Abstract
Background and objectives
Cardiac resynchronization therapy (CRT) is a well established heart failure treatment that has shown to improve renal function. However, landmark CRT trials excluded patients with severe renal dysfunction. Therefore, this study evaluated the effect of CRT on renal function and long-term prognosis in patients with stage 4 CKD.
Design, setting, participants, & measurements
This study evaluated 73 consecutive CRT patients (71±10 years) with stage 4 CKD who underwent echocardiographic and renal function evaluation at baseline and 6-month follow-up between 2000 and 2012. As a control group, 18 patients with stage 4 CKD who received an implantable cardioverter defibrillator (ICD) were selected. CRT recipients with ≥15% reduction in left ventricular end-systolic volume at 6-month follow-up were classified as CRT responders. During long-term follow-up (median, 33 months), appropriate defibrillator therapy, heart failure hospitalizations, and all-cause mortality (combined end point) were recorded.
Results
At 6-month follow-up, a significant reduction in left ventricular end-systolic volume was observed in CRT patients compared with patients with ICD (from 159±78 to 145±78 ml in CRT patients and from 126±54 to 119±49 ml in ICD patients; P=0.05), and CRT response was observed in 22 patients (30%). Compared with ICD patients, eGFR improved among CRT patients (from 25±4 to 30±9 ml/min per 1.73 m2; interaction time and group, P=0.04) and was more pronounced among CRT responders (25±3 to 34±9 ml/min per 1.73 m2; P<0.001). The combined end point was observed in 17 ICD and 62 CRT patients. CRT patients showed superior survival compared with ICD patients (log-rank P=0.03). More importantly, CRT response was independently associated with improved survival free from the combined end point (hazard ratio, 0.51; 95% confidence interval, 0.27 to 0.98; P=0.04) after adjustment for clinical and echocardiographic parameters.
Conclusions
Response to CRT occurs in approximately 30% of patients with stage 4 CKD, which is less than in the average CRT population. CRT was associated with better clinical outcome, and particularly, CRT response was associated with improvement in eGFR and better long-term prognosis.
Keywords: renal function, chronic kidney disease, congestive heart failure, chronic, heart failure, survival
Introduction
Renal dysfunction is a common comorbidity in patients with heart failure (HF) associated with poor prognosis (1–3). Cardiac resynchronization therapy (CRT) is a well established HF treatment for patients with broad QRS duration, improving left ventricular (LV) function, reducing HF symptoms, and improving long-term prognosis (4). In addition, CRT has demonstrated to be associated with improvement of renal function (5–11). However, landmark trials excluded patients with severe renal dysfunction, and only few data are available on the effect of CRT in this particular population (10). Therefore, the beneficial effect of CRT in patients with severe renal dysfunction remains still to be demonstrated. Accordingly, this study aimed to investigate in this specific subgroup of patients the effect of CRT on renal function, cardiac performance, and long-term outcome.
Materials and Methods
Patient Population
A total of 73 patients with severe renal dysfunction (defined by an eGFR of 15–29 ml/min per 1.73 m2) undergoing CRT at the Leiden University Medical Center between 2000 and 2012 were selected. All patients in hemodialysis, peritoneal dialysis, or previous kidney transplantation before device implantation were excluded. Patients received CRT devices (with a defibrillator function) according to the current guidelines on the basis of the presence of left ventricular ejection fraction (LVEF) ≤35%, HF symptoms as New York Heart Association (NYHA) classes II, III, and ambulatory IV despite optimal medical therapy, and a QRS duration ≥120 milliseconds (4). In addition, 18 patients with severe renal dysfunction undergoing implantable cardioverter defibrillator (ICD) implantation, matched for age, sex, and LVEF, were selected as the control group. All devices were conventionally implanted in the pectoral region as previously described (12,13).
The etiology of HF was considered ischemic in the presence of significant coronary artery disease (>50% stenosis in ≥1 major epicardial coronary artery) on coronary angiography and/or a history of myocardial infarction or previous revascularization. Patients with recent myocardial infarction (<3 months) or decompensated HF were excluded from analysis. Clinical data and all follow-up visits were prospectively collected in the departmental cardiology information system (EPD-Vision, Leiden University Medical Centre, Leiden, The Netherlands) and retrospectively analyzed.
Clinical Evaluation
Before device implantation, NYHA functional class was evaluated. Serum creatinine levels were routinely assessed before implantation and were used to calculate the eGFR according to the Modification of Diet in Renal Disease equation (14). An eGFR between 15 and 29 ml/min per 1.73 m2 corresponding with CKD stage 4 according to the National Kidney Foundation classification was considered as severe renal dysfunction (15). The change in renal function with an improvement to stage 3 CKD (eGFR between 30 and 59 ml/min per 1.73 m2) or a further deterioration of renal function (stage 5 CKD defined by eGFR <15 ml/min per 1.73 m2) was also evaluated.
Echocardiographic Evaluation
All patients were evaluated with two-dimensional transthoracic echocardiography using a commercially available system (Vivid 7 and E9; General Electric Vingmed Ultrasound, Horton, Norway). Standard two-dimensional and Doppler images were recorded and saved in cine-loop format for off-line analysis (EchoPac, version 110.0.0; GE-Vingmed, Horton, Norway). Echocardiographic analysis was performed according to the current recommendations and included quantification of LV end-diastolic volume, left ventricular end-systolic volume (LVESV), and LVEF by biplane Simpson’s method (16). The severity of mitral regurgitation was graded according to the most recent recommendations on the basis of a multiparametric approach (17). Right ventricular function was evaluated measuring the tricuspid annular plane systolic excursion, and the central venous pressure was evaluated by estimating the right atrial pressure using the inferior vena cava diameter and percentage of collapse during inspiration (18). LV dyssynchrony was assessed by the septal-to-lateral wall delay using color-coded tissue Doppler imaging in the apical four-chamber view as described previously (19).
Patients alive at the 6-month follow-up visit were re-evaluated, and changes in clinical (including renal function) and echocardiographic variables during follow-up were recorded.
Reduction of ≥15% in LVESV at 6-month follow-up echocardiography among CRT patients defined CRT response (20).
Long-Term Follow-Up Outcome
After 6-month follow-up, regular outpatient visits (every 3–6 months) were scheduled for all patients. Long-term follow-up was performed by medical chart review, telephone contact, or correspondence with the general practitioner and by retrieval of survival status through the municipal civil registries. A combined end point was used, including appropriate defibrillator therapy (antitachycardia pacing and defibrillator shocks), HF hospitalization, and all-cause mortality (whichever came first) (21). HF hospitalizations were adjudicated by the cardiologist responsible for the management of the patient during admission, whereas the appropriate defibrillator therapy was adjudicated by trained pacemaker technicians (and confirmed by a cardiologist) after device interrogation.
Statistical Analyses
Continuous variables are presented as mean±SD, and categorical data are presented as numbers and percentages. Unpaired t tests were used to compare continuous variables, and chi-squared tests were used to compare categorical variables. Differences in magnitude of change over time between ICD and CRT patients were compared; repeated-measures ANOVA was used for normally distributed continuous variables, and generalized estimating equations were used for ordinal/categorical variables. Wilcoxon matched-pairs signed-rank test was used to test the significance of the change in CKD stage and in NYHA class at follow-up compared with the baseline. Bivariate logistic analysis with Pearson’s test was used to evaluate the correlation between decrease in LVESV and increase in eGFR. The log-rank tests were used to compare the difference in Kaplan–Meier curves for the survival free from the combined end point between the groups. Furthermore, the predictors of the combined end point were evaluated with the Cox proportional hazards model; all clinically relevant variables or significant predictors (P<0.20) at the univariate analysis were introduced in the multivariate model. In case of collinearity, only one of these variables was entered in the multivariate model. Considering the limited number of events, backward stepwise elimination was used, and the least significant parameter was discarded from the model until appropriate number parameters remained (1 parameter per 12–15 events). All statistics were two-tailed. A P value <0.05 was considered statistically significant. All statistical analyses were performed using IBM PASW Statistics, version 20.0 (SPSS, Chicago, IL).
Results
Patient Population
The baseline clinical and echocardiographic characteristics of the 73 CRT and 18 ICD recipients with stage 4 CKD are summarized in Table 1. Despite similar age, sex, and LVEF, CRT patients had a worse NYHA functional class, more prolonged QRS duration, and more LV dyssynchrony as assessed by septal-to-lateral delay.
Table 1.
Baseline clinical and echocardiographic characteristics of the patient population
| Characteristic | ICD (n=18) | CRT (n=73) | P Value |
|---|---|---|---|
| Age, yr | 74±10 | 71±10 | 0.24 |
| Male | 12 (67) | 48 (66) | 0.94 |
| Ischemic etiology | 14 (78) | 48 (66) | 0.33 |
| QRS duration, ms | 130±21 | 168±28 | <0.001 |
| Atrial fibrillation | 6 (33) | 14 (19) | 0.19 |
| Diabetes | 7 (41) | 26 (36) | 0.67 |
| eGFR, ml/min per 1.73 m2 | 24±4 | 25±4 | 0.66 |
| Creatinine, mg/dl | 2.9±2.1 | 2.5±0.5 | 0.44 |
| Hemoglobin, g/dl | 12±1 | 12±2 | 0.21 |
| NYHA functional class | 1.5 (2–3) | 3 (3–3) | <0.001 |
| β-blockers | 10 (56) | 40 (55) | 0.95 |
| ACE-I/ARB-II | 11 (61) | 61 (84) | 0.04 |
| Diuretics | 16 (89) | 70 (96) | 0.24 |
| Amiodarone | 10 (56) | 24 (47) | 0.54 |
| LVEDV, ml | 172±60 | 205±87 | 0.13 |
| LVESV, ml | 126±54 | 159±78 | 0.09 |
| LVEF, % | 28±12 | 24±8 | 0.10 |
| Mitral regurgitation >2 | 7 (39) | 35 (48) | 0.49 |
| LV dyssynchrony, ms | 21±24 | 67±49 | <0.001 |
| TAPSE, mm | 14±4 | 16±6 | 0.31 |
| Right atrial pressure, mmHg | 7±5 | 6±4 | 0.42 |
Values are mean±SD, median (interquartile range), n (%), or as otherwise indicated. ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; NYHA, New York Heart Association; ACE-I/ARB-II, angiotensin-converting enzyme inhibitor/angiotensin II type I receptor blocker; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; LV, left ventricular; TAPSE, tricuspid annular plane systolic excursion; ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy.
Clinical and Echocardiographic Evaluation at 6 Months
As shown in Table 2, at 6-month follow-up, NYHA functional class improved from 3 (interquartile range [IQR], 3–3) to 2 (IQR, 2–3; Wilcoxon P<0.001) in CRT patients, whereas the ICD recipients showed a significant deterioration from 1.5 (IQR, 2–3) to 3 (IQR, 3–4; Wilcoxon P=0.02). The magnitude of change over time in NYHA functional class between the two groups was significantly different (interaction time and group P<0.001).
Table 2.
Clinical and echocardiographic changes compared between device groups (cardiac resynchronization therapy versus implantable cardioverter defibrillator) at 6-month follow-up
| Variable | ICD (n=18) | CRT (n=73) | Time and Group Interaction P Value | ||
|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | ||
| NYHA functional class | 1.5 (2–3) | 3 (3–4) | 3 (3–3) | 2 (2–3)a | <0.001 |
| eGFR, ml/min per 1.73 m2 | 24±4 | 24±13 | 25±4 | 30±9b | 0.04 |
| LVEDV, ml | 172±60 | 164±55 | 205±87 | 196±85b | 0.06 |
| LVESV, ml | 126±54 | 119±49 | 159±78 | 145±78b | 0.05 |
| LVEF, % | 28±12 | 29±11 | 24±8 | 28±11b | 0.30 |
| Mitral regurgitation >2, n (%) | 7 (39) | 2 (11) | 35 (48) | 23 (32)a | 0.20 |
| TAPSE, mm | 14±4 | 15±3 | 16±6 | 17±3 | 0.91 |
| Right atrial pressure, mmHg | 7±5 | 8±4 | 6±4 | 5±4 | 0.37 |
Values are mean±SD, median (IQR), or as otherwise indicated. ICD, implantable cardioverter defibrillator; CRT, cardiac resynchronization therapy; NYHA, New York Heart Association; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion.
Wilcoxon P value comparing baseline versus follow-up within the group is significant.
P value comparing baseline versus follow-up within the group is significant.
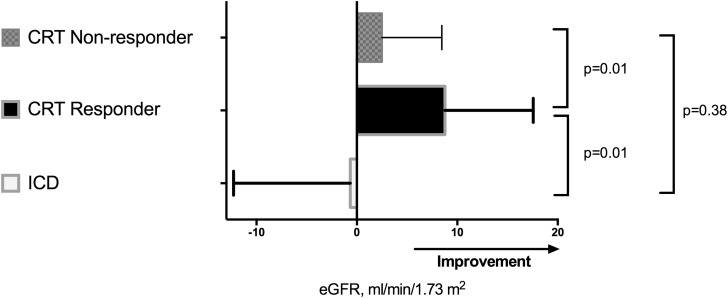
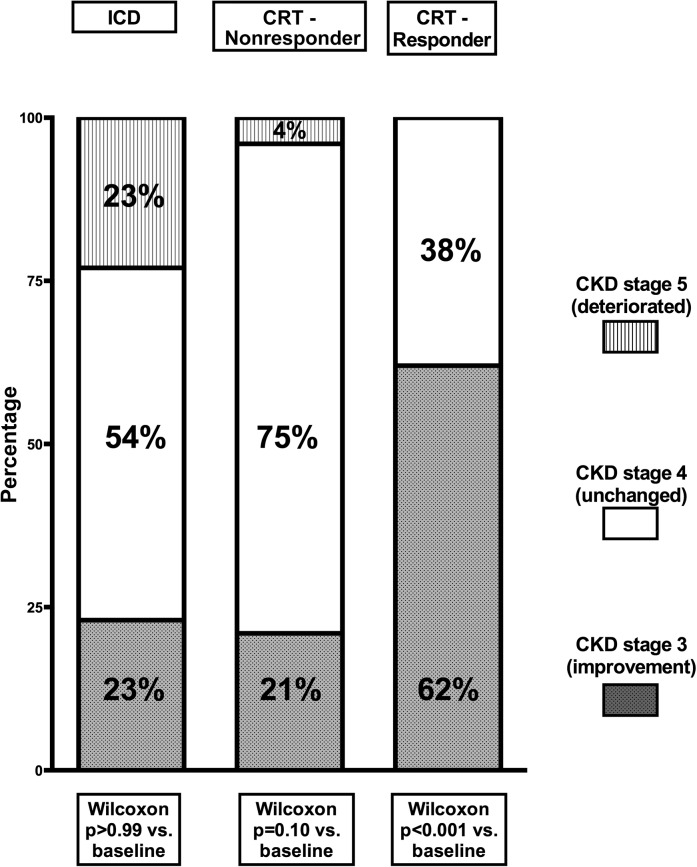
Similarly, renal function improved from 25±4 to 30±9 ml/min per 1.73 m2 (P<0.001) in CRT recipients, whereas the ICD patients showed no improvement (24±4 to 24±13 ml/min per 1.73 m2, P=0.84) after 6 months (Figure 1). The magnitude of change over time in eGFR between the two groups was significantly different (interaction time and group P=0.04). Furthermore, the change in renal dysfunction stage according to the National Kidney Foundation CKD classification was also evaluated. At 6 months after implantation, most of the ICD patients remained in stage 4 CKD (54%), whereas 23% improved to stage 3 CKD, and 23% deteriorated to stage 5 CKD (Wilcoxon P>0.99) (Figure 2). In the CRT group, a small group deteriorated further to stage 5 CKD (2%). More importantly, 58% remained in stage 4 CKD, whereas a relatively large group of patients improved to stage 3 CKD (40%), (Wilcoxon P<0.001). At 6-month follow-up, LV end-diastolic volume and LVESV decreased and LVEF increased among CRT recipients, whereas no significant changes were noticed in LV volumes or LV function among ICD recipients (Table 2). At 6-month follow-up, the reduction in LVESV was significantly different between CRT and ICD recipients (interaction group and time P=0.05) (Table 2).
Figure 1.
Comparison of mean changes±SD in eGFR 6 months after device implantation among implantable cardioverter defibrillator and cardiac resynchronization therapy recipients with further stratification for response to cardiac resynchronization therapy. Compared with baseline, eGFR improved significantly in CRT responders (P<0.001), whereas no significant changes were observed in CRT nonresponders (P=0.05) and ICD patients (P=0.84). CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator.
Figure 2.
Distribution of CKD stage at 6-month follow-up among implantable cardioverter defibrillator patients, cardiac resynchronization therapy responders, and cardiac resynchronization therapy nonresponders. CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator.
Response to CRT and Renal Function
According to the definition of response to CRT, 22 (30%) CRT patients were classified as responders at 6-month follow-up. As showed in Figure 1, improvement in renal function was statistically significant only among CRT responders (25±3 to 34±9 ml/min per 1.73 m2, P<0.001 versus 25±4 to 27±7 ml/min per 1.73 m2, P=0.05 in CRT nonresponders), and as a result, 62% of responders to CRT improved in CKD stage (from 4 to 3). The specific distribution of CKD stage at the 6-month follow-up in CRT responders and nonresponders is displayed in Figure 2. Furthermore, a significant difference in the changes (Δ) of eGFR was observed only between ICD patients and CRT responders (1±12 versus –9±9 ml/min per 1.73 m2, P=0.01), as shown in Figure 1. A correlation between the reduction in LVESV and the increase in eGFR was observed, but it did not reach statistical significance (Pearson correlation 0.26, P=0.09, two-tailed).
Long-Term Prognosis after CRT
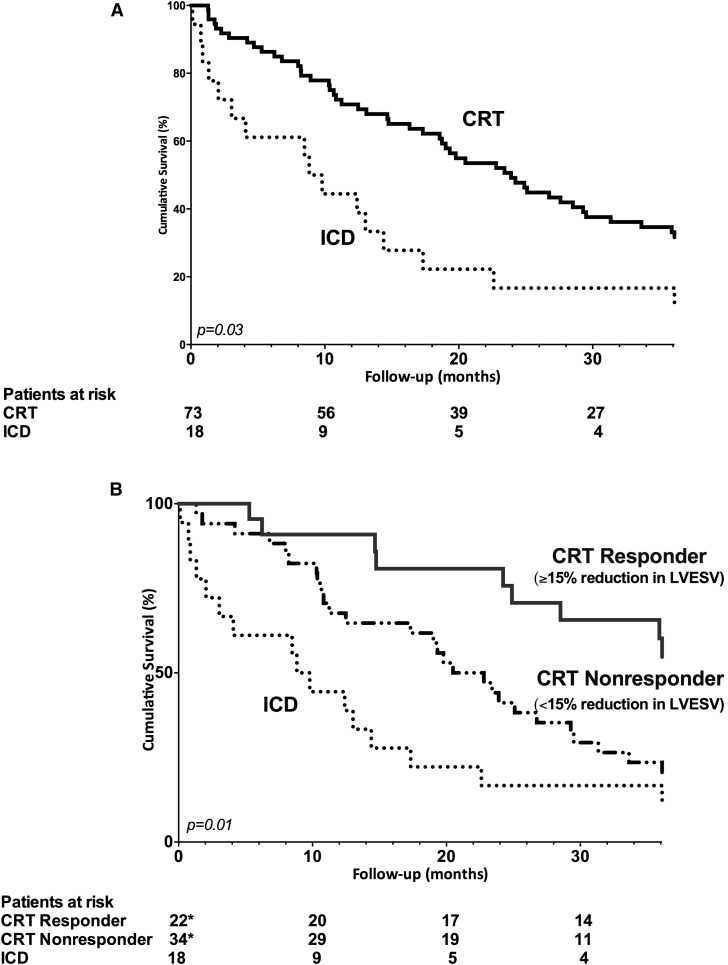
During a median follow-up of 33 months (IQR, 14–52 months), 74 patients died (81%; 59 CRT and 15 ICD patients). Five CRT (7%) and two ICD (11%) patients died before 6 months follow-up (chi-squared=0.19, log-rank P=0.89). During long-term follow-up, a total of 29 appropriate defibrillator therapy (32%) and 24 HF hospitalizations (26%) occurred together with 35 deaths. Most patients died because of HF progression (53%) and infections (14%). The other causes of death were sudden cardiac death (3%), malignancies (4%), major bleeding (1%), renal failure progression (1%), and unknown (4%). Specifically, the combined end point was observed in 17 ICD and 62 CRT patients. Compared with ICD patients, a superior survival free from the combined end point was observed among CRT patients (chi-squared=4.55, log-rank P=0.03) (Figure 3A). Furthermore, the cumulative incidence of the combined end point was significantly lower among CRT responders (chi-squared=8.55, log-rank P=0.01 for the comparison between CRT responders, CRT nonresponders, and ICD patients) (Figure 3B). More specifically, response to CRT was associated with a better long-term outcome compared with CRT nonresponse (chi-squared=4.56, log-rank P=0.03) and ICD patients (chi-squared=7.49, log-rank P=0.01).
Figure 3.
As compared to implantable cardioverter defibrillator patients, a superior survival free from the combined endpoint was observed among cardiac resynchronization therapy patients, particularly when significant left ventricular reverse remodeling occurred. (A) Kaplan–Meier curves comparing the survival free from defibrillator therapy, heart failure hospitalization, and all-cause mortality between ICD and CRT patients. (B) Kaplan–Meier curves comparing the survival free from defibrillator therapy, heart failure hospitalization, and all-cause mortality between ICD patients, CRT responders, and CRT nonresponders. *In 56 patients, response to CRT (LV reverse remodeling at 6-month follow-up) could be assessed. In 17 patients, response to CRT could not be defined because of death before 6-month follow-up or technical issues. CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LV, left ventricular.
Predictors of Long-Term Outcome
Univariate analysis performed in the CKD stage 4 patients indicated that CRT response was significantly related to the combined end point (Table 3). In the multivariate Cox analysis, CRT response was independently associated with better survival free from the combined end point after adjustment for relevant clinical and echocardiographic characteristics (sex, ischemic etiology, angiotensin-converting enzyme inhibitor/angiotensin II type I receptor blocker use, and LVEF) (Table 3). Age, atrial fibrillation, diabetes, β-blockers use, and hemoglobin were dispensed by the predefined backward stepwise elimination.
Table 3.
Cox regression analysis for the combined end point (of heart failure hospitalization, defibrillator therapy, and all-cause mortality) in patients with CKD stage 4 who underwent implantable cardioverter defibrillator or cardiac resynchronization therapy implantation
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age, per yr | 1.02 | 0.99 to 1.04 | 0.14 | |||
| Male | 1.29 | 0.80 to 2.07 | 0.30 | 1.20 | 0.68 to 2.08 | 0.55 |
| Ischemic etiology | 1.29 | 0.80 to 2.10 | 0.30 | 1.34 | 0.71 to 2.46 | 0.38 |
| Diabetes | 0.85 | 0.53 to 1.36 | 0.50 | |||
| Atrial fibrillation | 1.74 | 1.01 to 2.99 | 0.05 | |||
| QRS duration, per ms | 1.00 | 0.99 to 1.01 | 0.42 | |||
| eGFR, per ml/min per 1.73 m2 | 0.96 | 0.91 to 1.02 | 0.19 | |||
| Hemoglobin, per g/dl | 0.81 | 0.64 to 1.02 | 0.07 | |||
| ACE-I/ARB-II | 0.47 | 0.28 to 0.79 | 0.01 | 0.53 | 0.27 to 1.03 | 0.06 |
| β-blockers | 0.81 | 0.52 to 1.26 | 0.35 | |||
| LVEDV, per ml | 1.00 | 1.00 to 1.00 | 0.71 | |||
| LVESV, per ml | 1.00 | 1.00 to 1.00 | 0.69 | |||
| LVEF, per % | 1.00 | 0.97 to 1.02 | 0.71 | 1.01 | 0.98 to 1.04 | 0.47 |
| Mitral regurgitation >2 | 1.16 | 0.73 to 1.82 | 0.53 | |||
| LV dyssynchrony, per ms | 1.00 | 0.99 to 1.00 | 0.40 | |||
| CRT, nonresponse (reference) | 0.02a | 0.04a | ||||
| CRT, response (versus reference) | 0.55 | 0.30 to 1.01 | 0.06 | 0.51 | 0.27 to 0.98 | 0.04 |
| ICD (versus reference) | 1.50 | 0.82 to 2.75 | 0.19 | 1.18 | 0.60 to 2.23 | 0.64 |
HR, hazard ratio; 95% CI, 95% confidence interval; ACE-I/ARB-II, angiotensin-converting enzyme inhibitor/angiotensin II type I receptor blocker; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; LV, left ventricular; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator.
P value for the overall comparison among groups.
Discussion
The main findings of this study can be summarized as follows: CRT implantation in patients in stage 4 CKD was associated overall with an improvement in symptoms, in LV systolic performance, and in eGFR compared with ICD patients. The beneficial effect on renal function particularly was more pronounced among CRT responders compared with nonresponders and ICD patients. However, the response rate to CRT was relatively low in this category of very ill patients; nevertheless, when response to CRT occurred, it was associated with a better long-term outcome (including survival, HF hospitalization, and defibrillator therapy) compared with CRT nonresponse or ICD.
Effect of CRT on Renal Function in Patients with Severe Renal Dysfunction
HF and renal dysfunction often coexist (1). Treatment of HF with CRT has been associated with improved renal function potentially through several mechanisms (6–9). CRT often results in a significant improvement in LV systolic function, which therefore improves systemic hemodynamic status and prerenal circulation (6,22–29). In addition, CRT decreases central venous pressure, which is described to play an important role in the progression of renal failure (3,29,30). Finally, reduced sympathetic nerve and renin-angiotensin-aldosterone system activity and improved N-terminal pro-brain natriuretic peptide (NT-proBNP) levels are described after CRT (6,31,32). This favorable neurohormonal modulation further contributes to the CRT-derived improvement of renal function.
However, data on the effect of CRT in patients with severe renal dysfunction are scarce (5). Major CRT clinical trials excluded patients with severe renal dysfunction and only two small retrospective studies described the effect of CRT in this group of patients (5,10,11). Adelstein and coworkers evaluated the effect of CRT in patients with severe renal dysfunction (mixed group, including stages 4 and 5 CKD) in comparison with other CKD stages (<3) and ICD implantation alone (10). This study showed a larger improvement in renal function in patients with stage 4 CKD after CRT compared with other CKD stages (<3) but without significant improvement in LV function (LV reverse remodeling) compared with ICD alone (10). Similarly, our study showed an overall significant improvement in renal function (and CKD stage) in patients with stage 4 CKD, suggesting a favorable effect of CRT in these patients. However, this study also showed that the effect on renal function was significantly different between CRT responders and nonresponders. CRT responders showed a better improvement in eGFR compared with CRT nonresponders and ICD patients, suggesting that an increase in LV function (and overall in cardiac performance) is probably a key step for the improvement in renal function.
However, the rate of response to CRT in patients with stage 4 CKD was relatively low (30%) compared with that reported in a general HF population referred for CRT (7,10). Particularly, Van Bommel et al. reported a CRT response rate of 43% in patients with eGFR<60 ml/min per 1.73 m2, of 62% in patients with eGFR between 60 and 90 ml/min per 1.73 m2, and of 58% in patients with eGFR>90 ml/min per 1.73 m2 (7). Unfortunately, there are no data regarding CRT response among patients with CKD stage 4 to compare with. The limited reverse remodeling capacity of the myocardium after CRT in these patients may be caused by a chronic damage on the basis of uremic toxins, calcium/phosphate abnormalities, and hypervolemic damage (water and sodium retention) that could lead to myocardial fibrosis, capillary rarefraction, and endothelial cell dysfunction (5,7,13,33–35). Probably concomitant conditions, such as anemia, increased levels of catecholamines, activated renin-angiotensin-aldosterone system resulting in increased myocardial fibrosis, fluid retention, and increased afterload, might represent other important factors to limit the favorable effect of CRT in cardiac function (35).
Effect of CRT on Clinical Outcome among Patients with Severe Renal Dysfunction
Severe renal dysfunction in patients with HF is well known to be associated with poor survival (1–3). Data on long-term outcome in CRT patients with severe renal dysfunction are scarce and warranted (5). Adelstein and coworkers showed no additional benefit of CRT on long-term outcome compared with ICD in patients with severe renal dysfunction (10). However, the patient cohort evaluated by Adelstein et al. also included patients with stage 5 CKD, which were older compared with the ICD control group (median, 74 versus 65 years; P<0.05), and the authors did not distinguish between CRT response and nonresponse (10).
In line with the findings of Adelstein et al. we observed a high mortality rate in our cohort (55% at 3 years), emphasizing again the important prognostic value of significantly impaired renal function. Our study showed improved long-term outcome among CRT patients in comparison with ICD patients. However, this result was mainly driven by a superior clinical outcome in CRT responders. As shown by the difference in some baseline characteristics with ICD patients, CRT patients are in general more fragile patients with relatively more advanced HF, severely dilated LV volume, and a higher grade of mitral valve regurgitation, and a head-to-head comparison could lead to an underestimation of the positive effect of CRT compared with ICD. Furthermore, in this specific group of patients at high risk, an advantage in terms of symptoms/quality of life, an improvement in LV performance, and a reduction of the progression of renal dysfunction could represent an important end point on top of the advantage in terms of survival. Importantly, improvement in LV function (CRT response) seems a key step to a better long-term prognosis, emphasizing the need for a good selection of candidates referred to CRT (4).
Limitations
Several limitations of this study should be mentioned. A rather small population and a retrospective design are the main limitations. However, most the landmark trials on CRT and ICD have excluded patients with severe renal dysfunction, and this study represents one of the largest series so far. Therefore, this study should be considered hypothesis generating, and further studies with a larger patient population and longer follow-up are needed. Indication for cardiac device therapy was on the basis of the available guidelines at the moment of implantation. Therefore, characteristics of patients qualifying for ICD or CRT implantation represent a potential selection bias, which we tried to minimize matching for age, sex, and LVEF, but which should be taken into account in the interpretation of the result. Furthermore, the preexistent pathology of renal dysfunction preceding HF and the effect of HF medications on renal function in the follow-up could not be evaluated. Nonetheless, HF medication was optimized before device implantation in all patients. After 6 months of follow-up, measure of eGFR was also not systematically available; therefore, analysis on the effect on CRT on clinical outcome could not be corrected for this factor. Also, longer-term changes in renal function and in relation to cardiac function could not be evaluated. Between device implantation and 6-month follow-up, three hospitalizations occurred; the worsening of renal function caused by these hospitalizations could not be systematically assessed but was taken into account when measuring the renal function at the 6 months follow-up and including HF hospitalization in the end point. Finally in a total of eight patients, RRT was intended (median time to dialysis was 28 months; IQR, 13–45 months); however, the prognostic implications of RRT could not be tested considering the low number of patients.
Patients with severe renal dysfunction undergoing CRT showed an improved eGFR and CKD stage at 6-month follow-up compared with ICD patients. Improvement in renal function was more pronounced among CRT responders compared with CRT nonresponders; however, CRT response rate was relatively low (30%). More importantly, CRT response was independently associated with better long-term prognosis after adjustment for sex, etiology, angiotensin-converting enzyme inhibitor/angiotensin II type I receptor blocker use, and LVEF.
Disclosures
None.
Acknowledgments
The department of cardiology receives unrestricted grants from Biotronik (Berlin, Germany), Boston Scientific (Natick, Massachusetts), GE Healthcare (Buckinghamshire, United Kingdom), and Medtronic (Minneapolis, Minnesota). V. Delgado received consulting fees from Medtronic (Minneapolis, Minnesota) and St. Jude Medical (St. Paul, Minnesota).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Cardiorenal Resynchronization Therapy: Strengthening the Heart and Kidneys,” on pages 1705–1707.
References
- 1.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW: Renal insufficiency and heart failure: Prognostic and therapeutic implications from a prospective cohort study. Circulation 109: 1004–1009, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators : Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL: Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53: 582–588, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM, ESC Committee for Practice Guidelines (CPG) Document Reviewers : 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 34: 2281–2329, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Garg N, Thomas G, Jackson G, Rickard J, Nally JV, Jr, Tang WH, Navaneethan SD: Cardiac resynchronization therapy in CKD: A systematic review. Clin J Am Soc Nephrol 8: 1293–1303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerrigter G, Costello-Boerrigter LC, Abraham WT, Sutton MG, Heublein DM, Kruger KM, Hill MR, McCullough PA, Burnett JC, Jr: Cardiac resynchronization therapy improves renal function in human heart failure with reduced glomerular filtration rate. J Card Fail 14: 539–546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Bommel RJ, Mollema SA, Borleffs CJ, Bertini M, Ypenburg C, Marsan NA, Delgado V, Van Der Wall EE, Schalij MJ, Bax JJ: Impaired renal function is associated with echocardiographic nonresponse and poor prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 57: 549–555, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Cannizzaro LA, Piccini JP, Patel UD, Hernandez AF: Device therapy in heart failure patients with chronic kidney disease. J Am Coll Cardiol 58: 889–896, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Fung JW, Szeto CC, Chan JY, Zhang Q, Chan HC, Yip GW, Yu CM: Prognostic value of renal function in patients with cardiac resynchronization therapy. Int J Cardiol 122: 10–16, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Adelstein EC, Shalaby A, Saba S: Response to cardiac resynchronization therapy in patients with heart failure and renal insufficiency. Pacing Clin Electrophysiol 33: 850–859, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Lin G, Gersh BJ, Greene EL, Redfield MM, Hayes DL, Brady PA: Renal function and mortality following cardiac resynchronization therapy. Eur Heart J 32: 184–190, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Höke U, Auger D, Thijssen J, Wolterbeek R, van der Velde ET, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA: Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart 100: 960–968, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Höke U, Thijssen J, van Bommel RJ, van Erven L, van der Velde ET, Holman ER, Schalij MJ, Bax JJ, Delgado V, Marsan NA: Influence of diabetes on left ventricular systolic and diastolic function and on long-term outcome after cardiac resynchronization therapy. Diabetes Care 36: 985–991, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W, American Society of Echocardiography’s Nomenclature and Standards Committee. Task Force on Chamber Quantification. American College of Cardiology Echocardiography Committee. American Heart Association. European Association of Echocardiography, European Society of Cardiology : Recommendations for chamber quantification. Eur J Echocardiogr 7: 79–108, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL, European Association of Echocardiography : European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 11: 307–332, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB: Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23: 685–713, quiz 786–788, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ: Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 44: 1834–1840, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Bleeker GB, Bax JJ, Fung JW, van der Wall EE, Zhang Q, Schalij MJ, Chan JY, Yu CM: Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol 97: 260–263, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hinkle LE, Jr, Thaler HT: Clinical classification of cardiac deaths. Circulation 65: 457–464, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Inage T, Yoshida T, Hiraki T, Ohe M, Takeuchi T, Nagamoto Y, Fukuda Y, Gondo T, Imaizumi T: Chronic cardiac resynchronization therapy reverses cardiac remodelling and improves invasive haemodynamics of patients with severe heart failure on optimal medical treatment. Europace 10: 379–383, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Ypenburg C, Lancellotti P, Tops LF, Boersma E, Bleeker GB, Holman ER, Thomas JD, Schalij MJ, Piérard LA, Bax JJ: Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J 29: 757–765, 2008 [DOI] [PubMed] [Google Scholar]
- 24.St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR, Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group : Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 107: 1985–1990, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM, Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators : Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350: 2140–2150, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Steendijk P, Tulner SA, Bax JJ, Oemrawsingh PV, Bleeker GB, van Erven L, Putter H, Verwey HF, van der Wall EE, Schalij MJ: Hemodynamic effects of long-term cardiac resynchronization therapy: Analysis by pressure-volume loops. Circulation 113: 1295–1304, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Davis MK, Virani SA: Cardiac resynchronization therapy in the cardiorenal syndrome. Int J Nephrol 2011: 168461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullens W, Verga T, Grimm RA, Starling RC, Wilkoff BL, Tang WH: Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J Am Coll Cardiol 53: 600–607, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Nägele H, Azizi M, Castel MA: Hemodynamic changes during cardiac resynchronization therapy. Clin Cardiol 30: 141–143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH: Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53: 589–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamdan MH, Barbera S, Kowal RC, Page RL, Ramaswamy K, Joglar JA, Karimkhani V, Smith ML: Effects of resynchronization therapy on sympathetic activity in patients with depressed ejection fraction and intraventricular conduction delay due to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 89: 1047–1051, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Tarquini R, Guerra CT, Porciani MC, Michelucci A, Padeletti M, Ricciardi G, Chiostri M, Jelic S, Padeletti L: Effects of cardiac resynchronization therapy on systemic inflammation and neurohormonal pathways in heart failure. Cardiol J 16: 545–552, 2009 [PubMed] [Google Scholar]
- 33.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 1527–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Shanks M, Antoni ML, Hoke U, Bertini M, Ng AC, Auger D, Marsan NA, van Erven L, Holman ER, Schalij MJ, Bax JJ, Delgado V: The effect of cardiac resynchronization therapy on left ventricular diastolic function assessed with speckle-tracking echocardiography. Eur J Heart Fail 13: 1133–1139, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Schrier RW: Role of diminished renal function in cardiovascular mortality: Marker or pathogenetic factor? J Am Coll Cardiol 47: 1–8, 2006 [DOI] [PubMed] [Google Scholar]