Abstract
Background and objectives
Recombinant human erythropoietin (epoetin) is used routinely to increase blood hemoglobin levels in patients with ESRD and anemia. Although lower doses of epoetin are required to achieve equivalent hemoglobin responses when administered subcutaneously rather than intravenously, standard practice has been to administer epoetin to patients on hemodialysis intravenously. Randomized trials of alternative epoetin treatment regimens in patients with kidney failure have shown that risks of cardiovascular complications and death are related to the dose levels of epoetin used. Therefore, given the dose-sparing advantages of subcutaneous epoetin administration, the possibility that treatment of patients on hemodialysis with subcutaneous epoetin might be associated with more favorable outcomes compared with intravenous treatment was investigated.
Design, setting, participants, & measurements
A retrospective cohort study of 62,710 adult patients on hemodialysis treated with either intravenous or subcutaneous epoetin-α and enrolled in the Centers for Medicare and Medicaid Services ESRD Clinical Performance Measures Project from 1997 to 2005 was carried out. Risks of death and/or hospitalization for cardiovascular complications (adverse composite event outcomes) during 2 years of follow-up were determined in relationship to epoetin dose and route of administration (intravenous versus subcutaneous) by multivariate Cox proportional hazard modeling adjusted for demographics and clinical parameters.
Results
Epoetin doses used to achieve equivalent hemoglobin responses in study patients were, on average, 25% higher when epoetin was administered intravenously rather than subcutaneously (as expected). Moreover, adverse composite event outcomes were found to be significantly more likely to occur during follow-up for patients on hemodialysis managed with intravenous rather than subcutaneous epoetin (adjusted hazard ratio for adverse events within 1 year [intravenous versus subcutaneous] was 1.11 [95% confidence interval, 1.04 to 1.18]).
Conclusions
This study finds that treatment of patients on hemodialysis with subcutaneous epoetin is associated with more favorable clinical outcomes than those associated with intravenous epoetin treatment.
Keywords: anemia, ESRD, erythropoietin, epoetin, hemodialysis
Introduction
Recombinant human erythropoietin (epoetin-α) treatment increases blood hemoglobin levels in almost all patients with anemia of ESRD and has been a mainstay of managing these patients for decades (1–6). Both intravenous (iv) and subcutaneous (sc) epoetin effectively ameliorate anemia of kidney failure. However, epoetin pharmacokinetics and activity differ depending on the route of administration. Elevated epoetin blood levels after sc injection are much lower than after iv infusion of equivalent doses but more prolonged, and lower doses are required to achieve the same hemoglobin response when administered sc rather than iv (7–11).
Epoetin-α is expensive (>$1.7 billion in costs to Medicare in 2010) (12). Because of substantial cost savings that could be achieved without compromising quality of care, clinical guidelines issued by the National Kidney Foundation (NKF) in 1997 and 2000 (NKF Disease Outcomes Quality Initiative [DOQI]) recommended that sc rather than iv epoetin be used to treat patients with kidney failure (13–15). This recommendation also reflected the principle of limiting drug use to the minimum required to achieve a therapeutic goal. Nonetheless, standard practice in the United States outside of the Veterans Affairs Health Care System (16) has been to treat patients on hemodialysis with iv epoetin (17). Convenience, patient comfort, and concerns about rare cases of pure red cell aplasia (PRCA) caused by drug–induced antierythropoietin antibodies have been rationales for favoring iv epoetin in hemodialysis management (17–21). However, clinical care reimbursement policies also favored iv epoetin use. Before 2011, Medicare, which covers most costs of hemodialysis care in the United States, paid for epoetin treatment on a drug cost basis, and reimbursements for administering epoetin accounted for up to 25% of dialysis center income (22).
Furthermore, before 2006, there was no clear evidence of dose–related epoetin toxicities for patients with kidney failure. Since 2006, however, several large randomized trials evaluating epoetin and darbopoetin (a long-acting form of epoetin) treatment regimens in kidney disease found that risks of cardiovascular complications and early death were increased significantly when high doses of drug were used to maximize hemoglobin responses (23–26). These findings raised the possibility that sc epoetin might be safer than iv epoetin for patients on hemodialysis because of its dose-sparing advantages.
To explore relationships between epoetin dose, route of administration, and clinical outcomes in patients with ESRD, we conducted a retrospective cohort study of >60,000 patients on hemodialysis using data from the Centers for Medicare and Medicaid Services (CMS) ESRD Clinical Performance Measures (CPM) Project (www.cms.hhs.gov/cpmproject/) linked to CMS administrative data maintained by the US Renal Data System (USRDS; www.usrds.org). Clinical outcomes of patients on hemodialysis managed with iv versus sc epoetin were compared, and both dose and route of epoetin administration were evaluated as independent risk factors for adverse clinical outcomes.
Materials and Methods
Study Participants
Details of the CMS ESRD CPM have been reported previously (27). For this project, patients on hemodialysis (≥18 years old) stratified by regional ESRD Networks contracted to monitor quality of care were selected randomly from a census of all surviving patients undergoing hemodialysis on December 31st of successive years. Data used for this study encompass 9 years of the CPM Project (1997–2005) and represent information collected from patient records during 3-month periods immediately preceding each study year (i.e., 2005 data reflected care and observations recorded from October 1 to December 31, 2004). If a patient was randomly selected for the CPM Project more than one time, only data from the first year of selection were included for analysis. Data from CPM patient records were then linked to CMS administrative data maintained by the USRDS. Baseline clinical data, including epoetin-α treatment parameters, came from the CPM Project, whereas outcomes data (i.e., hospitalizations and mortality) were provided by the USRDS.
CPM Project data included records for 68,278 patients from 1997 to 2005 after elimination of duplicates. Patients were excluded if they could not be linked to USRDS patient files (471), did not receive epoetin (2858), lacked information about epoetin dosing or route of administration (1371), or had died or undergone kidney transplantation during the final 3 months of the year when selected for enrollment (69). For the remaining 63,509 patients, 57,602 patients received iv epoetin, 5108 patients received sc epoetin, and 799 patients received both and were excluded from the study. The remaining 62,710 patients constituted the study population for analysis.
Determination of Clinical Variables
Demographic information from the CPM Project included sex, age, race, and ethnicity. Clinical information included cause of ESRD, dialysis vintage, height and weight, type of vascular access, prescribed doses of epoetin, and route of administration. Laboratory measures included blood hemoglobin, transferrin saturation, ferritin concentration, and serum albumin as well as procedural data used to calculate spKt/V as a measure of dialysis adequacy. Epoetin doses, laboratory measurements, and spKt/V values for individual patients represented averages of those recorded during the 3-month periods when clinical information was obtained for the ESRD CPM Project (27). Characteristics of individual dialysis facilities providing patient data to the CPM Project (e.g., nonprofit versus for profit, hospital based versus nonhospital based) were obtained from USRDS records.
Determination of Clinical Outcomes and Data Analyses
Adverse composite event outcomes were examined as defined previously in randomized studies of patients with kidney disease (23,24). A composite event outcome represented the occurrence of one or more clinical events (death and/or hospitalization for congestive heart failure [CHF], acute myocardial infarction [AMI], and/or stroke [cerebrovascular accident (CVA)]) during the 2 years after a patient entered the CPM Project. Patients were classified as having had an AMI, CHF, or CVA if hospitalized with primary international classification of diseases (ICD-9) diagnosis codes of 410 (AMI), 428 (CHF), or 430–436 (CVA). Patient records were also screened for additional ICD-9 codes as described by Collins et al. (28) to detect possible patients with PRCA.
All study patients received epoetin-α and were treated exclusively with either iv or sc epoetin during periods of data collection. To control for unmeasured factors related to network and facility differences, the percentage of patients treated exclusively with sc epoetin was determined for each of 18 hemodialysis networks defined in the CPM Project, and these were grouped into four categories of sc epoetin administration (<4%, 4%–7%, 8%–11%, and ≥12% of patients). Bivariate analyses were conducted to compare groups using chi square statistics for categorical variables and the t test or Wilcoxon rank–sum test for continuous variables. Life table cumulative event rates were calculated using Kaplan–Meier estimates and evaluated by the log-rank test. Multivariate Cox proportional hazard analyses stratified by network group and adjusted for patient characteristics reported to affect clinical outcomes (e.g., nonblack race, older age, diabetes as the cause of ESRD, and low serum albumin [27,29,30]) as well as individual dialysis facility characteristics were carried out to determine whether variables of epoetin dose and route of administration were associated independently with differences in the incidence of adverse composite events. Separate regression analyses were done for 12 and 24 months of follow-up. Patients were censored at the time of transplant if they received a kidney transplant during follow-up. All data analyses were conducted using SAS, version 9.3 (SAS, Cary, NC).
Results
Clinical Characteristics of Patients on Hemodialysis Managed with iv Versus sc Epoetin-α
A majority (53%) of 62,710 adults with ESRD included in this study had been on dialysis for >2 years at the time of entry into the ESRD CPM Project (dialysis vintage >2.0 years) (Table 1). Overall, 8% (5108) were managed with sc epoetin, whereas 92% (57,602) received iv epoetin. The sc epoetin cohort was found to include greater proportions of patients with clinical characteristics associated with a poor prognosis (e.g., nonblack race, older age, diabetes as the cause of ESRD, and low serum albumin [29,30]) than the iv cohort (Table 1).
Table 1.
Characteristics of patients treated with intravenous versus subcutaneous epoetin
| Demographic and Clinical Variables of Study Patients | Route of Administration | |||
|---|---|---|---|---|
| Total (n=62,710) | Intravenous (n=57,602) | Subcutaneous (n=5108) | P Value | |
| Men (%) | 52.4 | 52.2 | 54.7 | <0.001 |
| Black (%) | 37.1 | 37.6 | 30.9 | <0.001 |
| Hispanic (%) | 12.6 | 12.6 | 12.6 | 0.97 |
| Age, yr | ||||
| Mean (SD) | 61.4 (15.3) | 61.3 (15.3) | 62.0 (15.2) | 0.004 |
| Median (interquartile range) | 63.3 (50.7–73.3) | 63.2 (50.7–73.3) | 64.1 (51.9–73.8) | |
| <65 yr old (%) | 46.0 | 45.8 | 47.9 | 0.004 |
| DM as the cause of ESRDa (%) | 42.2 | 42.0 | 44.7 | <0.001 |
| History of CHF, AMI, or stroke (%) | 25.9 | 26.1 | 23.7 | <0.001 |
| Dialysis vintage, yr (%) | ||||
| <0.5 | 12.2 | 12.0 | 14.4 | <0.001 |
| 0.5–0.9 | 14.2 | 14.1 | 15.2 | |
| 1.0–1.9 | 20.5 | 20.4 | 21.5 | |
| ≥2.0 | 53.1 | 53.4 | 48.9 | |
| Postdialysis BMI, kga | ||||
| Mean (SD) | 26.5 (7.00) | 26.5 (7.0) | 26.7 (6.8) | 0.07 |
| Median (interquartile range) | 25.2 (21.9–29.7) | 25.4 (21.9–29.7) | 25.4 (22.1–29.8) | |
| ≥25 (%) | 51.5 | 51.4 | 52.6 | 0.09 |
| Hgb | ||||
| Mean (SD) | 11.45 (1.23) | 11.45 (1.23) | 11.37 (1.22) | <0.001 |
| Median (interquartile range) | 11.53 (10.7–12.2) | 11.53 (10.8–12.2) | 11.47 (10.6–12.1) | |
| ≤11.5 (%) | 49.7 | 49.5 | 51.6 | 0.004 |
| TSAT<20a (%) | 23.6 | 23.4 | 26.2 | <0.001 |
| Serum ferritin <100 ng/ml (%) | 11.6 | 11.5 | 13.0 | 0.002 |
| Iron deficienta,b (%) | 5.2 | 5.1 | 6.8 | <0.001 |
| Prescribed intravenous irona (%) | 63.5 | 63.5 | 63.6 | 0.95 |
| Serum albumina ≥3.5/3.8 g/dlc (%) | 75.6 | 76.2 | 67.9 | <0.001 |
| Epoetin dose, units/kg per week | ||||
| Mean (SD) | 223 (158) | 227 (159) | 182 (133) | <0.001 |
| Median (interquartile range) | 183 (101–308) | 187 (103–314) | 146 (82–245) | <0.001 |
| <150 (%) | 40.7 | 39.8 | 51.0 | <0.001 |
| 150–299 (%) | 33.0 | 33.1 | 32.2 | |
| ≥300 (%) | 26.3 | 27.1 | 16.7 | |
| Facility (%) | ||||
| Nonprofit | 25.0 | 23.4 | 42.7 | <0.001 |
| Hospital based | 16.0 | 14.8 | 29.6 | <0.001 |
| Chain affiliated | 61.2 | 63.4 | 36.5 | <0.001 |
| <20 stations | 49.9 | 49.8 | 51.3 | 0.04 |
Continuous variables (with the exception of age and dialysis vintage) represent the mean of measurements recorded during 3-month study periods. DM, diabetes mellitus; CHF, congestive heart failure; AMI, acute myocardial infarction; BMI, body mass index; Hgb, hemoglobin; TSAT, transferrin saturation.
Numbers of patients for whom data were available (all, intravenous, and subcutaneous): DM as the cause of ESRD (62,698, 57,591, and 5107), BMI (61,994, 56,995, and 4999), TSAT (59,082, 54,442, and 4640), serum ferritin (59,890, 55,105, and 4785), iron deficient (57,489, 52,979, and 4510), prescribed iron (62,494, 57,404, and 5090), and serum albumin (62,394, 57,324, and 5070).
Mean TSAT <20% and mean serum ferritin <100 ng/ml.
Bromcresol green/bromcresol purple laboratory methods.
Differences in iv Versus sc Epoetin Doses
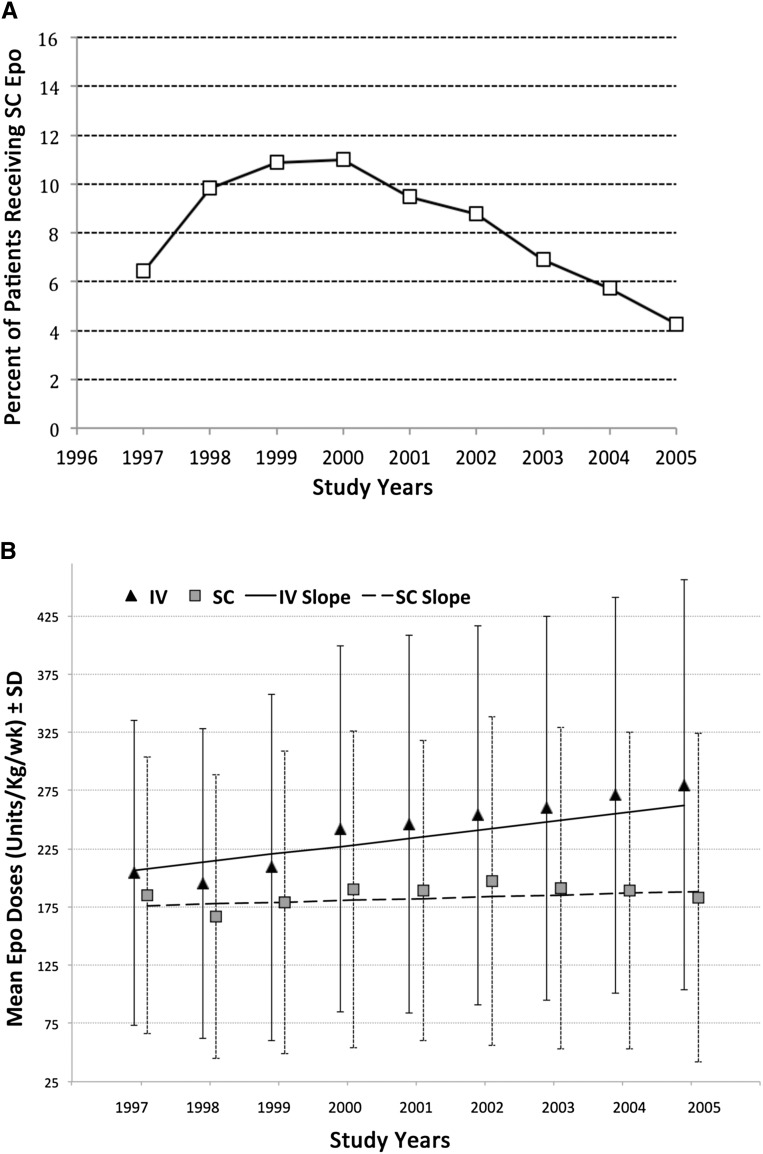
From 1997 to 2000, the use of sc epoetin increased slightly from 6.5% to 11.0% of patients but then declined to 4.3% by 2005 (Figure 1A). For patients managed with sc epoetin, average doses prescribed did not change substantially from 1997 to 2005. However, average iv epoetin doses overall were greater (P<0.001) than those prescribed for sc administration, which was expected (7,8), and they also increased progressively over time (P<0.001) (Figure 1B). Consequently, a greater proportion of patients managed with sc epoetin (51%) received relatively low doses (<150 units/kg per week) than those treated with iv epoetin (40%), and a greater proportion of patients treated with iv epoetin (27%) received high doses (>300 units/kg per week; representing the top quartile of epoetin doses) than patients treated with sc epoetin (17%; P<0.001).
Figure 1.
Route of epoetin (Epo) administration and Epo dosing changed from 1997 to 2005. (A) Percentage of patients on hemodialysis managed with subcutaneous (SC) versus intravenous (IV) Epo from study year 1997 to 2005. (B) Mean doses of Epo (±SD) used to manage study subjects from study year 1997 to 2005. Slopes calculated by linear regression indicate that the mean doses of IV epoetin increased by 7.0 units/kg per week per year (P<0.001), whereas mean doses of SC epoetin increased by 1.5 units/kg per week per year (P=0.06).
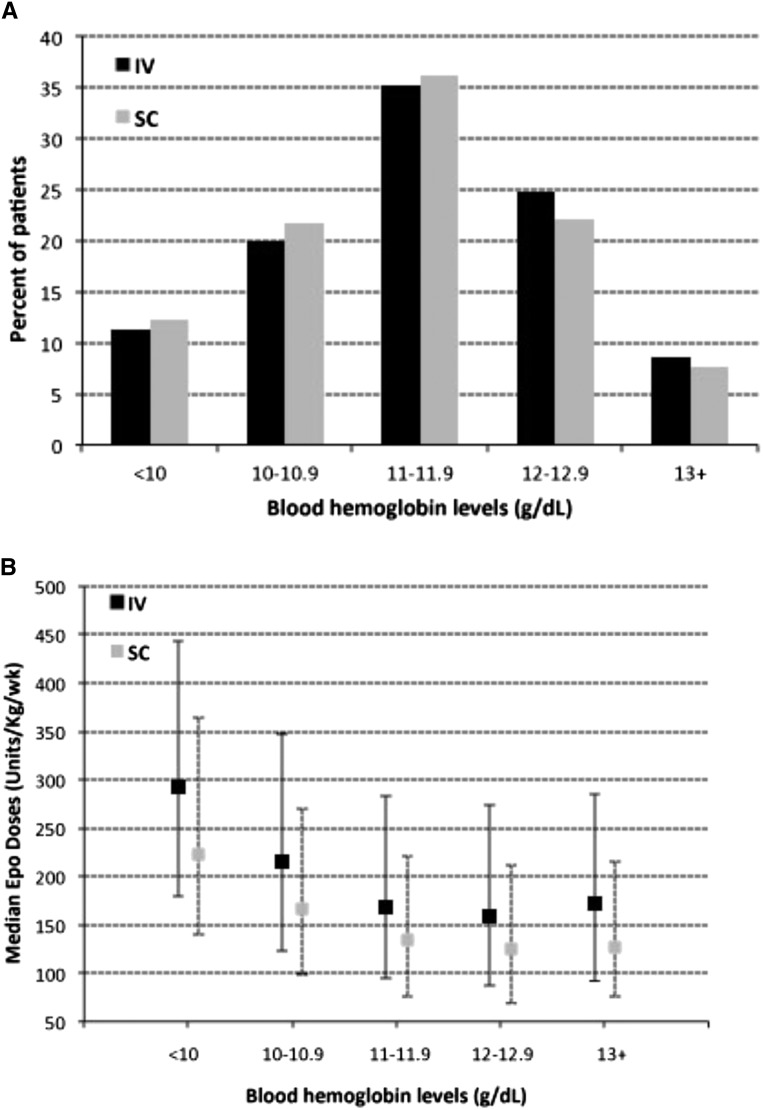
Average blood hemoglobin levels maintained in patients managed with sc versus iv epoetin were nearly identical (11.37±1.22 versus 11.45±1.23 g/dl; mean ± SD), and proportions of patients with blood hemoglobin levels maintained at each of varying levels from <10 to ≥13 g/dl were very similar for the iv and sc epoetin cohorts (Figure 2A). However, median epoetin doses (as well as the 25% and 75% quartile doses) at all levels of hemoglobin response were substantially lower (by 20%–28%) when epoetin was administered sc rather than iv (P<0.001) (Figure 2B).
Figure 2.
Proportions of patients managed with intravenous (IV) or subcutaneous (SC) epoetin (Epo), with blood hemoglobin levels maintained at varying levels, were very similar, but median doses of Epo used to achieve these hemoglobin levels were different. (A) Percentage of patients managed with SC or IV Epo whose blood hemoglobin levels were maintained at varying levels from <10 to ≥13 g/dl. Overall distributions of patients at varying hemoglobin levels were very similar but statistically different (P<0.01) for the cohorts managed with IV versus SC epoetin. (B) Medians and 25th and 75th percentiles (indicated by lines) of Epo doses (units per kilogram per week) used in patients managed with IV or SC Epo to achieve blood hemoglobin levels of varying levels from <10 to ≥13 g/dl. Median doses are lower for SC compared with IV within each hemoglobin category by 20%–28% (P<0.001 by the Wilcoxon rank–sum test).
Association of Epoetin Dose and Route of Administration with Adverse Clinical Outcomes
Study cohorts were compared with respect to incidence of adverse composite events, which as defined in the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) Study (23,24), included death and/or hospitalization for CHF, AMI, or CVA. High risks of cardiovascular events and death in patients with ESRD are well documented (29–36) and evident in this study. Overall, 28% of 62,710 study participants experienced an adverse composite event within 1 year of follow-up, and 40% experienced an adverse composite event within 2 years. Death was the most frequent adverse event (78% of all events occurring within 2 years and the only event in 58% of patients). Also, as observed in the CHOIR Study (23,24), CHF leading to hospitalization was more frequent (23%) than AMI (8%) or CVA (11%) as an initial adverse event.
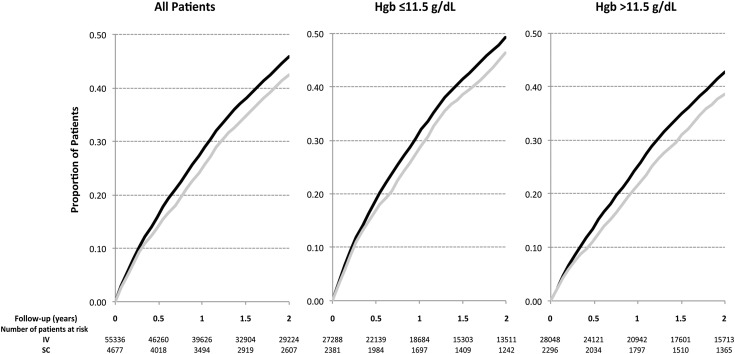
Kaplan–Meier life table analysis showed that the cumulative rate of adverse composite events was significantly greater overall for patients managed with iv versus sc epoetin (Figure 3A, P<0.001) as well as patient subgroups with hemoglobin levels either above or below the median of 11.5 g/dl (Figure 3, B, P<0.001 and C, P<0.001), representing patients who were relatively epoetin sensitive or resistant, respectively. Cumulative adverse event rates (Supplemental Figure 1) were also significantly greater for patients managed with iv versus sc epoetin at relatively low doses of epoetin (<150 units/kg per week; P=0.02) and intermediate doses (150–299 units/kg per week; P=0.01) but not those treated with the highest doses (≥300 units/kg per week; P=0.44).
Figure 3.
Cumulative adverse event rates are greater for patients treated with intravenous (IV) epoetin (Epo) versus subcutaneous (SC) Epo. Kaplan–Meier life table analysis of time to an adverse composite event (death or hospitalization for congestive heart failure, acute myocardial infarcation, or cerebrovascular accident) during 2 years of follow up for patients treated with IV (black lines) versus SC Epo (gray lines). (A) All patients (n=60,013; P<0.001). (B) Hemoglobin (Hgb) ≤11.5 g/dl (n=29,669; P<0.001). (C) Hgb>11.5 g/dl (n=30,344; P<0.001).
Multivariate Cox proportional hazard modeling adjusted for both patient characteristics known to affect clinical outcomes (e.g., nonblack race, older age, diabetes as the cause of ESRD, and low serum albumin [27,29,30]) and various dialysis facility characteristics (e.g., nonprofit versus for profit, etc.) also indicated that the risks of adverse events were higher for patients treated with iv versus sc epoetin (Table 2, all subjects). Adjusted hazard ratios (AHR) were highest and significantly elevated (95% confidence interval [95% CI], 1.01 to 1.26) for patients treated with low and intermediate doses of epoetin (<300 units/kg per week) representing approximately 75% of the study population (Table 2, AHR for iv versus sc epoetin by dose level) and for patients with hemoglobin levels maintained above 11.5 g/dl who were relatively epoetin sensitive (Table 2, AHR for iv versus sc epoetin by hemoglobin level); however, AHRs (iv versus sc epoetin) were elevated for all subgroups (AHR, 1.06 to 1.15) but not statistically different between epoetin dose and hemoglobin subgroups (P=0.38 and P=0.40, respectively). Higher risks of adverse events associated with iv versus sc epoetin were observed in ESRD networks that used sc epoetin both least frequently (<4% of patients; hazard ratio, 1.19; 95% confidence interval [95% CI], 1.04 to 1.36; n=16,476) and most frequently (>12% of patients; hazard ratio, 1.12; 95% CI, 1.03 to 1.23; n=9305). Moreover, higher adverse event rates and elevated AHRs for these events were observed consistently with iv versus sc epoetin independent of patient age, history of a prior event, albumin level, hemoglobin response, and the type of dialysis facility managing care (Supplemental Table 1).
Table 2.
Multivariate Cox proportional hazard model analysis of risk for 12- and 24-month composite events (hospitalization caused by acute myocardial infarction, congestive heart failure, or stroke and/or death during the 12-or 14-month follow–up periods)
| Demographic and Clinical Variables of Study Patients | 12-mo Composite Events AHR (95% CI)d | 24-mo Composite Events AHR (95% CI)d |
|---|---|---|
| All subjects | ||
| Intravenous versus subcutaneous epoetin | 1.11 (1.04 to 1.18) | 1.09 (1.04 to 1.14) |
| Men versus women | 1.03 (1.00 to 1.07) | 1.03 (1.01 to 1.06) |
| Black versus nonblack race | 0.85 (0.82 to 0.88) | 0.86 (0.83 to 0.88) |
| Hispanic versus non-Hispanic | 0.91 (0.86 to 0.95) | 0.89 (0.85 to 0.92) |
| Age, yra | 1.03 (1.02 to 1.03) | 1.03 (1.03 to 1.03) |
| Dialysis vintage, yrb | 1.01 (1.00 to 1.01) | 1.01 (1.00 to 1.01) |
| Diabetes mellitus as the cause of ESRD | 1.21 (1.18 to 1.25) | 1.26 (1.22 to 1.29) |
| Previous AMI, CHF, or stroke | 1.89 (1.83 to 1.96) | 1.78 (1.73 to 1.82) |
| BMI≥25 versus <25 | 0.82 (0.79 to 0.84) | 0.85 (0.82 to 0.87) |
| Hemoglobin ≤11.5 g/dl | 1.21 (1.18 to 1.25) | 1.17 (1.14 to 1.20) |
| Serum albumin ≥3.5/3.2 g/dlc | 0.65 (0.63 to 0.67) | 0.69 (0.67 to 0.71) |
| Epoetin dose 150–299 versus <150 units/kg per week | 1.18 (1.14 to 1.23) | 1.15 (1.12 to 1.19) |
| Epoetin dose ≥300 versus <150 units/kg per week | 1.51 (1.46 to 1.57) | 1.44 (1.40 to 1.49) |
| Facility: nonprofit | 0.93 (0.89 to 0.98) | 0.93 (0.89 to 0.96) |
| Facility: hospital based | 0.87 (0.82 to 0.92) | 0.87 (0.83 to 0.92) |
| Facility: chain affiliated | 1.04 (1.00 to 1.07) | 0.99 (0.96 to 1.02) |
| Facility: <20 stations | 1.00 (0.97 to 1.03) | 1.01 (0.98 to 1.03) |
| AHR for intravenous versus subcutaneous epoetin by dose level | ||
| Intravenous versus subcutaneous (<150 units/kg per week) | 1.12 (1.02 to 1.22) | 1.11 (1.03 to 1.19) |
| Intravenous versus subcutaneous (150–299 units/kg per week) | 1.13 (1.01 to 1.25) | 1.09 (1.00 to 1.18) |
| Intravenous versus subcutaneous (≥300 units/kg per week) | 1.06 (0.94 to 1.20) | 1.04 (0.94 to 1.15) |
| AHR for intravenous versus subcutaneous epoetin by hemoglobin level | ||
| Intravenous versus subcutaneous (hemoglobin ≤11.5 g/dl) | 1.08 (1.00 to 1.17) | 1.06 (1.00 to 1.13) |
| Intravenous versus subcutaneous (hemoglobin >11.5 g/dl) | 1.15 (1.05 to 1.26) | 1.12 (1.05 to 1.20) |
Continuous variables (with the exception of age and dialysis vintage) represent the mean of measurements recorded during 3-month study periods; n=60,013 after exclusion of 2697 with missing data. AHR, adjusted hazard ratio; 95% CI, 95% confidence interval; AMI, acute myocardial infarction; CHF, congestive heart failure; BMI, body mass index.
As of entry into the Clinical Performance Measures Project survey.
As of December 31st of the study period year.
Bromcresol green/bromcresol purple laboratory methods.
Stratified by network and grouped according to the frequency of use of subcutaneous epoetin.
Concerns about patients with rare drug–associated PRCA have led to recommendations that patients on hemodialysis be treated with iv rather than sc epoetin (17,19,21). We, therefore, examined CMS records for occurrences of PRCA among 62,710 study participants within 5 years after their entry into the ESRD CPM Project. Before 2008, no specific ICD-9 code was defined for PRCA; however, this diagnosis was included among other forms of acquired aplastic anemia (284.8). We, therefore, screened records for this ICD-9 code and then used other diagnosis, procedure, and treatment codes to detect possible cases of PRCA, using the criteria described by Collins et al. (28) (i.e., records of bone marrow examination, persistent anemia, and repeated red cell transfusions but not other causes of marrow failure). Of 57,602 study patients treated with iv epoetin, 92 (0.16%) had a 284.8 code recorded at some time within 5 years of entry into the CPM Project compared with 3 (0.06%) of 5108 patients treated with sc epoetin. However, of all patients, during 201,655 patient-years of follow-up, only 1 patient with possible PRCA (treated with iv epoetin) fulfilled the criteria of Collins et al. (28).
Discussion
This retrospective cohort study of >60,000 adult patients on hemodialysis confirms that higher doses of epoetin-α are required to achieve equivalent hemoglobin responses in patients with kidney failure when administered iv rather than sc. This study also finds that risks of early death and/or hospitalization for cardiovascular complications were significantly greater for patients managed with iv versus sc epoetin. Moreover, higher adverse event rates and significantly elevated AHRs for adverse events were observed consistently in association with iv versus sc epoetin administration for patients independent of age, history of a prior adverse event, albumin level, hemoglobin response, and type of dialysis facility managing care. Because patients treated with iv epoetin consistently required higher doses to achieve equivalent hemoglobin responses than those given sc epoetin, these findings support the conclusion that epoetin exposure per se affected clinical outcomes in a dose-dependent manner, as was indicated by a secondary analysis of the CHOIR Study (24).
Pharmacokinetic studies of iv and sc epoetin administration in healthy volunteers and patients with ESRD (9–11) have shown that peak epoetin blood levels attained after sc injection are substantially lower than those after iv infusion of identical doses, and it is possible that increased risks of adverse events associated with epoetin treatment occur primarily when epoetin blood levels exceed a certain threshold. Given the distinct pharmacokinetics of iv versus sc epoetin (9), lower doses of epoetin would be more likely to exceed such a threshold when administered iv rather than sc. However, because epoetin has greater erythropoietic bioactivity when given sc rather than iv, patients treated with high doses of sc epoetin (>300 units/kg per week) likely included a greater proportion of patients who were drug resistant than those who received the same doses iv. Because epoetin resistance is per se a surrogate risk factor for adverse clinical outcomes, this may explain why higher risks of adverse clinical outcomes with iv versus sc epoetin were less evident at doses >300 units/kg per week (Table 2, AHR for iv versus sc epoetin by dose level, Supplemental Figure 1).
There is long-standing evidence that expression of erythropoietin receptors is not restricted to erythroid precursors in the marrow but is also detectable in nonerythroid tissues, including neurons, adipocytes, and vascular endothelium (37). There is also evidence that erythropoietin affects vascular endothelial function and influences angiogenesis (38–42). Hence, nonerythropoietic effects of epoetin, particularly at high pharmacologic blood levels, could have a role in promoting adverse clinical events associated with epoetin treatment in patients with kidney disease.
Doses of epoetin and other erythropoiesis–stimulating agents (ESAs) used to manage patients with kidney disease in the United States have declined in recent years (USRDS 2013 information) (12,43,44). Evidence that aggressive ESA treatment regimens may be hazardous for these patients, reported since 2006, has likely prompted this change. However, financial considerations may also have had an influence. Medicare, which pays for most costs of hemodialysis care in the United States, discontinued separate reimbursements for ESA treatment on a drug cost basis in 2011 (12,43,44), including them instead in bundled payment formulae for dialysis care, thereby removing financial disincentives to limit ESA use. We were unable to determine whether the use of iv versus sc epoetin at dialysis centers has also changed in recent years, because information about the route of epoetin administration became unavailable through the CMS after 2006. However, the new Consolidated Renal Operations in a Web-enabled Network data reporting system for tracking ESRD management (http://projectcrownweb.org/assets/release_notes/Kidney_Data_Dictionary_Search.html) should provide this information in the future.
The choice of iv versus sc epoetin to treat patients on hemodialysis in the United States has been influenced by concerns about the immunogenic potential of epoetin and patients with rare cases of drug-associated PRCA. These concerns were cited as a basis for changes in NKF DOQI guidelines in 2006 and led to recommendations by Amgen and the Food and Drug Administration in 2005 and 2006 (17,19,21,45) that iv rather than sc epoetin be used to treat patients on hemodialysis, despite the fact that patients with rare cases of PRCA were observed primarily outside the United States and associated with Eprex, a form of epoetin not marketed in the United States (17–19). Although cases of PRCA have been documented in individuals treated with Epogen and Procrit (epoetin-α used in the United States), these cases have been extremely rare (incidence estimated at 0.2/100,000 patient-years) (18) and not exclusively associated with sc administration (18,19), consistent with our finding of only one possible patient with PRCA per 201,655 patient-years (associated with iv epoetin).
Several different ESAs are now available in the United States to treat anemia of ESRD (46). However, epoetin-α (Epogen) continues to be used widely in the United States. Although doses of epoetin and other ESAs used in hemodialysis management have declined, our study’s findings remain relevant to current practice, because observed differences in clinical outcomes associated with iv versus sc epoetin treatment were found to be most evident at lower dose levels. In conclusion, this study provides evidence that routine use of sc rather than iv epoetin to manage anemia in patients on hemodialysis could enhance survival and reduce hospitalizations for cardiovascular complications by minimizing epoetin dosing. Assuming that findings from this study accurately represent all patients with ESRD in the United States during the years 1997–2005, it can be estimated (47) that nearly 30,000 early deaths and/or hospitalizations for cardiovascular complications (approximately 3300 per year) might have been avoided had all patients been managed with sc epoetin. (This estimate is made on the basis of a number needed to treat of 27, calculated from observed adverse composite event rates within 12 months of 0.282 for patients treated with iv epoetin versus 0.249 for patients treated with sc epoetin, an AHR of 1.11 for adverse events [iv versus sc], and a total of 872,702 new patients on hemodialysis [USRDS] from 1997 to 2005, of whom 92% [802,886] were treated with iv epoetin.) However, because this is a retrospective, observational study, conclusions drawn from it must be qualified, because unmeasured hemodialysis facility and patient management variables not accounted for by multivariate analysis may have affected the results.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Diane Frankenfield for her contributions to the initial data analysis that led to the study before her retirement from the Office of Research, Development, and Information at the Centers for Medicare and Medicaid Services.
This study was supported by intramural programs of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01590215/-/DCSupplemental.
References
- 1.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, Korbet S, Krantz SB, Lundin AP, Nissenson AR, Ogden DA, Paganini EP, Rader B, Rutsky EA, Stivelman J, Stone WJ, Teschan P, Van Stone JC, Van Wyck DB, Kenneth Zuckerman K, Adamson JW: Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 111: 992–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW: Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med 321: 158–163, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Valderrábano F: Erythropoietin in chronic renal failure. Kidney Int 50: 1373–1391, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Jones M, Ibels L, Schenkel B, Zagari M: Impact of epoetin alfa on clinical end points in patients with chronic renal failure: A meta-analysis. Kidney Int 65: 757–767, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J: The effect of anemia treatment on selected health-related quality-of-life domains: A systematic review. Clin Ther 25: 1786–1805, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, Bommer J, Cruz JM, Kerr PG, Mendelssohn DC, Held PJ, Port FK: Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44: 94–111, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Kaufman JS, Reda DJ, Fye CL, Goldfarb DS, Henderson WG, Kleinman JG, Vaamonde CA, Department of Veterans Affairs Cooperative Study Group on Erythropoietin in Hemodialysis Patients : Subcutaneous compared with intravenous epoetin in patients receiving hemodialysis. N Engl J Med 339: 578–583, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Besarab A, Reyes CM, Hornberger J: Meta-analysis of subcutaneous versus intravenous epoetin in maintenance treatment of anemia in hemodialysis patients. Am J Kidney Dis 40: 439–446, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Brockmöller J, Köchling J, Weber W, Looby M, Roots I, Neumayer HH: The pharmacokinetics and pharmacodynamics of recombinant human erythropoietin in haemodialysis patients. Br J Clin Pharmacol 34: 499–508, 1992 [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon FG, Vargas R, Ryan M, Jain AK, Abels RI, Perry B, Smith IL: Pharmacokinetics and effects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood 76: 1718–1722, 1990 [PubMed] [Google Scholar]
- 11.Besarab A: Physiological and pharmacodynamic considerations for route of EPO administration. Semin Nephrol 20: 364–374, 2000 [PubMed] [Google Scholar]
- 12.USRDS : 2013 USRDS Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis 63[Suppl]: e1–e478, 2014 [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation : NFK-DOQI clinical practice guidelines for the treatment of anemia of chronic renal failure. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis 30[4 Suppl 3]: S192–S240, 1997 [PubMed] [Google Scholar]
- 14.National Kidney Foundation : IV. NKF-K/DOQI clinical practice guidelines for anemia of chronic kidney disease: Update 2000. Am J Kidney Dis 37[Suppl 1]: S182–S238, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hynes DM, Stroupe KT, Greer JW, Reda DJ, Frankenfield DL, Kaufman JS, Henderson WG, Owen WF, Rocco MV, Wish JB, Kang J, Feussner JR: Potential cost savings of erythropoietin administration in end-stage renal disease. Am J Med 112: 169–175, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Department of Veterans Affairs : FY97 Veterans Affairs Dialysis Case Mix Study 1998, Milwaukee, WI, National Center for Cost Containment, 1998 [Google Scholar]
- 17.Hynes DM, Stroupe KT, Kaufman JS, Reda DJ, Peterman A, Browning MM, Huo Z, Sorbara D: Adherence to guidelines for ESRD anemia management. Am J Kidney Dis 47: 455–461, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, McKoy JM, Kim B, Lyons EA, Trifilio SM, Raisch DW, Evens AM, Kuzel TM, Schumock GT, Belknap SM, Locatelli F, Rossert J, Casadevall N: Pure red-cell aplasia and epoetin therapy. N Engl J Med 351: 1403–1408, 2004 [DOI] [PubMed] [Google Scholar]
- 19.McKoy JM, Stonecash RE, Cournoyer D, Rossert J, Nissenson AR, Raisch DW, Casadevall N, Bennett CL: Epoetin-associated pure red cell aplasia: Past, present, and future considerations. Transfusion 48: 1754–1762, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershon SK, Luksenburg H, Coté TR, Braun MM: Pure red-cell aplasia and recombinant erythropoietin. N Engl J Med 346: 1584–1586, 2002 [DOI] [PubMed] [Google Scholar]
- 21.KDOQI. National Kidney Foundation : II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis 47[5 Suppl 3]: S16–S85, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Thamer M, Zhang Y, Kaufman J, Stefanik K, Cotter DJ: Factors influencing route of administration for epoetin treatment among hemodialysis patients in the United States. Am J Kidney Dis 48: 77–87, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G, McGee R, Nicolucci A, Tognoni G, Strippoli GF: Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 153: 23–33, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Frankenfield DL, Rocco MV, Roman SH, McClellan WM: Survival advantage for adult Hispanic hemodialysis patients? Findings from the end-stage renal disease clinical performance measures project. J Am Soc Nephrol 14: 180–186, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Collins AJ, Li S, Adamson JW, Gilbertson DT: Assessment of pure red cell aplasia in US dialysis patients: The limits of the Medicare data. Am J Kidney Dis 43: 464–470, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: Causes of death in dialysis patients: Racial and gender differences. J Am Soc Nephrol 5: 1231–1242, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Price DA, Owen WF, Jr.: African-Americans on maintenance dialysis: A review of racial differences in incidence, treatment, and survival. Adv Ren Replace Ther 4: 3–12, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Owen WF, Jr., Chertow GM, Lazarus JM, Lowrie EG: Dose of hemodialysis and survival: Differences by race and sex. JAMA 280: 1764–1768, 1998 [DOI] [PubMed] [Google Scholar]
- 32.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS, HEMO Study Group : Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Casserly LF, Reddy SM, Dember LM: Venous thromboembolism in end-stage renal disease. Am J Kidney Dis 36: 405–411, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Lindner A, Charra B, Sherrard DJ, Scribner BH: Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med 290: 697–701, 1974 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Thamer M, Cotter D, Kaufman J, Hernán MA: Estimated effect of epoetin dosage on survival among elderly hemodialysis patients in the United States. Clin J Am Soc Nephrol 4: 638–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arcasoy MO: The non-haematopoietic biological effects of erythropoietin. Br J Haematol 141: 14–31, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, Kobayashi T, Masuda S, Nagao M, Yoshimura N, Takagi H: Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med 353: 782–792, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT: Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A 91: 3974–3978, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bahlmann FH, De Groot K, Spandau JM, Landry AL, Hertel B, Duckert T, Boehm SM, Menne J, Haller H, Fliser D: Erythropoietin regulates endothelial progenitor cells. Blood 103: 921–926, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Beleslin-Cokic BB, Cokic VP, Yu X, Weksler BB, Schechter AN, Noguchi CT: Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104: 2073–2080, 2004 [DOI] [PubMed] [Google Scholar]
- 42.George J, Goldstein E, Abashidze A, Wexler D, Hamed S, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A: Erythropoietin promotes endothelial progenitor cell proliferative and adhesive properties in a PI 3-kinase-dependent manner. Cardiovasc Res 68: 299–306, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Miskulin DC, Zhou J, Tangri N, Bandeen-Roche K, Cook C, Ephraim PL, Crews DC, Scialla JJ, Sozio SM, Shafi T, Jaar BG, Boulware LE, DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators : Trends in anemia management in US hemodialysis patients 2004-2010. BMC Nephrol 14: 264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunelli SM, Monda KL, Burkart JM, Gitlin M, Neumann PJ, Park GS, Symonian-Silver M, Yue S, Bradbury BD, Rubin RJ: Early trends from the Study to Evaluate the Prospective Payment System Impact on Small Dialysis Organizations (STEPPS). Am J Kidney Dis 61: 947–956, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Brenner R: Amgen letter to Health Care Professionals Available at: http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM164481.pdf. Accessed
- 46.Drüeke TB: Anemia treatment in patients with chronic kidney disease. N Engl J Med 368: 387–389, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Altman DG, Andersen PK: Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 319: 1492–1495, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.