Abstract
Background and objectives
Anemia guidelines for CKD recommend withholding intravenous iron in the setting of active infection, although no data specifically support this recommendation. This study aimed to examine the association between intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection.
Design, setting, participants, & measurements
This was a retrospective observational cohort study using data from the US Renal Data System of 22,820 adult Medicare beneficiaries on in-center hemodialysis who had received intravenous iron in the 14 days preceding their first hospitalization for bacterial infection in 2010. In multivariable analyses, the association between receipt of intravenous iron at any point from the day of hospital admission to discharge and all-cause 30-day mortality, mortality in 2010, length of hospital stay, and readmission for infection or death within 30 days of discharge was evaluated.
Results
There were 2463 patients (10.8%) who received intravenous iron at any point from the day of admission to discharge. Receipt of intravenous iron was not associated with age, dialysis vintage, or comorbidities. There were 2618 deaths within 30 days of admission and 6921 deaths in 2010 (median follow-up 173 days; 25th and 75th percentiles, 78–271 days). The median length of stay was 7 days (25th and 75th percentiles, 5–12 days). Receipt of intravenous iron was not associated with higher 30-day mortality (odds ratio, 0.86; 95% confidence interval [95% CI], 0.74 to 1.00), higher mortality in 2010 (hazard ratio, 0.92; 95% CI, 0.85 to 1.00), longer mean length of stay (10.1 days [95% CI, 9.7 to 10.5] versus 10.5 days [95% CI, 10.3 to 10.7]; P=0.05), or readmission for infection or death within 30 days of discharge (odds ratio, 1.08; 95% CI, 0.96 to 1.22) compared with no receipt of intravenous iron.
Conclusions
This analysis does not support withholding intravenous iron upon admission for bacterial infection in hemodialysis patients, although clinical trials are required to make definitive recommendations.
Keywords: hemodialysis, iron, infection
Introduction
Intravenous iron is an important part of the optimal treatment of anemia of ESRD (1), but it is biologically plausible that iron may increase infection risk by impairing neutrophil (2,3) and T cell function (4) and serving as a growth factor for pathogens (4,5). Although an association between iron and viral and fungal infections has been described (5,6), the literature in hemodialysis patients has pertained largely to bacterial infections, with a particular emphasis on systemic infections (e.g., bacteremia) (7). Results from studies evaluating the association between intravenous iron and bacterial infection in hemodialysis patients have been conflicting, and prior studies have had limitations such as the use of serum ferritin as a predictor, lack of control for confounding and residual confounding, small sample size, short follow-up time, and publication bias (7).
Despite these inconsistent findings, guidelines for the treatment of anemia of CKD have considered intravenous iron to be a risk factor for infection (6). Canadian and Japanese guidelines have advised caution and careful weighing of the risks and benefits of intravenous iron use in the setting of infection (8,9). Kidney Disease Improving Global Outcomes guidelines included a nongraded recommendation to avoid intravenous iron in patients with active systemic infections (1). The strongest advisory was issued by the European Best Practice Guidelines, which recommended that intravenous iron be stopped in patients with ongoing bacteremia (10). However, no data specifically support the recommendation to withhold intravenous iron in the setting of active infection in hemodialysis patients. Our study aimed to evaluate the association between continued receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for bacterial infection who had been receiving intravenous iron as an outpatient.
Materials and Methods
Study Design and Data Source
We conducted a retrospective observational cohort study using data from the US Renal Data System. We obtained information from the 2010 core standard analytic files. The Committee on Human Research at the University of California, San Francisco did not consider the study to involve human subjects research.
Study Population and Variables
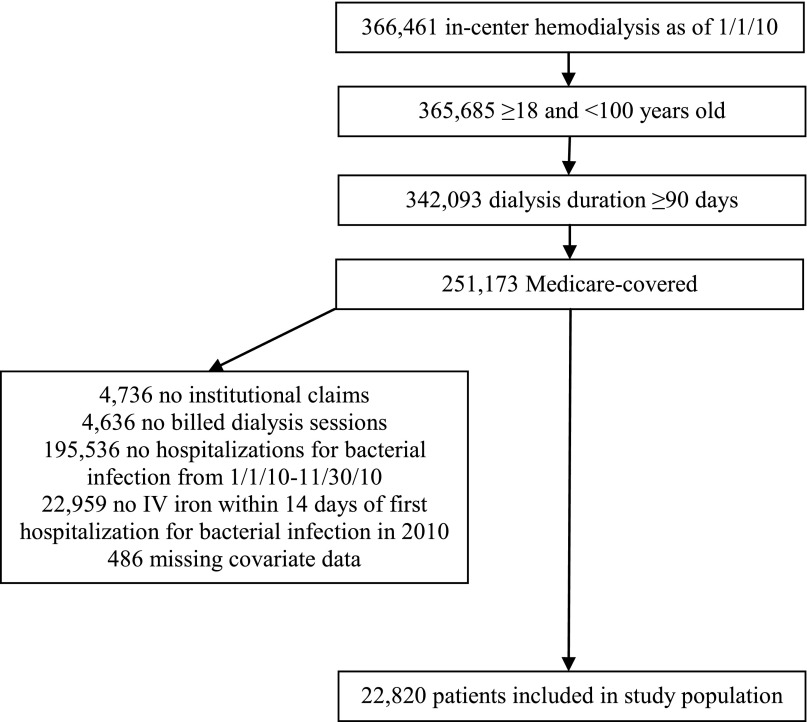
The study population (Figure 1) consisted of adult patients who had Medicare as their primary payer and had received at least 90 days of in-center hemodialysis as of January 1, 2010. We included patients who had at least one hospitalization for bacterial infection from January 1, 2010 to November 30, 2010. We defined a hospitalization as attributable to bacterial infection if the first diagnosis was on our extensive list of International Classification of Diseases, Ninth Revision, Clinical Modification codes for bacterial infection affecting various organ systems (Supplemental Table 1) and was indicated as the principal diagnosis. We excluded hospitalizations in which the dates of admission and discharge were the same.
Figure 1.
Cohort selection. Inclusion and exclusion criteria and selection of the cohort are shown. IV, intravenous.
We also required that patients had received intravenous iron of any dose, frequency, or product in the 14 days preceding admission for their first hospitalization for bacterial infection in 2010; this was intended to identify a population for whom intravenous iron was indicated and administered because our objective was to compare outcomes among those who continued to receive and did not continue to receive intravenous iron during the admission. We obtained information about intravenous iron treatment from the institutional claims files, including the specific iron formulation based on Healthcare Common Procedure Coding System codes (Supplemental Table 1), dose, and date of administration (11). We excluded patients who lacked Medicare claims or billed dialysis sessions in 2010 or who were missing covariate data (Figure 1).
The primary exposure of interest was receipt of intravenous iron of any dose, frequency, or product at any point from the day of hospital admission until discharge for the first hospitalization for bacterial infection in 2010. Secondary exposures of interest were total intravenous iron dose and iron product. Outcomes were defined with respect to the first hospitalization for bacterial infection in 2010 and consisted of all-cause mortality within 30 days of admission, all-cause mortality in 2010, length of stay (LOS), and the composite outcome of readmission for bacterial infection or death from any cause within 30 days of discharge.
Statistical Analyses
Demographic and clinical characteristics of the population are presented as means±SDs or medians (25th and 75th percentiles) for continuous variables and percentages for categorical variables. Comparisons between the groups that received and did not receive intravenous iron were performed with the t test, Wilcoxon rank-sum test, chi-squared test, and Fisher’s exact test as appropriate. For the primary analysis comparing receipt and no receipt of intravenous iron, logistic (30-day mortality; readmission for infection or death within 30 days of discharge), Cox (all-cause mortality in 2010), and generalized linear (LOS) models were constructed, adjusting for the following: demographics (age and duration of ESRD as continuous variables; sex, race [white, black, or other] and geographic location of ESRD network as defined by US Census geographic divisions as categorical variables), comorbidities (coronary artery disease, other cardiac disease, congestive heart failure, hypertension, peripheral vascular disease, diabetes mellitus, cerebrovascular disease, cancer, chronic obstructive pulmonary disease, alcohol dependence, drug dependence, and tobacco use as binary variables, indicating the presence or absence of each comorbidity), and the infected organ system (bacteremia; cardiovascular; catheter/graft; central nervous system; ear, nose, throat, dental; gastrointestinal/peritonitis; genitourinary; joint/bone; reproductive/breast; respiratory/thoracic; septicemia; skin, soft tissue, muscle; spleen/lymph nodes; tuberculosis; and other [Supplemental Table 1] as a categorical variable).
Because intravenous iron administration in the days immediately preceding hospital admission could exert effects that persist during hospitalization, we performed a sensitivity analysis excluding patients who had received intravenous iron within the week before the day of admission. We performed subgroup analyses evaluating the association between intravenous iron and adverse outcomes among patients hospitalized for catheter/graft infections and septicemia/bacteremia, the two most common infection subtypes. We also conducted secondary analyses according to total intravenous iron dose (≥100 mg versus none, <100 mg versus none, and ≥100 mg versus <100 mg), restricted to the day of admission in order to account for survival bias from patients living longer having the opportunity to accumulate higher dosing, and the two most commonly used iron products, iron sucrose and ferric gluconate (iron sucrose versus no intravenous iron, ferric gluconate versus no intravenous iron, and ferric gluconate versus iron sucrose).
To evaluate differences in subsequent anemia management, we compared the time to resumption of intravenous iron after discharge and receipt of red blood cell (RBC) transfusions and erythropoiesis-stimulating agents (ESAs) during hospitalization and 30 days after discharge among patients who received and did not receive intravenous iron during hospitalization. Analyses were performed using Stata software (version 12.1; StataCorp LP, College Station, TX).
Results
Intravenous Iron Receipt and Demographic Information
Our cohort consisted of 22,820 adult Medicare beneficiaries on in-center hemodialysis who had received intravenous iron in the 14 days preceding admission for their first hospitalization for bacterial infection in 2010 (Figure 1). The median age of the cohort was 63.9 years (25th and 75th percentiles, 52.6–74.0); 51.2% of patients were men and 57% were white. The groups that received and did not receive intravenous iron were similar in terms of their age, duration of ESRD, sex, race, and comorbidities (Table 1). There were statistically significant differences between the groups in terms of geographic location of ESRD network, infected organ system, and hematocrit, although these differences were small in magnitude (Table 1, Supplemental Table 2).
Table 1.
Demographic characteristics by receipt of intravenous iron
| Characteristic | Intravenous Iron Received (n=2463) | No Intravenous Iron Received (n=20,357) | P Valuea |
|---|---|---|---|
| Age (yr) | 63.0±14.7 | 62.7±14.9 | 0.42 |
| Duration of ESRD (yr) | 3.3 (1.4–5.9) | 3.1 (1.4–5.8) | 0.44 |
| Men | 51.0 | 51.2 | 0.87 |
| Race | |||
| White | 56.2 | 57.1 | 0.11 |
| Black | 39.9 | 38.3 | |
| Other | 3.9 | 4.6 | |
| Region | |||
| Northeast | 17.8 | 17.2 | <0.01 |
| South | 46.0 | 43.4 | |
| Midwest | 23.6 | 24.3 | |
| West | 12.6 | 15.1 | |
| Comorbidities | |||
| Cerebrovascular disease | 8.9 | 9.3 | 0.47 |
| Hypertension | 87.8 | 88.1 | 0.65 |
| Coronary artery disease | 18.8 | 20.1 | 0.12 |
| Congestive heart failure | 31.8 | 32.5 | 0.49 |
| Other cardiac disease | 11.0 | 12.7 | 0.02 |
| Peripheral vascular disease | 12.1 | 13.4 | 0.07 |
| Chronic obstructive pulmonary disease | 7.0 | 7.8 | 0.13 |
| Diabetes mellitus | 61.4 | 63.2 | 0.08 |
| Cancer | 4.6 | 4.8 | 0.70 |
| Tobacco use | 6.8 | 6.5 | 0.64 |
| Alcohol dependence | 1.6 | 1.4 | 0.48 |
| Drug dependence | 2.0 | 1.5 | 0.08 |
| Hematocritb | 33.4±3.9 | 33.6±3.8 | 0.02 |
Data are presented as means±SD, medians (25th and 75th percentiles), and percentages.
Comparisons performed with the t test, Wilcoxon rank-sum test, and chi-squared test as appropriate.
Value most proximal to and within 30 days before the day of admission.
Approximately 11% of the cohort (n=2463) received intravenous iron during hospitalization, 93% of whom received their only dose on the day of admission. The median total dose was 100 mg (25th and 75th percentiles, 100 mg). Iron sucrose was the most frequently administered product (86.6% of patients who received intravenous iron), followed by ferric gluconate (12.4%), ferumoxytol (0.8%), and iron dextran (0.2%).
Associations of Intravenous Iron with Adverse Outcomes
There were 2618 deaths (11.5% of the cohort) within 30 days of admission and 6921 deaths in 2010 (30.3% of the cohort) with a median follow-up time of 173 days (78–271). The median LOS was 7 days (5–12), which was not significantly different between the groups that received (7 days [4–11]) and did not receive intravenous iron (7 days [5–12]; P=0.29). There were 3220 patients (14.1% of the cohort) who were readmitted for infection or died from any cause within 30 days of discharge.
Receipt of intravenous iron was not associated with higher 30-day mortality (odds ratio [OR], 0.86; 95% confidence interval [95% CI], 0.74 to 1.00; P=0.04), higher mortality in 2010 (hazard ratio [HR], 0.92; 95% CI, 0.85 to 1.00; P=0.04), longer mean LOS (10.1 days [95% CI, 9.7 to 10.5] versus 10.5 days [95% CI, 10.3 to 10.7]; P=0.05), or readmission for infection or death within 30 days of discharge (OR, 1.08; 95% CI, 0.96 to 1.22; P=0.19) (Table 2). Excluding patients who had received intravenous iron within the week before the day of admission (Supplemental Table 3) and restricting the analysis to patients hospitalized for catheter/graft infections (Supplemental Table 4) or septicemia/bacteremia (Supplemental Table 5) did not generally change the interpretation of the results, although receipt of intravenous iron was associated with shorter mean LOS (9.6 days [95% CI, 9.0 to 10.1] versus 10.6 days [95% CI, 10.3 to 10.9]; P=0.001) among patients hospitalized for catheter/graft infections and with a higher odds of readmission for infection or death within 30 days of discharge (OR, 1.28; 95% CI, 1.05 to 1.56; P=0.01) and a shorter mean LOS (11.4 days [95% CI, 10.6 to 12.3] versus 12.5 days [95% CI, 12.1 to 12.8]; P=0.03) among patients hospitalized for septicemia/bacteremia.
Table 2.
Associations between receipt of intravenous iron and adverse outcomes
| Outcome | Estimated Adjusted Association or Length of Stay | P Value |
|---|---|---|
| All-cause mortality within 30 d of admissiona | 0.86 (0.74 to 1.00) | 0.04 |
| All-cause 2010 mortalityb | 0.92 (0.85 to 1.00) | 0.04 |
| Mean length of stay, d | 10.1 (9.7 to 10.5) versus 10.5 (10.3 to 10.7) | 0.05 |
| Readmission for infection or all-cause mortality within 30 d of dischargea | 1.08 (0.96 to 1.22) | 0.19 |
Estimated adjusted association and length of stay data are presented with 95% confidence intervals. Results show the comparison of receipt versus no receipt of intravenous iron adjusted for age, duration of ESRD, sex, race, geographic location of ESRD network, coronary artery disease, other cardiac disease, congestive heart failure, hypertension, peripheral vascular disease, diabetes mellitus, cerebrovascular disease, cancer, chronic obstructive pulmonary disease, alcohol dependence, drug dependence, tobacco use, and the infected organ system.
Odds ratio.
Hazard ratio.
Compared with not receiving intravenous iron, receipt of at least 100 mg on the day of admission was not associated with higher 30-day mortality (OR, 0.87; 95% CI, 0.75 to 1.02; P=0.08), higher mortality in 2010 (HR, 0.94; 95% CI, 0.86 to 1.02; P=0.14), longer mean LOS (10.0 days [95% CI, 9.6 to 10.4] versus 10.5 days [95% CI, 10.3 to 10.7]; P=0.04), or readmission for infection or death within 30 days of discharge (OR, 1.07; 95% CI, 0.94 to 1.21; P=0.31) (Table 3). There was no significant difference in adverse outcomes between patients who received at least 100 mg (n=2164) and <100 mg (n=155) of intravenous iron on the day of admission, although CIs were wide because of the small number of patients who received <100 mg of iron.
Table 3.
Associations between receipt of intravenous iron and adverse outcomes according to total intravenous iron dose on the day of admission
| Outcome | Estimated Adjusted Association or Length of Stay | P Value |
|---|---|---|
| All-cause mortality within 30 d of admissiona | ||
| ≥100 mg versus no IV iron | 0.87 (0.75 to 1.02) | 0.08 |
| <100 mg versus no IV iron | 1.23 (0.77 to 1.96) | 0.40 |
| ≥100 mg versus <100 mg | 0.71 (0.44 to 1.16) | 0.17 |
| All-cause 2010 mortalityb | ||
| ≥100 mg versus no IV iron | 0.94 (0.86 to 1.02) | 0.14 |
| <100 mg versus no IV iron | 0.97 (0.74 to 1.27) | 0.83 |
| ≥100 mg versus <100 mg | 0.97 (0.73 to 1.28) | 0.82 |
| Mean length of stay (d) | ||
| ≥100 mg versus no IV iron | 10.0 (9.6 to 10.4) versus 10.5 (10.3 to 10.7) | 0.04 |
| <100 mg versus no IV iron | 10.8 (9.4 to 12.3) versus 10.5 (10.3 to 10.7) | 0.66 |
| ≥100 mg versus <100 mg | 10.0 (9.6 to 10.4) versus 10.8 (9.4 to 12.3) | 0.29 |
| Readmission for infection or all-cause mortality within 30 d of dischargea | ||
| ≥100 mg versus no IV iron | 1.07 (0.94 to 1.21) | 0.31 |
| <100 mg versus no IV iron | 1.29 (0.84 to 1.96) | 0.24 |
| ≥100 mg versus <100 mg | 0.83 (0.54 to 1.28) | 0.40 |
Estimated adjusted association and length of stay data are presented with 95% confidence intervals. Results are adjusted for age, duration of ESRD, sex, race, geographic location of ESRD network, coronary artery disease, other cardiac disease, congestive heart failure, hypertension, peripheral vascular disease, diabetes mellitus, cerebrovascular disease, cancer, chronic obstructive pulmonary disease, alcohol dependence, drug dependence, tobacco use, and the infected organ system. IV, intravenous.
Odds ratio.
Hazard ratio.
Receipt of iron sucrose was not associated with higher 30-day mortality (OR, 0.84; 95% CI, 0.72 to 0.98; P=0.03), higher mortality in 2010 (HR, 0.90; 95% CI, 0.83 to 0.98; P=0.02), longer mean LOS (9.8 days [95% CI, 9.4 to 10.2] versus 10.5 days [95% CI, 10.3 to 10.7]; P=0.003), or readmission for infection or death within 30 days of discharge (OR, 1.04; 95% CI, 0.92 to 1.19; P=0.52) compared with not receiving iron (Table 4). Receipt of ferric gluconate, although much less commonly used than iron sucrose, was associated with a higher odds of readmission for infection or death within 30 days of discharge (OR, 1.40; 95% CI, 1.04 to 1.89; P=0.03) than not receiving iron; there were no significant differences with respect to other outcomes. Mean LOS was longer among patients who received ferric gluconate compared with iron sucrose (11.7 days [95% CI, 10.4 to 13.0] versus 9.8 days [95% CI, 9.4 to 10.2]; P=0.005); there were no significant differences with respect to other outcomes.
Table 4.
Associations between receipt of intravenous iron and adverse outcomes according to intravenous iron product
| Outcome | Estimated Adjusted Association or Length of Stay | P Value |
|---|---|---|
| All-cause mortality within 30 d of admissiona | ||
| IS versus no IV iron | 0.84 (0.72 to 0.98) | 0.03 |
| FG versus no IV iron | 1.05 (0.73 to 1.50) | 0.80 |
| FG versus IS | 1.25 (0.85 to 1.84) | 0.26 |
| All-cause 2010 mortalityb | ||
| IS versus no IV iron | 0.90 (0.83 to 0.98) | 0.02 |
| FG versus no IV iron | 1.06 (0.88 to 1.29) | 0.54 |
| FG versus IS | 1.18 (0.96 to 1.45) | 0.12 |
| Mean length of stay (d) | ||
| IS versus no IV iron | 9.8 (9.4 to 10.2) versus 10.5 (10.3 to 10.7) | 0.003 |
| FG versus no IV iron | 11.7 (10.4 to 13.0) versus 10.5 (10.3 to 10.7) | 0.07 |
| FG versus IS | 11.7 (10.4 to 13.0) versus 9.8 (9.4 to 10.2) | 0.005 |
| Readmission for infection or all-cause mortality within 30 d of dischargea | ||
| IS versus no IV iron | 1.04 (0.92 to 1.19) | 0.52 |
| FG versus no IV iron | 1.40 (1.04 to 1.89) | 0.03 |
| FG versus IS | 1.35 (0.98 to 1.85) | 0.07 |
Estimated adjusted association and length of stay data are presented with 95% confidence intervals. Results are adjusted for age, duration of ESRD, sex, race, geographic location of ESRD network, coronary artery disease, other cardiac disease, congestive heart failure, hypertension, peripheral vascular disease, diabetes mellitus, cerebrovascular disease, cancer, chronic obstructive pulmonary disease, alcohol dependence, drug dependence, and tobacco use and the infected organ system. IS, iron sucrose; IV, intravenous; FG, ferric gluconate.
Odds ratio.
Hazard ratio.
Outcomes Related to Anemia Management
Receipt of RBC transfusion was a rare event overall. Comparing the groups that received and did not receive intravenous iron, there was no significant difference in RBC transfusion receipt during (0.08% versus 0.13%; P=0.76) and 30 days after hospitalization (1.3% versus 1.4%; P=0.71). The majority of patients in our cohort did not receive any ESAs while hospitalized, although receipt of ESAs was significantly higher in the group that received intravenous iron compared with the group that did not (12.0% versus 2.3%; P<0.001). In the 30 days after hospitalization, receipt of ESAs was also significantly higher in the group that had received intravenous iron compared with the group that had not (86.1% versus 84.3%; P=0.02), but this difference was not clinically meaningful. More patients who had received intravenous iron during hospitalization resumed iron after discharge compared with those who had not received iron during the hospitalization (86.9% versus 83.4%; P<0.001), and the median time to resuming iron was significantly shorter (4 days [2–7] versus 4 days [2–11]; P<0.001), although these differences were not clinically meaningful.
Discussion
In our study of Medicare beneficiaries on in-center hemodialysis hospitalized with bacterial infection, we observed that continued receipt of intravenous iron during hospitalization was not associated with higher all-cause mortality, readmission for infection, or longer LOS. In fact, for most outcomes, the upper bound of the 95% CI excluded substantial harm. We also found that receipt of at least 100 mg of intravenous iron on the day of admission or iron sucrose, the most widely prescribed iron preparation in current United States practice (12), was not associated with adverse outcomes. Intravenous iron was associated with a significantly shorter LOS among patients hospitalized for catheter or graft infections and septicemia or bacteremia, the most common types of infection in our cohort. In addition, intravenous iron was associated with higher odds of readmission for infection among those hospitalized for septicemia or bacteremia. However, there was no association with mortality in either subgroup.
Our study was novel in its evaluation of outcomes associated with continued receipt of intravenous iron in the setting of active infection in hemodialysis patients. Recent large cohort studies have evaluated the association between intravenous iron and risk of subsequent infectious outcomes in hemodialysis patients and have observed differing results. In a cohort of 117,050 patients receiving hemodialysis, Brookhart et al. evaluated the association of intravenous iron dose (high versus low) and dosing strategy (bolus versus maintenance) over a 1-month exposure period on infectious hospitalization over the next 3 months (13). In multivariable-adjusted analyses, they observed a 3% higher hazard of infectious hospitalization (involving any major organ system) with high-dose iron (>200 mg per month) compared with low-dose iron (1–200 mg per month) (HR, 1.03; 95% CI, 1.01 to 1.06) and a 5% higher hazard of infectious hospitalization with bolus versus maintenance iron (HR, 1.05; 95% CI, 1.03 to 1.08). Using similar methods, these results were replicated by the same team of investigators in a cohort of 6605 hemodialysis patients from a small dialysis provider; bolus (versus maintenance) iron was associated with a 13% higher hazard of infectious hospitalization (involving any major organ system) (HR, 1.13; 95% CI, 1.03 to 1.24) (14).
However, other recent studies have challenged these findings. In two large cohort studies of patients initiating hemodialysis at Dialysis Clinic Inc. facilities, the investigators did not find an association between cumulative intravenous iron dose over 1-, 3-, or 6-month exposure windows and infectious hospitalization or mortality within the subsequent month (15,16). In an international prospective cohort of hemodialysis patients, a nonlinear association between intravenous iron and infection-related mortality was observed, wherein patients prescribed no iron or doses ≥200 mg per month were at nonstatistically significantly higher risk compared with patients prescribed lower-dose intravenous iron (17). Two small randomized trials involving iron repletion in hemodialysis patients collected data about infectious outcomes as part of their safety analysis and did not find a difference in infectious adverse events between the intervention and control groups (18,19).
Our study is also novel in its evaluation of the potential adverse consequences of withholding intravenous iron during active infection, particularly the possibility of increasing the rate of RBC transfusion, which may also pose risk for infection (20,21) and can lead to sensitization among patients awaiting kidney transplants (22), or higher use of ESAs. We found that transfusion was a rare event during hospitalization for infection, and we did not observe a higher risk of transfusion among patients who did not receive intravenous iron during hospital admission. ESA use during hospitalization was relatively uncommon, and we did not observe higher use of ESAs among patients who did not receive iron.
To our knowledge, no studies evaluating the association between intravenous iron and infection have focused specifically on outcomes among hemodialysis patients hospitalized for infection. A strength of our study is that it evaluates outcomes among patients known to have active infection, which is the clinical scenario that closely aligns with the setting in which guidelines have recommended caution in prescribing (8,9), avoidance (1), and withholding (10) of intravenous iron. In addition, the sample size is larger than that of most studies examining the topic of intravenous iron and infection risk in hemodialysis patients (7).
Our study also has several important limitations that must be acknowledged. As with any observational study, there is the possibility of residual confounding. In particular, it is possible that there could be confounding by indication, and relevant variables such as iron indices (e.g., ferritin, transferrin saturation) were not available in our database. The majority (93%) of intravenous iron was received on the day of admission, which limits our ability to make inferences about the longer-term use of intravenous iron during hospitalization for infection. Results from a sensitivity analysis limited to patients who received intravenous iron on the first day of admission were similar to those of the full cohort. We also acknowledge that we lack information regarding the circumstances (e.g., hospital-wide protocols or individualized assessment of patients) that informed the decision to administer intravenous iron. However, investigation of the association between receipt of intravenous iron at the start of an infectious hospitalization (indication notwithstanding) and subsequent outcomes may address a more clinically relevant concern than evaluation of the association with intravenous iron received at later time points when patients have presumably been treated and may no longer be actively infected. We did not include infections treated in the outpatient setting because our focus was on infections that were serious enough to warrant hospitalization. Ascertainment of hospitalizations for bacterial infection was limited by the use of International Classification of Diseases, Ninth Revision, Clinical Modification codes. However, it seems unlikely that misclassification of the outcomes would differ according to receipt of intravenous iron. The sample size was small in the subgroups receiving <100 mg on the day of admission and ferric gluconate, resulting in wide confidence intervals, which limits interpretation of some of the secondary analyses and warrants confirmation of these findings in future studies. We were also limited in our ability to evaluate the association between receipt of intravenous iron and RBC transfusion requirements because transfusions were rare events.
In summary, among hemodialysis patients hospitalized for bacterial infection, continued receipt of intravenous iron was not associated with higher all-cause mortality, readmission for infection, or longer hospital stay. The observational nature of our study precludes definitive conclusions and should not be interpreted as a recommendation to use intravenous iron in hemodialysis patients without discretion. Nevertheless, our analysis does not demonstrate clearcut benefit or harm from withholding intravenous iron upon admission for infection in hemodialysis patients and does not support any specific recommendation regarding the prescription of intravenous iron in the setting of active infection. However, further examination of the effect of intravenous iron administration and adverse outcomes using randomized controlled trials is necessary before recommending changes in intravenous iron prescribing practices for hemodialysis patients.
Disclosures
J.H.I. has received research funding from the American Society of Nephrology and Genentech/Roche. L.S.D. and B.A.G. have received research support from Dialysis Clinic Inc.
Supplementary Material
Acknowledgments
Because K.L.J. is a deputy editor of CJASN, she was not involved in the peer-review process for this article. Another editor oversaw the peer-review and decision-making process for this article.
This publication was made possible by grants from the American Society of Nephrology (to J.H.I.), the National Institute of Diabetes and Digestive and Kidney Diseases (K24-DK085153 to K.L.J., K23-DK093584 to L.S.D., and N01-DK7005 to K.L.J. and B.A.G.), and the University of California, San Francisco Clinical and Translational Science Institute (UL1-TR000004 to C.E.M. and B.A.G.).
The data reported here were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the United States government.
This study was presented in part as an oral presentation at the annual meeting of the American Society of Nephrology, held November 11–16, 2014, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Is Intravenous Iron Supplementation Safe to Administer to Patients on Hemodialysis with Active Infection—What Do We Know, and What More Do We Need to Know?,” on pages 1714–1715.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01090115/-/DCSupplemental.
References
- 1.Kidney Disease Improving Global Outcomes (KDIGO) Anemia Work Group : KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 2.Fishbane S: Review of issues relating to iron and infection. Am J Kidney Dis 34[Suppl 2]: S47–S52, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Sunder-Plassmann G, Patruta SI, Hörl WH: Pathobiology of the role of iron in infection. Am J Kidney Dis 34[Suppl 2]: S25–S29, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Mencacci A, Cenci E, Boelaert JR, Bucci P, Mosci P, Fè d’Ostiani C, Bistoni F, Romani L: Iron overload alters innate and T helper cell responses to Candida albicans in mice. J Infect Dis 175: 1467–1476, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Khan FA, Fisher MA, Khakoo RA: Association of hemochromatosis with infectious diseases: Expanding spectrum. Int J Infect Dis 11: 482–487, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Boelaert JR, Fenves AZ, Coburn JW: Deferoxamine therapy and mucormycosis in dialysis patients: Report of an international registry. Am J Kidney Dis 18: 660–667, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Ishida JH, Johansen KL: Iron and infection in hemodialysis patients. Semin Dial 27: 26–36, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madore F, White CT, Foley RN, Barrett BJ, Moist LM, Klarenbach SW, Culleton BF, Tonelli M, Manns BJ, Canadian Society of Nephrology : Clinical practice guidelines for assessment and management of iron deficiency. Kidney Int Suppl 74[S110]: S7–S11, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T: 2008 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 14: 240–275, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S, European Best Practice Guidelines Working Group : Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19[Suppl 2]: ii1–ii47, 2004 [DOI] [PubMed] [Google Scholar]
- 11.US Renal Data System : Researcher’s Guide, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 12.Hirth RA, Turenne MN, Wheeler JR, Nahra TA, Sleeman KK, Zhang W, Messana JA: The initial impact of Medicare’s new prospective payment system for kidney dialysis. Am J Kidney Dis 62: 662–669, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV: Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 24: 1151–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freburger JK, Ellis AR, Kshirsagar AV, Wang L, Brookhart MA: Comparative short-term safety of bolus versus maintenance iron dosing in hemodialysis patients: A replication study. BMC Nephrol 15: 154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangri N, Miskulin DC, Zhou J, Bandeen-Roche K, Michels WM, Ephraim PL, McDermott A, Crews DC, Scialla JJ, Sozio SM,Shafi T, Jaar BG, Meyer K, Ebony Boulware L, DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators : Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: A comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant 30: 667–675, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miskulin DC, Tangri N, Bandeen-Roche K, Zhou J, McDermott A, Meyer KB, Ephraim PL, Michels WM, Jaar BG, Crews DC, Scialla JJ, Sozio SM, Shafi T, Wu AW, Cook C, Boulware LE, Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Network Patient Outcomes in End Stage Renal Disease Study Investigators : Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 9: 1930–1939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, Mason N, Tong L, Locatelli F, Marshall MR, Inaba M, Robinson BM: Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 87: 162–168, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Besarab A, Amin N, Ahsan M, Vogel SE, Zazuwa G, Frinak S, Zazra JJ, Anandan JV, Gupta A: Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 11: 530–538, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR, DRIVE Study Group : Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Bordin JO, Heddle NM, Blajchman MA: Biologic effects of leukocytes present in transfused cellular blood products. Blood 84: 1703–1721, 1994 [PubMed] [Google Scholar]
- 21.Klein HG: Immunologic aspects of blood transfusion. Semin Oncol 21[Suppl 3]: 16–20, 1994 [PubMed] [Google Scholar]
- 22.Leffell MS, Kim D, Vega RM, Zachary AA, Petersen J, Hart JM, Rossert J, Bradbury BD: Red blood cell transfusions and the risk of allosensitization in patients awaiting primary kidney transplantation. Transplantation 97: 525–533, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.