Abstract
Background and objectives
The prevalence of ESRD among Hispanics/Latinos is 2-fold higher than in non-Hispanic whites. However, little is known about the prevalence of earlier stages of CKD among Hispanics/Latinos. This study estimated the prevalence of CKD in US Hispanics/Latinos.
Design, setting, participants, & measurements
This was a cross-sectional study of 15,161 US Hispanic/Latino adults of Cuban, Dominican, Mexican, Puerto Rican, Central American, and South American backgrounds enrolled in the multicenter, prospective, population-based Hispanic Community Health Study/Study of Latinos (HCHS/SOL). In addition, the prevalence of CKD in Hispanics/Latinos was compared with other racial/ethnic groups in the 2007–2010 National Health and Nutrition Examination Survey (NHANES). Prevalent CKD was defined as an eGFR <60 ml/min per 1.73 m2 (estimated with the 2012 Chronic Kidney Disease Epidemiology Collaboration eGFR creatinine-cystatin C equation) or albuminuria based on sex-specific cut points determined at a single point in time.
Results
The overall prevalence of CKD among Hispanics/Latinos was 13.7%. Among women, the prevalence of CKD was 13.0%, and it was lowest in persons with South American background (7.4%) and highest (16.6%) in persons with Puerto Rican background. In men, the prevalence of CKD was 15.3%, and it was lowest (11.2%) in persons with South American background and highest in those who identified their Hispanic background as “other” (16.0%). The overall prevalence of CKD was similar in HCHS/SOL compared with non-Hispanic whites in NHANES. However, prevalence was higher in HCHS/SOL men and lower in HCHS/SOL women versus NHANES non-Hispanic whites. Low income, diabetes mellitus, hypertension, and cardiovascular disease were each significantly associated with higher risk of CKD.
Conclusions
Among US Hispanic/Latino adults, there was significant variation in CKD prevalence among Hispanic/Latino background groups, and CKD was associated with established cardiovascular risk factors.
Keywords: CKD, prevalence, Hispanics
Introduction
Hispanics/Latinos are the largest minority group in the United States and this population is projected to become one-third of the US population by 2060 (1). In addition, Hispanics/Latinos are culturally, socioeconomically, and genetically heterogeneous and represent a wide variety of national origins (2). CKD is a major health problem in the United States and recent evidence suggests that Hispanics/Latinos are disproportionately affected. Over 90,000 US Hispanics/Latinos with ESRD were treated by hemodialysis in 2011, and the rate of incident ESRD among Hispanics/Latinos is 50% greater than in non-Hispanics (3). However, little is known about the prevalence of earlier stages of CKD. Estimates of the prevalence of CKD in the United States are principally derived from the National Health and Nutrition Examination Survey (NHANES). Because NHANES studied mainly Mexican Americans (one-half of which were US born), our understanding of differences in CKD prevalence among major Hispanic/Latino background groups is incomplete. We studied the prevalence and risk factors associated with CKD in a group of Hispanic Americans with a broad range of ethnic backgrounds who were enrolled in a community-based cohort representative of the US Hispanic/Latino population.
Materials and Methods
Study Participants
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a population-based cohort of 16,415 Hispanics/Latinos aged 18–74 years from randomly selected households in four US field centers (Chicago, Illinois; Miami, Florida; Bronx, New York; and San Diego, California) with baseline examination (2008–2011) and yearly telephone follow-up assessment. Participants self-reported their background as Cuban, Dominican, Mexican, Puerto Rican, or Central or South American. The category “other” was used for participants belonging to a group not listed or to more than one group. The sample design and cohort selection were previously described (4,5). Briefly, a stratified two-stage area probability sample of household addresses was selected in each field center. The first sampling stage randomly selected census block groups with stratification based on Hispanic/Latino concentration and proportion of high/low socioeconomic status. The second sampling stage randomly selected households, with stratification, from US Postal Service registries that covered the randomly selected census block groups. Finally, the study oversampled the group aged 45–74 years (n=9714, 59.2%) to facilitate examination of target outcomes. Sampling weights were generated to reflect the probabilities of selection at each stage. Of 39,384 individuals who were screened and selected and who met eligibility criteria, 41.7% were enrolled, representing 16,415 persons from 9872 households. This study adheres to the Declaration of Helsinki and was approved by the institutional review boards at each field center where all participants gave written consent.
Data Collection and Variable Definition
The baseline study examination included clinical measurements, questionnaires, and fasting venous blood and urine specimens. Demographic factors, socioeconomic status, acculturation, cigarette smoking, and medical history were obtained using standard questionnaires. Medication use was ascertained by conducting an inventory of all currently used medications. BPs were defined as the average of the second and third of three repeat seated measurements obtained after a 5-minute rest. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertensive medication. Diabetes mellitus was defined as fasting plasma glucose of ≥126 mg/dl, 2-hour postload glucose levels of ≥200 mg/dl, a hemoglobin A1c level of ≥6.5%, or use of antidiabetes medication. The presence of cardiovascular disease was self-reported. CKD awareness was ascertained by asking the following question: “Has a doctor ever said that you have kidney problems?”
Kidney Function Measurements
Creatinine was measured in serum and urine on a Roche Modular P Chemistry Analyzer using a creatinase enzymatic method (Roche Diagnostics, Indianapolis, IN). Serum creatinine measurements are isotope dilution mass spectrometry traceable. Urine albumin was measured using an immunoturbidimetric method on the ProSpec nephelometric analyzer (Dade Behring GmbH, Marburg, Germany). Serum cystatin C was measured using a turbidimetric method on the Roche Modular P Chemistry Analyzer (Gentian AS, Moss, Norway). We estimated GFR using three different equations, which were developed from pooling of 5352 participants from 13 studies: (1) Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine, (2) CKD-EPI cystatin C, and (3) CKD-EPI creatinine-cystatin C (eGFRcreat-cyst) (6). CKD was defined by either a low eGFR (<60 ml/min per 1.73 m2) or the presence of albuminuria based on spot urine samples using sex-specific cutoffs (urine albumin/creatinine ratio ≥17 mg/g in men and ≥25 mg/g in women) (7).
Statistical Analyses
Summary statistics, prevalence estimates, and odds ratios (ORs) were weighted to adjust for sampling probability and nonresponse as previously described (4,5). All analyses account for cluster sampling and the use of stratification in sample selection. Low eGFR, albuminuria, and CKD prevalence were computed by sex and for all participants by Hispanic/Latino background with and without adjustment for age. Survey-specific procedures were used to compute 95% confidence intervals (95% CIs) to account for the two-stage sampling design, stratification, and clustering. Comparisons across Hispanic/Latino groups were performed using the overall Wald test. Multivariable survey logistic regression analyses were used to examine associations of sociodemographic and clinical factors with prevalence of low eGFRcreat-cyst, albuminuria, and CKD. ORs with 95% CIs were computed. All statistical tests were two sided at a significance level of 0.05. No adjustments were made for multiple comparisons. All analyses were performed using SAS software (version 9.2; SAS Institute). Additional analyses were conducted to estimate the prevalence of low eGFR (using the CKD-EPI creatinine equation given that cystatin C was not available in 2007–2010 NHANES participants), albuminuria, and CKD among Mexican Americans (n=2007), non-Hispanic whites (n=4507), and non-Hispanic blacks (n=1938) in the 2007–2010 NHANES. We followed recommendations from the National Center for Health Statistics to account for stratification and clustering of the survey design, as well as oversampling of ethnic minorities and elderly persons (8). Age-adjusted estimates were calculated using the mean age of participants in HCHS/SOL and NHANES (the mean age for HCHS/SOL participants and NHANES participants was 41 years).
Results
Of the 16,415 HCHS/SOL participants, 15,161 (92.4%) were included in analyses to estimate prevalence of low eGFR, albuminuria, and CKD (9032 women and 6129 men); 1001 participants (6.1%) were excluded because of missing data on serum creatinine (n=168), serum cystatin C (n=116), or urine albumin (n=717), and 253 participants were excluded due to missing data regarding race or Hispanic/Latino background group. In addition, 1114 participants were excluded from regression analyses as a result of missing covariate data. Compared with individuals included in the study, those excluded due to missing data were of similar age (mean 41 years), sex (women: 49.9% versus 52.5%, P=0.12), and Hispanic/Latino background. The prevalence of CKD and albuminuria was higher among excluded compared with included participants (22.7% versus 13.4% and 16.1% versus 12.1%, respectively, P<0.001 for each comparison).
Participant Characteristics
The mean age was 41 years (Table 1). Compared with persons without CKD, individuals with CKD were more likely to be older (48 versus 40 years, P<0.001), have annual household income <$20,000 (48% versus 41%, P<0.001), attain less than a high school education (39% versus 31%, P<0.001), and have health insurance (60% versus 49%, P<0.001). Participants with CKD were also more likely to be obese (50% versus 38%, P<0.001), to have diabetes (38% versus 11%, P<0.001), and to have BP >140/90 mmHg (30% versus 8%, P<0.001). Participants with low eGFR had significantly more albuminuria.
Table 1.
Clinical and demographic characteristics of study participants by CKD status
| Characteristic | Overall | CKD | No CKD | P Value | Albuminuria | No Albuminuria | P Value | eGFRcreat-cyst | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| <60 | ≥60 | |||||||||
| Number of participants | 14,035 | 2112 | 11,923 | 1900 | 12,135 | 386 | 13,649 | |||
| Age, yr | 41.1 (40.6 to 41.6) | 48.2 (47.0 to 49.4) | 40.0 (39.5 to 40.5) | <0.001 | 46.6 (45.4 to 47.9) | 40.3 (39.8 to 40.8) | <0.001 | 62.5 (61.1 to 63.9) | 40.6 (40.1 to 41.1) | <0.001 |
| Women | 52.5 | 50.9 | 52.7 | 0.29 | 50.2 | 52.8 | 0.14 | 48.3 | 52.6 | 0.23 |
| ≥10 yr in United States | 72.3 | 77.4 | 71.5 | <0.001 | 77.4 | 71.6 | <0.001 | 79.3 | 72.1 | 0.06 |
| US born | 23.0 | 19.5 | 23.5 | 0.01 | 20.4 | 23.3 | 0.05 | 12.7 | 23.2 | <0.001 |
| Annual income <$20,000 | 41.8 | 47.5 | 40.9 | <0.001 | 46.6 | 41.1 | 0.003 | 56.9 | 41.4 | <0.001 |
| Less than high school education | 32.2 | 39.4 | 31.1 | <0.001 | 38.5 | 31.3 | <0.001 | 47.6 | 31.8 | <0.001 |
| Health insurance | 50.7 | 59.8 | 49.3 | <0.001 | 57.7 | 49.8 | <0.001 | 78.7 | 50.1 | <0.001 |
| Current smoker | 21.1 | 21.1 | 21.1 | 0.99 | 21.0 | 21.2 | 0.89 | 21.9 | 21.1 | 0.80 |
| Cardiovascular disease | 6.0 | 12.5 | 5.0 | <0.001 | 11.9 | 5.2 | <0.001 | 23.0 | 5.6 | <0.001 |
| Hypertension | 21.8 | 49.2 | 17.6 | <0.001 | 46.9 | 18.4 | <0.001 | 74.2 | 20.6 | <0.001 |
| Systolic BP, mmHg | 119.8 (119.3 to 120.4) | 130.6 (129.2 to 132.1) | 118.2 (117.7 to 118.6) | <0.001 | 130.6 (129.0 to 132.1) | 118.4 (117.9 to 118.8) | <0.001 | 137.1 (134.2 to 140.1) | 119.4 (118.9 to 119.9) | <0.001 |
| BP >130/80 mmHg | 22.0 | 45.0 | 18.4 | <0.001 | 45.1 | 18.8 | <0.001 | 56.2 | 21.2 | <0.001 |
| BP >140/90 mmHg | 11.1 | 30.0 | 8.2 | <0.001 | 30.4 | 8.4 | <0.001 | 37.1 | 10.5 | <0.001 |
| BMI ≥30 kg/m2 | 39.9 | 49.6 | 38.4 | <0.001 | 49.5 | 38.6 | <0.001 | 52.1 | 39.6 | <0.001 |
| Waist circumference, cm | 97.4 (97.0 to 97.8) | 102.0 (100.9 to 103.0) | 96.7 (96.2 to 97.1) | <0.001 | 101.6 (100.5 to 102.8) | 96.8 (96.4 to 97.3) | <0.001 | 105.4 (103.3 to 107.5) | 97.2 (96.8 to 97.6) | <0.001 |
| Diabetes mellitus | 14.6 | 37.7 | 11.0 | <0.001 | 36.6 | 11.6 | <0.001 | 54.1 | 13.7 | <0.001 |
| Hemoglobin A1c, % | 5.7 (5.7 to 5.8) | 6.5 (6.4 to 6.6) | 5.6 (5.6 to 5.6) | <0.001 | 6.5 (6.4 to 6.6) | 5.6 (5.6 to 5.6) | <0.001 | 6.5 (6.3 to 6.7) | 5.7 (5.7 to 5.7) | <0.001 |
| Triglycerides, mg/dl | 114 (79 to 160) | 132 (91 to 187) | 112 (77 to 159) | — | 131 (90 to 187) | 112 (78 to 159) | — | 140 (108 to 188) | 113 (79 to 162) | — |
| Total cholesterol, mg/dl | 193.3 (192.2 to 194.4) | 195.6 (193.2 to 198.0) | 193.0 (191.8 to 194.1) | <0.001 | 195.4 (192.9 to 197.8) | 193.1 (191.9 to 194.2) | <0.001 | 195.5 (189.9 to 201.1) | 193.3 (192.2 to 194.4) | <0.001 |
| LDL cholesterol, mg/dl | 119.7 (118.8 to 120.7) | 119.7 (117.8 to 121.7) | 119.7 (118.7 to 120.8) | <0.001 | 119.5 (117.5 to 121.6) | 119.8 (118.8 to 120.8) | <0.001 | 119.3 (114.3 to 124.2) | 119.8 (118.8 to 120.7) | <0.001 |
| HDL cholesterol, mg/dl | 48.8 (48.5 to 49.2) | 47.6 (46.8 to 48.5) | 49.0 (48.6 to 49.3) | <0.001 | 47.8 (46.9 to 48.7) | 48.9 (48.6 to 49.3) | <0.001 | 45.7 (44.5 to 47.0) | 48.9 (48.5 to 49.2) | <0.001 |
| C-reactive protein, mg/L | 3.9 (3.7 to 4.1) | 5.8 (4.9 to 6.7) | 3.6 (3.4 to 3.8) | <0.001 | 5.8 (4.9 to 6.8) | 3.6 (3.4 to 3.8) | <0.001 | 6.6 (5.4 to 7.9) | 3.8 (3.6 to 4.0) | <0.001 |
| Use of ACEi/ARB | 8.5 | 19.6 | 6.8 | <0.001 | 18.4 | 7.2 | <0.001 | 33.0 | 8.0 | <0.001 |
| CKD awareness | — | 17.8 | — | — | 17.2 | 23.1 | 0.17 | 33.9 | 14.3 | <0.001 |
| Urine ACR ≥300 mg/g | 1.4 | 10.6 | — | — | 11.7 | — | — | 18.6 | 1.0 | <0.001 |
For continuous variables, weighted mean (95% confidence interval) values are presented unless otherwise specified; for categorical variables, weighted proportions are reported. eGFR, ml/min per 1.73 m2; BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotenin receptor blocker; ACR, albumin/creatinine ratio; –, not applicable.
Prevalence of CKD
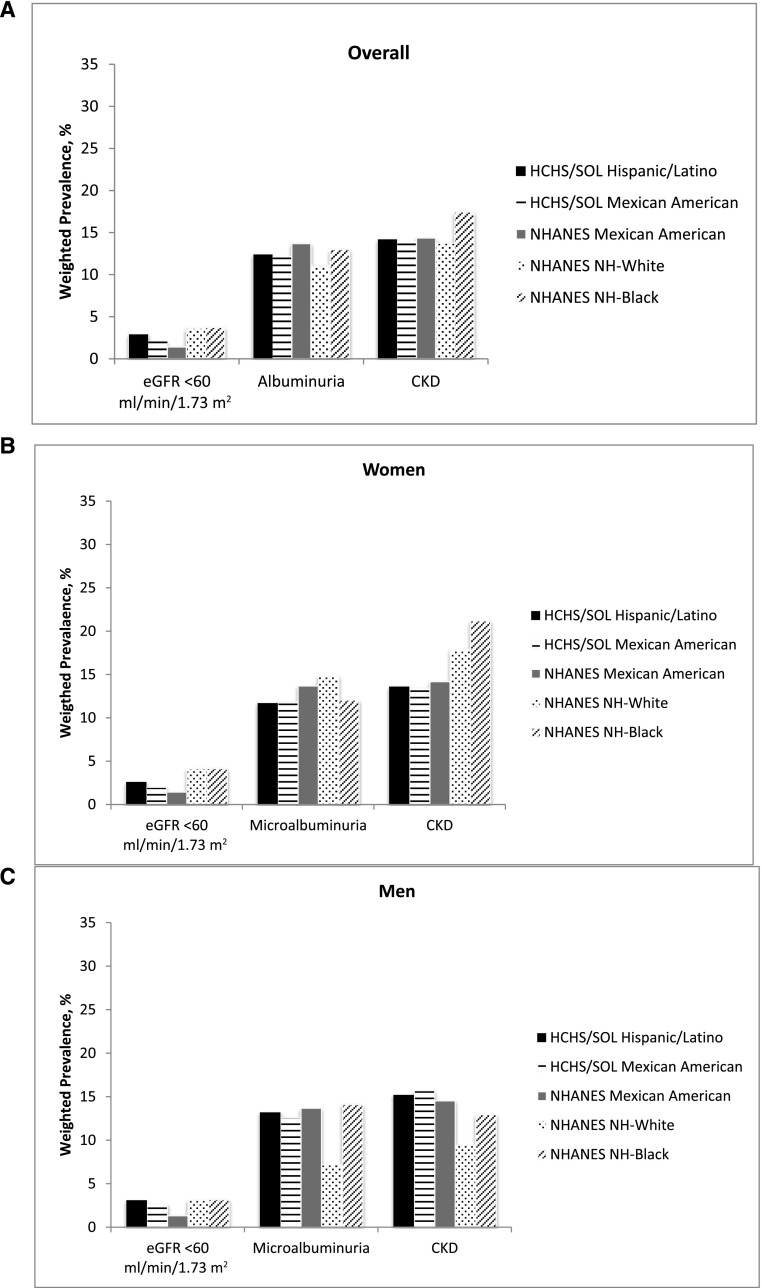
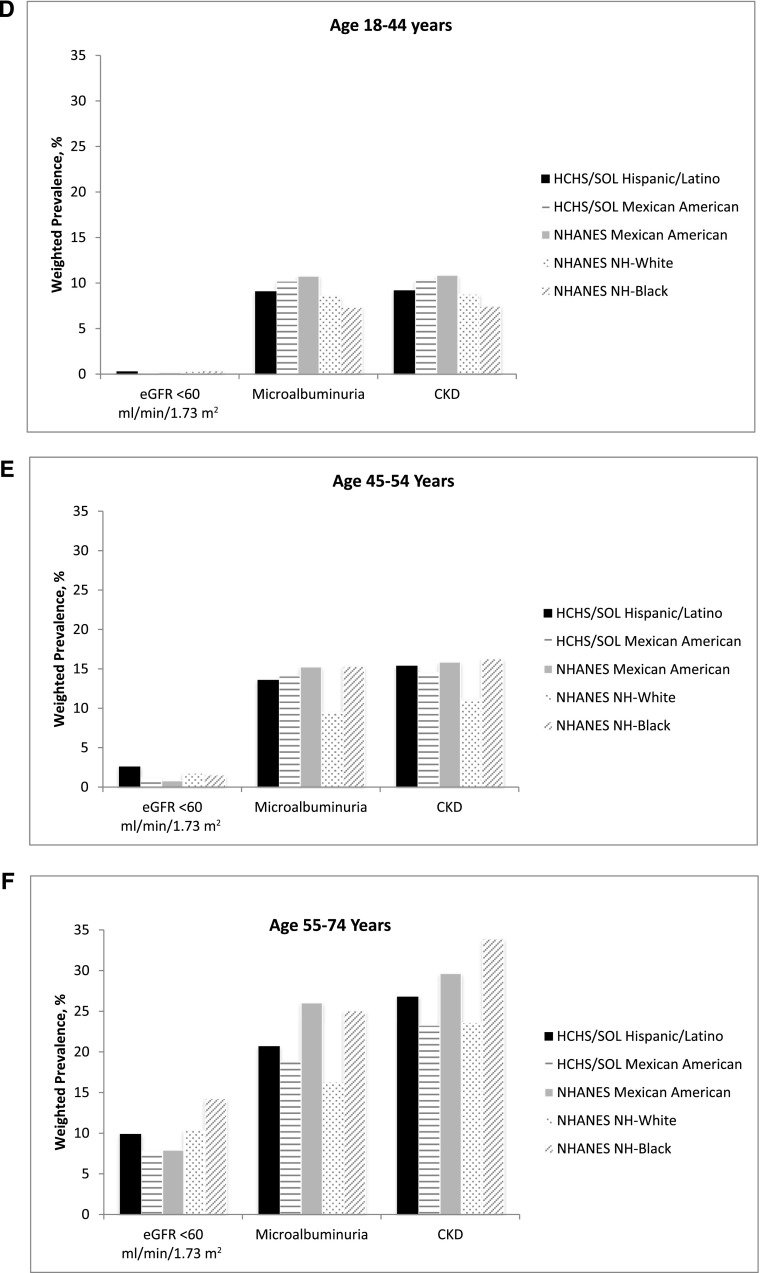
The overall age-adjusted prevalence of CKD was 13.7% (based on the CKD-EPIcreat-cyst estimating equation). Among women, the overall age-adjusted CKD prevalence was 13.0%; prevalence was lowest in persons with South American background (7.4%) and highest in persons with Puerto Rican background (16.6%) (Table 2). In men, the overall age-adjusted prevalence of CKD was 14.8% and was lowest (11.2%) in persons with South American background and highest (16.0%) in those with other Hispanic background (Table 2). CKD awareness was reported by 18% of participants who met our study criteria for CKD and by 34% of participants with eGFR <60 ml/min per 1.73 m2 (Table 1). Overall, based on the CKD-EPI creatinine GFR estimating equation (cystatin C not available in 2007–2010 NHANES participants), the age-adjusted prevalence of CKD in Hispanics/Latinos from HCHS/SOL was 14.2% (95% CI, 13.4 to 15.0) compared with 13.7% (95% CI, 12.6% to 14.8%) in non-Hispanic whites and 17.4% (95% CI, 15.6 to 19.3) in non-Hispanic blacks from the 2007–2010 NHANES (Figure 1A). Among women, the age-adjusted CKD prevalence in Hispanics/Latinos from HCHS/SOL was 13.6% (95% CI, 12.4 to 14.7) compared with 17.9% (95% CI, 16.2 to 19.5) in non-Hispanic whites and 21.2% (95% CI, 18.0 to 24.4) in non-Hispanic blacks from NHANES (Figure 1B). Among men, the age-adjusted prevalence of CKD in Hispanics/Latinos from HCHS/SOL was 15.2% (95% CI, 14.1 to 16.3) compared with 9.4% (95% CI, 8.2 to 10.7) in non-Hispanic whites and 13.0% (95% CI, 10.8 to 15.1) in non-Hispanic blacks from NHANES (Figure 1C). CKD prevalence stratified by age is presented in Figure 1, D–F. Of note, the presence of albuminuria was the main determinant of CKD in participants aged 18–44 years, whereas low eGFR was more common among individuals aged 55–74 years. Mean serum creatinine, cystatin C, eGFR, and age-unadjusted CKD prevalence, as well as the percentage of the US Hispanic/Latino population by eGFR and albuminuria category using the Kidney Disease Improving Global Outcomes 2009 definition and classification of CKD (9) are presented in Supplemental Tables 1–3.
Table 2.
CKD-related parameters by sex and Hispanic background group
| Parameter | All | Central American | Cuban | Dominican | Mexican | Puerto Rican | South American | Other | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Women | |||||||||
| Number of participants | 9032 | 951 | 1155 | 899 | 3707 | 1498 | 574 | 248 | |
| Urine ACR, mg/g | 7.4 (5.2, 13.2) | 7.3 (5.0, 12.9) | 7.3 (5.1, 12.7) | 7.1 (5.0, 12.5) | 7.7 (5.3, 13.5) | 7.8 (5.2, 15.7) | 6.5 (4.9, 10.9) | 6.8 (4.7, 9.8) | |
| Urine ACR ≥25 mg/g | 11.7 (10.8 to 12.7) | 12.6 (9.1 to 16.1) | 11.3 (9.3 to 13.3) | 11.0 (8.2 to 13.8) | 11.8 (10.1 to 13.4) | 14.6 (12.0 to 17.3) | 7.2 (4.7, 9.6) | 8.2 (4.4, 12.0) | <0.001 |
| Age-adjusted CKD prevalence | |||||||||
| CKD-EPI creatinine | 13.6 (12.4 to 14.7) | 13.1 (9.7 to 16.4) | 11.9 (9.6 to 14.2) | 13.3 (10.4 to 16.2) | 13.4 (11.8 to 15.1) | 17.4 (14.6 to 20.2) | 7.7 (5.1, 10.4) | 16.6 (5.8, 27.3) | <0.001 |
| CKD-EPI cystatin C | 13.4 (12.4 to 14.4) | 13.0 (9.6 to 16.4) | 12.6 (10.4 to 14.9) | 12.5 (9.6 to 15.5) | 13.8 (12.1 to 15.4) | 16.8 (14.0 to 19.5) | 7.2 (4.6, 9.9) | 10.5 (6.9, 14.1) | <0.001 |
| CKD-EPI creatinine + cystatin C | 13.0 (12.0 to 13.9) | 12.7 (9.4 to 16.1) | 11.7 (9.6 to 13.9) | 12.5 (9.6 to 15.4) | 13.3 (11.6 to 14.9) | 16.6 (13.8 to 19.4) | 7.4 (4.8, 10.1) | 10.2 (6.6, 13.9) | <0.001 |
| Men | |||||||||
| Number of participants | 6129 | 629 | 1040 | 479 | 2289 | 1069 | 415 | 208 | |
| Urine ACR, mg/g | 5.3 (3.8, 10.0) | 5.3 (3.7, 9.6) | 5.6 (3.8, 11.0) | 5.2 (3.6, 10.2) | 5.2 (3.8, 9.1) | 5.7 (4.0, 12.4) | 5.1 (3.7, 8.8) | 5.2 (3.8, 8.4) | |
| Urine ACR ≥17 mg/g, % | 13.2 (12.1 to 14.4) | 13.1 (10.5 to 15.8) | 14.2 (11.5 to 17.0) | 13.1 (9.4 to 16.7) | 12.6 (10.6 to 14.6) | 14.7 (12.2 to 17.3) | 10.2 (6.4 toss 14.0) | 10.8 (6.0 to 15.6) | 0.45 |
| Age-adjusted CKD prevalence | |||||||||
| CKD-EPI creatinine | 15.2 (14.1 to 16.3) | 15.6 (12.9 to 18.3) | 14.0 (11.5 to 16.5) | 15.8 (12.2 to 19.3) | 15.7 (13.7 to 17.8) | 16.1 (13.6 to 18.5) | 11.1 (7.4 to 14.9) | 15.9 (11.2 to 20.6) | 0.40 |
| CKD-EPI cystatin C | 15.6 (14.4 to 16.7) | 15.6 (12.9 to 18.3) | 14.5 (12.0 to 17.1) | 16.0 (12.4 to 19.5) | 15.4 (13.4 to 17.5) | 18.2 (15.5 to 20.9) | 10.8 (7.0 to 14.5) | 16.1 (11.3 to 20.9) | 0.09 |
| CKD-EPI creatinine + cystatin C | 14.8 (13.7 to 15.9) | 15.4 (12.7 to 18.1) | 13.8 (11.2 to 16.3) | 15.0 (11.6 to 18.4) | 15.3 (13.2 to 17.3) | 15.7 (13.3 to 18.2) | 11.2 (7.4 to 14.9) | 16.0 (11.2 to 20.8) | 0.51 |
Data are presented as medians (interquartile ranges) or percentages (95% confidence intervals). ACR, albumin/creatinine ratio. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Figure 1.
Age-adjusted, weighted prevalence of CKD in Hispanic/Latino HCHS/SOL participants and Mexican-American, non-Hispanic black, and non-Hispanic white 2007–2010 NHANES participants. (A) Overall prevalence. (B and C) Prevalence for women (B) and men (C). (D–F) Prevalence for participants aged 18–44 years (D), 45–54 years (E), and 55–74 years (F). HCHS/SOL, Hispanic Community Health Study/Study of Latinos; NH, non-Hispanic; NHANES, National Health and Nutrition Examination Survey.
Baseline Clinical and Demographic Factors Associated with CKD
The adjusted prevalence odds for CKD were higher among Hispanics/Latinos with health insurance (OR, 1.25; 95% CI, 1.08 to 1.46), diabetes mellitus (adjusted OR, 1.64; 95% CI, 1.33 to 2.04), hypertension (OR, 1.67; 95% CI, 1.37 to 2.04), and self-reported cardiovascular disease (OR, 1.38; 95% CI, 1.07 to 1.77). Annual family income >$50,000 was associated with lower odds of CKD (OR, 0.71; 95% CI, 0.54 to 0.94) (Table 3). Hispanic/Latino background group, living in the United States for ≥10 years, educational attainment, and body mass index were not significantly associated with prevalent CKD. Similar patterns were observed separately for low eGFRcreat-cyst and albuminuria (Table 3). In addition, for each 10-year unit increment in age, the odds of low eGFR were nearly 3-fold higher (OR, 2.88; 95% CI, 2.32 to 3.57), US-born Hispanics/Latinos had higher odds of low eGFRcreat-cyst (OR, 2.41; 95% CI, 1.51 to 3.84), and female sex was associated with lower odds of low eGFRcreat-cyst (OR, 0.73; 95% CI, 0.54 to 0.99).
Table 3.
Multivariable logistic regression ORs for prevalent CKD, low eGFR (eGFRcreat-cyst), and albuminuria
| Parameter | CKD | Albuminuria | eGFRcreat-cyst <60 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Background | ||||||
| Mexican | Reference | Reference | Reference | |||
| Central American | 0.88 (0.70 to 1.12) | 0.30 | 0.92 (0.72 to 1.17) | 0.48 | 0.81 (0.43 to 1.51) | 0.50 |
| Cuban | 0.92 (0.74 to 1.13) | 0.42 | 0.86 (0.69 to 1.08) | 0.19 | 1.04 (0.64 to 1.70) | 0.86 |
| Dominican | 0.82 (0.64 to 1.06) | 0.13 | 0.83 (0.63 to 1.08) | 0.16 | 1.04 (0.63 to 1.72) | 0.87 |
| Puerto Rican | 0.94 (0.76 to 1.15) | 0.52 | 0.92 (0.74 to 1.13) | 0.42 | 1.20 (0.76 to 1.89) | 0.44 |
| South American | 0.76 (0.55 to 1.04) | 0.08 | 0.75 (0.54 to 1.04) | 0.09 | 0.91 (0.45 to 1.85) | 0.80 |
| Other/mixed | 0.74 (0.50 to 1.10) | 0.14 | 0.76 (0.51 to 1.14) | 0.18 | 1.33 (0.46 to 3.89) | 0.60 |
| Age (per 10-yr increment) | 1.02 (0.95 to 1.10) | 0.56 | 0.92 (0.85 to 0.99) | 0.02 | 2.88 (2.32 to 3.57) | <0.001 |
| Women (versus men) | 0.95 (0.82 to 1.11) | 0.54 | 0.96 (0.82 to 1.11) | 0.58 | 0.73 (0.54 to 0.99) | 0.04 |
| Years in United States ≥10 (versus <10) | 0.93 (0.76 to 1.12) | 0.43 | 0.96 (0.79 to 1.17) | 0.70 | 0.73 (0.48 to 1.11) | 0.15 |
| US born (yes versus no) | 1.23 (0.98 to 1.54) | 0.08 | 1.14 (0.91 to 1.44) | 0.25 | 2.41 (1.51 to 3.84) | <0.001 |
| Income ($, per yr) | ||||||
| 20,000 | Reference | Reference | Reference | |||
| 20,001–50,000 | 0.95 (0.80 to 1.12) | 0.53 | 0.98 (0.83 to 1.17) | 0.85 | 0.73 (0.50 to 1.05) | 0.09 |
| >50,000 | 0.71 (0.54 to 0.94) | 0.02 | 0.73 (0.55 to 0.98) | 0.03 | 0.54 (0.29 to 0.97) | 0.04 |
| Other (missing/not available) | 0.87 (0.67 to 1.14) | 0.28 | 0.86 (0.65 to 1.13) | 0.28 | 0.89 (0.51 to 1.55) | 0.68 |
| Education, high school or greater (yes versus no) | 0.96 (0.82 to 1.13) | 0.64 | 0.98 (0.83 to 1.15) | 0.80 | 1.02 (0.71 to 1.45) | 0.92 |
| Health insurance (yes versus no) | 1.25 (1.08 to 1.46) | 0.003 | 1.20 (1.02 to 1.40) | 0.03 | 1.55 (1.08 to 2.23) | 0.02 |
| Current smoker (yes versus no) | 1.01 (0.84 to 1.22) | 0.88 | 0.98 (0.82 to 1.18) | 0.87 | 1.47 (0.98 to 2.21) | 0.06 |
| BMI ≥30 kg/m2 (yes versus no) | 1.03 (0.86 to 1.23) | 0.75 | 1.06 (0.88 to 1.28) | 0.52 | 0.87 (0.61 to 1.24) | 0.45 |
| Waist circumference (per 1-cm increment) | 1.00 (1.00 to 1.01) | 0.34 | 1.00 (0.99 to 1.01) | 0.71 | 1.02 (1.01 to 1.03) | 0.003 |
| Hypertension (yes versus no) | 1.67 (1.37 to 2.04) | <0.001 | 1.52 (1.23 to 1.89) | <0.001 | 1.90 (1.23 to 2.94) | 0.004 |
| Systolic BP (per 10-mmHg increment) | 1.25 (1.20 to 1.32) | <0.001 | 1.31 (1.25 to 1.38) | <0.001 | 1.04 (0.95 to 1.14) | 0.40 |
| Diabetes (yes versus no) | 1.64 (1.33 to 2.04) | <0.001 | 1.52 (1.24 to 1.87) | <0.001 | 1.87 (1.23 to 2.84) | 0.003 |
| HbA1c (per 1% increment) | 1.28 (1.20 to 1.36) | <0.001 | 1.33 (1.25 to 1.42) | <0.001 | 0.97 (0.84 to 1.11) | 0.64 |
| Cardiovascular disease (yes versus no) | 1.38 (1.07 to 1.77) | 0.01 | 1.36 (1.05 to 1.78) | 0.02 | 1.52 (1.08 to 2.15) | 0.02 |
| LDL-C ≥100 mg/dl (yes versus no) | 0.89 (0.76 to 1.05) | 0.17 | 0.89 (0.75 to 1.06) | 0.18 | 0.90 (0.65 to 1.26) | 0.54 |
| CRP (per 1-mg/L increment) | 1.02 (1.01 to 1.03) | <0.001 | 1.02 (1.01 to 1.03) | <0.001 | 1.02 (1.01 to 1.03) | <0.001 |
eGFR, ml/min per 1.73 m2; OR, odds ratio; eGFRcreat-cyst, CKD-EPI creatinine-cystatin C eGFR estimating equation; BMI, body mass index; HbA1c, hemoglobin A1c; LDL-C, LDL cholesterol; CRP, C-reactive protein; 95% CI, 95% confidence interval.
Discussion
The burden of CKD in the US population of Hispanics/Latinos remains uncertain, because the most informative prior national estimate included primarily Mexican Americans and was performed about a decade ago (10). Similarly, whether the higher prevalence of ESRD among Hispanics/Latinos is a result of greater prevalence of earlier stages of CKD or more rapid CKD progression (lower competing risk for death before ESRD) also remains unknown. Our study, the first systematic evaluation of the prevalence of CKD in a large diverse contemporary cohort of Hispanics/Latinos, adds important insights into each of these important questions. We found that the prevalence of CKD, defined as either an eGFRcreat-cyst <60 ml/min per 1.73 m2 or albuminuria was 14%, varied markedly across Hispanic/Latino background groups and was similar to the prevalence of non-Hispanic whites in NHANES. Correlates of CKD included lower annual income, hypertension, diabetes mellitus, and prevalent cardiovascular disease.
According to the 2013 US Renal Data System report, the rate of incident ESRD among Hispanics/Latinos is 50% greater than in non-Hispanics (3). This striking disparity in the burden of ESRD in Hispanics/Latinos underscores the importance of understanding the epidemiology of earlier stages of CKD in this population. However, very little is known about the epidemiology of CKD in Hispanics/Latinos before the onset of dialysis (11). By design, NHANES examines predominantly Mexican Americans and therefore does not provide insights into the prevalence of CKD in other Hispanic/Latino background groups.
Our findings of significant variation in the prevalence of CKD between Hispanic/Latino background groups have important public health implications. Prevalence was 17% in women of Puerto Rican background compared with 7% in women of South American background. This is consistent with a prior finding from HCHS/SOL of substantial differences in cardiovascular risk factor burden among Hispanic/Latino background groups (cardiovascular risk factors are also risk factors for CKD) (12,13). After adjusting for clinical and demographic factors, the risk of CKD was similar across all Hispanic/Latino background groups. By contrast, the Hispanic Health and Nutrition Examination Survey reported a higher risk for CKD (defined as estimated creatinine clearance < 60 ml/min per 1.73 m2) in mainland Puerto Ricans and Cuban Americans compared with Mexican Americans (14). However, this survey was conducted >30 years ago and did not include an assessment of urinary albumin or serum cystatin C. Our findings in a larger, contemporaneous, and diverse cohort suggest that differences in CKD prevalence across background groups are explained by modifiable factors, including diabetes mellitus, hypertension, and cardiovascular disease. Nonetheless, future work is needed to explore the importance of additional risk factors, including genetic susceptibility (e.g., the presence of at-risk variants of apolipoprotein L1, which have been associated with increased risk of progression to ESRD in Hispanics with a greater degree of African ancestry) (15).
Although the prevalence of ESRD is higher in Hispanics/Latinos compared with non-Hispanic whites, we found the overall prevalence of CKD to be similar between these two groups. This suggests that Hispanics/Latinos may be at increased risk for CKD progression or alternatively that the mortality rate before the onset of ESRD is higher in non-Hispanic whites compared with Hispanics/Latinos. However, this hypothesis is not supported by analyses of data from NHANES III (16). Future work is needed to better understand risk factors associated with progression of CKD in this population and issues related to the competing risks of death and progression to ESRD. The ongoing National Institute of Diabetes and Digestive and Kidney Diseases–sponsored Hispanic Chronic Renal Insufficiency Cohort study, which includes Hispanics/Latinos with mild to moderate CKD, is expected to provide additional insights into this issue (17).
We also found that the prevalence of CKD was higher in men than women. Furthermore, in multivariable analyses, the odds of eGFRcreat-cyst <60 ml/min per 1.73 m2 were lower in women than men. Interestingly, this is the opposite of what has been found in non-Hispanic whites (10). Reasons for these differences are not clear and need further investigation. It is possible that these contrasting findings may be related to the lack of studies examining the validity of the eGFR equations in Hispanic/Latino background groups. In addition, we found that the HCHS/SOL participants with CKD were socioeconomically disadvantaged and displayed a high burden of cardiovascular risk factors and other comorbidities. More than one-half had an annual household income <$20,000, 40% lacked medical insurance, 49% had hypertension, 38% had diabetes, and one-half were obese. Furthermore, we found a prevalence of current smoking of 21%, which is concerning given the known association between smoking and adverse CKD outcomes such as progression to ESRD, cardiovascular events, and death (18). Interestingly, mean LDL cholesterol was 120 mg/dl, which is higher than that reported among 2001–2010 NHANES participants with CKD (111 mg/dl) and without CKD (117 mg/dl) (19). Despite the presence of multiple cardiovascular risk factors, only 20% of individuals with CKD were prescribed either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, medications that are known to decrease CKD progression risk (20). Also alarming is our finding that only 18% of individuals with CKD and 34% of those with eGFRcreat-cyst <60 ml/min per 1.73 m2 were aware of having CKD, which is consistent with prior reports (21,22). These findings suggest that there is considerable opportunity to improve CKD management in Hispanics/Latinos, and there is an urgent need to improve CKD awareness among patients who might be missing important preventive and therapeutic opportunities that may decrease their CKD progression risk.
To estimate GFR in this study, we used three different equations (creatinine and cystatin C based) that have been validated in large and diverse US populations (6); however, they did not include a significant number of Hispanics/Latinos in the cohorts used to establish them. Despite this shortcoming, the CKD prevalence estimates were similar for each of these equations. Future work is needed to validate GFR estimating equations in Hispanics/Latinos.
Our findings confirmed the strong association of established risk factors (e.g., hypertension and diabetes mellitus) with prevalent CKD in the Hispanic/Latino population. Similar to other studies (23–26), we found that lower annual household income was associated with prevalent CKD. We found a positive association between having health insurance and CKD; the reason for this finding is not clear, but we suspect that this could be because individuals with CKD have comorbid conditions (e.g., hypertension, diabetes mellitus) and therefore may be more likely to seek insurance coverage. We also examined issues related to acculturation, which are particularly relevant to the Hispanic/Latino population. Although place of birth and length of residence in the United States were not associated with increased risk for CKD (defined as albuminuria or eGFRcreat-cyst <60 ml/min per 1.73 m2), birth in the United States was associated with a >2-fold higher adjusted odds of having an eGFRcreat-cyst <60 ml/min per 1.73 m2. This potentially suggests that the Western lifestyle adopted by US-born Hispanics/Latinos may be associated with increased risk for CKD and is consistent with an analysis from NHANES III, which found an association between birth in the United States and heightened cardiovascular risk (27).
Our study has a number of positive features. First, this was a community sample and provides an opportunity to establish population estimates of prevalence. Second, we studied Hispanics/Latinos with diverse backgrounds. Third, the sample size was relatively large and participants were recruited from across the United States. Our findings should be considered with the following limitations. First, the classification of persons with CKD was based on single measurements of serum creatinine, cystatin C, and urine albumin. Second, the GFR estimating equations we used have not been yet validated in Hispanics/Latinos. Third, the cross-sectional study design limits interpretation of these associations. Finally, we used a second, nonconcurrent study, 2007–2010 NHANES, to compare prevalence of CKD in HCHS/SOL participants with non-Hispanics.
In summary, we found significant variation in CKD prevalence among Hispanic/Latino background groups. In addition, Hispanics/Latinos with CKD have low rates of health insurance, poor control of hypertension, low angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use, and low awareness of CKD. Correlates of CKD included low income, hypertension, diabetes mellitus, and cardiovascular disease. Our findings have important implications for public health policy targeted at improving access to care and chronic disease management for this rapidly growing population. Future research is needed to better identify modifiable risk factors for CKD progression in Hispanics/Latinos.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the HCHS/SOL staff and participants for their important contributions. A complete list of staff and investigators was provided by Lavange et al. (Ann Epidemiol. 20: 642–649, 2010) and is also available on the study website (http://www.cscc.unc.edu/hchs/).
HCHS/SOL was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes, centers, or offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: the National Institute on Minority Health and Health Disparities, the National Institute on Deafness and Other Communications Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Neurological Disorders and Stroke, and the Office of Dietary Supplements. A.C.R. and J.P.L are funded by grants from the NIDDK (K23-DK094829 to A.C.R. and K24-DK092290 to J.P.L.). N.F. is supported by grants from the National Institutes of Health (R21-HL123677-01, 1R01-ES021367-01, and 1R01-HL118305-01A1).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The Role of Ethnic Variation and CKD,” on pages 1708–1710.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02020215/-/DCSupplemental.
References
- 1.US Census Bureau: U.S. Census Bureau projections show a slower growing, older, more diverse nation a half century from now, 2102. Available at http://www.census.gov/newsroom/releases/archives/population/cb12-243.html. Accessed Septembers 11, 2014
- 2.González Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, Arena JF, Kittles R, Perez-Stable EJ, Ziv E, Risch N: Latino populations: A unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health 95: 2161–2168, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 4.Lavange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP: Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 20: 642–649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, Lavange L, Chambless LE, Heiss G: Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 20: 629–641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics : Analytical and Reporting Guidelines: The Third National Health and Nutrition Examination Survey, 1988–1994, Hyattsville, MD, National Center for Health Statistics; 1996 [Google Scholar]
- 9.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Peralta CA, Shlipak MG, Fan D, Ordoñez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J: Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 308: 1775–1784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez RA, Hernandez GT, O’Hare AM, Glidden DV, Pérez-Stable EJ: Creatinine levels among Mexican Americans, Puerto Ricans, and Cuban Americans in the Hispanic Health and Nutrition Examination Survey. Kidney Int 66: 2368–2373, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Tzur S, Rosset S, Skorecki K, Wasser WG: APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 27: 1498–1505, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra R, Kermah D, Fried L, Adler S, Norris K: Racial differences in mortality among those with CKD. J Am Soc Nephrol 19: 1403–1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP, CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, Fink JC, Frydrych A, Jensvold NG, Lustigova E, Nessel LC, Porter AC, Rahman M, Wright Nunes JA, Daviglus ML, Lash JP, CRIC Study Investigators : Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 65: 412–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuznik A, Mardekian J, Tarasenko L: Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: An analysis of National Health and Nutritional Examination Survey data, 2001-2010. BMC Nephrol 14: 132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Plantinga LC, Boulware LE, Coresh J, Stevens LA, Miller ER, 3rd, Saran R, Messer KL, Levey AS, Powe NR: Patient awareness of chronic kidney disease: Trends and predictors. Arch Intern Med 168: 2268–2275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Wen XJ, Pavkov ME, Zhao G, Balluz LS, Ford ES, Williams D, Gotway CA: Awareness of kidney disease among US adults: Findings from the 2011 behavioral risk factor surveillance system. Am J Nephrol 39: 306–313, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vart P, Gansevoort RT, Coresh J, Reijneveld SA, Bültmann U: Socioeconomic measures and CKD in the United States and The Netherlands. Clin J Am Soc Nephrol 8: 1685–1693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce MA, Beech BM, Crook ED, Sims M, Wyatt SB, Flessner MF, Taylor HA, Williams DR, Akylbekova EL, Ikizler TA: Association of socioeconomic status and CKD among African Americans: The Jackson Heart Study. Am J Kidney Dis 55: 1001–1008, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR: Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis 55: 992–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta CA, Ziv E, Katz R, Reiner A, Burchard EG, Fried L, Kwok PY, Psaty B, Shlipak M: African ancestry, socioeconomic status, and kidney function in elderly African Americans: A genetic admixture analysis. J Am Soc Nephrol 17: 3491–3496, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Sundquist J, Winkleby MA: Cardiovascular risk factors in Mexican American adults: A transcultural analysis of NHANES III, 1988-1994. Am J Public Health 89: 723–730, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.