Abstract
Background and objectives
Mortality and CKD risk have not been described in military casualties with post-traumatic AKI requiring RRT suffered in the Iraq and Afghanistan wars.
Design, setting, participants, & measurements
This is a retrospective case series of post-traumatic AKI requiring RRT in 51 military health care beneficiaries (October 7, 2001–December 1, 2013), evacuated to the National Capital Region, documenting in-hospital mortality and subsequent CKD. Participants were identified using electronic medical and procedure records.
Results
Age at injury was 26±6 years; of the participants, 50 were men, 16% were black, 67% were white, and 88% of injuries were caused by blast or projectiles. Presumed AKI cause was acute tubular necrosis in 98%, with rhabdomyolysis in 72%. Sixty-day all-cause mortality was 22% (95% confidence interval [95% CI], 12% to 35%), significantly less than the 50% predicted historical mortality (P<0.001). The VA/NIH Acute Renal Failure Trial Network AKI integer score predicted 60-day mortality risk was 33% (range, 6%–96%) (n=49). Of these, nine died (mortality, 18%; 95% CI, 10% to 32%), with predicted risks significantly miscalibrated (P<0.001). The area under the receiver operator characteristic curve for the AKI integer score was 0.72 (95% CI, 0.56 to 0.88), not significantly different than the AKI integer score model cohort (P=0.27). Of the 40 survivors, one had ESRD caused by cortical necrosis. Of the remaining 39, median time to last follow-up serum creatinine was 1158 days (range, 99–3316 days), serum creatinine was 0.85±0.24 mg/dl, and eGFR was 118±23 ml/min per 1.73 m2. No eGFR was <60 ml/min per 1.73 m2, but it may be overestimated because of large/medium amputations in 54%. Twenty-five percent (n=36) had proteinuria; one was diagnosed with CKD stage 2.
Conclusions
Despite severe injuries, participants had better in-hospital survival than predicted historically and by AKI integer score. No patient who recovered renal function had an eGFR<60 ml/min per 1.73 m2 at last follow-up, but 23% had proteinuria, suggesting CKD burden.
Keywords: acute renal failure, chronic kidney disease, dialysis, mortality risk, military casualties
Introduction
Mortality and CKD risk have not been reported in American military casualties with post-traumatic AKI (PTAKI) requiring RRT suffered in the Iraq and Afghanistan wars. PTAKI-associated mortality in casualties requiring RRT has been 63%–75% since the Korean conflict (1,2). Although mortality is unchanged, casualties are increasingly severe, associated with improved resuscitation, surgical, and evacuation capabilities (3–9). Civilian mortality is similar at 36%–73% (10–13). In Korea and Vietnam, survivors were reported to recover fully, but follow-up was sparse, and quantification of renal function not available. In civilian series, most do not require dialysis at discharge; however, in one cohort, 27% had not normalized serum creatinine (sCr) (11). Because even a single episode of AKI may increase future CKD risk, patients with PTAKI requiring RRT who recover renal function may be at risk for subsequent CKD, with associated morbidity and mortality (14–16). We performed a retrospective case series of United States military casualties from the Iraq and Afghanistan wars with PTAKI requiring RRT, ultimately evacuated to the National Capital Region, describing 60-day and long-term mortality, ESRD incidence, and CKD incidence. Observed mortality was compared to historic mortality for military and civilian PTAKI patients requiring RRT and to mortality predicted by the VA/NIH Acute Renal Failure Trial Network AKI integer risk score, developed in critically ill patients who received RRT for AKI (17,18). We hypothesized that this young, healthy cohort would have lower mortality than historically reported, and evidence of CKD in long-term follow-up.
Materials and Methods
Participants were male and female military health care beneficiaries ≥18 years with PTAKI requiring RRT resulting from injuries sustained during the Iraq or Afghanistan wars from October 7, 2001–December 1, 2013, managed at one of the hospitals with RRT support in the National Capital Region. (Before September 2011 these were Walter Reed Army Medical Center [WRAMC] in Washington, DC, and National Naval Medical Center in Bethesda, Maryland. In September 2011, these were consolidated into one hospital—Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland.) Trauma was defined as an externally applied injury, which preceded and precipitated AKI. Those who had burns requiring subspecialty burn unit care were excluded and have been reported separately (6).
Potential participants were identified by searching the WRNMMC Cache transactional database (InterSystems, Vienna, VA), which included inpatient (Essentris) and outpatient (Composite Health Care System (CHCS)/AHLTA) electronic medical records. Essentris inpatient records were distinct for each hospital (WRAMC and National Naval Medical Center) before September 2011 and integrated afterward. Outpatient records were available through the single CHCS/AHLTA system throughout the time period. We used International Classification of Diseases, 9th Revision (ICD9) codes for acute renal failure, dialysis-associated Current Procedural Terminology (CPT) codes, nephrology and dialysis clinic identifiers, appointment categories, demographic identifiers (age and active duty status), Defense Enrollment Eligibility Reporting System eligibility dates, and the WRAMC/WRNMMC nephrology procedure log (documenting continuous RRT [CRRT] and dialysis catheter placement) to identify potential participants.
There were 741 individual admission records screened by four investigators (J.A.B., C.M.Y., D.J.L., and S.W.O.) for inclusion and exclusion criteria. Fifty-one patients meeting criteria were entered into the study. Individual data collection sheets were populated by one investigator (K.C.A., C.M.Y., and J.A.B.), and entries were verified by a second investigator (C.M.Y. and D.J.L.). Data were obtained from Essentris and AHLTA electronic medical records, which include clinical records from military theater, including Landstuhl Regional Medical Center (LRMC), Veterans’ Administration outpatient clinics, and other United States–based military medical facilities. Follow-up was through January 1, 2014. Deidentified data were entered into an Excel spreadsheet for analysis.
Sixty-day all-cause mortality was defined as death by day 60 after beginning RRT, whether before or after hospital discharge (17). The VA/NIH Acute Renal Failure Trial Network AKI integer risk score was manually calculated using the arithmetic sum of points for each of 21 parameters, and 60-day mortality risk was determined (18). Two patients, both of whom died, did not have sufficient data for integer risk score calculation.
All participants were seen in consultation by nephrology (intensivists did not independently provide RRT in the National Capital Region). Clinical diagnosis of AKI cause(s) recorded at the time of consultation was used as the diagnosis, unless subsequent biopsy (one subject) indicated otherwise. RRT initiation date was determined from in-theater, LRMC, or National Capital Region medical records, dialysis treatment records, and transfer summaries. RRT indication(s) was that recorded by the provider at initiation. The latest recorded sCr was used to determine eGFR using the Chronic Kidney Disease Epidemiology Collaboration formula. Proteinuria was assessed using the latest recorded urinalysis, urine protein/creatinine ratio, or 24-hour urine protein, if available. Proteinuria was deemed present if >150 mg protein/g creatinine, >150 mg protein/24 hours, or ≥1+(30 mg/dl) protein on urine dipstick. Hypertension (HTN) was identified on the basis of a diagnosis in follow-up with or without antihypertensive therapy. Normal BP was deemed present if the last 2–3 BP determinations were <140/90 mmHg in a subject not receiving antihypertensive medication. ESRD was defined as the requirement for permanent RRT (including transplant) at last follow-up. CKD was defined to be present if eGFR was <60 ml/min per 1.73 m2, proteinuria was present as previously defined, or if the patient had a diagnosis of CKD, ESRD, or kidney transplant at last follow-up (19).
Amputation was defined as small (one or less trans-knee amputation); medium (bilateral trans-knee amputation or unilateral above-the-knee amputation with or without a trans-knee amputation); or large (bilateral above-the-knee amputation or higher) (20). Date and cause of injury and details of initial trauma were determined from in-theater and LRMC transfer summaries and arrival history. Number of units of packed red blood cells administered before arrival in the National Capital Region was determined from in-theater and LRMC transfer summaries and admission history. The estimated number of surgeries from the time of injury to death or discharge was determined from in-theater and LRMC transfer summaries, admission, discharge, and death summaries. Outpatient serum calcium, phosphate, and hemoglobin were the latest available. Maximum creatine phosphokinase was the highest creatine phosphokinase (units/L) recorded before RRT initiation.
Data are presented as mean±SD or as median (range). Descriptive statistics and transformations were performed in Excel. Comparisons were made using t test or Fisher exact test (QuickCalcs, GraphPad Software; available at: http://www.graphpad.com/quickcalcs/). Data not normally distributed were transformed as described in the text and tables before use of t test. A P value <0.05 was considered to be significant. AKI integer score–estimated mortality risk for those who died within 60 days versus those who survived was compared using the Wilcoxon rank-sum test (STATA 12.1; StataCorp, College Station, TX). Observed 60-day mortality was compared with expected historical mortality of 50% (the lower range of historical reports for military casualties with PTAKI requiring dialysis, and the weighted average mortality observed in more recent civilian case series [10–13]) (binomial distribution; QuickCalcs). It was also compared with AKI integer score–estimated mortality risk using the Hosmer–Lemeshow goodness of fit test in the 49 participants who had sufficient information to calculate the score (programmed in R by S.R.H.; available at: http://www.r-project.org, using the Resource Selection package from CRAN, for five subgroups and tested for stability from three to ten subgroups [Supplemental Material 1]) (21). Sensitivity analysis was performed by adding two fictional patients (accounting for those who were excluded from analysis because of insufficient data), with predicted mortality risk drawn from 0% to 100% in 1% increments (Supplemental Material 2). Receiver operator characteristic (ROC) analysis assessing performance of the AKI integer score estimation of mortality risk (in the same 49 patients) versus performance of the AKI integer risk score as reported in Figure 2 of Demirjian et al. (18) was also programmed in R (S.R.H.), using the pROC package from CRAN (22). ROC curves include 95% bootstrap confidence bands, with the area under the curve (AUC) reported with 95% confidence interval [95% CI] confidence, and the two AUCs were compared by the method of DeLong et al. (23). Kaplan–Meier survival estimate analysis in all 51 participants was performed in STATA 12.1 (C.M.Y.).
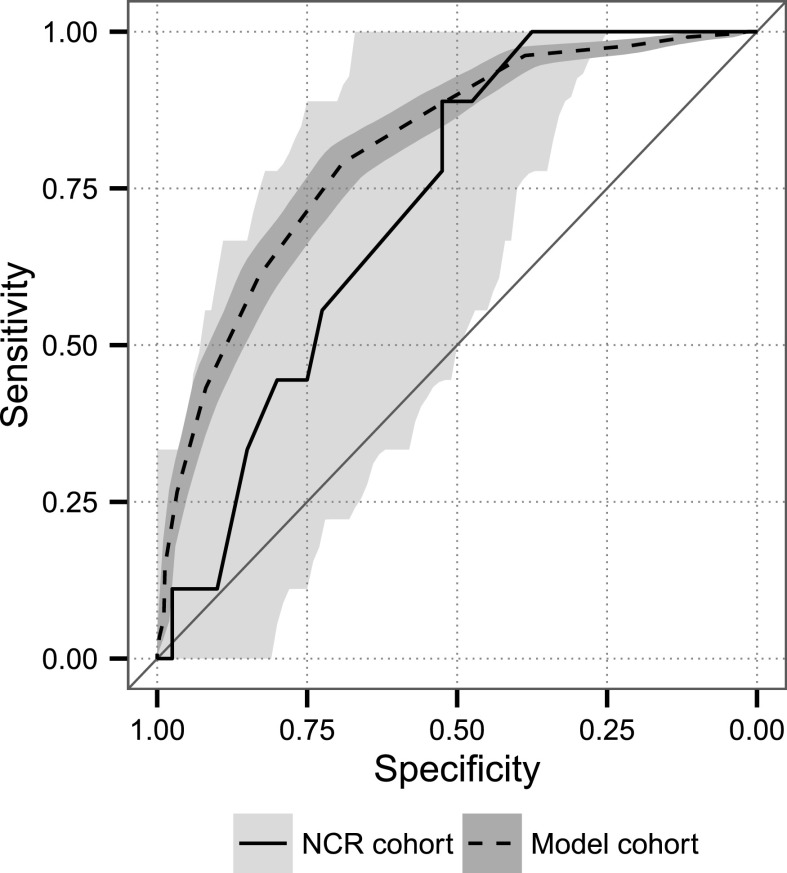
Figure 2.
Receiver operator characteristic analysis of 49 participants in whom AKI integer score mortality risk could be determined versus reference AKI integer score cohort (18). The area under the curve (C-statistic) was 0.72 (95% confidence interval, 0.56 to 0.88) for the National Capital Region cohort. The 95% confidence bands are shown for both curves, and they were not significantly different (P=0.27). Model cohort, reference AKI integer score cohort; NCR cohort, participants in whom AKI integer score mortality risk could be determined.
The WRNMMC Institutional Review Board approved the study (#394030) as minimal risk, with waiver of informed consent, and adherent to the Declaration of Helsinki. The manuscript was approved by the WRNMMC Department of Research Programs and Office of Public Affairs.
Results
Demographic features and clinical characteristics of initial injury, hospital course, and RRT are shown for the 51 participants (Table 1). Participants were predominantly men, aged 26±6 years. Of these, 88% were injured by explosive devices or projectiles, and 98% required ventilator support. Seventy-five percent had bacteremia with at least one organism, and 35% had bacteremia with >1 organism. There were 18% that had Acinetobacter bacteremia, and 12% had mucormycosis. Seventy-one percent ultimately experienced amputation.
Table 1.
Demographics and characteristics of wounded warriors requiring RRT for AKI managed in the National Capital Region (n=51)
| Characteristic | Value |
|---|---|
| Demographic features | |
| Age at injury (y) | 26±6 |
| Sex, male:female | 50:1 |
| Race | |
| White | 34 (67) |
| Black | 8 (16) |
| Other | 9 (18) |
| Injury features | |
| Type of injury | |
| IED | 35 (69) |
| GSW | 6 (12) |
| Rocket/RPG/mortar | 4 (8) |
| Poisoning | 3 (6) |
| Immobilization trauma | 2 (4) |
| Cardiac arrest/shock | 1 (2) |
| Rhabdomyolysis | 2 (4) |
| Postconcussive seizures | 1 (2) |
| Physical assault/ingestion | 1 (2) |
| Vehicular | 1 (2) |
| Units of PRBCs downrange (n=47) | 24 (0–200) |
| Estimated surgeries until death or discharge | 10 (0–36) |
| Amputation | 36 (71) |
| Maximum CPK (n=49) (units/L) | 14,550 (155–1,900,000) |
| Urinary tract trauma/injurya | 24 (47) |
| Kidney(s) | 5 (20) |
| Ureter(s) | 5 (20) |
| Bladder | 12 (50) |
| Urethra | 14 (58) |
| Total hospital days in National Capital Region | 51 (6–312) |
| Clinical features of AKI | |
| Presumed Cause of AKIb | |
| ATN | 50 (98) |
| Sepsis/hypotension | 46 (92) |
| Rhabdomyolysis | 36 (72) |
| IV contrast | 3 (6) |
| Cortical necrosis (by biopsy) | 1 |
| 60-d mortality | 11 (22) |
| Death on RRT | 8 (16) |
| Days from injury to RRT | 4 (1–66) |
| Dialysis initiated before reaching CONUS | 30 (59) |
| Serum creatinine at initiation (n=49) (mg/dl) | 5.58±2.85 |
| AKI integer score (n=49) | 22 (14–37) |
| Probability of death from AKI integer score (%) | 33 (6–96) |
| ESRD | 1 (2) |
| Indications for dialysisc | |
| Hyperkalemia | 35 (69) |
| Volume overload | 25 (49) |
| Acidosis | 23 (45) |
| Azotemia | 26 (51) |
| Other | 2 (4) |
| Days on RRT to death or recovery (n=50) | 17 (1–51) |
| RRT treatments per week | 5.0±1.4 |
| RRT modalities: 31 (61%) were treated with >1 modality | |
| iHD | 50 (98) |
| SLEDD | 25 (49) |
| CRRT | 13 (26) |
| PD | 0 (0) |
| Serum creatinine at hospital discharge (n=40) (mg/dl) | 1.03±1.51 |
| On dialysis at hospital discharge (n=40) | 1 (2.5) |
Data are presented as mean±SD, median (range), or count (%). IED, improvised explosive device; GSW, gunshot wound; RPG, rocket-propelled grenade; PRBCs, packed red blood cells; CPK, creatine phosphokinase; IV, intravenous; CONUS, continental United States; iHD, intermittent hemodialysis; SLEDD, slow low efficiency daily dialysis; CRRT, continuous RRT; PD, peritoneal dialysis.
Individual patients could have more than one site of urologic injury.
Individual patients with clinical acute tubular necrosis could be assigned more than one potential etiology.
Individual patients could have more than one indication for RRT.
Urologic trauma occurred in 47%; most commonly this was bladder and urethral. There were 50 patients who had clinically diagnosed acute tubular necrosis; 72% with concurrent rhabdomyolysis. One patient did not recover function, had biopsy-proven cortical necrosis, and was successfully transplanted.
Median time to RRT from initial injury was 4 days. Hyperkalemia was the most frequent indication. RRT was initiated before arrival in the United States in 30 patients; 26 began RRT at LRMC. Median time on RRT to death or recovery was 17 (range, 1–51) days. The number of RRT treatments per week was 5.0±1.4. Intermittent hemodialysis was used in 98%, slow low efficiency daily dialysis was used in 49%, and CRRT was used in 28%. No patient received peritoneal dialysis.
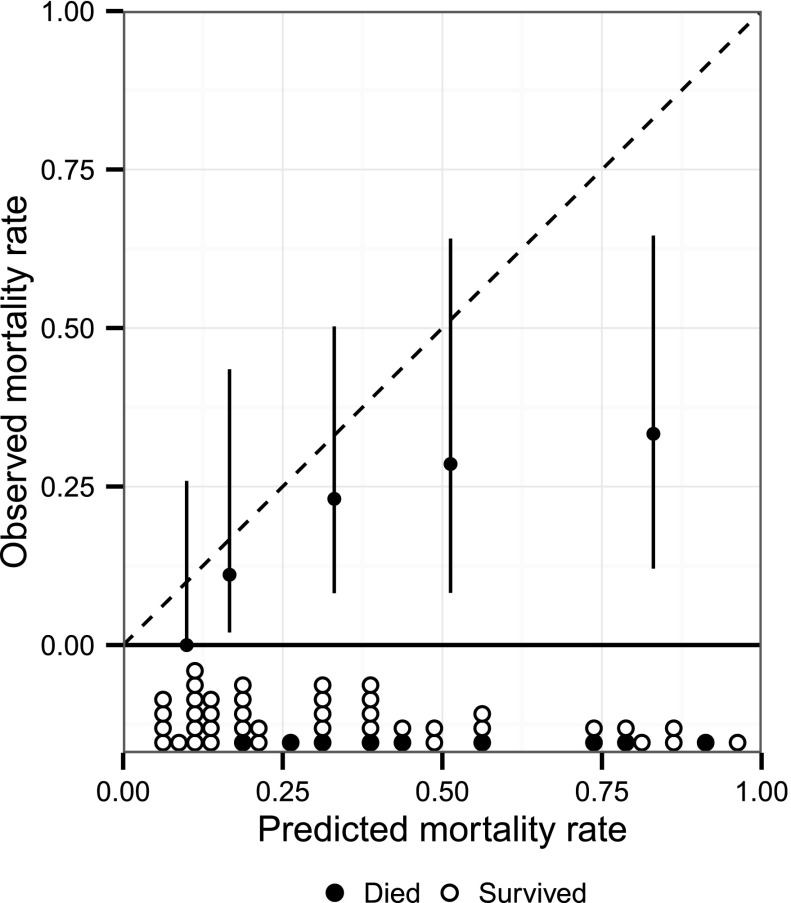
On Kaplan–Meier survival analysis, median follow-up time from date of injury was 987 days (range, 14–3318 days), or 2.7 years. Twelve died, one at 605 days after injury. Of the remaining 11 patients, all of whom died within 60 days of dialysis initiation, median time from injury to death was 20 days (range, 14–55 days), and median time from dialysis initiation to death was 12 days (range, 1–54). Sixty-day mortality was 22% (95% CI, 12% to 35%), significantly less than the predicted historical mortality of 50% (P<0.001, binomial distribution). AKI integer score–predicted median mortality risk was 33% (range, 6%–96%), among the 49 participants with sufficient data to calculate the score. Nine of these 49 patients died, yielding a 60-day mortality of 18% (95% CI, 10% to 32%). Predicted risks were significantly miscalibrated (H-statistic 19.3, 3 df, P<0.001). The validation plot of observed versus predicted mortality for the AKI integer score is shown in Figure 1, demonstrating predicted mortality diverged from observed mortality. Sensitivity analysis showed inclusion of two additional patients would not substantially affect significance (P=0.005, worst-case scenario). The area under the ROC curve (Figure 2) for the AKI integer score in our cohort was 0.72 (95% CI, 0.56 to 0.88), not significantly different than that observed for the model cohort developed from the VA/NIH Acute Renal Failure Trial Network study (P=0.27) (17,18).
Figure 1.
Observed versus predicted 60-day mortality in 49 participants in whom AKI integer score mortality risk could be determined. (Hosmer–Lemeshow goodness of fit test with five subgroups [3 df]: H-statistic 19.3; P<0.001). Vertical bars indicate 95% confidence interval.
Of the 40 survivors, 32 had outpatient follow-up through January 1, 2014. Seven had a last follow-up date of late July 2013–December 31, 2013. One patient died 1 year after hospital discharge, of unknown causes. Table 2 shows the demographic characteristics of the survivors and their follow-up course. Age at last follow-up was 29±6 years, and median time from injury to last follow-up was 1326 (range, 219–3318) days, or 3.63 years.
Table 2.
Follow-up clinical characteristics of wounded warriors requiring RRT for AKI managed in the National Capital Region who survived hospitalization (n=40)
| Demographic Features | Value | ||
|---|---|---|---|
| Age at injury (y) | 25±5 | ||
| Age at last follow-up (y) | 29±6 | ||
| Death during follow-up | 1 (2.5) | ||
| Time from injury to last follow-up (d) | 1326 (219–3318) | ||
| Sex, male:female | 39:1 | ||
| Race | |||
| White | 26 (65) | ||
| Black | 8 (15) | ||
| Other | 6 (20) | ||
| ESRD (present at hospital discharge) | 1 (2.5) | ||
| Kidney function (n=39, without ESRD at hospital discharge) | |||
| Serum creatinine at hospital discharge (mg/dl) | 0.80±0.36 | ||
| Time from injury to last serum creatinine (d) | 1158 (99–3316) | ||
| Serum creatinine at last follow-up | 0.85±0.24 | ||
| eGFR (CKD-EPI) at last follow-up (ml/min per 1.73 m2) | 118±23 | ||
| eGFR≤60 ml/min per 1.73 m2 | 0 (0) | ||
| HTN present at last follow-up | 8 (21) | ||
| Proteinuria present at last follow-up (n=36) | 9 (25) | ||
| Calcium at last follow-up (n=32) (mg/dl) | 9.6±0.7 | ||
| Phosphate at last follow-up (n=13) (mg/dl) | 3.7±0.9 | ||
| Hemoglobin at last follow-up (n=26) (g/dl) | 13.8±2.4 | ||
| Nephrolithiasis by history or imaging | 7 (18) | ||
| Neurogenic bladder | 7 (18) | ||
| Amputation and Renal Function (n=39, without ESRD at hospital discharge) | |||
| Degree of Amputation | Serum Creatinine at Last Follow-Up (mg/dl) | eGFR (CKD-EPI) at Last Follow-Up (ml/min per 1.73 m2) | |
| None (n=12) | 0.86±0.30 | 117±27 | |
| Small (n=6) | 1.00±0.18 | 107±20 | |
| Medium (n=8) | 0.82±0.14 | 121±11 | |
| Large (n=13) | 0.81±0.26 | 122±26 | |
Data are presented as mean±SD, median (range), or count (%). CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; HTN, hypertension.
Of the 39 survivors not at ESRD, the time from injury to last sCr was 1158 (range, 99–3316) days; sCr was 0.85±0.24 mg/dl. None had an eGFR≤60 ml/min per 1.73 m2, but four patients had an eGFR<80 ml/min per 1.73 m2. Of the 36 patients who had a measurement, nine (25%) had proteinuria. Two had proteinuria quantified at ≥150 mg/24 h or per gram creatinine. The remainder had 30 mg/dl (n=3) or greater (n=4) on dipstick urinalysis. Of these, two patients had associated HTN, and one had an eGFR<80 ml/min per 1.73 m2. This patient, the only one with a CKD diagnosis, had angiotensin receptor blocker–treated HTN, 266 mg proteinuria/24 h, and an eGFR of 74 ml/min per 1.73 m2.
Of the patients, 69% (Table 2) had amputations; 13 (33%) of which were large (i.e., bilateral above-the-knee amputations or higher). Despite this loss of muscle mass and creatinine source, sCr and eGFR were not different in large amputees versus those without amputation (P=0.67 and P=0.60, respectively). This was also true for all amputees versus those with no amputation (P=0.97 and P=0.81, respectively). There were 18% who had nephrolithiasis by history or imaging and 18% who had neurogenic bladder, although of varying severity.
Table 3 compares demographic and clinical characteristics of those who died in-hospital versus those who survived. Those who died had significantly fewer hospital days (16±7 versus 90±70, P=0.001), surgical procedures, and days on RRT. They had more RRT treatments per week and lower sCr at RRT initiation (3.66±1.07 versus 6.01±2.96 mg/dl, P=0.01). AKI integer score mortality risk in participants who died was significantly greater than in survivors (44% [range, 18%–91%] versus 23% [range, 6%–96%], P=0.04; Wilcoxon rank-sum test); however, the score could be calculated in only nine of the 11 patients who died.
Table 3.
Comparison of demographic and clinical characteristics of wounded warriors requiring RRT for AKI managed in the National Capital Region on the basis of in-hospital mortality
| Demographic Features | Survived (n=40) | Died (n=11) | P Value |
|---|---|---|---|
| Age at injury (y) | 25±5 | 28±6 | 0.10 |
| Sex (male) | 39 (98) | 11 (100) | >0.99 |
| Race | |||
| White | 26 (65) | 8 (73) | 0.73 |
| Black | 8 (20) | 0 (0) | 0.19 |
| Other | 6 (15) | 3 (27) | 0.39 |
| Injury features | |||
| Type of injury (IED) | 26 (65) | 9 (81.8) | 0.47 |
| Units of PRBCs downrangea | 24 (0–159) | 26 (0–200) | 0.32 |
| Hospital days in National Capital Region | 90±70 | 16±7 | 0.001 |
| Estimated surgeries until death or discharge | 13±9 | 7±3 | 0.04 |
| Amputation | 27 (68) | 9 (82) | 0.47 |
| Maximum CPK (units/L)b | 15,053 (155–200,000) | 11,574 (691–1,900,000) | 0.95 |
| Clinical features of AKI | |||
| Presumed cause of AKI | ATN 39 | ATN 11 | >0.99 |
| Rhabdo 29 | Rhabdo 7 | ||
| Other 1 | Other 0 | ||
| Days from injury to RRT | 8±11 | 7±7 | 0.78 |
| Serum creatinine at initiation | 6.01±2.96 | 3.66±1.07 | 0.01 |
| AKI integer scorec | 20 (14–37) (n=40) | 24 (19–34) (n=9) | 0.04 |
| Probability of mortality from integer scorec (%) | 23 (6–96) (n=40) | 44 (18–91) (n=9) | 0.04 |
| Indications for dialysis | |||
| Hyperkalemia | 27 (68) | 8 (73) | >0.99 |
| Volume overload | 21 (53) | 4 (36) | 0.50 |
| Acidosis | 17 (43) | 6 (55) | 0.51 |
| Azotemia | 22 (55) | 4 (36) | 0.32 |
| Other | 2 (5) | 0 (0) | >0.99 |
| Days on RRT | 20±11 | 12±11 | 0.04 |
| RRT treatments per week | 4.7±1.1 | 6.0±1.2 | 0.001 |
| RRT modality | |||
| iHD | 39 (98) | 11 (100) | >0.99 |
| SLEDD | 20 (50) | 5 (46) | >0.99 |
| CRRT | 8 (20) | 5 (46) | 0.12 |
| CRRT as initial RRT modality | 6 (15) | 4 (36) | 0.19 |
Data are presented as mean±SD, median (range), count (%), or as otherwise indicated. IED, improvised explosive device; PRBCs, packed red blood cells; CPK, creatine phosphokinase; ATN, acute tubular necrosis; iHD, intermittent hemodialysis; SLEDD, slow low efficiency daily dialysis; CRRT, continuous RRT.
Data were transformed using a square-root transformation before t test.
Data were transformed using a log transformation before t test.
Wilcoxon rank-sum test.
Discussion
In this retrospective case series of young, healthy military casualties with PTAKI requiring RRT during the Iraq and Afghanistan wars, 60-day mortality was 22% (95% CI, 12% to 35%), significantly less than the 63%–75% mortality reported during Korea and Vietnam (1–3,5,7–9). Participants were demographically similar to historical cohorts, but medical and surgical management have changed substantially, with increased number and severity of casualties surviving to receive definitive care (24). Potential explanations for improved survival include shorter evacuation times because of definitive air superiority and better resuscitation, surgery, and dialysis techniques (3,4,25–28). Casualties requiring specialized burn care, treated at San Antonio Military Medical Center, were not included. Stewart et al. reported 65% mortality among the burn casualties with AKI requiring RRT (6), and others have reported that PTAKI burn patients have higher mortality (13).
Civilian outcomes for PTAKI requiring RRT likely reflect improvements in medical and surgical capabilities, but they include few young patients without comorbidities. Observed mortality is approximately 40%–80% in civilian trauma series (10–12,29,30). Preexisting chronic conditions and increasing age have been associated with higher mortality in some series (10,12), However, Machemehl et al. reported mortality was independent of age, albeit in an older population with comorbidities (13). Among children in the Prospective Pediatric CRRT registry, mortality was 42%, but PTAKI was not a reported cause of AKI (31).
We used the VA/NIH Acute Renal Failure Trial Network AKI integer risk score (17,18) to compare observed versus expected mortality in the 49 participants in whom it could be calculated. Median mortality risk predicted by the AKI integer risk score was significantly greater than observed mortality. Although the integer score differentiated between survivors and those who died within 60 days of RRT initiation, it was significantly miscalibrated. The ROC curve developed in our cohort was not significantly different than that of the original model cohort (17,18). To our knowledge, this is the first study using the AKI integer score to predict mortality risk. Although mortality estimates for our cohort were miscalibrated, the integer score performed reasonably well, especially because the model cohort was older (59.7±15.3 years) and had multiple comorbidities. The maximum AKI integer score possible is 59, with four parameters (age >50 years, chronic hypoxemia, malignancy, and cardiovascular disease) contributing a maximum 14 points. None of the participants in our cohort received these points. The maximum possible score was 45; the maximum observed score was 37.
All but one of the survivors (98%) recovered renal function and discontinued dialysis. Renal recovery was similar to that previously reported in military casualties (1–3,5,7–9). In civilian series, >90% of patients recover renal function before discharge, even though many had comorbidities that increased ESRD risk (10–12,14,29,30).
Even one episode of AKI is associated with future CKD risk; however, few, if any, studies describe CKD incidence in young, healthy persons without preexisting comorbidities or underlying kidney disease after AKI requiring RRT (15,16,32–34). In children who survived neonatal extracorporeal membrane oxygenation-associated AKI, 32% had evidence of mild CKD (35). Of the survivors in our cohort, 23% had proteinuria in follow-up; however, urine protein quantification may not have been done in first-morning void specimens and therefore may overestimate albuminuria prevalence (36). One survivor had diagnosed stage 2 CKD with proteinuria and eGFR<80 mg/min per 1.73 m2. Some of the amputees may have undiagnosed CKD, despite apparently normal eGFR. Creatinine-based eGFR is inaccurate after amputation, overestimating function (20).
Ninety-eight percent were treated with intermittent hemodialysis; however, many also received slow low efficiency daily dialysis and/or CRRT. Peritoneal dialysis was not used, consistent with previous experience (37). The most common RRT indication was hyperkalemia (69%), followed by azotemia (51%), volume overload (49%), and acidosis (45%). This was consistent with the clinical situation—rhabdomyolysis, large-volume resuscitation with blood products (38,39), hypercatabolism, and frequent (often daily) surgery. Often, RRT was required within days of initial injury; 59% required initiation before reaching the United States. Intensity was high, particularly early on: 5.0±1.4 treatments per week overall. CRRT, as an initial RRT modality, was not associated with survival.
As a case series, similar to all previous reports, our study is descriptive, not permitting definitive statistical inferences. There is no internal comparison group, and historical comparisons cannot account for potential confounding variables. The number of participants is small. It is, however, the only report of the characteristics and long-term outcomes of PTAKI-associated RRT for the nation’s longest war. It is difficult to study AKI in randomized controlled trials, and prospective cohort studies (40,41) include few patients with PTAKI and few young patients. Our data do not allow determination of PTAKI incidence. Non-RRT requiring PTAKI was not studied. The Combat Casualty Critical Care database contains 6011 critically injured combat casualties who required intensive care unit admission and survived to be evacuated from Iraq or Afghanistan to the LRMC between 2002 and 2011 and may yield further information on PTAKI and associated RRT (42). The study of PTAKI will continue to rely on observational analyses, which can inform the design of much-needed prospective trials. The AKI integer risk score supplied a predicted mortality risk not dependent on historic reports. We suggest its routine use in future case series.
In summary, mortality in this young and otherwise healthy cohort with PTAKI requiring RRT was 22%, significantly less than historically predicted. Mortality predicted by AKI integer risk score was closer to that observed, but it is still an overestimate (18). Renal recovery occurred in 98% of survivors, but residual mild CKD is likely, with 23% having proteinuria at last follow-up, and creatinine-based eGFR is likely overestimated in amputees (20). Our observations are pertinent to civilian patients with similar demographics and may inform prognosis.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank Robin S. Howard and Minoo Rouhanian, Department of Research Programs, WRNMMC, for statistical advice. We gratefully acknowledge the many expert health care professionals who cared for the patients described, from the time of initial injury through rehabilitation and ongoing follow-up care.
Preliminary data were presented in abstract form at the American Society of Nephrology Kidney Week 2014 (Philadelphia, PA).
The views expressed in this report are those of the authors and do not reflect the official policy of the Department of the Army, the Department of the Navy, the Department of Defense, or the United States Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00890115/-/DCSupplemental.
References
- 1.Teschan PE, Post RS, Smith LH, Jr, Abernathy RS, Davis JH, Gray DM, Howard JM, Johnson KE, Klopp E, Mundy RL, O’Meara MP, Rush BF, Jr: Post-traumatic renal insufficiency in military casualties. I. Clinical characteristics. Am J Med 18: 172–186, 1955 [DOI] [PubMed] [Google Scholar]
- 2.Smith LH, Jr, Post RS, Teschan PE, Abernathy RS, Davis JH, Gray DM, Howard JM, Johnson KS, Klopp E, Mundy RL, O’Meara MP, Rush BF, Jr: Post-traumatic renal insufficiency in military casualties. II. Management, use of an artificial kidney, prognosis. Am J Med 18: 187–198, 1955 [DOI] [PubMed] [Google Scholar]
- 3.Welch PG: Deployment dialysis in the U.S. Army: History and future challenges. Mil Med 165: 737–741, 2000 [PubMed] [Google Scholar]
- 4.Chung KK, Perkins RM, Oliver JD, 3rd: Renal replacement therapy in support of combat operations. Crit Care Med 36[Suppl]: S365–S369, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Butkus DE: Post-traumatic acute renal failure in combat casualties: A historical review. Mil Med 149: 117–124, 1984 [PubMed] [Google Scholar]
- 6.Stewart IJ, Tilley MA, Cotant CL, Aden JK, Gisler C, Kwan HK, McCorcle J, Renz EM, Chung KK: Association of AKI with adverse outcomes in burned military casualties. Clin J Am Soc Nephrol 7: 199–206, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Lordon RE, Burton JR: Post-traumatic renal failure in military personnel in Southeast Asia. Experience at Clark USAF hospital, Republic of the Philippines. Am J Med 53: 137–147, 1972 [DOI] [PubMed] [Google Scholar]
- 8.Stone WJ, Knepshield JH: Post-traumatic acute renal insufficiency in Vietnam. Clin Nephrol 2: 186–190, 1974 [PubMed] [Google Scholar]
- 9.Iaina A, Reisin E, Eliahou H: Acute renal failure in combat injuries. J Trauma 15: 281–284, 1975 [DOI] [PubMed] [Google Scholar]
- 10.Morris JA, Jr, Mucha P, Jr, Ross SE, Moore BF, Hoyt DB, Gentilello L, Landercasper J, Feliciano DV, Shackford SR: Acute posttraumatic renal failure: A multicenter perspective. J Trauma 31: 1584–1590, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Brown CV, Dubose JJ, Hadjizacharia P, Yanar H, Salim A, Inaba K, Rhee P, Chan L, Demetriades D: Natural history and outcomes of renal failure after trauma. J Am Coll Surg 206: 426–431, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Beitland S, Moen H, Os I: Acute kidney injury with renal replacement therapy in trauma patients. Acta Anaesthesiol Scand 54: 833–840, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Machemehl T, Hsu P, Pahad H, Williams P, Yilmaz TH, Vassiliu P, Boffard KD, Degiannis E, Doll D: Haemodialysis for post-traumatic acute renal failure - factors predicting outcome. S Afr Med J 103: 652–657, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Stads S, Fortrie G, van Bommel J, Zietse R, Betjes MG: Impaired kidney function at hospital discharge and long-term renal and overall survival in patients who received CRRT. Clin J Am Soc Nephrol 8: 1284–1291, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafrance JP, Miller DR: Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21: 345–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P, VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirjian S, Chertow GM, Zhang JH, O’Connor TZ, Vitale J, Paganini EP, Palevsky PM, VA/NIH Acute Renal Failure Trial Network : Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol 6: 2114–2120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease; Chapter 1: Definition and classification of CKD. Kidney Int Suppl 3: 19–62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurlow JS, Abbott KC, Linberg A, Little D, Fenderson J, Olson SW: SCr and SCysC concentrations before and after traumatic amputation in male soldiers: A case-control study. Am J Kidney Dis 63: 167–170, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW: Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 21: 128–138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M: pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44: 837–845, 1988 [PubMed] [Google Scholar]
- 24.Leland A, Oboroceanu MJ: American war and military operations casualties: Lists and statistics. Congressional Research Service 7-5700, 2010. RL32492. Availbable at: http://www.au.af.mil/au/awc/awcgate/crs/rl32492.pdf. Accessed July 18, 2015
- 25.Perkins R, Simon J, Jayakumar A, Neff R, Cohen I, Bohen E, Oliver J, Kumke K, Older S, Perkins J, Grathwohl K, Yuan C, Abbott K: Renal replacement therapy in support of Operation Iraqi Freedom: A tri-service perspective. Mil Med 173: 1115–1121, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Manring MM, Hawk A, Calhoun JH, Andersen RC: Treatment of war wounds: A historical review. Clin Orthop Relat Res 467: 2168–2191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belmont PJ, Jr, Goodman GP, Zacchilli M, Posner M, Evans C, Owens BD: Incidence and epidemiology of combat injuries sustained during “the surge” portion of operation Iraqi Freedom by a U.S. Army brigade combat team. J Trauma 68: 204–210, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB: Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 61: 1366–1372, discussion 1372–1373, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Vivino G, Antonelli M, Moro ML, Cottini F, Conti G, Bufi M, Cannata F, Gasparetto A: Risk factors for acute renal failure in trauma patients. Intensive Care Med 24: 808–814, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Ala-Kokko T, Ohtonen P, Laurila J, Martikainen M, Kaukoranta P: Development of renal failure during the initial 24 h of intensive care unit stay correlates with hospital mortality in trauma patients. Acta Anaesthesiol Scand 50: 828–832, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Symons JM, Chua AN, Somers MJ, Baum MA, Bunchman TE, Benfield MR, Brophy PD, Blowey D, Fortenberry JD, Chand D, Flores FX, Hackbarth R, Alexander SR, Mahan J, McBryde KD, Goldstein SL: Demographic characteristics of pediatric continuous renal replacement therapy: A report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2: 732–738, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Bucaloiu ID, Kirchner HL, Norfold ER, Hartle JE, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Zwiers AJ, IJsselstijn H, van Rosmalen J, Gischler SJ, de Wildt SN, Tibboel D, Cransberg K: CKD and hypertension during long-term follow-up in children and adolescents previously treated with extracorporeal membrane oxygenation. Clin J Am Soc Nephrol 9: 2070–2078, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saydah SH, Pavkov ME, Zhang C, Lacher DA, Eberhardt MS, Burrows NR, Narva AS, Eggers PW, Williams DE: Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009-2010. Clin Chem 59: 675–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yong K, Dogra G, Boudville N, Pinder M, Lim W: Acute kidney injury: Controversies revisited. Int J Nephrol 2011: 762634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkins RM, Aboudara MC, Abbott KC, Holcomb JB: Resuscitative hyperkalemia in noncrush trauma: A prospective, observational study. Clin J Am Soc Nephrol 2: 313–319, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Aboudara MC, Hurst FP, Abbott KC, Perkins RM: Hyperkalemia after packed red blood cell transfusion in trauma patients. J Trauma 64[Suppl]: S86–S91, discussion S91, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Chertow GM, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL, PICARD : Reasons for non-enrollment in a cohort study of ARF: The Program to Improve Care in Acute Renal Disease (PICARD) experience and implications for a clinical trials network. Am J Kidney Dis 42: 507–512, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Stewart IJ, Fang R, Cannon JW, Zonies DH, Morrow BD, Orman JA, Oliver JD, Abbott KC, Jones JA, Chung KK: Derivation of candidates for the combat casualty critical care (C4) database. Mil Med 179: 370–374, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.