Abstract
Rapid PCR-based influenza tests are increasingly used as point-of-care diagnostics in hospitals and clinics. To our knowledge, no prior studies have described clinical outcomes with implementation of rapid PCR-based influenza tests in hospitalized adult inpatients. Electronic medical records were used to assess differences in laboratory testing time and antiviral use among a subset of 175 consecutive adult inpatients tested for influenza in two respiratory seasons before and after implementation of rapid PCR-based influenza testing at an academic medical center. Of the 350 hospitalized inpatients included in this analysis, 96 (27%) were over 65 years of age and 308 (88%) had a comorbid condition. The overall time to result decreased significantly from 25.2 to 1.7 hr (P<0.001) after implementation of rapid PCR-based influenza testing. Among influenza-negative patients, the frequency of oseltamivir initiation remained unchanged (before: 43% vs. after: 45%; P=0.60), though the median duration of oseltamivir was significantly decreased from 1.1 to 0.0 days (P<0.001). By providing an earlier result to clinicians, rapid PCR-based influenza tests may decrease unnecessary antiviral use among adult inpatients who test negative for influenza.
Keywords: influenza, PCR, oseltamivir, infection control
INTRODUCTION
Early diagnosis of influenza decreases unnecessary antibiotic use and reduces additional diagnostic testing in both adult and pediatric populations [Bonner et al., 2003]. Antiviral therapy with oseltamivir initiated within 48 hr of symptom onset may reduce duration of symptoms and viral shedding [Treanor et al., 2000]. Currently, the Centers for Disease Control (CDC) recommends initiation of antiviral therapy in high-risk patients, including all hospitalized patients, with suspected influenza without awaiting test results [Fiore et al., 2011]. This is in part because of the low sensitivity of antigen-based rapid influenza diagnostic tests (RIDTs) [Chan et al., 2014]. Influenza reverse-transcriptase polymerase chain reaction (RT-PCR) tests are highly sensitive and specific, but take several hours to days to provide results. Rapid PCR-based influenza tests have been shown to be as sensitive and specific as traditional influenza RT-PCR assays [Woodberry et al., 2013], and have the additional advantage of providing results within 1 hr. These have the potential to be used in settings such as emergency departments and inpatient hospital wards, where reliable and rapid influenza test results in high-risk patients may impact patient care and clinical outcomes. Recently, the Food and Drug Administration issued a Clinical Laboratory Improvement Amendments (CLIA) waiver to expand the use of nucleic-acid based influenza testing to physician offices and clinics [Food and Drug Administration, 2014]. To our knowledge, no prior studies have described clinical outcomes with implementation of rapid PCR-based influenza tests in hospitalized adult inpatients.
METHODS
We performed electronic chart review of a subset of 175 consecutive patients from February to March 2012 in Season 1 (pre-rapid testing), and 175 consecutive patients from January 2013 in Season 2 (rapid testing), immediately after implementation of rapid PCR-based influenza testing at the University of Washington and Harborview Medical Centers in Seattle, WA, USA on January 1, 2013 (Fig. 1). This represented 11% of 1659 total patients tested from September 2011 to August 2012 and 7% of 2535 total patients tested from September 2012 to August 2013. We selected the time periods in the two seasons to correspond to periods of heightened seasonal influenza activity in the Pacific Northwest region (http://depts.washington.edu/rspvirus/respiratory.htm). We included patients age 18 years or older who were hospitalized within 48 hours of influenza testing or were inpatients at the time of testing.
Fig. 1.
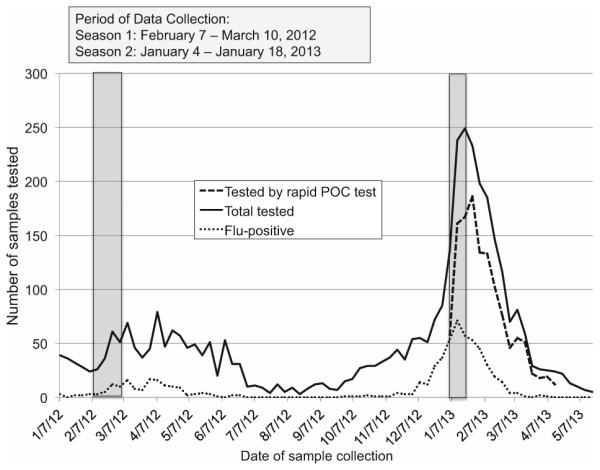
Graph showing period of data collection during two seasons before and after implementation of rapid PCR-based influenza testing.
Briefly, nasal swabs were collected from patients with respiratory illness in the hospital or emergency department for influenza testing at the discretion of the clinician. Using laboratory records, we identified patients who had testing performed for influenza using the laboratory-developed traditional RT-PCR assay in Season 1 and using the rapid PCR-based influenza test in Season 2 according to previously published methods [Woodberry et al., 2013]. The rapid PCR-based influenza assay (Simplexa Flu A/B & RSV Direct kit, Focus Diagnostics, Cypress, CA) combines nucleic acid extraction and real-time RT-PCR for detection of influenza A/B and RSV using primers and probes targeting the conserved regions of the viral genomes. Results are provided in one hour, and include both a qualitative result as well as a cycle threshold, a semiquantitative measure of viral load. The number of doses of oseltamivir received as an inpatient was obtained from electronic pharmacy records. Influenza vaccine receipt was assessed by electronic chart review of immunization records or as documented in the inpatient medical record. Beginning October 2012, both hospitals instituted a policy whereby a patient who was tested for influenza was automatically initiated on oseltamivir treatment and placed in droplet precautions through a bundled electronic order set. Prior to this, influenza testing and oseltamivir treatment were separately ordered at the discretion of the clinician. Antibiotic receipt was classified as being administered for respiratory illness or fever per provider documentation in the medical record with no evidence on chart review of other potential sites of infection requiring antibiotics.
Data were entered into Project REDCap [Harris et al., 2009], and analyzed using Stata 12.0 (College Station, TX). Fisher’s exact and Wilcoxon rank-sum tests were used for comparison of categorical and continuous variables, respectively. Statistical significance was defined as two sided P-values <0.05. Multivariate regression analysis was used to compare the time from sample collection to testing result, number of doses of oseltamivir received as an inpatient, receipt of antibiotics for fever and/or respiratory illness, and days of hospitalization. Log transformation was performed to normalize values. Factors imbalanced at baseline between the two cohorts (pregnancy, receipt of influenza vaccine, immunocompromising condition defined as receipt of solid organ transplant [SOT], receipt of hematopoietic stem cell transplant [HSCT], or malignancy, location of sample collection, and age) were adjusted for in multivariate analyses. This study was approved by the University of Washington Institutional Review Board. The requirement for informed consent was waived.
RESULTS
Of the subset of 350 hospitalized patients with influenza testing performed over both seasons, 96 (27%) were over 65 and 308 (88%) had a comorbid condition including 69 (20%) with chronic obstructive pulmonary disease, 125 (36%) with HSCT, SOT, or malignancy, 73 (21%) with diabetes, and/or 12 (3%) who were pregnant (Table I). Baseline characteristics in the two cohorts differed significantly by age, pregnancy status, immunocompromising condition, influenza vaccine receipt, and location of sample collection.
TABLE I.
Baseline Characteristics of the Two Cohorts
| Baseline characteristics | Season 1 (n=175) | Season 2 (n=175) | P-value |
|---|---|---|---|
| Age, mean [SD]a | 52.2 [15.9] | 57.4 [19.7] | 0.007 |
| Male sexb | 105 | 98 | 0.59 |
| Caucasianb | 131 | 123 | 0.40 |
| Comorbiditiesb | 158 | 150 | 0.25 |
| Chronic obstructive pulmonary disease | 35 | 34 | 1.00 |
| Malignancy | 48 | 26 | 0.006 |
| Hematopoietic stem cell transplant | 26 | 1 | <0.001 |
| Solid organ transplant | 15 | 9 | 0.29 |
| Pregnant or given birth within 2 weeksb | 0 | 12 | <0.001 |
| Sample collected in emergency departmentb | 46 | 88 | <0.001 |
| Influenza vaccinationb | 76 | 112 | <0.001 |
Tested using Wilcoxon rank-sum test.
Tested using Fisher’s exact or χ2 test.
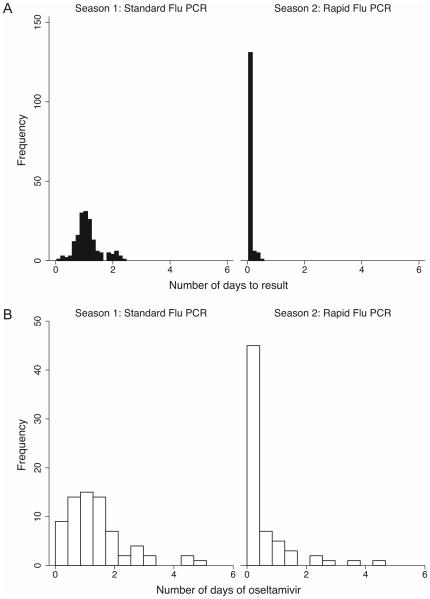
Eight (5%) patients in Season 1 and 32 (18%) in Season 2 had influenza detected by RT-PCR, all of whom were started on oseltamivir therapy (Table II). The median time between sample collection and laboratory result in Season 1 was 25.2 hr (range: 2.7–55.9) as compared to 1.7 hr (range: 0.97–11.4) in Season 2 (P<0.001; Fig. 2A). Among all patients, oseltamivir was more frequently initiated in Season 2 (n=97; 55%) as compared to Season 1 (n=79; 45%; P=0.05), but this effect was due primarily to more patients with influenza detected in Season 2. Among patients who were influenza-negative, no differences were observed between frequency of oseltamivir initiation in the two seasons [Season 1: n=71 (43%) vs. Season 2: n=65 (45%); P=0.60]. Overall, the median duration of oseltamivir in Season 1 was 1.3 days (range: 0–9.4) as compared to 0.36 days (range: 0–15.2) in Season 2 (P<0.001). Among influenza-positive patients, median duration did not differ significantly between the two seasons [Season 1: 2.8 days (range: 0.98–9.4) vs. Season 2: 2.4 days (range: 0–15.2); P=0.81]. Among patients who were influenza-negative, the median duration of oseltamivir was 1.1 days (range: 0–4.7) in Season 1 as compared to 0 days (range: 0–4.5) in Season 2 (P<0.001; Figure 2B). Patients in Season 2 were overall less likely to receive antibiotics for fever or respiratory illness [Season 1: n=133 (76%) vs. Season 2: n=110 (63%); P=0.008], with the effect predominantly observed in patients who were influenza-negative [Season 1: n=128 (77%) vs. n=90 (63%); P=0.01]. No significant differences were observed between the two seasons in proportions of patients admitted to the intensive care unit (P=0.19), respiratory illness-associated mortality (P=0.68), or receipt of discharge antibiotics for respiratory infection (P=0.17).
TABLE II.
Clinical Outcomes Among Patients in Season 1 Versus Season 2
| Clinical outcomes | Season 1 (n=175) Median (range); n(%) |
Season 2 (n=175) Median (range); n(%) |
P-value |
|---|---|---|---|
| Influenza detecteda | 8 (5) | 32 (18) | <0.001 |
| Time to result in hoursb | 25.2 (2.7–55.9) | 1.7 (0.97–11.4) | <0.001 |
| Antibiotics administered for respiratory | 133 (76) | 110 (63) | 0.008 |
| illness or fevera | |||
| Influenza-positive | 5 (63) | 20 (63) | 1.00 |
| Influenza-negative | 128 (77) | 90 (63) | 0.01 |
| Days of hospitalizationb | 5 (0–117) | 4 (1–164) | 0.33 |
| Influenza-positive | 4 (1–11) | 4 (1–126) | 0.92 |
| Influenza-negative | 5 (0–117) | 5 (1–164) | 0.42 |
| Number of total patients started on oseltamivira |
79 (45) | 97 (55) | 0.05 |
| Influenza-positive | 8 (100) | 32 (100) | 1.00 |
| Influenza-negative | 71 (43) | 65 (45) | 0.60 |
| Doses of oseltamivir receivedb | 3 (1–23) | 2 (1–29) | 0.004 |
| Influenza-positive | 6.5 (4–23) | 6 (1–29) | 0.72 |
| Influenza-negative | 3 (1–8) | 1 (1–9) | <0.001 |
| Days of oseltamivir receivedb | 1.3 (0–9.4) | 0.36 (0–15.2) | <0.001 |
| Influenza-positive | 2.8 (0.98–9.4) | 2.4 (0–15.2) | 0.81 |
| Influenza-negative | 1.1 (0–4.7) | 0 (0–4.5) | <0.001 |
| Discharge antibiotic prescription for respiratory illnessa |
61 (35) | 49 (28) | 0.17 |
| Influenza-positive | 1 (13) | 9 (28) | 0.65 |
| Influenza-negative | 60 (24) | 40 (28) | 0.15 |
| Intensive care unit admissiona | 43 (25) | 55 (31) | 0.19 |
| Death due to respiratory illnessa | 4 (0.02) | 2 (0.01) | 0.68 |
Tested using Fisher’s exact or χ2 test.
Tested using Wilcoxon rank-sum test.
Fig. 2.
Histogram of days from sample collection to result in Seasons 1 and 2 (A). Histogram of duration of oseltamivir therapy in influenza-negative patients in Seasons 1 and 2 (B).
On multivariate analysis adjusting for baseline characteristics imbalanced between the two cohorts, similar results were found when comparing the two seasons in time to result (P<0.001) and receipt of antibiotics (P<0.001). Duration of oseltamivir among all patients did not differ significantly between the seasons (P=0.99). However, duration of oseltamivir in influenza-negative patients was significantly reduced in season 2 (P=0.02).
We additionally examined the cost of using the rapid PCR-based influenza test as compared to traditional PCR tests. Using the current costs at our institution for both laboratory testing and antiviral use, we assume a cost of $14.47 per 75 mg tablet of oseltamivir. We assume that the cost for the rapid PCR test is $94.14 as compared to $83.70 for the cost of traditional flu PCR testing. We also assume that among the subset of patients who are flu-negative and receive oseltamivir, those who undergo rapid testing receive 1.1 fewer doses than those who have traditional testing. We find that the cost savings per patient sample varies substantially by the proportion of patients who test flu-positive as well as the percentage of flu-negative patients who receive oseltamivir (Fig. 3). At our institution, approximately 2000 tests are performed per year, with 15% who are flu-positive and 50% of flu-negative patients who receive oseltamivir. In this scenario, the cost savings with use of rapid testing is $2.29 per patient sample tested for a total savings of $4580.
Fig. 3.
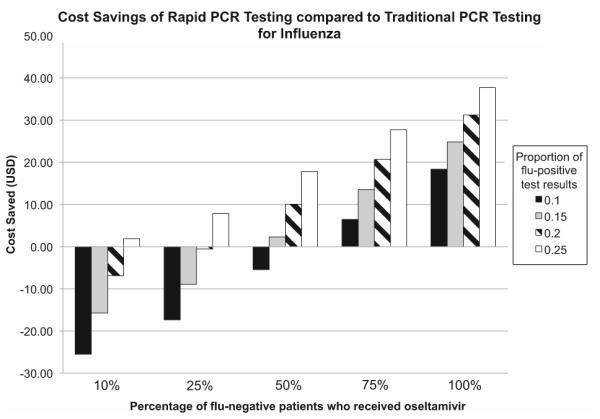
Chart showing cost savings per patient sample tested using rapid PCR-based influenza tests as compared to traditional PCR tests. The x-axis represents the percentage of flu-negative patients who receive oseltamivir. The y-axis represents the numbers of dollars saved per patient sample tested. The bars represent the proportion of overall patients who test positive for influenza.
DISCUSSION
Results of influenza testing performed after implementation of rapid PCR-based influenza testing were available in significantly less time than in the previous respiratory season with traditional influenza RT-PCR tests. Among hospitalized patients who tested influenza-negative, implementation of rapid influenza PCR-based testing was also associated with decreased duration of antiviral use.
Antigen-based RIDTs provide timely results, can be performed on-site with limited training of personnel, and reduce antibiotic use in adult inpatients who test influenza negative [Falsey et al., 2007]. The use of RIDTs in emergency departments reduces ordering of ancillary tests and antibiotic prescriptions and increases antiviral use in patients who test positive for influenza [Blaschke et al., 2014]. However, the sensitivity of these tests is low, ranging from 38% to 60% as compared to RT-PCR testing [Peci et al., 2014]. Traditional influenza RT-PCR tests, which provide sensitive and specific results that can guide clinical decision making, often require several hours to complete, highly trained technical expertise, and a higher cost compared to rapid antigen testing [Kuypers et al., 2006]. The long turn-around time makes them less useful in the acute inpatient setting where a delay in diagnosis may impact patient care. Because of an inability to quickly and reliably diagnose influenza in high-risk patients and the benefit of timely initiation of oseltamivir, the CDC recommends initiation of antiviral therapy without awaiting a testing result. This recommendation has the potential to expose many patients without influenza to unnecessary antiviral therapy and prolong duration of contact and droplet precautions on the inpatient wards.
Rapid PCR-based influenza tests are as sensitive and specific as traditional RT-PCR tests [Woodberry et al., 2013]. Because they require minimal training and equipment and provide results in as little as one hour, they can be performed on an as-needed basis. We show that use of this testing method in a high-risk inpatient population provides results within two hours from time of sample receipt, as compared to over 24 hr with traditional RT-PCR testing performed once daily. At our institution, traditional RT-PCR testing for influenza is performed off-site, resulting in additional delays between sample collection and testing result. This, however, is similar to many institutions where influenza RT-PCR testing is a send-out test. With routine implementation in high-risk settings, it might be possible to consider a change in policy to test for influenza first before initiating antiviral therapy. However, use of rapid PCR-based influenza testing rather than treatment initiation alone is unlikely to be cost-effective based on drug costs alone, as demonstrated by prior modeling studies [Dugas et al., 2013]. In a typical season at our institution, the cost savings is only $4580; however, this does not include a potential reduction in hospital costs associated with earlier discontinuation of isolation precautions in those with rapid testing, which we were not able to measure in this retrospective study. Oseltamivir treatment is relatively inexpensive (cost per dose at our institution: $14.47) with few side effects and drug resistance is unlikely to arise in the setting of a short treatment course. We were unable to assess the effect of rapid testing on duration of isolation precautions and ordering of ancillary tests, all of which could be potential cost benefits of an earlier influenza diagnosis. Implementation of contact and droplet precautions at our hospital is performed by nursing staff and often does not directly correspond to the time that the order is initiated in the electronic medical record. To our knowledge, only one study has examined the impact of influenza testing on clinical outcomes in adult inpatients, and this study was performed using RIDTs [Falsey et al., 2007]. In this study, they found that antibiotic use was lower in inpatients who were antigen-positive, although no difference was found in length of hospital stay between the two groups.
We did not find a decrease in initial prescription rates for oseltamivir in the rapid testing season, as would be expected if clinicians were able to have results within one hour. This is likely due to implementation of the “bundled” approach to the initial management of patients with suspected influenza prior to the rapid testing season. This order set included influenza testing, initiation of oseltamivir treatment, and droplet isolation.
Our study has several limitations. First we analyzed only a small subset of the total patients tested during each season who may not be representative of all the patients during the entire year. Additionally, because of the retrospective nature of the study, we are unable to directly attribute change in antiviral and antibiotic prescribing to implementation of the rapid influenza RT-PCR test alone. As compared to the first season, the rapid testing season had significantly more total cases of influenza. However, adjustments were made in regression analysis for differences between the two seasons with no significant change in results. In addition, we were also unable to match the two seasons in terms of time of testing for patient selection due to the fact that the influenza season peaked in March-April in 2012 as compared to January 2013. Finally, it is possible that availability of the rapid influenza RT-PCR test itself impacted provider behavior.
In conclusion, implementation of rapid influenza RT-PCR testing decreases time to laboratory result by approximately one day and is associated with decreased use of antivirals among hospitalized patients who tested influenza negative. Use of these tests may allow clinicians to rapidly and accurately diagnose influenza in high-risk inpatients, and potentially decreases unnecessary antiviral use in patients without influenza.
ACKNOWLEDGMENTS
We would like to acknowledge Anne Cent for providing data regarding respiratory viral testing results and Reggie Sampoleo, Rohit Shankar, Isabel Palileo, Greg Boughton, and Janet Crowley in the molecular virology laboratory. We would like to acknowledge the patients who participated in this study.
Grant sponsor: National Institutes of Health; Grant number: K23 AI103105.
Prior presentation of results: These results have been presented in part at the Infectious Disease Society of America annual meeting in Philadelphia, PA in October 2014.
Footnotes
CONFLICTS OF INTEREST
JAE has received research support from Gilead, Chimerix, GlaxoSmithKline, and Roche, has received payment for lectures from Abbvie, and serves as a consultant for GlaxoSmithKline and Gilead. HYC has received research support from GlaxoSmithKline. KRJ has received research support from Roche. All other authors declare no conflicts of interest. All other authors declare no sources of financial support.
REFERENCES
- Blaschke AJ, Shapiro DJ, Pavia AT, Byington CL, Ampofo K, Stockmann C, Hersh AL. A national study of the impact of rapid influenza testing on clinical care in the emergency department. J Pediatric Infect Dis Soc. 2014;3:112–118. doi: 10.1093/jpids/pit071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: Results of a randomized, prospective, controlled trial. Pediatrics. 2003;112:363–367. doi: 10.1542/peds.112.2.363. [DOI] [PubMed] [Google Scholar]
- Chan MC, Lee N, Ngai KL, Leung TF, Chan PK. Clinical and virologic factors associated with reduced sensitivity of rapid influenza diagnostic tests in hospitalized elderly patients and young children. J Clin Microbiol. 2014;52:497–501. doi: 10.1128/JCM.02316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas AF, Coleman S, Gaydos CA, Rothman RE, Frick KD. Cost-utility of rapid polymerase chain reaction-based influenza testing for high-risk emergency department patients. Ann Emerg Med. 2013;62:80–88. doi: 10.1016/j.annemergmed.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167:354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- FDA grants first CLIA waiver for nucleic acid-based flu diagnostic test. 2015 Jan 6; Retrieved June 1, 2015, from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm429127.htm.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, Morrow R. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peci A, Winter AL, King EC, Blair J, Gubbay JB. Performance of rapid influenza diagnostic testing in outbreak settings. J Clin Microbiol. 2014;52:4309–4317. doi: 10.1128/JCM.02024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: A randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- Woodberry MW, Shankar R, Cent A, Jerome KR, Kuypers J. Comparison of the Simplexa FluA/B & RSV direct assay and laboratory-developed real-time PCR assays for detection of respiratory virus. J Clin Microbiol. 2013;51:3883–3885. doi: 10.1128/JCM.02395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]