotus ostreatus(2) basidiome tissue blocks treated with pure MT aliquots (1A and 2A = H2O; 1B and 2B = 50 μg; 1C and 2C = 100 μg; 1D and 2D = 250 μg; 1E and 2E = 500 μg; 1F = 750 μg; 1G and 2F = 1,000 μg; 2G = 1,500 μg).
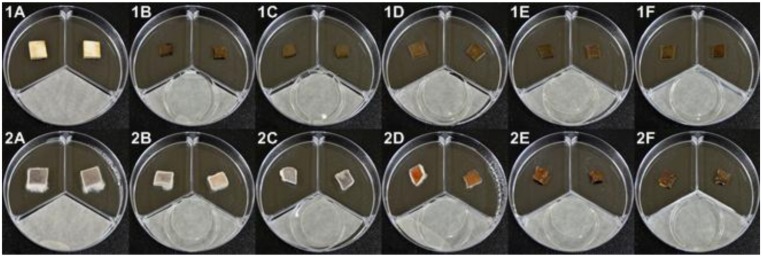
Aerial mycelia growth reduction, yellowing, brown discoloration and deliquescence of A. bisporus and P. ostreatus tissue blocks were observed when they were treated with DMDS aliquots, even though P. ostreatus tissue resulted, also in this case, less sensitive than A. bisporus (Figure 10). The yellowing of A. bisporus tissue blocks was observed with 0.156 μg of DMDS per Petri dish treatment (Figure 10-1B), while the tissue deliquescence was observed with doses ≥0.625 μg (Figure 10-1D). Treatments with 1.25 μg of DMDS caused, on P. ostreatus tissue blocks, a reduction of the aerial mycelia growth (Figure 10-2C), whereas 2.5 μg caused brown discoloration (Figure 10-2D). A marked depressed brown discoloration and deliquescence of tissue was observed with doses of DMDS ≥20 μg (Figure 10-2G).
FIGURE 10.
Agaricus bisporus(1) and Pleurotus ostreatus(2) basidiome tissue blocks treated with pure DMDS aliquots (1A and 2A = H2O; 1B = 0.156 μg; 1C = 0.312 μg; 1D and 2B = 0.625 μg; 1E and 2C = 1.25 μg; 1F and 2D = 2.5 μg; 1G and 2E = 5 μg; 2F = 10 μg; 2G = 20 μg).
1-undecene treatments also caused aerial mycelia growth reduction, yellowing, brown discoloration, and deliquescence of A. bisporus and P. ostreatus tissue blocks and, in this case too, P. ostreatus tissue was found less sensitive than A. bisporus (Figure 11). In fact, A. bisporus tissue blocks showed marked depressed brown discoloration and deliquescence of tissue at all 1-undecene doses used in this work (Figure 11-1). On the other side, P. ostreatus tissue blocks at doses ≤5 μg of the VOC caused aerial mycelia growth reduction (Figure 11-2B,C), at doses of 10 μg determined brown discoloration (Figure 11-2D), and at doses ≥25 μg induced a marked depressed brown discoloration and deliquescence of tissue (Figure 11-2E,F).
FIGURE 11.
Agaricus bisporus(1) and Pleurotus ostreatus(2) basidiome tissue blocks treated with pure 1-undecene aliquots (1A and 2A = H2O; 1B and 2B = 2.5 μg; 1C and 2C = 5 μg; 1D and 2D = 10 μg; 1E and 2E = 25 μg; 1F and 2F = 50 μg).
Seed Bioassays
Methanethiol and DMDS at doses >500 and 5 μg per Petri dish, respectively, inhibited broccoli seeds germination. At lower doses, different effects on seedlings growth were observed (Figure 12). Doses of 500, 250, and 100 μg of MT led to highly significant reduction (P < 0.001) of whole seedling (27, 52, and 57%), epicotyl (18, 48, and 46%), and main rootlet growth (32, 54, and 64%), respectively, when compared to the control (100%) (Figure 12A). Treatment with 50 μg of MT caused a significant reduction (P ≤ 0.002) of the whole seedling (9%) and epicotyl (24%) growth but the main rootlet growth was not significantly different (99%) from the control (Figure 12A).
FIGURE 12.
Average growth (%) of whole seedlings ( ), epicotyls (
), epicotyls ( ), and main rootlets (
), and main rootlets ( ) of broccoli in presence of pure MT (A), DMDS (B), and 1-undecene (C) aliquots. Bars on the columns correspond to the standard error of the mean in percentage.
) of broccoli in presence of pure MT (A), DMDS (B), and 1-undecene (C) aliquots. Bars on the columns correspond to the standard error of the mean in percentage.
Significant reduction (P < 0.001) of whole seedling (19%), epicotyl (22%) and main rootlet (17%) growth, in comparison with water seed treatment (100%) used as control (Figure 12B), was observed after seeds treatment with 2.5 μg of DMDS. Doses of 1.25 μg of DMDS caused significant decrease (P < 0.001) of the whole seedling (86%) and epicotyl length (85%) while the main rootlet growth (86%) was not statistically different (P > 0.05) from the control (Figure 12B). Highly significant increase (P ≤ 0.002) of seedlings growth (whole seedling, epicotyl, and main rootlet) was obtained by treatments with 0.625 μg (165, 158, and 170%, respectively) and 0.312 μg (169, 140, and 184%, respectively) of DMDS, compared to the control (Figure 12B).
Broccoli seedlings growth stimulation was also noticed in 1-undecene treatments (Figure 12C). In fact, significant increase of whole seedlings, epicotyls and main rootlets growth was caused by treatments with 10 μg (161, 180, and 149%, respectively, with P ≤ 0.013), 25 μg (150, 147, and 151%, respectively, with P ≤ 0.021), and 50 μg (173, 175, and 171%, respectively, with P ≤ 0.05) of 1-undecene, compared to the control (Figure 12C).
Discussion
In this paper, it was demonstrated that P. tolaasii, an ubiquitous bacterium mainly recognized as a mushroom pathogen but also reported as associated to plant in either parasitic or commensal positions (Rainey et al., 1991; Zdor and Anderson, 1992; Noval et al., 1993; Shirata et al., 1995; Talame and Piccirillo, 1995; Tanprasert and Reed, 1997), is able to produce in vitro volatiles which were identified by GC–MS. They were demonstrated to affect mushrooms, seed germination, and seedling growth features confirming previous evidences (Shirata, 1996). Indeed, in assays performed in non-sealed Petri dishes, P. eryngii and P. ostreatus strains mycelia growth was greatly reduced when exposed to P. tolaasii strains volatile metabolites. In the same experimental conditions P. tolaasii volatiles toxicity was explicated by pronounced A. bisporus and P. ostreatus basidiome tissue blocks brown lesions or yellowing. On the other side, P. tolaasii volatiles in sealed Petri dishes, did not have toxic effect: P. eryngii and P. ostreatus mycelia showed a significant growth increase and A. bisporus and P. ostreatus blocks presented even mycelia proliferation on their surfaces. These apparently divergent and contradictory results may be explained as follows. In in vitro experimental systems performed, there is the strictly aerobic bacterium P. tolaasii and Agaricus and Pleurotus sp. which are able to live also in limited oxygen conditions (Rast and Bachofen, 1967a,b; San Antonio and Thomas, 1972; Zadražil, 1975). In fact, in the latter condition Pleurotus sp. mycelia increase their biomasses because of their ability to assimilate CO2 when available in the environment at high concentrations (Rast and Bachofen, 1967a,b; San Antonio and Thomas, 1972; Zadražil, 1975). This seems the case of the sealed Petri dishes assay conditions. On the other hand the oxygen limitation may lead the bacterium to produce VOCs, which levels may be under the toxicity threshold; it could also be that the simultaneous accumulation of CO2, produced by both organisms, may counteract VOCs toxic effects (Rast and Bachofen, 1967a,b; San Antonio and Thomas, 1972; Zadražil, 1975).
Pseudomonas tolaasii, when grown in non-sealed Petri dishes, expresses its aerobic metabolism and inorganic and organic volatile compounds are produced better than the bacterium could do in limited oxygen conditions (sealed plates) (Ferchichi et al., 1986). In such state, despite the fact that part of the volatile compounds produced may be dispersed outside of the Petri dish, the VOCs still reach toxic concentrations for Pleurotus sp. mycelia and A. bisporus and P. ostreatus basidiome tissue blocks.
Lettuce and broccoli seeds exposure to bacterial volatiles led to negative effect on both germination and seedling growth. The highest effects were observed when the assays were carried out in sealed Petri dishes. In non-sealed dishes, with normal oxygen concentrations and gas exchange, seeds germination is just partly inhibited due to the possible sub-lethal volatiles dose achievement. In sealed plates system, the toxic effects of volatiles, since there is a progressive decrease of oxygen concentration and an accumulation of gaseous catabolites, considering also that both aerobic organisms are stressed in this conditions, may depend on possible additional/synergistic action of volatiles (Segal and Starkey, 1969; Ferchichi et al., 1986; van Leerdam et al., 2008). Moreover, the above results suggest roots as being the most sensitive plantlet part to the P. tolaasii toxic action volatiles since they showed some brown-necrotic areas beside growth reduction. Furthermore, despite the fact that P. tolaasii produced high quantities of CO2 and it is known that this compound is able to stimulate plant growth (Kai and Piechulla, 2009), no beneficial effect on plant biostimulation was observed.
The GC–MS analysis, despite the fact that in non-sealed conditions no volatiles were detected probably because of their dispersion, indicated that P. tolaasii strains are able to produce different volatiles, among which MT, DMDS, and 1-undecene have been produced by all the three strains of the bacterium. Moreover, GC–MS analysis of non-inoculated KBA or MEA and Pleurotus sp. strains inoculated on MEA clearly demonstrated that no one of the above mentioned volatiles were detected, confirming their exclusive origin from P. tolaasii. The same GC-MS analysis stated, as expected, the presence of a quite important level of CO2 produced by the growing microorganisms.
Finally, all P. tolaasii strains did not produce HCN, although this substance is produced by several bacteria including some Pseudomonas sp. inhabiting plants rhizosphere (Blom et al., 2011; Weise et al., 2013). However, P. tolaasii strains produce NH3 and this volatile compound has a significant role on the plants and fungi growth (Mikeš et al., 1994; Weise et al., 2013). In fact, it is known that NH3 inhibits the growth of Arabidopsis thaliana through the alkalization of the neighboring plant medium and, on the other hand, it stimulates the fungal growth via its assimilation by the glutamine synthetase and glutamate dehydrogenase NAD-dependent enzymes (Mikeš et al., 1994; Weise et al., 2013). This may contribute to explain the growth inhibition of broccoli and lettuce seedlings and increased mycelia growth in biological assays performed in this study.
On the basis of these results the VOCs MT, DMDS, and 1-undecene were selected for further assays.
The in vitro assays results, performed using pure VOCs showed that MT, DMDS, and 1-undecene, whose toxic action on pathogenic fungi is well known (Lewis, 1985; Fritsch, 2005; Groenhagen et al., 2013; Wang et al., 2013; Popova et al., 2014; Hunziker et al., 2015), are also toxic for the P. eryngii and P. ostreatus mycelia and A. bisporus and P. ostreatus tissue blocks. Furthermore, higher MT and DMDS doses (from 50 to 500 μg and ≥1.25 μg, respectively) inhibited the broccoli seeds germination while the DMDS and 1-undecene (at doses ≤0.625 and ≥10 μg, respectively) have also caused an increase of whole broccoli seedlings growth. The results concerning the bioactivity of the above mentioned VOCs and, in particular, on plant growth stimulation action by 1-undecene have here been reported, to the best of our knowledge, for the first time.
These findings clearly indicate that MT and DMDS have an important role in the bioactivity of bacterial volatiles natural blend toward fungal and plant systems. The toxic effect of these VOCs may have cellular respiration as target, since it is known that MT and DMDS are able to inhibit mitochondrial activity (Waller, 1977; Dugravot et al., 2003). Our recent electron microscopy studies (Giorgio et al., 2015) revealed that DMDS and other VOCs, such as 2-nonanone and DL-limonene, are also able to determine structural alterations of cell membranes on phytopathogenic fungi that can contribute, along with the alteration of mitochondrial activity, to cell death. DMDS also proved capable, at doses ≤0.625 μg, of stimulating the growth broccoli seedlings. These results are not very different from the ones obtained by other authors. In fact, Kai et al. (2009) showed that DMDS inhibits A. thaliana (L.) Heynh growth and Groenhagen et al. (2013) have highlighted that the same molecule, at doses ranging from 1 ng to 1 mg, significantly increased the same plant biomass. Recently, Meldau et al. (2013) demonstrated that DMDS is able to make available its organic sulfur to the roots of Nicotiana attenuata Torr. ex S. Watson favoring its growth.
1-undecene’s ability to inhibit Pleurotus sp. strains growth, to alter A. bisporus and P. ostreatus blocks and increase the growth of broccoli seedlings are, to our knowledge, new biological properties for this compound. Antimicrobial activity of 1-undecene has been only recently determined (Kai et al., 2009; Groenhagen et al., 2013; Wang et al., 2013; Popova et al., 2014) on some target microorganisms [Agrobacterium tumefaciens Smith & Townsend, Synechococcus sp., Rhizoctonia solani (Cooke) Wint. and Fusarium culmorum (Wm. G. Sm.) Sacc.] among which only the fungus F. culmorum was weakly inhibited; biostimulation ability on plants of this molecule, on the other side, remains totally unexplored. Some authors (Nisenbaum et al., 2013) have previously shown that hydrocarbons, including 1-undecene, can be assimilated or transformed by microorganisms, but to our knowledge, no one has proven whether broccoli or plants in general, are able to utilize 1-undecene as carbon source. This outcome certainly highlights the need for further studies to understand this result.
Conclusion
Pseudomonas tolaasii volatile blend, as well as pure VOCs used in the present work, may have an important role in the interaction between pathogen and cultivated mushrooms. Shirata (1996) reported that P. tolaasii volatiles were able to contribute to the disease symptoms and interfere with mushrooms mycelia development, during substrate colonization, and thus, indirectly on mushrooms production. The fact that MT, DMDS and 1-undecene showed antifungal activity toward cultivated mushrooms mycelia and were toxic toward basidiome tissue blocks suggest the mentioned VOCs as P. tolaasii new potential virulent factors since they were able to reproduce, at least in part, brown blotch and yellowing typical symptoms caused by bacteria on A. bisporus and P. ostreatus, respectively. However, it is necessary to consider that, in some of the case, the above VOCs reproduced the biological effect of the whole volatile blend when applied in high, probably non-natural, concentrations. New studies would unravel whether the bacterium is able to produce in vivo VOCs among resulting toxic to mushrooms and if VOCs synergic actions may occur. P. tolaasii mutants availability in producing VOCs may highlight their contribution, beside tolaasins, on the virulence of the pathogen. Moreover, since P. tolaasii is also reported to be associated to plants rhizospheres (Zdor and Anderson, 1992), it is not to be excluded that VOCs, possibly produced in those niches, may interfere with the behavior of organisms dwelling the same habitat. In this regard, P. tolaasii, could present typical biocontrol agents traits since it is able to produce lipodepsipeptides and VOCs which are toxic for fungi. Studies to verify the rhizosphere competence of the bacterium would be very useful to assess his candidacy as a biocontrol agent.
Nevertheless, our results on these VOCs biological activity indicate that they may represent alternatives to methyl bromide for fumigation of soils infected by soil-borne fungal pathogens. As a matter of fact, DMDS is already used as a novel pre-planting soil fumigant under the commercial name PALADIN. Furthermore, it has been recently established the ability of DMDS to control plant pathogenic fungi (Fritsch, 2005; Kai et al., 2009), either directly or via the induction of systemic resistance (Huang et al., 2012), nematodes (Coosemans, 2005) and weeds (Freeman et al., 2009). Finally, since DMDS and 1-undecene were able to increase plant growth, they can also be candidate as potential plant biostimulators/fertilizers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Daniele Sisto, Università degli Studi di Bari, Italy and Puglia Fungo, Rutigliano, Bari, Italy are acknowledged for generously providing A. bisporus, P. ostreatus, and P. eryngii strains, and fresh picked mushrooms basidiomes. The authors would also like to thank Maurizio D’Auria and Rocco Racioppi, Università degli Studi della Basilicata, for the advice and support provided in GC–MS analysis.
References
- Afsharmanesh H., Ahmadzadeh M., Sharifi-Tehrani A. (2006).Biocontrol of Rhizoctonia solani, the causal agent of bean damping-off by fluorescent pseudomonads. Commun. Agric. Appl. Biol. Sci. 71 1021–1029. [PubMed] [Google Scholar]
- Blom D., Fabbri C., Eberl L., Weisskopf L. (2011). Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl. Environ. Microbiol. 77 1000–1008. 10.1128/AEM.01968-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccino J. G., Sherman N. (2010). Microbiology: A Laboratory Manual. California: The Benjamin/Cummings Publishing Co. [Google Scholar]
- Coosemans J. (2005). Dimetyl disulphide (DMDS): a potential novel nematicide and soil disinfectant. Acta Hortic. 689 57–64. [Google Scholar]
- Cutri S. S., Macauley B. J., Roberts W. P. (1984). Characteristics of pathogenic non-fluorescent (smooth) and nonpathogenic fluorescent (rough) forms of Pseudomonas tolaasii and Pseudomonas gingeri. J. Appl. Bacteriol. 57 291–298. 10.1111/j.1365-2672.1984.tb01393.x [DOI] [Google Scholar]
- Dugravot S., Grolleau F., Macherel D., Rochetaing A., Hue B., Stankiewicz M., et al. (2003). Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect KATP channels. J. Neurophysiol. 90 259–270. 10.1152/jn.01096.2002 [DOI] [PubMed] [Google Scholar]
- Effmert U., Kalderás J., Warnke R., Piechulla B. (2012). Volatile mediated interactions between bacteria and fungi in the Soil. J. Chem. Ecol. 38 665–703. 10.1007/s10886-012-0135-5 [DOI] [PubMed] [Google Scholar]
- Ercolani G. L. (1970). Primi risultati di osservazioni sulla maculatura batterica dei funghi coltivati [Agaricus bisporus (Lange) Imbach] in Italia: identificazione di Pseudomonas tolaasii Paine. Phytopathol. Mediterr. 9 59–61. [Google Scholar]
- Farag M. A., Ryu C. M., Summer L. W., Pare P. W. (2006). GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67 2262–2268. 10.1016/j.phytochem.2006.07.021 [DOI] [PubMed] [Google Scholar]
- Ferchichi M., Hemme D., Bouillanne C. (1986). Influence of oxygen and pH on methanethiol production from L-methionine by Brevibacterium linens CNRZ 918. Appl. Environ. Microbiol. 51 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermor T. R. (1987). “Bacterial diseases of edible mushrooms and their control”, in Developments in Crop Science, Cultivating Edible Fungi, 10, eds Wuest P. J., Royse D. J., Beelman R. B. (Amsterdam: Elsevier Science Publishing; ), 361–370. [Google Scholar]
- Ferri F. (1985). I Funghi. Micologia, Isolamento Coltivazione. Bologna: Edagricole. [Google Scholar]
- Freeman J., Rideout S., Wimer A. (2009). Dimetyl disulphide use for bacterial wilt management and weed control in Virginia tomatoes. Hort. Sci. 44 571. [Google Scholar]
- Fritsch J. (2005). Dimethyl disulfide as a new chemical potential alternative to methyl bromide in soil disinfestation in France. Acta Hortic. 698 71–76. [Google Scholar]
- Gandy D. G. (1979). The pathogens of Agaricus bitorquis. Ann. Rep. Glasshouse Crops Res. Inst. 142. [Google Scholar]
- Gill W. M. (1995). Bacterial diseases of Agaricus Mushrooms. Rep. Tottori Mycol. Inst. 33 34–55. [Google Scholar]
- Giorgio A., De Stradis A., Lo Cantore P., Iacobellis N. S. (2015). Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 6:1056 10.3389/fmicb.2015.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S. I. S., Han B., Johnstone K. (1995). Identification and characterization of a locus which regulates multiple functions in Pseudomonas tolaasii, the cause of brown blotch disease of Agaricus bisporus. J. Bacteriol. 177 4658–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenhagen U., Baumgartner R., Bailly A., Gardiner A., Eberl L., Schulz S., et al. (2013). Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 39 892–906. 10.1007/s10886-013-0315-y [DOI] [PubMed] [Google Scholar]
- Haas D., Défago G. (2005). Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3 307–319. 10.1038/nrmicro1129 [DOI] [PubMed] [Google Scholar]
- Howell C. R., Beier R. C., Stipanovic R. D. (1988). Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Ecol. Epidemiol. 78 1075–1078. 10.1094/Phyto-78-1075 [DOI] [Google Scholar]
- Huang C.-J., Tsay J.-F., Chang S.-Y., Yang H.-P., Wu W.-S., Chen C.-Y. (2012). Dimethyl disulfide is an induced systemic resistance-elicitor produced by Bacillus cereus C1L. Pest Manag. Sci. 68 1306–1310. 10.1002/ps.3301 [DOI] [PubMed] [Google Scholar]
- Hunziker L., Bönisch D., Groenhagen U., Bailly A., Schulz S., Weisskopf L. (2015). Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 81 821–830. 10.1128/AEM.02999-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis N. S. (2011). “Recent advances on bacterial diseases of cultivated mushrooms”, in Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, (ICMBMP7), eds Savoie J.-M., Foulongne-Oriol M., Largeteau M., Barroso G. Arcachon: 452–460. [Google Scholar]
- Kai M., Crespo E., Cristescu S. M., Harren F. J. M., Francke W., Piechulla B. (2010). Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 88 965–976. 10.1007/s00253-010-2810-1 [DOI] [PubMed] [Google Scholar]
- Kai M., Effmert U., Berg G., Piechulla B. (2007). Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 187 351–360. 10.1007/s00203-006-0199-0 [DOI] [PubMed] [Google Scholar]
- Kai M., Haustein M., Molina F., Petri A., Scholz B., Piechulla B. (2009). Bacterial volatiles and their action potential. Appl. Microbiol. Biotechnol. 81 1001–1012. 10.1007/s00253-008-1760-3 [DOI] [PubMed] [Google Scholar]
- Kai M., Piechulla B. (2009). Plant growth promotion due to rhizobacterial volatiles – an effect of CO2? FEBS Lett. 583 3473–3477. 10.1016/j.febslet.2009.09.053 [DOI] [PubMed] [Google Scholar]
- King E. O., Ward M. K., Raney D. E. (1954). Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44 301–307. [PubMed] [Google Scholar]
- Largeteau M. L., Savoie J.-M. (2010). Microbially induced diseases of Agaricus bisporus: biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 86 63–73. 10.1007/s00253-010-2445-2 [DOI] [PubMed] [Google Scholar]
- Lee H. U., Lee M. H., Cho D. J., Shin W. K., Moon B. J. (1996). Influence of Pseudomonas species causing bacterial blotch of mushrooms on the mycelial growth in Pleurotus ostreatus and Flammulina velutipes. J. Agric. Sci. 38 887–892. [Google Scholar]
- Lewis B. A. (1985). Inhibition of Candida albicans by methanethiol produced by Brevibacterium linens. Microbiologica 8 387–390. [PubMed] [Google Scholar]
- Lo Cantore P., Lazzaroni S., Coraiola M., Dalla Serra M., Cafarchia C., Evidente A., et al. (2006). Biological characterization of WLIP produced by Pseudomonas “reactans” NCPPB1311. Mol. Plant Microbe Interact. 19 1113–1120. 10.1094/MPMI-19-1113 [DOI] [PubMed] [Google Scholar]
- Lorck H. (1948). Production of hydrocyanic acid by bacteria. Physiol. Plant 1 142–146. 10.1111/j.1399-3054.1948.tb07118.x [DOI] [Google Scholar]
- Meldau D. G., Meldau S., Hoang L. H., Underberg S., Wünsche H., Baldwin I. T. (2013). Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuate growth by enhancing sulfur nutrition. Plant Cell 25 2731–2747. 10.1105/tpc.113.114744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikeš V., Zofall M., Chytil M., Fulneček J., Scháněl L. (1994). Ammonia-assimilating enzymes in the basidiomycete fungus Pleurotus ostreatus. Microbiology 140 977–982. 10.1099/00221287-140-4-977 [DOI] [Google Scholar]
- Morath S. U., Hung R., Bennett J. W. (2012). Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol. Rev. 26 73–83. 10.1016/j.fbr.2012.07.001 [DOI] [Google Scholar]
- Nisenbaum M., Sendra G. H., Gilbert G. A. C., Scagliola M., González J. F., Murialdo S. E. (2013). Hydrocarbon biodegradation and dynamic laser speckle for detecting chemotactic responses at low bacterial concentration. J. Environ. Sci. 25 613–625. [DOI] [PubMed] [Google Scholar]
- Noval C., Seisdedos M. T., De La Cruz J. I. (1993). Estudio de la posible relación entre la caída de yemas en peral y la capacidad bacteriana para formar núcleos de hielos u originar podredumbre banda. Bol. Sanid. Veg. Plagas 19 649–661. [Google Scholar]
- Nutkins J. C., Mortishire Smith R. J., Packman L. C., Brodey C. L., Rainey P. B., Johnstone K., et al. (1991). Structure determination of tolaasin, an extracellular lipodepsipeptide produced by the mushroom pathogen Pseudomonas tolaasii Paine. J. Am. Chem. Soc. 113 2621–2627. 10.1021/ja00007a040 [DOI] [Google Scholar]
- Paine S. G. (1919). A brown blotch disease of cultivated mushrooms. Ann. Appl. Biol. 5 206–219. [Google Scholar]
- Park C. J., Oh S. K., Chun U. H. (1994). Identification of mushroom brown blotch causing agent from Pseudomonas tolaasii culture broth. Agric. Chem. Biotechnol. 37 392–396. [Google Scholar]
- Popova A. A., Koksharova O. A., Lipasova V. A., Zaitseva J. V., Katkova-Zhukotskaya O. A., Eremina S. I., et al. (2014). Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed. Res. Int. 2014 1–11. 10.1155/2014/125704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey P. B., Brodey C. L., Johnstone K. (1991). Biological properties and spectrum of activity of tolaasin, a lipodepsipeptide toxin produced by the mushroom pathogen Pseudomonas tolaasii. Physiol. Mol. Plant Pathol. 39 57–70. 10.1016/0885-5765(91)90031-C [DOI] [Google Scholar]
- Rainey P. B., Brodey C. L., Johnstone K. (1992). Biology of Pseudomonas tolaasii, cause of brown blotch disease of the cultivated mushrooms. Adv. Plant Pathol. 8 95–117. [Google Scholar]
- Rast D., Bachofen R. (1967a). Carboxylierungsreaktionen in Agaricus bisporus. I. Der endogene CO2-acceptor. Arch. Microbiol. 57 392–405. [PubMed] [Google Scholar]
- Rast D., Bachofen R. (1967b). Carboxylierungsreaktionen in Agaricus bisporus. II. Aceton als ein CO2-acceptor. Arch. Microbiol. 58 339–356. [PubMed] [Google Scholar]
- Rodriguez E. A. E., Royse D. J. (2007). Yield, size and bacterial blotch resistance of Pleurotus eryngii grown on cottonseed hull/oak sawdust supplemented with manganese, copper and whole ground soybean. Bioresource Technol. 98 1898–1906. 10.1016/j.biortech.2006.07.027 [DOI] [PubMed] [Google Scholar]
- Ryu C.-M., Farag A. F., Hu C., Reddy M. S., Kloepper J. W., Pare P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134 1017–1026. 10.1104/pp.103.026583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C.-M., Farag M. A., Hu C.-H., Reddy M. S., Wei H.-X., Paré P. W., et al. (2003). Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100 4927–4932. 10.1073/pnas.0730845100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Antonio J. P., Thomas R. L. (1972). Carbon dioxide stimulation of hyphal growth of the cultivated mushroom, Agaricus bisporus (Lange) sing. Mushroom Sci. 8 623–629. [Google Scholar]
- Segal W., Starkey R. L. (1969). Microbial decomposition of methionine and identity of the resulting sulfur products. J. Bacteriol. 98 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirata A. (1996). Production of volatile components by Pseudomonas tolaasii and their toxic activity. Ann. Phytopathol. Soc. Jpn. 62 185–193. [Google Scholar]
- Shirata A., Sugaya K., Takasugi M., Monde K. (1995). Isolation and biological activity of toxins produced by a Japanese strain of Pseudomonas tolaasii, the pathogen of bacterial rot of cultivated oyster mushroom. Ann. Phytopathol. Soc. Jpn. 61 493–502. [Google Scholar]
- Soler-Rivas C., Jolivet S., Arpin N., Olivier J. M., Wichers H. J. (1999). Biochemical and physiological aspects of brown blotch disease of Agaricus bisporus. FEMS Microbiol. Rev. 23 591–614. 10.1111/j.1574-6976.1999.tb00415.x [DOI] [PubMed] [Google Scholar]
- Talame M., Piccirillo F. (1995). I nemici del cavolfiore. Inf. Agrar. 51 25–33. [Google Scholar]
- Tanprasert P., Reed B. M. (1997). Detection and identification of bacterial contaminants from strawberry runner explants. In Vitro Cell. Dev. Biol. Plant 33 221–226. [Google Scholar]
- Tolaas A. G. (1915). A bacterial disease of cultivated mushrooms. Phytopathology 5 51–54. [Google Scholar]
- Tsuneda A., Suyama K., Murakami S., Ohira I. (1995). Occurrence of Pseudomonas tolaasii on fruiting bodies of Lentinula edodes formed on Quercus logs. Mycoscience 36 283–288. 10.1007/BF02268603 [DOI] [Google Scholar]
- van Leerdam R. C., Bonilla-Salinas M., de Bok F. A. M., Bruning H., Lens P. N. L., Stams A. J. M., et al. (2008). Anaerobic methanethiol degradation and methanogenic community analysis in an alkaline (pH 10) biological process for liquefied petroleum gas desulfurization. Biotechnol. Bioeng. 101 691–701. 10.1002/bit.21933 [DOI] [PubMed] [Google Scholar]
- Vespermann A., Kai M., Piechulla B. (2007). Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl. Environ. Microbiol. 73 5639–5641. 10.1128/AEM.01078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R. L. (1977). Methanethiol inhibition of mitochondrial respiration. Toxicol. Appl. Pharmacol. 42 111–117. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang Z., Qiao X., Li Z., Li F., Chen M.et al. (2013). Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 341 45–51. 10.1111/1574-6968.12088 [DOI] [PubMed] [Google Scholar]
- Weise T., Kai M., Piechulla B. (2013). Bacterial ammonia causes significant plant growth inhibition. PLoS ONE 8:e63538 10.1371/journal.pone.0063538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. C., Preece T. F. (1980). Pseudomonas tolaasii in mushroom crops: a note on primary and secondary sources of the bacterium on a commercial farm in England. J. Appl. Bacteriol. 49 305–314. 10.1111/j.1365-2672.1980.tb05129.x [DOI] [Google Scholar]
- Zadražil F. (1975). Influence of CO2 concentration on the mycelium growth of three Pleurotus species. Eur. J. Appl. Microbiol. 1 327–335. [Google Scholar]
- Zdor R. E., Anderson A. J. (1992). Influence of root colonizing bacteria on the defense responses of bean. Plant Soil 140 99–107. [Google Scholar]