Abstract
Aim:
To conduct a systematic review and meta-analysis to assess the current evidence available regarding the promoting blood circulation and removing blood stasis (PBCRBS) therapy for Chinese patients with acute intracerebral hemorrhage (ICH).
Methods:
Six databases were searched from their inception to November 2013. The studies assessed in ≥4 domains with 'yes' were selected for detailed assessment and meta-analysis. The herbal compositions for PBCRBS therapy for acute ICH patients were also assessed.
Results:
From the 6 databases, 292 studies claimed randomized-controlled clinical trials (RCTs). Nine studies with 798 individuals were assessed in ≥4 domains with 'yes' by using the Cochrane RoB tool. Meta-analysis showed that PBCRBS monotherapy and adjuvant therapy for acute ICH could improve the neurological function deficit, reduce the volume of hematoma and perihematomal edema, and lower the mortality rate and dependency. Moreover, there were fewer adverse effects when compared with Western conventional medication controls. Xueshuantong Injection and Fufang Danshen Injection, Buyang Huanwu Decoction and Liangxue Tongyu formula, and three herbs (danshen root, sanqi and leech) were the most commonly used Chinese herbal patent injections, herbal prescriptions and single herbs, respectively.
Conclusion:
Despite the apparently positive findings, it is premature to conclude that there is sufficient efficacy and safety of PBCRBS for ICH because of the high clinical heterogeneity of the included studies and small number of trials in the meta-analysis. Further large sample-sizes and rigorously designed RCTs are needed.
Keywords: acute intracerebral hemorrhage, traditional Chinese medicine, promoting blood circulation for removing blood stasis, systematic review, meta-analysis
Introduction
Spontaneous intracerebral hemorrhage (ICH) is one of the most detrimental subtypes of stroke. It accounts for 10%–15% of all strokes and is an important public health problem that leads to significant morbidity and mortality1. The epidemiological perspective on ICH remains bleak. ICH affects 24.6 per 100 000 person-years; the median case fatality within 1 month was 40.4%, and the functional dependency after ICH was approximately 75%2. In China, a nation of 1.4 billion, which accounts for almost one-fifth of the world's total population, stroke is already the leading cause of death3. In addition, a recent systematic review showed that the Chinese have higher overall stroke incidence. ICH accounts for a higher proportion of stroke in Chinese than the white population4.
Currently, no specific therapies or treatments improve the outcome after ICH. The updated evidence-based guidelines for the management of ICH from the American Heart Association/American Stroke Association remain multifaceted; most recommendations are symptomatic and supportive5. In the trial in surgery, the results of spontaneous supratentorial lobar intracerebral hematomas (STICH II) confirmed that early surgery did not increase the rate of death or disability at 6 months. This suggested that there is a small survival advantage for patients with ICH who did not have intraventricular hemorrhage6. Acute hemostatic management is not usually recommended to control bleeding in ICH guidelines; the exception is rare cases, such as those including oral anticoagulants, coagulation factor deficiencies, and platelet abnormalities, in which underlying hemostatic abnormalities may contribute to ICH7. Although hemostatic therapy with rFVIIa can limit the extent of hematoma expansion in noncoagulopathic ICH patients, there is an increase in thromboembolic risk with rFVIIa, and the survival or functional outcome after ICH is not increased8. Faced with the limitations of the present available treatments, complementary and/or alternative medicine (CAM) is thus increasingly sought to treat stroke worldwide.
In China, traditional Chinese medicine (TCM), including various forms of herbal medicine, acupuncture, massage (Tuina), exercise (qigong), and dietary therapy, has been used to treat stroke patients for over 2000 years9. In fact, TCM was the major available method of healthcare in the western pacific region of the world before modern Western medicine was introduced. However, stroke was divided into ischemic and hemorrhagic until the end of the Qing Dynasty (1616–1911). A brief history of TCM application in acute ICH has been summarized by Zheng et al10as follows: (1) before the 1950s–1960s, the pathogenesis of acute ICH emphasized up-stirring of the liver and adverse-rising of both blood and qi; (2) from the 1970s, the central pathogenesis of acute ICH has been considered as a blocked passage of the middle Jiao, a disorder of qi in ascending and descending and abnormal flow of qi and blood; (3) from the 1980s, it was claimed that the vital pathogenesis of acute ICH was blood stasis; (4) in recent years, theories of endogenous toxins and deficient vital qi have been developed. However, blood stasis syndrome can be found throughout the pathological process of ICH under the TCM theory of 'abnormal flow of the blood is blood stasis' in Xuezheng Lun (On Blood Syndromes), written by Tang Rong-chuan during the Qing Dynasty. Thus, the key point of the treatment method for ICH was promoting blood circulation for removing blood stasis (PBCRBS). Blood stasis, known as 'Oketsu' in Japanese, 'Xueyu' in Chinese and 'Eohyul' in Korean, refers to whenever the circulation of blood is not smooth or the blood flow is stagnant and forms stasis11. Although the consensus among the interviewed experts was that the definition of blood stasis is rather complicated and that there is no gold standard marker for detecting blood stasis12, blood stasis refers to a group of distinct syndromes. Over the following three decades, there have been a number of clinical trials to evaluate the efficacy and safety of the PBCRBS method for acute ICH. Therefore, the objective of the present systematic review is to assess the current evidence available regarding the PBCRBS method for patients suffering from acute ICH.
Methods
Standard protocol registration
This systematic review was registered in PROSPERO, and the registration identifier of the protocol is CRD4201400900313.
Study criteria
Types of studies
Only randomized controlled clinical trials (RCTs), which evaluate the efficacy and safety of the PBCRBS prescription for acute ICH, were included in the qualitative analyses, regardless of blinding, publication status or language. We included the RCTs assessed in ≥4 domains with 'yes' for the analyses using the Cochrane RoB tool14,15. Quasi-RCTs, taking those using the admission sequence for treatment allocation as example, were not considered.
Types of participants: patients of any gender, age, or race/ethnicity with ICH within 14 d from the onset were included. The ICH was diagnosed according to the Chinese national criteria in Diagnostic Essentials of Various Cerebrovascular Diseases revised at the Fourth National Conference of the China Society of Medicine on Cerebrovascular Diseases in 199516. The diagnosis of ICH was confirmed by CT scan or MRI.
Types of interventions
The patients of the control groups were given Western conventional medication (WCM), WCM plus stereotactic microsurgery (SM), or placebo alone. The patients at the treatment groups were given PBCRBS intervention as add-on therapy, which included PBCRBS prescriptions, Chinese patent herbal oral preparations, and Chinese patent herbal injections. PBCRBS therapy was defined as the use of common blood-invigorating and stasis-removing herbal prescriptions, based on the Eight Principles plus differentiation between qi and blood11, and any Chinese patent herbal preparation that comes from commonly used blood-invigorating and stasis-removing herbs based on both TCM theory and Western medicine. Studies comparing different forms of TCM were excluded. The clinical trials were included regardless of the dosage or duration of treatment. The mode of delivery was the oral route or injection route.
Types of outcome measurements
The primary outcome measures were mortality and dependency at the end of the treatment course or at the end of follow-up. Dependency was defined as needing assistance in the activity of daily living scale (ADL), using the Barthel Index (BI) and modified Rankin Standard (mRS). The secondary outcome measures were the clinical effective rate, the neurological deficit improvement, volume of hematoma, volume of perihematomal edema and adverse events. The neurological deficit improvement was assessed using the Chinese Clinical Neurological Deficit Scale (CCNDS) and National Institutes of Health Stroke Scale (NIHSS) score after treatment.
Literature search
A comprehensive literature search was conducted in CENTRAL (The Cochrane Library), PubMed, EMBASE, Chinese National Knowledge Infrastructure (CNKI), VIP Journals Database and the Wanfang database from inception to December 2013. The search terms used were '(Promoting Blood Circulation OR Removing Blood Stasis) AND (intracerebral hemorrhage OR hemorrhagic stroke)' in English and (Huoxue OR Huayu) AND (Naochuxue OR Chuxuezhongfeng) in Chinese pinyin in the Chinese databases. The search was restricted to clinical trials or reviews. No limitation was placed on publication date, country or language. The reference lists of all relevant articles were also searched for appropriate studies.
Study selection and data collection process
All articles were screened by 2 independent reviewers, who extracted data from the articles according to a standardized data extraction form, including study design, patients' characteristics (age, gender, and onset of ICH), PBCRBS treatment protocol, control intervention, and outcome parameters. The reasons for inclusion and exclusion of studies were recorded at all stages. For eligible studies, 2 authors of this work extracted the data independently. The missing data were obtained by contacting the authors of the original studies. Disagreements were settled through discussion or consultation with corresponding authors.
Risk of bias and grading the quality of evidence
The risk of bias was assessed using the 7 criteria recommended by the Cochrane Handbook17. We included the RCTs assessed in ≥4 domains with 'yes' for the analyses; ie, we excluded those cases assessed in ≥3 domains with 'unclear' or 'no' which were classified as having a high risk of bias14,15. The level of evidence was assessed by the updated GRADE system18. We classified evidence into 4 grades: high quality, moderate quality, low quality and very low quality. The low and very low quality of evidence showed a serious limitation in the study design, study quality, consistency, directness of the evidence and precision of the results.
Description of the herbal medicine and herbal prescription
The selection criteria of high-frequency herbs and herbal prescriptions in the treatment of ICH were those with cumulative frequencies of over 50%.
Data analysis
All data analyses were performed using Review Manager 5.1.0, compiled by the Cochrane Collaboration. The risk ratio (RR), with a 95% confidence interval (CI), was calculated for dichotomous outcomes, whereas weighted mean differences (WMD) or standardized mean differences (SMD) were used for continuous outcomes. Heterogeneity was examined by the chi-square test at a significance level of 0.05. An I2 statistic was also calculated to estimate variation among studies as follows: I2 values of 25%, 50%, and 75% correspond to low, moderate, and high level of heterogeneity, respectively. However, on account of the clinical heterogeneity, all meta-analyses were performed using a random-effect model. Publication bias was detected by funnel plot analyses. Two-tailed P values less than 0.05 were considered statistically significant.
Results
Description of the selection process
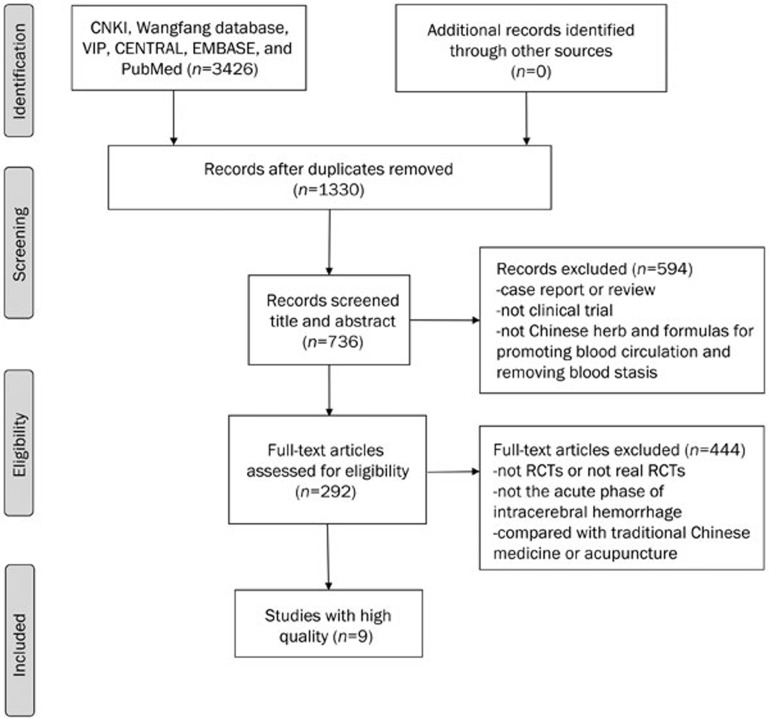
We identified 3426 potentially relevant articles from 6 databases. After removal of duplicates, 1330 records remained. After going through the titles and abstracts, we excluded 594 studies for at least one of following reasons: (1) the study was a case report or review, (2) not a clinical trial, or (3) did not include Chinese herbs and formulas for promoting blood circulation and removing blood stasis. By reading the full texts of the remaining 736 articles, we excluded 444 studies for at least one of following reasons: (1) there were no RCTs or no real RCTs, (2) the study did not include the acute phase of intracerebral hemorrhage, or (3) the study compared TCM or acupuncture. The remaining 292 studies examining the efficacy of Chinese herbs and formulas were included for qualitative analysis. Of the 292 studies, 9 studies assessed ≥4 domains with 'yes'; these were selected for detailed assessment and meta-analysis. The flow diagram is shown in Figure 1.
Figure 1.
PRISMA 2009 flow diagram.
Description of the studies
The sample sizes of the 9 studies ranged from 21 to 213, with a total of 830 subjects (Table 1). All studies were carried out in China and published in Chinese language journals from 2000 to 2013. The criteria used in these 9 studies for diagnosis of ICH were the Chinese Cerebrovascular Disease Diagnosis Standard 1995 (CCDDS 1995). Of the 9 studies, 5 studies compared PBCRBS plus WCM with WCM alone, 2 studies19,20 compared PBCRBS plus WCM plus SM with WCM plus SM, one21 compared PBCRBS plus WCM plus SM with WCM alone, and only one22 used a placebo control. The subjects' durations of ICH reported in the 9 studies were all within 3 d. The course of PBCRBS treatment lasted 10 d to 8 weeks in the 9 studies. Seven of the 9 studies reported follow-up; follow-up was from 30 d to one year after finishing treatment.
Table 1. The characteristics of included studies.
| Included trials | Country/type of case | Eligibility criteria | Study design | Gender (male/female); mean age (years) |
Mean hematoma volume (trial/control) mL (range) | Time of onset | Interventions (n) drug/dosage |
Course of treatment | Follow-up | Course of treatment outcomes | Intergroup differences | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | Control | Trial | Control | ||||||||||
| Wang YQ, 2013 | China/inpatient with intracerebral hemorrhage | CCDDS 1995 | RCT | 21/8, 63.28±10.19 | 30/11, 63.37±9.84 | 28.77±5.68/28.77±5.68 (15–40) | <24 h | XZD+WCM | WCM | 4 w | 1 year | 1. Volume of hematoma (1 month) 2. CCNDS score (3 month, 1 year | 1. P<0.05 2. P<0.05 |
| Zhang SQ, 2012 | China/inpatients with intracerebral hemorrhage | CCDDS 1995 | RCT | 25/20; 55.70±12.16 | 26/19; 56.10±11.45 | 28.20±5.30/28.85±3.25 (10–40) | 1–3 d | BHHD+WCM | WCM | 8 w | 90 d | 1. Volume of hematoma (7, 14, 28 d) 2. Volume of perihematomal edema (7, 14, 28 d) 3. NIHSS score (14, 28, 60, 90 d) 4. Barthel Index (90 d) 5. Clinical efficacy | 1. P<0.05 2. P<0.05 3. P<0.05 4. P<0.05 5. P<0.05 |
| Wang ZF, 2011 | China/inpatient with hypertensive cerebral hemorrhage | CCDDS 1995 | RCT | 55/35, 60.78±5.69 | 53/36, 61.24±5.68 | 24.36±4.25/24.75±4.11 (4–40) | <72 h | THD+WCM | WCM | 14 d | 90 d | 1. Volume of hematoma (14 d) 2. CCNDS score (90 d) 3. Barthel Index (90 d) | 1. P<0.05 2. P<0.01 3. P<0.05 |
| Huang JL, 2010 | China/inpatients with severe cerebellopontine hemorrhage | CCDDS 1995 | RCT | 8/2; 57 | 9/2; 56 | 11.5/11.2 (7–21) | 6–12 h | SM+XHLP+WCM | WCM | 1 m | 6 month | 1. GOS score 2. Time being awake and awake rate 3. Incidence of complications | 1. P<0.01 2. P<0.01 3. P<0.01 |
| Chen SH, 2010 | China/inpatient with intracerebral hemorrhage | CCDDS 1995 | RCT | 108>45 | 105<45 | >5 | <72 h | ZXOS+WCM | Placebo+WCM | 30 d | 60 d | 1. Mortality rate 2. The number of modified rankin score more than 4 3. The number of NIHSS score less than 1 (90 d) 4. GOS score (90 d) 5. The number of modified rankin score less than 2 (90 d) 6. Barthel Index (90 d) | 1. P<0.05 2. P<0.05 3. P<0.05 4. P<0.05 5. P<0.05 6. P<0.05 |
| Sun JH, 2008 | China/inpatient with intracerebral hemorrhage | CCDDS 1995 | RCT | 28/17, 58.36±16.54 | 25/19, 59.62±15.93 | (30–49) | 7–72 h | SM+WCM+SMI | SM+WCM | 21 d | 30 d | 1. Volume of perihematomal edema (14, 21 d) 2. NIHSS score (14, 30 d) | 1. P<0.05 2. P<0.05 |
| Dai MX, 2002 | China/inpatient with hypertensive cerebral hemorrhage | CCDDS 1995 | RCT | 26/14, 58.6±10.8 | 27/13, 58.8±10.5 | 50.8±11.5/50.5±10.8 (30–90) | <24 h | SM+WCM+ZXD | SM+WCM | 10 d | 6 month | 1. Clinical efficacy 2. CCNDS score 3. The number of hematoma dissipation 4. The number of hospitalization days | 1. P<0.05 2. P<0.05 3. P<0.05 4. P<0.05 |
| He D, 2002 | China/inpatient with intracerebral hemorrhage | CCDDS 1995 | RCT | 7/5, 69.20±14.32 | 8/4, 67.22±13.83 | (10–30) | <48 h | XST+WCM | WCM | 14 d | No report | 1. Volume of hematoma (10, 21 d) 2. Volume of cerebral edema (4, 10, 21 d) 3. ESS score (4, 10, 21 d) | 1. P<0.05 2. P<0.05 3. P<0.05 |
| Fan Y, 2000 | China/inpatient with intracerebral hemorrhage | CCDDS 1995 | RCT | 19/13, 64.44±12.12 | 17/15, 61.31±11.91 | 19.22±13.39/19.16±9.82 | <2 d | LTOS+WCM | WCM | 4 week | Not report | 1. Total clinical efficacy 2. Barthel Index (28 d) 3. Grade of cerebral edema | 1. P<0.05 2. P<0.01 3. P<0.01 |
Note: CCDDS, Chinese Cerebrovascular Disease Diagnosis Standard 1995; RCT, Randomized Controlled Trial; BHHD, Bushen Huoxue Huatan Decoction; SM, Stereotactic Microsurgery; WCM, Western Conventional Medication; XHLP, Xingnao Huoxue Lishui Prescription; LTOS, Liangxue Tongyu Oral Solution; SMI, Salviae Miltiorrhizae Injection; XZD, Xuefu Zhuyu Decoction; XST, Xue Shuan Tong; ZXOS, Zhongfeng Xingnao Oral Solution; THD, Tongqiao Huoxue Decoction; ZXD, Zhuyu Xiaozhong Decoction; NIHSS: National Institutes of Health Stroke Scale; ADL, Activities of Daily Living; GOS, Glasgow Outcome Score; ESS, European Stroke Scale; CCNDS, Chinese Clinical Neurological Deficit Scale (1995); d, day.
WCM refer to the combination of needed therapies of the following aspects: (1) General supportive care mainly include: (A) airway, ventilatory support and supplemental oxygen, (B) cardiac monitoring and treatment, (C) temperature, (D) blood pressure, (E) blood glucose and (F) nutrition; (2) No Specialized care included; (3) Treatment of acute complications mainly include: (A) brain edema and elevated intracranial pressure, (B) seizures, (C) pneumonia, (D) deep vein thrombosis prophylaxis, (F) stress ulcer. (4) Intensive care units mainly include: (A) surveillance and monitoring of ICP, cerebral perfusion pressure and hemodynamic function; (B) titration and implementation of protocols for management of ICP, BP, mechanical ventilation, fever, and serum glucose; and (C) prevention of complications of immobility through positioning, airway maintenance, and mobilization within physiological tolerance.
Assessment by the Cochrane's risks of bias
Eight of the 9 studies had at least one domain rated as high risk of bias19,20,21,23,24,25,26,27 (Table 2). In the double-blind study, none of the domains were rated as having high risks of bias. In the 2 single-blind studies, the blinding procedure was not described, and there was a high risk of bias in concealment of allocation. In the 6 open-label studies, there were high risks of bias both in concealment of allocation and blinding: neither the subjects nor the evaluators were blinded.
Table 2. Assessment of study quality and risk of bias.
| First author, year | 7-item criteria |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | ||
| Wang YQ, 2013 | + | − | − | ? | + | + | + | 4 |
| Zhang SQ, 2012 | + | − | − | ? | + | + | + | 4 |
| Wang ZF, 2011 | + | − | − | ? | + | + | + | 4 |
| Chen SF, 2010 | + | + | + | + | + | + | + | 7 |
| Huang JL, 2010 | + | − | − | ? | + | + | + | 4 |
| Sun JH, 2008 | + | − | − | ? | + | + | + | 4 |
| Dai MX, 2002 | + | − | − | ? | + | + | + | 4 |
| He D, 2002 | + | − | ? | ? | + | + | + | 4 |
| Fan Y, 2000 | + | − | ? | ? | + | + | + | 4 |
A to G, the 7-Item criteria. A, adequate sequence generation; B, concealment of allocation; C, blinding of participants and personnel; D, blinding of out-come assessment; E, incomplete out-come data; F, selective reporting; G, other bias; +: low risk of bias, −: high risk of bias, ?: uncertain risk of bias.
Description of the PBCRBS herbs and prescriptions
A total of 41 standardized Chinese herbal formulas were examined in 229 (78.4%) of the 292 studies, while the other 63 studies used an individualized approach. The top 8 herbal formulas were used in 142 (48.6%) of the 292 reviewed studies (Table 3). These included Xueshuantong Injection (11.3%), an extract from Sanqi (Radix Notoginseng); Fufang Danshen Injection/Xiangdan Injection (10.3%), composition of Danshen (Radix Salviae miltiorrhizae) and Jiangxiang (Lignum Dalbergiae Odoriferae); Danshen Injection (7.9%), an extract from Danshen root; Shuxuetong Injection (5.5%), composition of Shuizhi (Hirudo) and Dilong (Lumbricus); Shuxuening injection/Yinxingye Preparations (4.1%), an extract from Yinxingye (Folium Ginkgo); Dengzhan Xixin Injection/Dengzhan Huashu Injection (3.8%), an extract from Dengzhan Xixin (Herba Erigerontis); Buyang Huanwu decoction (3.1%), and Liangxue Tongyu Injection/Liangxue Tongyu oral liquid (2.7%). These commonly used prescriptions corresponded to syndromes, including the syndrome of blood stasis (due to bleeding), syndrome of stagnation of qi and blood stasis, syndrome of blood stasis due to blood deficiency, syndrome of intermingled phlegm and blood stasis, syndrome of blood stasis, syndrome of blood stasis due to coagulated cold, syndrome of blood stasis due to qi deficiency, and syndrome of blood stasis due to heat toxicity, respectively (Table 3). Seven out of the 8 prescriptions are Chinese herbal patent preparations, which are quality controlled for manufacturing methods. In addition, 6 Chinese herbal patent preparations all have injection preparations, which are more convenient during a stroke emergency. The remaining preparation is a famous Chinese herbal prescription, Buyang Huanwu decoction, which is specifically used to treat stroke, according to the theory of qi deficiency and blood stasis recorded in Yilin Gaicuo (Correction of Errors in Medical Classics), written by Wang Qingren in 1830.
Table 3. The most commonly used prescription corresponding to syndrome and pharmacological study for acute intracerebral hemorrhage.
| Prescription: N/292 | Compositions | Function | Syndrome | Pharmacology |
|---|---|---|---|---|
| Xueshuantong Injection: 33 (11.3%) | Sanqi (Radix Notoginseng) | Promoting blood circulation for hemostasis | Syndrome of blood stasis (due to bleeding) | Ameliorate brain edema by inhibiting the expression of AQP-430 and decreasing thrombin generation31, protect neurons and abate neuronal apoptosis by decreasing the expression of Bax and increasing Bcl-232, protect neurons by regulating excitatory amino acid receptors33. |
| Fufang Danshen Injection/Xiangdan Injection: 30 (10.3%) | Danshen (Radix Salviae Miltiorrhizae), Jiangxiang (Lignum Dalbergiae Odoriferae) | Activating qi flowing and promoting blood circulation | Syndrome of stagnation of qi and blood stasis | Ameliorate brain edema by decreasing MDA and increasing SOD activity34, protect neurons and abate neuronal apoptosis by deceasing caspase-335. |
| Danshen Injection: 23 (7.9%) | Danshen (Radix Salviae Miltiorrhizae) | Promoting blood circulation and nourishing blood | Syndrome of blood stasis due to blood deficiency | |
| Shuxuetong Injection: 16 (5.5%) | Shuizhi (Hirudo), Dilong (Lumbricus) | Drastically removing blood stasis and removing obstruction in collaterals | Syndrome of intermingled phlegm and blood stasis | Ameliorate brain edema as a thrombin inhibitor36, improve the development of hyperlasia of capillary, glial cells and their activities37. |
| Shuxuening injection/Yinxingye Preparations: 12 (4.1%) | Yinxingye (Folium Ginkgo) | Promoting blood circulation for removing blood stasis | Syndrome of blood stasis | Abate neuronal apoptosis by decreasing the expression of Bax and caspase-3 and increasing Bcl-238, protect brain against inflammatory injury by the inhibition of IL-8 and ICAM-1 mediated neutrophil infiltration39. |
| Dengzhan Xixin Injection/Dengzhan Huashu Injection: 11 (3.8%) | Dengzhan Xixin (Herba Erigerontis) | Dispelling cold and activating blood circulation | Syndrome of blood stasis due to coagulated cold | Protect neurons by decreasing inflammatory factors including TNF-α and IL-8 in brain areas around hemorrhagic focus40, alleviate secondary nerve damage by repressing the expression of ICAM-141. |
| Buyang Huanwu Decoction: 9 (3.1%) | Danshen (Radix Salviae Miltiorrhizae), Chishao (Radix Paeoniae Rubra), Danggui (Radix Angelicae Sinensis), Chuanxiong (Rhizoma Ligustici Chuanxiong), Huangqi (Radix Astragali seu Hedysari), Taoren (Semen Persicae), Honghua (Flos Cartham), Niuxi (Radix Achyranthis Bidentatae), Dilong (Lumbricus) | Benefiting qi for activating blood circulation | Syndrome of blood stasis due to qi deficiency | Improve the neurological function deficits and alleviate brain edema through inhibition of AQP4 expression42, prohibit neuronal apoptosis and promote absorption of the hematoma through decreasing the expression of activated caspase-343, enhance angiogenesis by promoting the expression of VEGF44. |
| Liangxue Tongyu Injection/Liangxue Tongyu oral liquid: 8 (2.7%) | Shuniujiao (Cornu Bubali), Dahuang (Radix et Rhizoma Rhei), Shengdi (Radix Rehmanniae Recens), Mudanpi (Cortex Moutan Radicis), Sanqi (Radix Notoginseng), Shichangpu (Rhizoma Acori Tatarinowii), Zhizi (Fructus Gardeniae) | Cooling blood and removing blood stasis, purging fu-viscera, clearing heat-toxicity | Syndrome of blood stasis due to heat-toxicity | Ameliorate brain edema and facilitate hematoma removal through up-regulation of t-PA and tissue inhibitor of metalloproteinase-1, and down-regulation of matrix metalloproteinase-945,46. |
Note: AQP-4, aquaporins-4; MDA, malondialdehyde; SOD, superoxide dismutase; IL-8, interleukin-8; ICAM-1, intercellular adhesion molecule-1; VEGF, vascular endothelial growth factor; t-PA, tissue plasminogen activator; TNF-α, tumor necrosis factor-α.
The numbers of Chinese herbs in the formulas varied from 1 to 9. The top 15 single herbs are shown in Table 4. Danshen (Radix Salviae miltiorrhizae) was the most frequently used single herb; it was used in 109 (37.3%) of the 292 studies. This was followed by Sanqi (Radix Panax notoginseng) (36.3%), Shuizhi (Hirudo) (30.1%), Chuanxiong (Rhizoma Ligustici Chuanxiong) (27.4%), Taoren (Semen Persicae) (23.3%), Chishao (Radix Paeoniae rubra) (20.2%), Honghua (Flos Cartham) (19.9%), Dilong (Lumbricus) (18.8%), Danggui (Radix Angelicae sinensis) (16.8%), Chuanniuxi (Radix Cyathulae) (13.0%), Jiangxiang (Lignum Dalbergiae Odoriferae) (12.0%), Huangqi (Radix Astragali seu Hedysari) (6.8%), Yujin (turmeric root tuber, Curcuma longa) (5.5%), Shexiang (musk) (5.1%), and Quanxie (scorpion) (4.5%).
Table 4. The most commonly used herbs for acute intracerebral hemorrhage.
| Chinese name | English name | Latin name | Family | N/292 (%) |
|---|---|---|---|---|
| Dan Shen | Danshen root | Radix Salviae Miltiorrhizae | Salvia miltiorrhiza Bge | 109 (37.3) |
| San Qi | Sanqi | Radix Notoginseng | Panax notoginseng (Burk) F H Chen | 106 (36.3) |
| Shui Zhi | Leech | Hirudo | Hirudo nipponica Whitman | 88 (30.1) |
| Chuan xiong | Sichuan lovage rhizome | Rhizoma Ligustici Chuanxiong | Ligusticum chuanxiong Hort | 80 (27.4) |
| Tao Ren | Peach seed | Semen Persicae | Amygdalus persica L | 68 (23.3) |
| Chi Shao | Peony root | Radix Paeoniae Rubra | Paeonia lactiflora Pall | 59 (20.2) |
| Hong Hua | Safflower | Flos Carthami | Carthamus tinctorius L | 58 (19.9) |
| Di Long | Earthworm | Lumbricus | Pheretima aspergillum (E Perrier) | 55 (18.8) |
| Dang Gui | Chinese angelica | Radix Angelicae Sinensis | Angelica sinensis (Oliv) Diels | 49 (16.8) |
| Chuan Niu Xi | Medicinal cyathula root | Radix Cyathulae | Cyathula officinalis Kuan | 38 (13.0) |
| Jiang Xiang | Rosewood | Lignum Dalbergiae Odoriferae | Dallbergia odarifera T Chen | 35 (12.0) |
| Huang qi | Milkvetch root | Radix Astragali seu Hedysari | Astragalus membranaceus (Fisch) Bge var Mongolicus (Bge) Hsiao | 20 (6.8) |
| Yujin | Turmeric root tuber | Radix Curcumae | Curcuma wenyujin YH Chen et C Ling, Curcuma longa L, Curcuma kwangsiensis S G Lee et C F Liang, Curcuma phaeocaulis Val | 16 (5.5) |
| Shexiang | Musk | Moschus | Moschus berezovskii Flerov, Moschus sifanicus Przewalski, Moschus moschiferus Linnaeus | 15 (5.1) |
| Quanxie | Scorpion | Scorpion | Buthus martensii Karsch | 13 (4.5) |
Efficacy assessment
Mortality
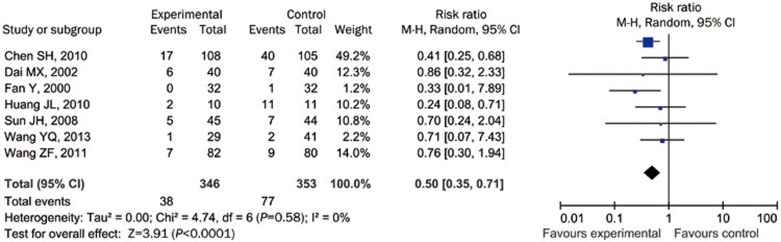
The mortality was reported in 7 of the 9 studies. Meta-analysis showed that PBCRBS treatment significantly reduced the mortality rate in the trial group compared with the control group. The risk ratio in the 7 studies varied from 0.24 to 0.86, with an overall risk ratio of 0.50 (95% CI: 0.35 to 0.71, P<0.05, I2=0%, Figure 2). In the double-blind placebo control study22, the risk ratio was 0.41, which was lower than the overall risk ratio.
Figure 2.
The forest plot: mortality rate.
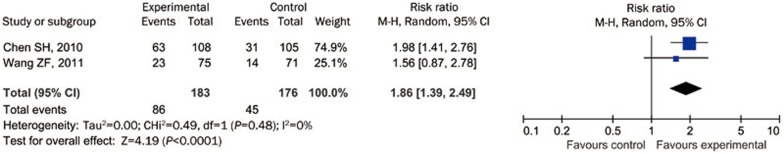
ADL score
The Barthel Index was used in 2 studies22,27 and evaluated at 90 d after PBCRBS treatment. There were significant differences between the PBCRBS group and the control group (1.86, 95% CI: 1.39 to 2.49, P<0.05, I2= 0%, Figure 3). In addition, the mRS was used in Chen's study22. At the last time point investigated, there were 60 patients (55.5%) who achieved a good outcome (mRS 0, 1, 2) in the PBCRBS group and 30 patients (28.5%) in the control group; there were significant differences among these studies, according to the Cochran-Mantel-Hansel (CMH) test (P=0.048). For the subjects who began treatment within 24 h after an attack, there were 5 patients (4.6%) who had severe disability (mRS 4, 5, 6) in the PBCRBS group and 9 patients (8.6%) in the control group; there were no significant differences between these groups, according to the CMH test (P=0.741). For the subjects who began treatment between 24 and 48 h after the attack, there were 5 patients (5.1%) who had severe disability (mRS 4, 5, 6) in the PBCRBS group and 15 patients (16.7%) in the control group; there was a significant difference between these two groups, according to the CMH test (P=0.048). For the subjects who began treatment between 48 and 72 h after an attack, there were 7 patients (7.5%) who had severe disability (mRS 4, 5, 6) in the PBCRBS group and 19 patients (21.2%) in the control group; there were significant differences between these groups, according to the CMH test (P=0.031).
Figure 3.
The forest plot: the barthel index.
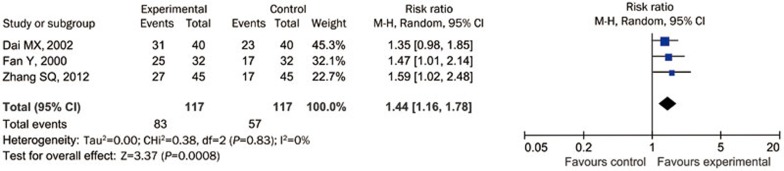
Clinical effective rate
The clinical efficacy was reported in 3 of the 9 studies. The definition of effective rate was not standardized. It was defined according to CCNDS 1995 in 2 studies20,26 and according to the clinical guidelines for new drugs for TCM28 in the remaining 1 study24. The risk ratio of clinical efficacy in the 3 studies varied from 1.35 to 1.59, with an overall risk ratio of 1.44 (95% CI: 1.16 to 1.78, P<0.05, I2=0%, Figure 4); for the SM group20, the risk ratio was 1.35; for the non-SM groups24,26, the risk ratios were 1.47 and 1.59, respectively.
Figure 4.
The forest plot: Clinical effective rate.
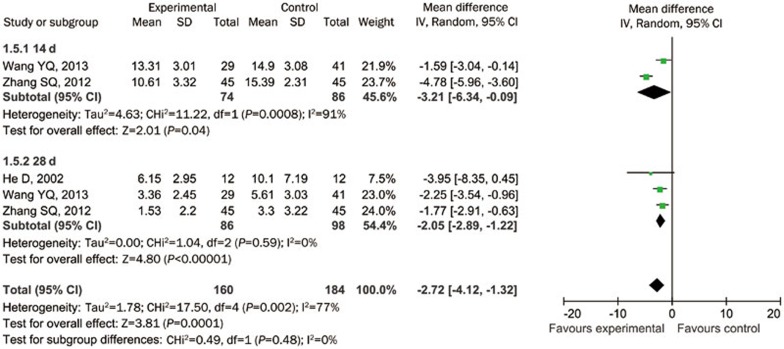
Volume of hematoma
The volume of hematoma was used as an outcome measure in 3 of the 9 studies; the volume was evaluated at 14 and 28 d after PBCRBS treatment. Meta-analysis showed that PBCRBS treatment significantly reduced the volume of hematoma in the trial group when compared with the control group (−2.72, 95% CI: −4.12 to −1.32, P<0.05, I2=77%). The volume of hematoma in the trial group was more significantly reduced than that in the control group at 28 d (−2.05, 95% CI: −2.89 to −1.22, P<0.05, I2=0%) after PBCRBS treatment, but not at 14 d (−3.21, 95% CI: −6.34 to −0.09, P=0.04, I2=91%, Figure 5).
Figure 5.
The forest plot: volume of hematoma.
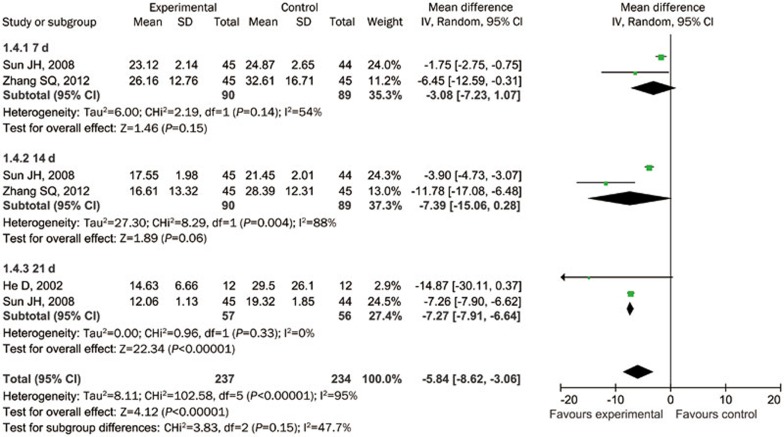
Volume of perihematomal edema
The volume of perihematomal edema was used as an outcome measure in 3 of the 9 studies and evaluated at 7, 14, and 21 d after PBCRBS treatment. Meta-analysis showed that PBCRBS treatment significantly reduced the volume of perihematomal edema in the trial group compared with the control group (−5.84, 95% CI: −8.62 to −3.06, P<0.05, I2=95%). After PBCRBS treatment, significant differences between the trial group and control group were detected at 21 d (−7.27, 95% CI: −7.91 to −6.64, P<0.05, I2=0%), but not at 7 d (−3.08, 95% CI: −7.23 to 1.07, P=0.15, I2=54%) or 14 d (−7.39, 95% CI: −15.06 to 0.28, P=0.06, I2=88%, Figure 6).
Figure 6.
The forest plot: volume of perihematomal edema.
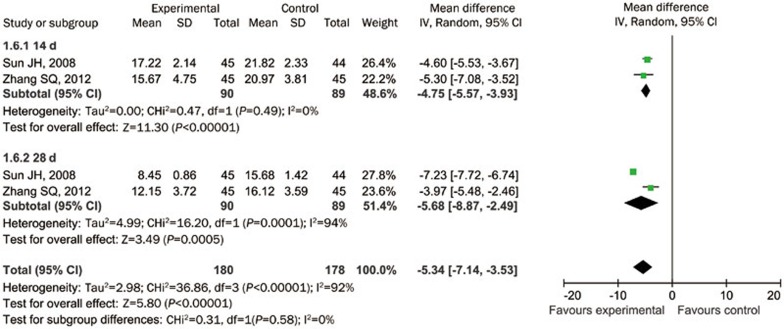
NIHSS score
Two19,26 of the 9 studies used NIHSS scores to determine the effect of PBCRBS on the neurological function of the patients. The NIHSS score was assessed at 14 and 28 d after PBCRBS treatment. The overall results indicate that the PBCRBS group had a measurably better recovery of neurological functions than the control group (−5.34, 95% CI: −7.14 to −3.53, P<0.05, I2=92%). Furthermore, the NIHSS score in the PBCRBS group was significantly lower when compared with the control group at 14 d (−4.75, 95% CI: −5.57 to −3.93, P<0.05, I2=0%) and 28 d (−5.68, 95% CI: −8.87 to −2.49, P<0.05, I2=94%, Figure 7).
Figure 7.
The forest plot: national institute of health stroke scale (NIHSS) score.
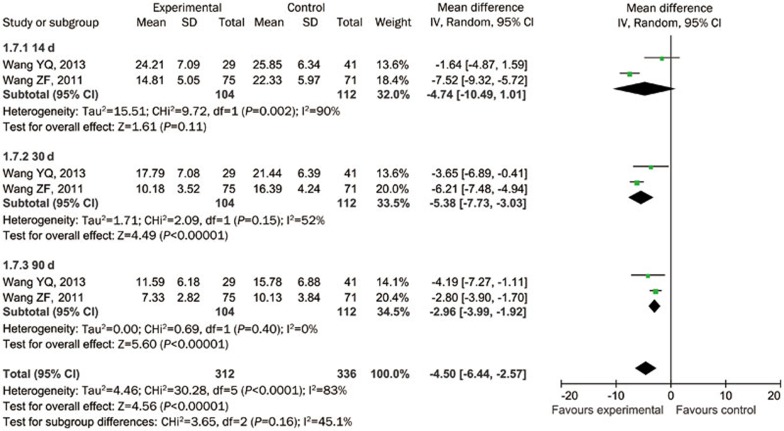
CCNDS score
The CCNDS score was used in 2 studies25,27 and assessed at 14, 30 and 90 d following PBCRBS treatment. Meta-analysis showed that PBCRBS treatment significantly reduced the CCNDS score in the trial group compared with the control group (−4.50, 95% CI: −6.44 to −2.57, P<0.05, I2=83%). After PBCRBS treatment, significant differences between the trial group and control group were detected at 30 d (−5.38, 95% CI: −7.73 to −3.03, P<0.05, I2=52%) and 90 d (−2.96, 95% CI: −3.99 to −1.92, P<0.05, I2=83%), but not at 14 d (−4.74, 95% CI: −10.49 to 1.01, P=0.11, I2=90%, Figure 8).
Figure 8.
The forest plot: Chinese clinical neurological deficit scale (CCNDS) score.
Adverse event reporting
Adverse events were reported in 2 of the 9 studies and not mentioned in the remaining 7 studies. Chen et al22 reported that there were 24 cases of adverse events related to drugs and 2 cases of serious adverse events in the trial group, but there were 41 cases of adverse events and 6 cases of serious adverse events in the control group. The frequency of adverse events in this study was 26/108 in the trial group and 47/105 in the control group. Fan et al24 reported 7 types of adverse events, including 2 cases of mild diarrhea, 3 cases of mild epigastric discomfort, 3 cases of transient renal damage, 5 cases of electrolyte imbalances, and 1 case of melena in the trial group; there were 8 cases of transient renal damage, 17 cases of electrolyte imbalances, 3 cases of transient liver damage, 6 cases of melena and 1 case of hematemesis in the control group.
GRADE profile evidence
Quality assessment of the evidence is shown in Table 5. The quality of evidence in the outcomes of the clinical effectiveness rate, Barthel Index (90 d), volume of perihematomal edema (21 d), volume of hematoma (28 d), and neurological deficit scores (NIHSS score and CCNDS score) were high; the quality of evidence in the outcomes of mortality rate, volume of perihematomal edema (7 d and 14 d), and volume of hematoma (14 d) were moderate.
Table 5. The updated GRADE profile.
| Quality assessment |
Summary of findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No of patients |
|||||||||
| No of studies (design) | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Experimental | Control | Relative risk (95% CI) | Quality |
| The clinical effective rate | |||||||||
| 3 (RCT) | Concealment and blinding not clear in most studies1 | No serious inconsistency | No serious indirectness | No serious imprecision | Funnel plot asymmetrical2 | 83/117 | 57/117 | RR 1.46 (1.17 to 1.81) | ⊕⊕⊕⊕ High3 |
| Mortality rate | |||||||||
| 7 (RCT) | Concealment and blinding not clear in most studies4 | No serious inconsistency | No serious indirectness | Serious imprecision5 | Funnel plot asymmetrical2 | 38/346 | 77/353 | RR 0.5 (0.35 to 0.7) | ⊕⊕⊕O Moderate due to imprecision6 |
| Barthel Index 90 d | |||||||||
| 2 (RCT) | Lack of concealment and blinding7 | No serious inconsistency | No serious indirectness | No serious imprecision | Funnel plot asymmetrical2 | 84/183 | 45/176 | RR 1.4 (1.22 to 1.55) | ⊕⊕⊕⊕ High3 |
| Volume of perihematomal edema8 - 7 d | |||||||||
| 2 (RCT) | Lack of concealment and blinding7 | No serious inconsistency | No serious indirectness | Serious imprecision5 | Funnel plot asymmetrical2 | 90 | 89 | −8 | ⊕⊕⊕O Moderate due to imprecision6 |
| Volume of perihematomal edema8 - 14 d | |||||||||
| 2 (RCT) | Lack of concealment and blinding7 | CI show no overlap and P-value on test for heterogeneity <0.0001, I2=88%9 | No serious indirectness | No serious imprecision | Funnel plot asymmetrical2 | 90 | 89 | −8 | ⊕⊕⊕O Moderate due to inconsistency10 |
| Volume of perihematomal edema8 - 21 d | |||||||||
| 2 (RCT) | Concealment and blinding not clear in most studies1 | No serious inconsistency | No serious indirectness | No serious imprecision | Funnel plot asymmetrical2 | 57 | 56 | −8 | ⊕⊕⊕⊕ High3 |
| Volume of hematoma8 - 14 d | |||||||||
| 2 (RCT) | Lack of concealment and blinding7 | CI show no overlap and P-value on test for heterogeneity <0.0008, I2=91%9 | No serious indirectness | No serious imprecision | undetected | 74 | 86 | −8 | ⊕⊕⊕O Moderate due to inconsistency10 |
| Volume of hematoma8 - 28 d | |||||||||
| 3 (RCT) | concealment and blinding not clear in most studies1 | No serious inconsistency | No serious indirectness | No serious imprecision | Funnel plot asymmetrical2 | 86 | 98 | −8 | ⊕⊕⊕⊕ High3 |
| Neurological deficit scores8 - NIHSS score | |||||||||
| 2 (RCT) | Lack of concealment and blinding7 | No serious inconsistency | No serious indirectness | No serious imprecision | Undetected | 90 | 89 | −8 | ⊕⊕⊕⊕ High11 |
| Neurological deficit scores8 - CCNDS score | |||||||||
| 3 (RCT) | Lack of concealment and blinding7 | P-value on test for heterogeneity <0.008, I2=79%9 | No serious imprecision | No serious indirectness | Funnel plot asymmetrical2 | 138 | 145 | −8 | ⊕⊕⊕⊕ High12 |
RCT, random clinical trail; CI, confidence interval; MD, mean difference.
1 Unclear allocation concealment in all studies, participants and personnel blinded in only one study, outcome assessors not blinded in any study. Final decision was not to rate down for risk of bias.
2 Funnel plot was asymmetrical, while number of included trials were limited and further research may change the estimate. Final decision was not to rate down for risk of bias.
3 Although there also was concern about a risk of bias and publication bias, we did not further rate down the quality of evidence because not every criterion appeared to justify rating down by one level.
4 Allocation concealment in only one study, participants and personnel blinded in two studies, outcome assessors blinded in only one study. Final decision was not to rate down for risk of bias.
5 The confidence interval (CI) included no effect.
6 We rated down for imprecision. Although there also was concern about a risk of bias and publication bias, we did not further rate down the quality of evidence because not every criterion appeared to justify rating down by one level.
7 Unclear allocation concealment and blinding in all studies. Final decision was not to rate down for risk of bias.
8 Continuous outcome, therefore no relative effect is given.
9 CI show no overlap and the statistical test for heterogeneity shows a low P-value and the I2 is large. Final decision was rate down for inconsistency.
10 We rated down for inconsistency. Although there also was concern about a risk of bias and publication bias, we did not further rate down the quality of evidence because not every criterion appeared to justify rating down by one level.
11 Although there also was concern about a risk of bias, we did not further rate down the quality of evidence because not every criterion appeared to justify rating down by one level.
12 Although there also was concern about a risk of bias, inconsistency and publication bias, we did not further rate down the quality of evidence because not every criterion appeared to justify rating down by one level.
Discussion
Summary of evidence
This study is an updated systematic review and meta-analysis of the efficacy and safety of PBCRBS for ICH. Two hundred and 29 studies claimed RCTs. Nine better quality studies with 798 individuals assessed in ≥4 domains with 'yes' were identified based on the Cochrane RoB tool. The main findings were that PBCRBS monotherapy and adjuvant therapy for acute ICH could improve neurological function deficits, reduce the volume of hematoma and perihematomal edema, and lower the mortality rate and dependency; there were fewer adverse effects in comparison with WCM controls. The quality of the evidence was mostly moderate to high, according to the quality assessment, using the GRADE methodology and profiler. Despite the apparently positive findings, it is premature to conclude that there is increased efficacy and safety of PBCRBS for ICH because of the high clinical heterogeneity of the included studies and small number of trials in the meta-analysis.
Limitations
There are several limitations to this review. First, none of included studies in this review had been formally registered. Thus, protocols were not available to confirm free of selective reporting29. Second, although the strength of this systematic review and meta-analysis was that all the RCTs included were of better quality and assessed in ≥4 domains with 'yes', we did acknowledge some methodological weaknesses in the primary studies, such as allocation concealment and blindedness. Research outcomes could possibly be influenced by either the selection bias or the observer bias. Third, among the 9 included studies, only one22 used a formal placebo control. Because of the lack of placebo controls, the interpretation of the positive findings of treatment with Chinese herbal medicine (CHM) should be made with caution. Fourth, most of the included studies were of relatively small sample size and did not include a formal sample size estimation. Trials with inadequate sample sizes often run the risk of overestimating intervention benefits30. Fifth, the clinical heterogeneity compromised the validity of the included studies. There were large variations in the formulation, dosage, administration, and duration of treatments of the CHM in the included studies.
Implications for practice
Based on a brief overview of TCM application history of acute ICH10, the main methods for ICH were the following: (1) subduing the liver yang, calming down the internal wind and clearing heat; (2) purging the Fu organ and the harmony of qi and blood; (3) PBCRBS; and (4) anti-toxin or benefiting vital qi. However, PBCRBS is the essence of the TCM treatment method for ICH because blood stasis syndrome can be found throughout the pathological process of ICH. On a practical level, this systematic review provides premature evidence for the efficacy and safety of PBCRBS therapy after acute ICH; the evidence remains limited because of the high clinical heterogeneity and small number of trials included. However, it should be remembered that a lack of scientific evidence does not necessarily mean that the treatment is ineffective31.
Modern pharmacological studies further supported the potential use of PBCRBS therapy for acute ICH as follows
(1) Sanqi (Radix Notoginseng) could ameliorate brain edema by inhibiting the expression of AQP-432 and decreasing thrombin generation33, protect neurons and abate neuronal apoptosis by decreasing the expression of Bax and increasing Bcl-234, and protect neurons by regulating excitatory amino acid receptors35; (2) Danshen (Radix Salviae miltiorrhizae) could ameliorate brain edema by decreasing malondialdehyde (MDA) and increasing superoxide dismutase (SOD) activity36, and protect neurons and abate neuronal apoptosis by deceasing caspase-337; (3) Shuizhi (Hirudo) could ameliorate brain edema as a thrombin inhibitor38, improve the development of hyperplasia of the capillaries, glial cells and their respective activities39; (4) an extract from Yinxingye (Folium Ginkgo biloba) could abate neuronal apoptosis by decreasing the expression of Bax and caspase-3 and increasing Bcl-240, protect the brain against inflammatory injury by the inhibition of interleukin (IL)-8 and intercellular adhesion molecule (ICAM)-1 mediated neutrophil infiltration41; (5) an extract from Dengzhan Xixin (Herba Erigerontis) could protect neurons by decreasing inflammatory factors, including TNF-α and IL-8 in the brain areas around the hemorrhagic focus42, alleviate secondary nerve damage by repressing the expression of ICAM-143; (6) Buyang Huanwu decoction could improve the neurological function deficits and alleviate brain edema through inhibition of AQP-4 expression44, prohibit neuronal apoptosis and promote absorption of the hematoma through decreasing the expression of activated caspase-345, enhance angiogenesis by promoting the expression of VEGF46; and (7) Liangxue Tongyu preparations could ameliorate brain edema and facilitate hematoma removal through up-regulation of t-PA and tissue inhibitor of metalloproteinase-1, and down-regulation of matrix metalloproteinase-947,48. These herbal preparations may clinically contribute to further combating ICH.
It is worth noting that PBCRBS for acute ICH may raise a concern regarding the enlargement of the hematoma because doctors are afraid of potentially increasing bleeding. In actuality, hematoma size and hematoma expansion is an independent predictor of mortality and functional outcomes49. It has been well established that initial hemorrhage volume in approximately one-third of spontaneous ICH patients is not static but frequently progresses, usually within the early 6 h after the ictus50. Thus, the selection of patients at high risk for hematoma enlargement is crucial for PBCRBS treatment. Although most studies reporting PBCRBS therapy did not increase or reduce hemorrhage volume and mortality rate, an issue of hematoma expansion may still warrant consideration. In addition, Nie et al51 reported that Panax Notoginseng saponins were used in treating ICH patients with large amounts of hematoma at super-early stages, which may worsen brain edema and increase the nerve defect score, although it could promote the absorptance of the hematoma. Thus, we suggest several aspects for safety considerations as follows: (1) the contrast extravasation on CT angiography (CTA) is a well-established imaging predictor for subsequent hematoma expansion that may be used as a useful imaging marker to guide therapies; (2) a number of risk factors have been associated with hematoma progression, such as an irregularly shaped hematoma, coagulation abnormalities, hyperglycemia, hypertension, and anticoagulation. These risk factors should be controlled when the levels are compatible; (3) PBCRBS therapy is best to use 24 h, or at least 6 h, after ICH onset; (4) the most common herbs and prescriptions, especially their method of treatment, that are identified in the present study may be used prior to the clinic visit; (5) intensive monitoring of adverse reactions related to PBCRBS use in ICH patients should be performed.
Implications for further research
This work identifies some key areas for further research. Firstly, PBCRBS is widely used in the treatment of ICH. The most common herbal preparation is a promising candidate for further mechanism study and clinical trial of ICH. For example, thrombin plays dual roles both in brain injury and in brain protection after ICH; its deleterious effects come either from resident neural cells or from prothrombin in the blood52. Hirudin, a thrombin inhibitor, is a naturally occurring peptide in the salivary glands of medicinal leeches (such as Hirudo medicinalis) that has a blood anticoagulant property. An upcoming random double-blind controlled clinical trial will investigate the safety and efficacy of acute ICH treated with Hirudo and Tabanus PBCRBS therapy. The question whether the traditional methods can influence brain hematoma enlargement must be verified. This study is currently recruiting participants53. Secondly, disease-syndrome combination mainly refers to the idea and theory of disease differentiation in Western medicine as well as syndrome differentiation in TCM. The syndrome is not only the core of TCM basic theory and syndrome differentiation but also the bridge to associate disease and prescription, ie, prescription corresponding to syndrome54. Based on each individual syndrome, a precisely tailored Chinese herbal prescription for individuals can help to improve the efficacy of the selected TCM herbal prescription (Fufang) intervention55. One high-quality study published in JAMA56indicated that using individualized CHM for the treatment of irritable bowel syndrome is more effective than prescribing a common hypnotic prescription. The research on blood stasis syndrome and corresponding PBCRBS therapy is one of the most active areas regarding syndrome identification in China57. In recent years, the essence of blood stasis syndrome was mostly investigated in the form of disease-syndrome combination. Interesting, all patients suffering from ICH may be considered as having a blood stasis syndrome according to the TCM theory of 'the blood flow outside the vessels is the blood stasis'; this also takes advantage of the utility of a CT scan or MRI in the diagnosis of ICH. Thus, all ICH patients can be treated with PBCRBS therapy. However, the syndrome summarizes the nature, location, and syndrome of diseases, which is traditionally identified from a comprehensive analysis of clinical information from four main diagnostic TCM methods: observation, listening, questioning, and pulse analyses58. Whether modern blood stasis is different from traditional blood stasis and whether their correlation with prescriptions corresponds to the syndrome requires further clarification; this may contribute to not only the essence of the blood stasis syndrome but also evidence-based syndrome identification in ICH. Thirdly, although the PBCRBS evaluated in this review was well tolerated by ICH patients, the safety of PBCRBS could not be confirmed because only 2 studies reported the safety of interventions or investigated adverse events. Investigators of these studies might have underestimated possible adverse events. In addition, the safety of herbal patent injection itself has become a major concern to both national health authorities and the general public59. A standard reporting format for adverse drug reactions (ADR) has been developed60, and we suggest that improvement of the reporting of adverse events and ADRs of PBCRBS should be closely followed. Fourthly, improvement in the methodological quality of primary RCTs is still crucial for future clinical studies. We recommend that specific guidelines, such as the CONSORT 2010 statement61, guidelines for RCTs investigating CHM62 and CONSORT for TCM63, should be used as a combined guideline when designing and reporting RCTs for CHM.
Conclusion
Despite the apparently positive findings, it is premature to conclude that there is increased efficacy and safety of PBCRBS for ICH because of the high clinical heterogeneity in the included studies and small number of trials in the meta-analysis. However, this work identifies some key areas for further research. Further large sample size and rigorously designed RCTs are still needed.
Author contribution
Ji-ping FAN, Guo-qing ZHENG, Hui-qin LI, and Jing-jing WEI designed the research; Hui-qin LI, Jing-jing WEI, Wan XIA, Ji-huang LI, Ai-ju LIU, Su-bing YIN, Chen WANG, Liang SONG, and Yan WANG performed the research; Hui-qin LI, Jing-jing WEI, Wan XIA, Ji-huang LI, Ai-ju LIU, Su-bing YIN, Chen WANG, Liang SONG, and Yan WANG analyzed the data; Hui-qin LI, Jing-jing WEI, Guo-qing ZHENG, and Ji-ping FAN wrote the paper.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (81173395/H2902); the young and middle-aged university discipline leaders of Zhejiang province, China (2013277); the Project of Wenzhou Municipal Science and Technology Bureau in Zhejiang province (Y20110031); and the Administration of Traditional Chinese Medicine of Zhejiang Province (2011ZB094).
References
- 1Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009; 373: 1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnicorigin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–76. [DOI] [PubMed] [Google Scholar]
- 3Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke 2011; 42: 3651–4. [DOI] [PubMed] [Google Scholar]
- 4Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 2013; 81: 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Morgenstern LB, Hemphill JC 3rd, Anderson C, Becker K, Broderick JP, Connolly ES Jr, et al. American heart association stroke council and council on cardiovascular nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010; 41: 2108–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013; 382: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Toyoda K, Steiner T, Epple C, Kern R, Nagayama M, Shinohara Y, et al. Comparison of the European and Japanese guidelines for the acute management of intracerebral hemorrhage. Cerebrovasc Dis 2013; 35: 419–29. [DOI] [PubMed] [Google Scholar]
- 8Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med 2008; 358: 2127–37. [DOI] [PubMed] [Google Scholar]
- 9Wang Y, Fan YC, Xie CL, Zheng GQ. History of post–stroke epilepsy in ancient China. J Neurol 2011; 258: 1555–8. [DOI] [PubMed] [Google Scholar]
- 10Zheng GQ, Huang PX. Origin and development of hemorrhagic stroke. Chin J Med Hist 2005; 35: 25–8. [PubMed] [Google Scholar]
- 11Chen KJ. Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy. Chin J Integr Med 2012; 18: 891–6. [DOI] [PubMed] [Google Scholar]
- 12Choi TY, Jun JH, Lee JA, Park B, You S, Jung J, et al. Expert opinions on the concept of blood stasis in China: an interview study. Chin J Integr Med 2014. doi: 10.1007/s11655-014-1983-3. [DOI] [PubMed]
- 13Li HQ, Liu AJ, Xia W, Li JH, Wei JJ, Yin SB, et al. Promoting blood circulation for removing blood stasis as therapy for acute intracerebral hemorrhage: a systematic review and meta-analysis [database on the Internet]. York: University of York (UK). c2014 - [cited 2014 Jun 18]. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014009003 [DOI] [PMC free article] [PubMed]
- 14Higgins JP, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Chung JH, Lee JW, Jo JK, Kim KS, Lee SW. A quality analysis of randomized controlled trials about erectile dysfunction. World J Mens Health 2013; 31: 157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Chen QT. Classification, diagnostic criteria and evaluation of neurological impairment for stroke patients. Chin J Neurol 1996; 29: 379. [Google Scholar]
- 17Cochrane.org [www.cochrane-handbook.org]. London: The Cochrane Collaboration (UK); c2008 [updated March 2011; cited 2014 Jun 18]. Available from: www.cochrane-handbook.org
- 18Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013; 66: 151–7. [DOI] [PubMed] [Google Scholar]
- 19Sun JH, Chen W. Clinical study of minimally traumatic puncture drainage of hematoma combined with Salviae Miltiorrhizae injection in treatment of hypertensive cerebral hemorrhage. Chin J Integr Tradit And Western Med 2008; 15: 28–30. Chinese. [Google Scholar]
- 20Dai MX, Xiao YJ, Wang JP, Xiao HL, Tang CH, et al. Clinical efficacy of zhuyuxiaozhong decoction combined with Stereotactic microsurgery therapy in treating hypertensive cerebral hemorrhage. J New Chin Med 2002; 3: 40–2. Chinese. [Google Scholar]
- 21Huang JL, Li YH, Lin ZP. Therapeutic efficacy of stereotactic microsurgery combined with therapy of inducing resuscitation, activating blood and promoting diuresis in treating severe cerebellopontine hemorrhage. J Guangzhou Univ Tradit Chin Med 2010; 27: 445–8. Chinese. [Google Scholar]
- 22Chen SH, Zhang XY, Li XG, Wang J, Long CG, Xiong Y, et al. Clinical efficacy of zhongfengxingnao oral solution in Chinese subjects with acute cerebral hemorrhage: a randomized, double-blind, placebo-controlled trial [database on the Internet]. Beijing: Tsinghua Tongfang Knowledge Network Technology Co, Ltd. c2010 - [cited 2014 Jun 18]. Available from: http://cpfd.cnki.com.cn/Article/CPFDTOTAL-ZHZY201008004006.htm.
- 23He D, Liu QR, Zhao JX. Therapeutic efficacy of xueshuantong injection on patients with acute intracerebral hemorrhage in early stage. Chin J Integr Tradit And West Med Intens and Crit Care 2002; 9: 27–9. Chinese. [Google Scholar]
- 24Fan Y, Zhou ZY, Jin MW. The influence of liangxuetongyu oral solution on early rehabilitation of hypertensive cerebral hemorrhage. Chin J Integr Tradit West Med 2000; 20: 138–40. Chinese. [Google Scholar]
- 25Wang YQ, Shi Q, Wang WP. Clinical study of xuefuzhuyu decoction on hypertensive intracerebral hemorrhage. J Emerg Tradit Chin Med 2013; 22: 1686–7, 1689. Chinese. [Google Scholar]
- 26Zhang SQ, Zhang CP, Chen ML, Xu XY, Hou B. Clinical study of bushenhuoxuehuatan decoction on acute cerebral hemorrhage. J Emerg Tradit Chin Med 2012; 4: 529–30, 557. Chinese. [Google Scholar]
- 27Wang ZF, Wang JY. Efficacy observation of the sequential therapy of traditional Chinese medicine for hypertensive cerebral hemorrhage. China Pharm 2011; 3: 263–5. Chinese. [Google Scholar]
- 28Ministry of Health of the People's Republic of China. Clinical guideline of new drugs for traditional Chinese medicine. 1993. 35–6.
- 29De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, et al. International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal. New Engl J Med 2004; 351: 1250–1. [DOI] [PubMed] [Google Scholar]
- 30Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001; 135: 982–9. [DOI] [PubMed] [Google Scholar]
- 31Kotsirilos V. Complementary and alternative medicine. Part 2-evidence and implications for GPs. Aust Fam Physician 2005; 34: 689–91. [PubMed] [Google Scholar]
- 32Meng LQ, Huang RY, Zhu CL, Wei SG, Li XB, Huang JM. The effect of Panax Notoginseng Saponins on expression of AQP4 and brain edema after intracerebral hemorrhage in rats. Chin J Geriatr Heart Brain Vessel Dis 2007; 9: 53–6. Chinese. [Google Scholar]
- 33Zhao XS, Chen ZG, Gao F, Xu ZF. Effect of Panax Notoginseng saponins on cerebral water content and thrombin of intracerebral hemorrhage rats. Chin J Inform on TCM 2014; 21: 46–8. Chinese. [Google Scholar]
- 34Zhu JH, Wang DK, Dong KQ, Dai XW, He LF. Effects of total panax notoginseng saponins on ultrastructure of mitochondria for intracerebral hemorrhage focus in rats. Chin J Neuroanat 2014; 30: 60–4. Chinese. [Google Scholar]
- 35Si YC, Li JW, Zhu PC, Zhang LJ. Effects of Panax Notoginseng saponins on expression of excitatory amino acid receptor GluR2 subunit of AMPA receptor in rat forebrain after intracerebral hemorrhage. Chin J Anat 2005; 28: 535–8. Chinese. [Google Scholar]
- 36Zhang ZZ, Zhang BH, Chen M. The study of Danshen Injection for prevention and treatment of brain edema in acute intracerebral hemorrhage. Zhejiang J Integr Tradit Chin West Med 2003; 13: 355–7. Chinese. [Google Scholar]
- 37Liu HY, Zhang HY, Ma QR, Wang DK, Sun Z, Han JL, et al. Effect of Danshen Injection on neurons apoptosis that surround intracerebral hemorrhage in rats. Chin Tradit Patent Med 2012; 34: 731–3. Chinese. [Google Scholar]
- 38He YP, Huang JZ, Ying RB, Pan GC. Study on correlation of thrombin and cerebral edema following intracerebral hemorrhage and effects of treated with hirudo powder. Chin J Emerg Med 2002; 11: 172–5. Chinese. [Google Scholar]
- 39Wu Q, Chen GH, Yang F, Chen ZW. Pathological study of treatment effect of hirudo on experimental intracerebral hemorrhage. J Clin Exp Pathol 1999; 15: 51–3. Chinese. [Google Scholar]
- 40Wang DK, Zhang HY, Sun Z, Liu HY, Li Q, Zhang LX, et al. Effects of Ginkgo Biloba extract on apoptotic neurons around the early hematoma after intracerebral hemorrhage in rats. J Ningxia Med Univ 2011; 33: 537–9. Chinese. [Google Scholar]
- 41Ke JF, Du QC, Zhang HY, Qin Y, Li L, Li Q. Effects of injection of ginkgo biloba leave extract on content of IL-8 and ICAM-1in brain of intracerebral hemorrhage in rats. Ningxia Med J 2010; 32: 46–8.Chinese. [Google Scholar]
- 42Hu JH, Chen HJ, Liu HY, Che P, Qing Y, Zhang LX. The protective effect of erigeron on hemorrhagic brain injury in rats. Chin J Neuroanat 2013; 29: 209–13. Chinese. [Google Scholar]
- 43Dai XW, Zhang HY, Wang DK, Qin Y, Zhang Y. Effects of Breviscapine on cerebral edema and expression of intercellular adhension molecular-1 in rats of cerebral hemorrhage. Chin J Neuroanat 2011; 27: 665–9. Chinese. [Google Scholar]
- 44Sun H, Guo FQ, Wang DZ, Zhang HY, Wang JY. Effects of Buyang Huanwu decoction on expression of aquaporin-4 in brain tissue of intracerebral hemorrhage rats. J Clin Neurol (China) 2012; 25: 115–8. Chinese. [Google Scholar]
- 45Luo JK, Tang T, Li XQ. Effect of Buyang Huanwu decoction on activated caspase-3 after intracerebral hemorrhage in rats. J Tradit Chin Med Univ Hunan 2006; 26: 12–4. Chinese. [Google Scholar]
- 46Liu XJ, Tang T, Luo JK, Huang JF, Yang QD, Xing ZH, et al. Effect of Buyang Huanwu decoction on expression of VEGF mRNA in rat brains following cerebral hemorrhage. Tradit Chin Drug Res Clin Pharmacol 2007; 18: 100–3. Chinese. [Google Scholar]
- 47He CY, Huang JH, Zhou XP, Jin MW, Ye F, Wu HT, et al. Effect of Liangxue Tongyu Formula on t-PA and MMP-9 expressions in rats with intracerebral hemorrhage. Chin J Tradit Chin Med Pharm 2011; 26: 50–3. Chinese. [Google Scholar]
- 48He CY, Huang JH, Wang WJ, Jin MW, Ye F, Wu HT, et al. Effect of Liangxue Tongyu Formula on brain edema and expressions of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in rats with intracerebral hemorrhage. J Chin Integr Med 2010; 8: 347–51. Chinese. [DOI] [PubMed] [Google Scholar]
- 49Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66: 1175–81. [DOI] [PubMed] [Google Scholar]
- 50Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007; 38: 1257–62. [DOI] [PubMed] [Google Scholar]
- 51Nie YX, Wang D, Zhang X. Effect of panax notoginseng saponins injection on brain edema in intracerebral hemorrhage rats. Chin J Integr Tradit West Med 2006; 26: 922–5. Chinese [PubMed] [Google Scholar]
- 52Xue M, Hollenberg MD, Demchuk A, Yong VW. Relative importance of proteinase-activated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke 2009; 40: 2199–204. [DOI] [PubMed] [Google Scholar]
- 53Guangzhou University of Traditional Chinese Medicine. Clinical re-evaluation of removing blood stasis therapy in treating acute cerebral hemorrhage safety and efficacy [database on the Internet]. Bethesda: National Library of Medicine (US). c2013 - [cited 2014 Jun 18]. Available from: http://clinicaltrials.gov/show/NCT01918722
- 54Wang J, Xiong X. Current situation and perspectives of clinical study in integrative medicine in china. Evid Based Complement Alternat Med 2012; 2012: 268542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Jiang M, Lu C, Zhang C, Yang J, Tan Y, Lu A, et al. Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol 2012; 140: 634–42. [DOI] [PubMed] [Google Scholar]
- 56Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A, Ngu M. Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA 1998; 280: 1585–9. [DOI] [PubMed] [Google Scholar]
- 57Li SM, Xu H, Chen KJ. The diagnostic criteria of blood-stasis syndrome: considerations for standardization of pattern identification. Chin J Integr Med 2014; 20: 483–9. [DOI] [PubMed] [Google Scholar]
- 58Lu A, Jiang M, Zhang C, Chan K. An integrative approach of linking traditional Chinese medicine pattern classification and biomedicine diagnosis. J Ethnopharmacol 2012; 141: 549–56. [DOI] [PubMed] [Google Scholar]
- 59Wang L, Yuan Q, Marshall G, Cui X, Cheng L, Li Y, et al. Adverse drug reactions and adverse events of 33 varieties of traditional Chinese medicine injections on national essential medicines List (2004 edition) of China: an overview on published literatures. J Evid Based Med 2010; 3: 95–104. [DOI] [PubMed] [Google Scholar]
- 60Bian ZX, Tian HY, Gao L, Shang HC, Wu TX, Li YP, et al. Improving reporting of adverse events and adverse drug reactions following injections of Chinese materia medica. J Evid Based Med 2010; 3: 5–10. [DOI] [PubMed] [Google Scholar]
- 61Schulz KF, Altman DG. Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63: 834–40. [DOI] [PubMed] [Google Scholar]
- 62Flower A, Witt C, Liu JP, Ulrich-Merzenich G, Yu H, Lewith G. Guidelines for randomised controlled trials investigating Chinese herbal medicine. J Ethnopharmacol 2012; 140: 550–4. [DOI] [PubMed] [Google Scholar]
- 63Bian Z, Liu B, Moher D, Wu T, Li Y, Shang H, et al. Consolidated standards of reporting trials (CONSORT) for traditional Chinese medicine: current situation and future development. Front Med 2011; 5: 171–7. [DOI] [PubMed] [Google Scholar]