Abstract
Background and objectives
Recent animal experiments suggest that dysregulation of the EGF receptor pathway plays a role in the pathophysiology of autosomal dominant polycystic kidney disease (ADPKD). Research on EGF receptor ligands in humans with ADPKD is lacking. EGF receptor ligands were measured in patients with ADPKD at baseline and after treatment with a vasopressin V2 receptor antagonist (V2RA) because this information might provide a rationale for future V2RA combination therapy.
Design, setting, participants, & measurements
Blood and urine concentrations of the EGF receptor ligands heparin-binding (HB)-EGF, EGF, and TGF-α were measured by ELISAs in 27 patients with ADPKD who participated in a single-center study investigating a V2RA in 2011–2013 and in 27 controls who were selected from a general population–based observational study. Cyst fluid concentrations were also measured. In patients with ADPKD, ligands were measured at baseline, after 3-week treatment with a V2RA, and 3 weeks after drug withdrawal. The measured GFR (mGFR) was determined by iothalamate infusion, and total kidney volume was measured by magnetic resonance imaging.
Results
Urinary HB-EGF excretion and plasma concentration were higher in patients with ADPKD than in controls (median, 1.4 [interquartile range, 1.2–1.9] versus 0.6 [0.4–0.8] µg/24 hours [P<0.001] and 157.9 [83.1–225.9] versus 77.2 [37.2–174.3] pg/ml [P=0.04]). In contrast, urinary EGF excretion and plasma EGF concentration were lower in patients with ADPKD, whereas TGF-α did not differ between patients and controls. Higher HB-EGF excretion was correlated with more severe disease, assessed as lower mGFR (r=−0.39; P=0.05), higher total kidney volume (r=0.39; P=0.05), and higher urinary excretion of albumin and heart-type fatty acid–binding protein, whereas higher EGF excretion and TGF-α excretion were negatively correlated with disease severity. During V2RA treatment, HB-EGF excretion increased (from 1.4 [1.2–1.9] to 2.4 [2.1–3.1] µg/24 hours; P<0.001).
Conclusion
In patients with ADPKD, higher urinary HB-EGF excretion is correlated with more severe disease. Whether this association is causal needs to be investigated in intervention studies.
Keywords: ADPKD, HB-EGF, V2 receptor antagonist, EGF receptor
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by proliferation of renal tubular cells leading to cyst formation in both kidneys. These cysts progressively expand and compress viable renal tissue, thereby causing renal insufficiency (1–3). For a long time there was no proven renoprotective treatment for this disease.
Recently, however, the vasopressin V2 receptor antagonist (V2RA) tolvaptan has been shown to slow the rate of growth in total kidney volume (TKV) and the rate of renal function loss in patients with relatively early ADPKD (4). Despite these positive findings, use of this agent has limitations. First, although the V2RA was proven effective in the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes Trial, disease still progressed during treatment. Second, some patients cannot tolerate the drug. Third, experimental data suggest that this drug may be less efficacious in later-stage ADPKD (5). Finally, the US Food and Drug Administration has not yet approved tolvaptan for treatment of ADPKD, in contrast to the Japanese, Canadian, and European regulatory authorities. Tolvaptan is therefore not universally clinically available at present. Additional treatments are therefore needed.
In this respect, intervention in the EGF receptor pathway may be of interest because this pathway is involved with growth, migration, and proliferation of renal tubular cells (6). Dysregulation of this pathway has been suggested to play a role in the pathogenesis of ADPKD (7). The EGF receptor pathway is of special interest since agents have been developed to block its activation.
The urinary excretion of the EGF(ErbB1) receptor ligands is expected to be representative for the activity of the EGF receptor pathway because binding of the ligands to the EGF receptor will activate this pathway (6). Excretion of EGF receptor ligands in human ADPKD has not been investigated yet. Activation of the EGF receptor by binding with its ligands results in cyst formation in vitro and in mice (8,9). These data form a rationale for our interest in the EGF receptor ligands in human ADPKD. We therefore measured urinary excretion of the EGF receptor ligands (EGF, transforming growth factor alpha [TGF-α], and heparin-binding EGF-like growth factor [HB-EGF]) in patients with ADPKD and controls, and we correlated these values with disease severity. We hypothesized that these ligands would be increased compared with controls as determinants of rate of renal tubular cell proliferation in ADPKD. We also measured these ligands during treatment with a V2RA to investigate activity of the EGF receptor pathway during treatment because this information could provide a rationale for future combination therapy. Because tolvaptan is the first treatment proven to ameliorate the rate of disease progression in ADPKD, it is of interest to investigate whether this drug has any additional effects on the EGF receptor pathway.
Material and Methods
Study Design and Population
We included 27 patients with ADPKD who all completed a single-center interventional trial investigating the short-term renal hemodynamic effects of the V2RA tolvaptan that was performed between 2011 and 2013 (8). ADPKD was diagnosed using the modified Ravine criteria (9). Patients were 18–70 years of age and were divided into three groups, each consisting of nine patients with ADPKD, stratified for eGFR (>60, 30–60, and <30 ml/min per 1.73 m2). Patients were studied at baseline, after 3 weeks of treatment with tolvaptan (uptitration to 90/30 mg per day, split dose), and 3 weeks after withdrawal of tolvaptan. Exclusion criteria have been described previously (8). Controls were randomly chosen from the Prevention of Renal and Vascular ENd-stage Disease (PREVEND) study, a general population–based, prospective, observational cohort study in which participants visit an outpatient clinic for detailed measurement of health status (10). In addition, cyst fluid was collected from seven patients with ADPKD during a nephrectomy, which was performed to allow sufficient space for a renal transplantation. Cyst fluid was obtained during surgery by fine-needle aspiration before clipping of the renal artery. These patients were included at three centers in The Netherlands (UMC Utrecht, MST Enschede, and UMC Groningen).
This study was approved by the local ethics committee and performed in adherence to the Declaration of Helsinki. All participants gave written informed consent.
Measurements
Concentrations of EGF receptor ligands (HB-EGF, EGF, and TGF-α) in urine and plasma, as well as in cyst fluid, were measured with Duoset ELISA kits (R&D Systems, Minneapolis, MN). These assays were optimized to allow measurement in the lower range as further described in the Supplemental Material. The ELISAs were carried out at Haemoscan in The Netherlands. Urinary EGF receptor ligand excretion was calculated by multiplying urinary concentration of these ligands with 24-hour urine volume and expressed as ratio to 24-hour creatinine clearance to obtain fractional clearances.
Measured GFR (mGFR) was measured as urinary iothalamate clearance (11) and total kidney volume (TKV) by magnetic resonance imaging. In addition, several markers indicating damage to the various parts of the nephron were measured in urine samples (12). For the methods used, see the Supplemental Material.
To study HB-EGF stability in urine, six additional patients with ADPKD and seven additional controls collected a spot urine sample. HB-EGF concentration was measured in these samples immediately and after 24 hours' storage at 3°C to assess recovery.
Statistical Analyses
Baseline characteristics are shown for patients with ADPKD and controls. Parametric variables are displayed as mean±SD and nonparametric variables as median and interquartile range. Differences in baseline characteristics between patients and controls were calculated with an unpaired-samples t test or a Mann–Whitney U test in case of nonparametric data and a chi-squared test for categorical variables. For comparisons between treatment periods, the paired-samples t test was used; for nonparametric data, the Wilcoxon signed-rank test was used.
Orthogonal regression analysis was used to investigate whether EGF receptor ligands correlated with each other, with mGFR, effective renal plasma flow, TKV, and urinary excretion of biomarkers indicating damage to different segments of the nephron. Effective renal plasma flow, TKV, and biomarker excretions were logarithmic transformed to achieve equal distribution of residuals. Scatterplots were made showing the associations of mGFR and TKV with urine excretion and plasma concentration of the EGF receptor ligands. For significant associations the regression line is depicted.
As sensitivity analysis to test the robustness of our findings the aforementioned analyses were repeated stratified for sex and antihypertensive medication use.
All statistical analyses were performed using SPSS software, version 20.0 (IBM, Inc., Armonk, NY). A P value <0.05 was considered to represent statistical significance, and all statistical tests were two tailed.
Results
The baseline characteristics of the 27 patients with ADPKD and controls are shown in Table 1. There were no significant differences between patients with ADPKD and controls except that patients were on average 10 years younger than controls, had higher diastolic BP and 24-hour urine volume, and had lower urine osmolality and eGFR, reflecting their disease status.
Table 1.
Characteristics of patients at baseline and controls
| Characteristics | Patients | Controls |
|---|---|---|
| Participants (n) | 27 | 27 |
| Men (%) | 51.9 | 51.9 |
| Age (yr) | 45.7±9.7 a | 57.6±9.6 |
| Weight (kg) | 81.2±18.4 | 79.2±16.1 |
| Height (cm) | 179±12 | 174±9 |
| Body surface area (m2) | 2.0±0.3 | 1.9±0.2 |
| Systolic BP (mmHg) | 130±11 | 126±21 |
| Diastolic BP (mmHg) | 81±8 a | 74±10 |
| AHT (%) | 77.8 a | 11.1 |
| Serum creatinine (mg/dl) | 1.89±1.13 a | 0.92±0.15 |
| eGFR (ml/min per 1.73 m2) | 52±30 a | 76±10 |
| Urine volume (L/24 hr) | 2.58±0.84 a | 1.69±0.64 |
| Urine osmolality (mOsm/kg) | 359 (281–410) a | 503 (361–649) |
| Urinary urea excretion (g/24 hr) | 23.6 (17.6–29.4) | 20.9 (15.3–25.1) |
| Urinary sodium excretion (mmol/24 hr) | 155 (122–192) | 148 (87–198) |
| mGFR (ml/min) | 70±39 | |
| ERPF (ml/min) | 222±110 | |
| Total kidney volume (ml) | 2147 (1100–2767) |
Values are percentages, means±SD, or medians with interquartile range. AHT, antihypertensives; mGFR, measured GFR; ERPF, effective renal plasma flow.
P<0.05 versus controls.
Table 2 shows that in patients with ADPKD, plasma concentration of HB-EGF and urinary HB-EGF excretion were higher than in controls, whereas plasma concentration of EGF and urinary EGF excretion were lower. For TGF-α, no significant differences were observed between the two study groups. EGF had a fractional excretion that exceeded 100%, whereas HB-EGF and TGF-α had lower fractional excretions. In a multivariable regression analysis that adjusted for differences in baseline characteristics between patients and controls (i.e., age, sex, BP, and eGFR), similar findings were obtained. Patients with an eGFR of 60 ml/min per 1.73 m2 or higher (n=11) had similar renal function compared with the control group (76 versus 82 ml/min per 1.73 m2, respectively; P=0.16), but the results with respect to the EGF receptor ligand excretions were similar to those in the overall group of patients with ADPKD. In addition, no correlations were observed between eGFR and HB-EGF, EGF, and TGF-α in the control group (P=0.47, P=0.42, and P=0.95, respectively), in contrast to observations in the patient group (see below).
Table 2.
Urinary excretion, plasma concentration, and fractional clearance of EGF receptor ligands in controls and in patients with autosomal dominant polycystic kidney disease at baseline, after treatment with a vasopressin V2 receptor antagonist, and 3 weeks after stopping treatment (washout)
| Variable | Controls | Patients with ADPKD | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | V2RA | Washout | Controls versus Baseline | Treatment versus Baseline | Washout versus Treatment | Washout versus Baseline | ||
| Excretion (ng/24 hr) | ||||||||
| HB-EGF | 606 (440–806) | 1421 (1158–1854) | 2399 (2090–3114) | 1400 (1083–1866) | <0.001 | <0.001 | <0.001 | 0.66 |
| EGF | 32,939 (26,049–63,420) | 11,345 (345–26,367) | 3282 (1.2–27,006) | 6206 (456–23,253) | <0.001 | 0.44 | 0.67 | 0.70 |
| TGF-α | 3.9 (1.6–6.2) | 1.9 (0.1–7.0) | 0.1 (0.1–0.2) | 1.5 (0.1–4.2) | 0.14 | 0.01 | 0.002 | 0.21 |
| Plasma concentration (pg/ml) | ||||||||
| HB-EGF | 77.2 (37.2–174.3) | 157.9 (83.1–225.9) | 149.5 (34.4–319.1) | 122.4 (40.1–379.1) | 0.04 | 0.07 | 0.64 | 0.13 |
| EGF | 63.4 (54.9–116.2) | 24.5 (22.7–33.6) | 23.9 (21.2–30.1) | 22.9 (21.0–29.2) | <0.001 | 0.58 | 0.96 | 0.39 |
| TGF-α | 2.6 (0.4–7.4) | 4.4 (1.1–11.0) | 1.9 (0.02–6.6) | 1.4 (0.02–11.4) | 0.46 | 0.007 | 0.71 | 0.05 |
| Fractional clearance (%) | ||||||||
| HB-EGF | 4.5 (2.0–9.0) | 9.7 (2.5–25.2) | 20.7 (8.8–56.2) | 15.6 (2.9–41.9) | 0.02 | <0.001 | 0.005 | 0.11 |
| EGF | 324.4 (163.0–547.7) | 305.6 (28.9–602.7) | 170.2 (0.08–791.1) | 279.7 (32.3–517.9) | 0.46 | 0.89 | 0.92 | 0.77 |
| TGF-α | 1.0 (0.1–6.4) | 0.3 (0.0–2.2) | 0.1 (0.0–4.8) | 1.4 (0.2–22.8) | 0.19 | 0.60 | 0.004 | 0.08 |
Unless otherwise noted, values are the median (interquartile range). HB-EGF, heparin-binding EGF; ADPKD, autosomal dominant polycystic kidney disease; V2RA, vasopressin V2 receptor antagonist.
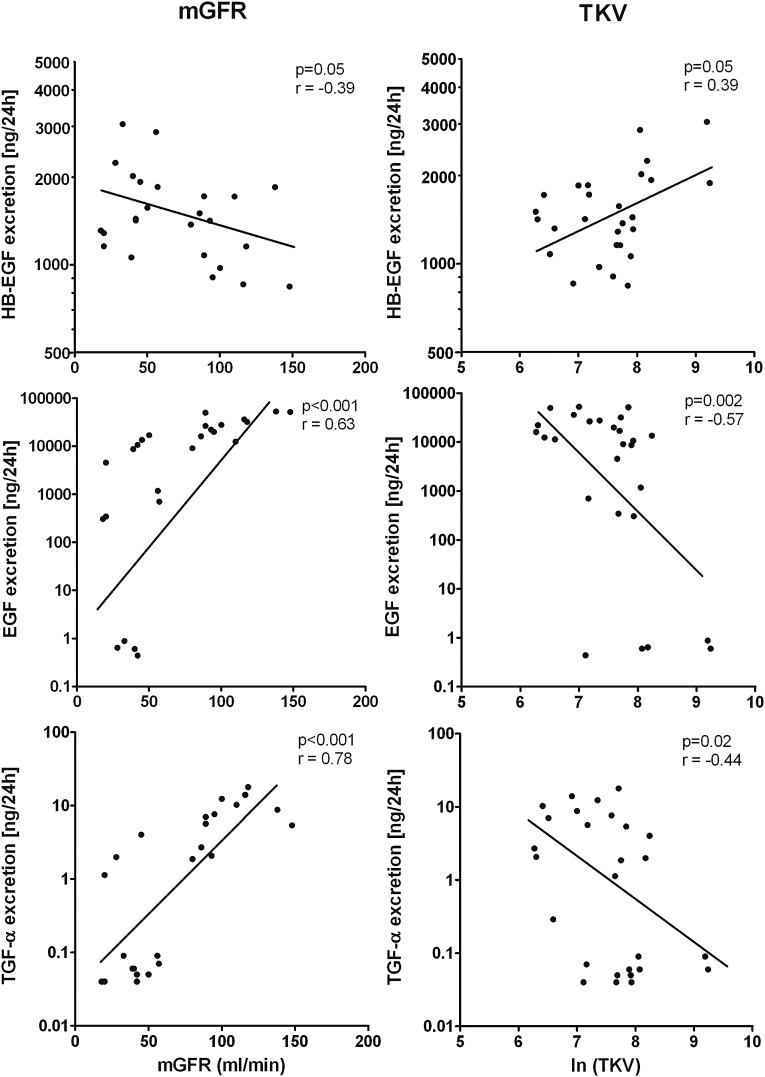
At baseline, we investigated in patients with ADPKD associations between urinary EGF receptor ligand excretions and markers of disease severity (Table 3). When these parameters indicate more severe disease (i.e., a higher TKV and lower mGFR), HB-EGF was higher. For EGF and TGF-α, opposite findings were seen (Figure 1). Of note, at baseline mGFR and TKV were highly correlated (R=−0.53; P<0.01). We found various associations between the urinary excretions of the EGF receptor ligands and biomarkers indicating damage to various parts of the nephron (Table 3). A positive association was noted between HB-EGF and albuminuria, which may reflect glomerular or tubular damage. For HB-EGF a positive association was noted with heart-type fatty acid–binding protein, a marker for damage to the distal tubule. EGF and heart-type fatty acid–binding protein showed an inverse association. No associations were found between plasma concentrations of the EGF receptor ligands and the aforementioned markers of disease severity.
Table 3.
Associations between urinary excretion of EGF receptor ligands and measures of autosomal dominant polycystic kidney disease severity at baseline
| Variable | HB-EGF Excretion | EGF Excretion | TGF-α Excretion | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient | P Value | Correlation Coefficient | P Value | Correlation Coefficient | P Value | |
| mGFR (ml/min) | −0.39 | 0.05 | 0.63 | <0.001 | 0.78 | <0.001 |
| ERPF (ml/min) | −0.37 | 0.07 | 0.64 | <0.001 | 0.75 | <0.001 |
| TKV (ml) | 0.39 | 0.05 | −0.57 | 0.002 | −0.44 | 0.02 |
| Albumin excretion (mg/24h) | 0.40 | 0.05 | −0.38 | 0.06 | −0.31 | 0.13 |
| IgG excretion (μg/24h) | 0.18 | 0.40 | −0.23 | 0.26 | −0.02 | 0.94 |
| KIM-1 excretion (μg/24h) | 0.14 | 0.49 | −0.15 | 0.47 | −0.03 | 0.89 |
| HFABP excretion (μg/24h) | 0.44 | 0.03 | −0.60 | 0.002 | −0.32 | 0.12 |
| NGAL excretion (μg/24h) | 0.10 | 0.63 | −0.32 | 0.12 | −0.46 | 0.02 |
| MCP-1 excretion (μg/24h) | 0.21 | 0.31 | 0.18 | 0.39 | 0.40 | 0.05 |
HB-EGF, heparin-binding EGF; TGF-α, transforming growth factor alpha; mGFR, measured GFR; ERPF, effective renal plasma flow; TKV, total kidney volume; KIM, kidney injury molecule; HFABP, heart-type fatty acid–binding protein; NGAL, neutrophil gelatinase–associated lipocalin; MCP, monocyte chemoattractant protein.
Figure 1.
Correlations between urinary excretion of EGF receptor ligands and measures of severity of autosomal dominant polycystic kidney disease (measured GFR [mGFR] and total kidney volume (TKV). HB-EGF, heparin-binding EGF.
When investigating associations between the different EGF receptor ligands, we found a negative correlation between HB-EGF excretion and EGF as well as TGF-α excretion (R=−0.63 [P<0.001] and R=−0.50 [P<0.001], respectively). In contrast, there was a positive correlation between EGF and TGF-α excretion (R=0.71; P<0.001).
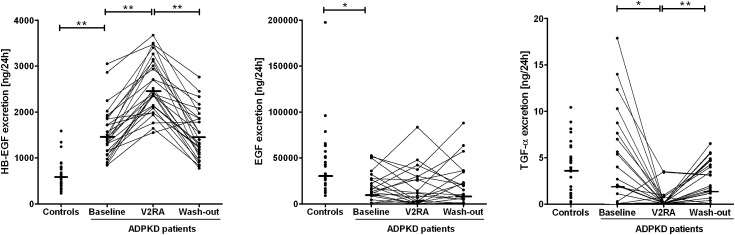
Table 2 also shows the values of EGF receptor ligands in blood and urine in patients with ADPKD during tolvaptan treatment. In general there were no differences in plasma concentrations of the EGF receptor ligands during treatment when compared with baseline. Only TGF-α levels decreased minimally. Urinary excretion changed significantly during treatment for HB-EGF and TGF-α. Figure 2 shows the individual responses in urinary excretion of the EGF receptor ligands in patients with ADPKD during tolvaptan treatment. In all patients, HB-EGF excretion increased during treatment with tolvaptan. This increase was not associated with sex or age, or with baseline BP, mGFR, or TKV (P=0.48, P=0.77, P=0.90, P=0.44, and P=0.26, respectively). In contrast, percentage change in HB-EGF excretion was positively associated with percentage change in urine volume (P=0.001). During treatment, EGF excretion increased in some patients and decreased in others, whereas TGF-α excretion decreased strongly in all but two patients. In addition, percentage change in TGF-α excretion was negatively associated with percentage change in urine volume (P<0.01). After drug withdrawal (washout), urinary excretion of HB-EGF, EGF, and TGF-α returned toward pretreatment values, with washout values being highly correlated with baseline values (R=0.65 [P<0.001], R=0.88 [P<0.001], and R=0.55 [P=0.003], respectively).
Figure 2.
Urinary excretion of EGF receptor ligands in controls and in patients with autosomal dominant polycystic kidney disease (ADPKD) at baseline, after 3 weeks' treatment with a vasopressin V2 receptor antagonist (V2RA), and 3 weeks after stopping treatment (washout). HB-EGF, heparin-binding EGF. *P<0.05; **P<0.001.
Finally, we investigated the concentration of EGF receptor ligands in cyst fluid from seven patients with ADPKD. HB-EGF was found in a concentration of 89.1 (interquartile range, 0.8–120.9) pg/ml, whereas EGF and TGF-α were below the detection limit in all and in five out of seven patients, respectively.
Urinary HB-EGF stability was tested and proven in six additional patients with ADPKD and seven controls; recovery of HB-EGF concentration in spot urine samples after 24 hours storage at 3°C was 93%±28% and 95±21%, respectively, compared with values that were measured immediately after these participants provided their urine samples.
The sensitivity analyses in which the aforementioned analyses were repeated, stratified for sex and antihypertensive medication use, rendered essentially similar results as the main analyses. In line, no significant interaction was found for sex and medication use in the regression analyses. After exclusion of values that were below the limit of detection (for EGF, 22 of 108 samples; for TGF-α, 42 of 108 samples), all correlations that are described above were still significant, except for the correlations of EGF and TGF-α with TKV at baseline.
Discussion
Our study shows that in patients with ADPKD, HB-EGF is detectable in cyst fluid and that the concentration of HB-EGF is increased in plasma and urine compared with concentrations in controls. In contrast, EGF and TGF-α concentrations in cyst fluid are very low, and EGF plasma concentration and urinary excretion are lower in patients with ADPKD than in controls. In addition, HB-EGF excretion is significantly higher in patients with more severe disease, whereas EGF and TGF-α excretion was inversely associated with disease severity.
Signaling by EGF receptors is essential for growth, migration, and proliferation of cells (6). The EGF receptor family (ErbB receptors) consists of four receptors: the EGF/ErbB1, HER-2, ErbB3, and ErbB4 receptors. Activation of these receptors by binding with a ligand leads to upregulation of downstream signal transduction cascades, which causes cell proliferation, thereby modulating cell migration, adhesion, and proliferation (13). Ligands for the EGF receptor are EGF, TGF-α, and HB-EGF. Recently, experimental studies have shown that activation of this EGF receptor by binding with the ligands EGF or TGF-α results in cyst formation in vitro (8) and in vivo (9). Inhibition of EGF receptor tyrosine kinase activity blocked tubular cyst formation in an in vitro system (14), blockade of EGF receptor resulted in attenuation of cystic disease in animal models (15,16), whereas ErbB4 knockout mice showed an accelerated cyst progression and renal function deterioration (17). Together, these experimental studies provide compelling evidence that the EGF receptor pathway is involved with disease progression in ADPKD.
Despite the recent interest in the EGF receptor pathway, studies in human ADPKD are scarce. Two older small scale studies, investigating cystic epithelia of patients with ADPKD, described a modest increase in TGF-α and EGF receptor expression (18,19). Another study described a negative association between EGF concentration in cyst fluid and serum creatinine (20). We found in cyst fluid very low EGF and TGF-α concentrations, whereas HB-EGF was present in a similar concentration range as in plasma, and an increased HB-EGF urinary excretion in patients with ADPKD compared with controls. On the basis of these data, and the association of HB-EGF excretion with disease severity, we hypothesize that HB-EGF may have a detrimental role in ADPKD. Importantly, HB-EGF concentration has not been measured in these previous human studies. In line with our study are the findings in a Pkd1 model showing that the major ligand for the EGF receptor in renal cysts was HB-EGF and not EGF (21). Of note, in the subgroup of patients with ADPKD with normal kidney function (CKD eGFR stages 1 and 2), eGFR was similar as in controls, yet these patients still had significantly higher HB-EGF excretion and decreased TGF-α excretion. In addition, no correlations were observed between eGFR and HB-EGF, EGF, and TGF-α in the control group. This suggests that the correlation in patients with ADPKD between kidney function and the urinary EGF receptor ligands is reflecting the disease process, and not kidney function per se.
HB-EGF is known to be a more potent mitogen for renal epithelia than EGF (13,22). Whether the increased urinary HB-EGF excretion is the result of increased filtration of plasma derived HB-EGF or of local intrarenal production is not exactly known, because fractional excretion is below 100%. Molecular weight of HB-EGF is 23 kD, which suggests that only a fraction plasma HB-EGF will be filtrated. However, the fact that we found associations of various markers of ADPKD severity with urinary HB-EGF excretion and not with plasma HB-EGF concentration, and that tolvaptan increased urinary excretion of HB-EGF and not of plasma HB-EGF, indicates that local production is more likely. The negative correlation between HB-EGF excretion and EGF excretion, as well as TGF-α excretion and positive correlation between EGF excretion and TGF-α excretion, could theoretically suggest that EGF and TGF-α are downregulated by the increased levels of HB-EGF.
We observed that V2RA treatment caused an increase in urinary HB-EGF excretion, and that after cessation of treatment, HB-EGF excretion returned toward baseline values. In addition, the drug-induced increase in urine flow was associated with an increase in HB-EGF excretion. The fact that these observations were made after only 3 weeks of treatment suggests that the changes in excretion of EGF receptor ligands are caused by physiologic drug-induced mechanisms, rather than by structural changes in the polycystic kidneys, because these changes take longer to occur.
There are four potential explanations for this increase in HB-EGF. First, vasopressin stimulates intracellular cAMP production in collecting duct cells. Both cAMP and EGF receptor activation stimulate the proliferation of tubular cells to form cysts by the RAS/RAF/MEK/ERK pathway (23). Cross-talk between these two pathways could exist (i.e., between the EGF receptor and the vasopressin-cAMP pathways [24–26]), resulting in an increase in HB-EGF during treatment with the V2RA. Second, V2RA treatment could induce short-term ischemia, causing an increase in HB-EGF excretion. Interestingly, in the Tolvaptan Efficacy and Safety in Managment of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes 3:4 trial, urinary excretion of the inflammation marker monocyte chemoattractant protein-1 increased after 3 weeks of treatment with tolvaptan, whereas after longer treatment a gradual and significant decrease occurred. Another study showed that 3 weeks treatment with tolvaptan already led to a decrease in urinary excretion of kidney injury markers (except kidney injury molecule-1) (27). These data indicate that there may be differences between short-term and long-term effects of this medication and that there may be differences in the outcome parameter under investigation. Future studies are therefore warranted to investigate whether longer-term V2RA treatment will also result in an increased HB-EGF excretion. Third, the increase in HB-EGF during tolvaptan use may not be unique to patients with ADPKD. Because there are no data on HB-EGF concentration or excretion in patients who use tolvaptan for other indications, it is of interest to study this issue in other patients groups. Finally, upregulation of the EGF receptor pathway during V2RA treatment could also be a feedback mechanism that just reflects the renoprotective effect of this treatment. Notwithstanding this theoretical consideration, we hypothesize that V2RA may be less renoprotective than might be expected from its primary effect on cAMP because of concomitant stimulation of intrarenal HB-EGF production. If true, combining a V2RA with an agent that interferes with the EGF receptor pathway could be a logical choice to optimize the renoprotective effect of the V2RA.
At the moment, two studies are investigating the efficacy of a tyrosine kinase inhibitor in ADPKD (clintrials.gov NCT01233869 and NCT01559363). Both are relatively small-scale studies and apply nonspecific tyrosine kinase inhibitors (KD019 and bosutinib). Because EGF receptor signaling has widespread functions, unselected long-term inhibition of EGF receptor signaling may result in considerable adverse events. Therefore, in our opinion future research should be directed to develop a more targeted approach that would limit the number and seriousness of adverse effects. HB-EGF could be such a target. In addition, measuring the urinary HB-EGF excretion could have also value in clinical practice to assess disease severity and prognosis in patients with ADPKD. Future research is needed to establish this potential role.
Some limitations of this study need to be addressed. First, the relatively small number of participants that was included (i.e., 27 patients with ADPKD and 27 controls), which limits the power of our study. Nonetheless, we found significant differences between patients and controls and associations for EGF receptor ligands with ADPKD severity, suggesting that the power of our study is sufficient. Second, controls were not matched to the patients with ADPKD regarding age and eGFR. However, multivariate analyses, which adjusted for differences in characteristics between patients and controls, confirmed the significant differences between both groups in urinary EGF receptor ligands excretion. Third, the association between ADPKD severity and HB-EGF might also exist because HB-EGF is increased as a result of another mechanism (e.g., in reaction to activation of repair or inflammatory processes) and as such does not contribute to the disease. However, previous findings on the EGF receptor pathway in ADPKD tissue and our present results indicate that the increase in HB-EGF might be causally related to disease progression. Finally, it is unknown whether the higher HB-EGF excretion is specific for ADPKD or can also be found in patients with CKD who do not have ADPKD. Future studies should investigate this issue.
Strengths of this study include the improvement in the sensitivity of commercially available ELISAs for the various EGF receptor ligands, in such a way that urinary concentrations of HB-EGF, EGF, and TGF-α could be measured, and the use of gold standard measurements to assess mGFR and TKV. In addition, to our knowledge this is the first study to investigate the influence of drug treatment (i.e., treatment with a V2RA) on plasma and urine concentrations of EGF receptor ligands.
In conclusion, in patients with ADPKD, higher urinary HB-EGF excretion is associated with more severe disease. Furthermore, we observed that treatment with a V2RA is associated with an increase in urinary HB-EGF excretion. Additional research is needed to investigate whether this association is causal, for instance by studying whether interventions directed at blocking HB-EGF activity will result in renoprotection in ADPKD.
Disclosures
R.T.G. is a consultant for Otsuka, manufacturer of tolvaptan. None of the other authors declared any conflict of interest.
Supplementary Material
Acknowledgments
An abstract of this manuscript was selected for a poster presentation at the 2014 Annual American Society of Nephrology conference.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09941014/-/DCSupplemental.
References
- 1.Rizk D, Chapman AB: Cystic and inherited kidney diseases. Am J Kidney Dis 42: 1305–1317, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Chang MY, Ong AC: Autosomal dominant polycystic kidney disease: Recent advances in pathogenesis and treatment. Nephron, Physiol 108: 1–7, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Bankir L, Grantham JJ: A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol 4: 1140–1150, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 5.Meijer E, Gansevoort RT, de Jong PE, van der Wal AM, Leonhard WN, de Krey SR, van den Born J, Mulder GM, van Goor H, Struck J, de Heer E, Peters DJ: Therapeutic potential of vasopressin V2 receptor antagonist in a mouse model for autosomal dominant polycystic kidney disease: optimal timing and dosing of the drug. Nephrol Dial Transplant 26: 2445–2453, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, van Goor H: Epidermal growth factor receptor signaling in the kidney: Key roles in physiology and disease. Hypertension 52: 987–993, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Torres VE, Harris PC: Mechanisms of disease: Autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55, quiz 55, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Neufeld TK, Douglass D, Grant M, Ye M, Silva F, Nadasdy T, Grantham JJ: In vitro formation and expansion of cysts derived from human renal cortex epithelial cells. Kidney Int 41: 1222–1236, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Lowden DA, Lindemann GW, Merlino G, Barash BD, Calvet JP, Gattone VH, 2nd: Renal cysts in transgenic mice expressing transforming growth factor-alpha. J Lab Clin Med 124: 386–394, 1994 [PubMed] [Google Scholar]
- 10.Boertien WE, Meijer E, de Jong PE, Bakker SJ, Czerwiec FS, Struck J, Oberdhan D, Shoaf SE, Krasa HB, Gansevoort RT: Short-term renal hemodynamic effects of tolvaptan in subjects with autosomal dominant polycystic kidney disease at various stages of chronic kidney disease. Kidney Int 84: 1278–1286, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM: Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343: 824–827, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Diercks GF, Janssen WM, van Boven AJ, Bak AA, de Jong PE, Crijns HJ, van Gilst WH: Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]). Am J Cardiol 86: 635–638, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Aplin AE, Howe A, Alahari SK, Juliano RL: Signal transduction and signal modulation by cell adhesion receptors: The role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev 50: 197–263, 1998 [PubMed] [Google Scholar]
- 14.Pugh JL, Sweeney WE, Jr, Avner ED: Tyrosine kinase activity of the EGF receptor in murine metanephric organ culture. Kidney Int 47: 774–781, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Torres VE, Sweeney WE, Jr, Wang X, Qian Q, Harris PC, Frost P, Avner ED: EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int 64: 1573–1579, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED: Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest 101: 935–939, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng F, Miyazawa T, Kloepfer LA, Harris RC: Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice. Kidney Int 86: 538–547, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DC, Chan KW, Chan SY: Expression of transforming growth factor alpha and epidermal growth factor receptor in adult polycystic kidney disease. J Urol 159: 291–296, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Klingel R, Dippold W, Störkel S, Meyer zum Büschenfelde KH, Köhler H: Expression of differentiation antigens and growth-related genes in normal kidney, autosomal dominant polycystic kidney disease, and renal cell carcinoma. Am J Kidney Dis 19: 22–30, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Munemura C, Uemasu J, Kawasaki H: Epidermal growth factor and endothelin in cyst fluid from autosomal dominant polycystic kidney disease cases: Possible evidence of heterogeneity in cystogenesis. Am J Kidney Dis 24: 561–568, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Jiang ST, Chiou YY, Wang E, Lin HK, Lin YT, Chi YC, Wang CK, Tang MJ, Li H: Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol 168: 205–220, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horikoshi S, Kubota S, Martin GR, Yamada Y, Klotman PE: Epidermal growth factor (EGF) expression in the congenital polycystic mouse kidney. Kidney Int 39: 57–62, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ: Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Jansson K, Nguyen AN, Magenheimer BS, Reif GA, Aramadhaka LR, Bello-Reuss E, Wallace DP, Calvet JP, Blanco G: Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am J Physiol Renal Physiol 303: F982–F990, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiermayer S, Biondi RM, Imig J, Plotz G, Haupenthal J, Zeuzem S, Piiper A: Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol Biol Cell 16: 5639–5648, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumaz N, Marais R: Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft für Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J 272: 3491–3504, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Boertien WE, Meijer E, de Jong PE, Ter Horst GJ, Renken RJ, van der Jagt EJ, Kappert P, Ouyang J, Engels GE, van Oeveren W, Struck J, Czerwiec FS, Oberdhan D, Krasa HB, Gansevoort RT: Short-term effects of tolvaptan in individuals with autosomal dominant polycystic kidney disease at various levels of kidney function. Am J Kidney Dis 65: 833–841, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.