Opinion Statement
TNM stage remains the key determinant of patient prognosis after surgical resection of colorectal cancer (CRC), and informs treatment decisions. However, there is considerable stage-independent variability in clinical outcome that is likely due to molecular heterogeneity. This variability underscores the need for robust prognostic and predictive biomarkers to guide therapeutic decision-making including the use of adjuvant chemotherapy. Although the majority of CRCs develop via a chromosomal instability pathway, approximately 12-15% have deficient DNA mismatch repair (dMMR) which is characterized in the tumor by microsatellite instability (MSI). Tumors with the dMMR/MSI develop from a germline mutation in an MMR gene (MLH1, MSH2, MSH6, PMS2), i.e., Lynch syndrome, or more commonly from epigenetic inactivation of MLH1 MMR gene. CRCs with dMMR/MSI status have a distinct phenotype that includes predilection for the proximal colon, poor differentiation, and abundant tumor infiltrating lymphocytes. Consistent data indicate that these tumors have a better stage-adjusted survival compared to proficient MMR or microsatellite stable (MSS) tumors, and may respond differently to 5-fluorouracil-based adjuvant chemotherapy. To increase the identification of dMMR/MSI patients in clinical practice that includes those with Lynch Syndrome, it is recommended that all resected CRCs to be analyzed for MMR status. Available data indicate that patients with stage II dMMR CRCs have an excellent prognosis and do not benefit from 5-FU-based adjuvant chemotherapy which supports their recommended management by surgery alone. In contrast, the benefit of standard adjuvant chemotherapy with the FOLFOX regiment in stage III dMMR CRC patients awaits further study and therefore, all patients should be treated with standard adjuvant FOLFOX.
Keywords: colorectal cancer, DNA mismatch repair, microsatellite instability, adjuvant chemotherapy
Introduction
TNM stage remains the gold standard for informing patient prognosis and guiding management after resection for non-metastatic colorectal cancer (CRC). Despite the same disease stage, however, CRC patients exhibit considerable variability in clinical outcome that is likely related to molecular tumor heterogeneity. Therefore, the molecular classification of CRC may identify patient subgroups at varying risk of recurrence and death and for who personalized approaches to therapy may beneficial. The majority of CRCs develop via the chromosomal instability pathway (CIN), whereas 12-15% arise from the microsatellite instability (MSI) pathway that is a consequence of deficient (d) DNA mismatch repair (MMR). Deficient MMR can develop from an inherited germline mutation in a MMR gene (MLH1, MSH2, MSH6, PMS2) i.e., Lynch Syndrome, or more commonly due to epigenetic inactivation of the MLH1 gene and the CpG island methylator phenotype (CIMP). These sporadic dMMR tumors carry somatic mutations in the BRAF oncogene in approximately half of cases. Studies have shown that dMMR tumors have phenotypic features including poor differentiation, proximal colon location, and abundant tumor-infiltrating lymphocytes. Furthermore, dMMR tumors have been consistently associated with a better stage-adjusted survival compared to proficient MMR (pMMR) tumors.
Among early stage CRCs, the survival advantage of dMMR status appears to be greater among stage II compared to stage III patients. In patients with stage II colon cancers and dMMR, studies demonstrate a lack of benefit of adjuvant 5-FU-based chemotherapy. Among patients with stage III disease, the predictive impact of MMR status for adjuvant chemotherapy remains controversial. Multiple prior studies have demonstrating a lack of benefit for 5-fluorouracil (FU) as adjuvant chemotherapy, although only limited data exist for patients with stage III dMMR CRCs treated with standard the adjuvant FOLFOX regimen. In contrast to 5-FU, in vitro data indicate that dMMR/MSI CRC cell lines display sensitivity to oxaliplatin and accordingly, this agent may provide benefit in patients with dMMR CRCs.
DNA Mismatch Repair and Microsatellite Instability
The Cancer Genome Atlas (TCGA) Research Network has revealed a comprehensive characterization of the genomes of 224 cancerous colorectal tumors and normal pairs (1). Among CRCs studied, 16% of were found to be hypermutated, and 77% of these tumors displayed high frequency microsatellite instability (MSI-H) that was generally associated with hypermethylation and MLH1 gene. The remaining hypermutated tumors were primarily characterized by having mutations in somatic MMR pathways and in polymerase epsilon (POLE)[1].
The DNA MMR system repairs base-base mispairs introduced into microsatellites during DNA synthesis to maintain genomic stability (2). Microsatellites are short, tandemly-repeated sequences that occur throughout the genome and are used as markers of deficient (d) MMR. The DNA MMR system is composed of 4 MMR genes and their encoded proteins (MLH1, MSH2, MSH6, PMS2). Inactivation of MLH1 and MSH2 account for over 90% of dMMR cases. Deficiency of MMR results in production of a truncated, nonfunctional protein or loss of a protein that causes MSI. Therefore, dMMR is frequently analyzed by testing for loss of an MMR protein or for MSI using a PCR-based assay.
MSI testing
MSI testing can be performed on fresh, frozen or paraffin-embedded tumor tissue using a PCR-based assay for detection of instability (3, 4).
The National Cancer Institute Workshop agreed on five microsatellite markers necessary to determine MSI (5) that include two mononucleotide – BAT25/26 and three dinucleotide markers – D2S123, D5S346, and D17S250. Interpretation of the profiles requires a comparison with normal DNA from each patient. An alternative molecular method based exclusively on quasi-monomorphic mononucleotide markers was developed to avoid the analysis of matching normal DNA. This method has been proven to be more specific and sensitive than the original NCI panel (5).
On the basis of the MSI status, CRCs can be classified into three groups: MSI-H, if two or more of the five microsatellite markers show instability; MSI-L (low-frequency MSI), if only one of five markers shows instability; and microsatellite stable (MSS) if none of the markers show instability (6).
MMR Protein Expression: Immunohistochemistry (IHC)
Analysis of MMR protein expression by IHC is an alternative test that is widely available with the advantage of not requiring a molecular laboratory, and the ability to identify the affected gene by detecting loss of its protein product.
Another advantage of IHC testing is that loss of a specific mismatch gene product (MLH1, MSH2, MSH6, and PMS2) can direct germline testing to that specific gene, and assists in the identification of patients with LS (4).
MSI testing and IHC are complimentary, and loss of MMR protein expression by IHC has been shown to be highly concordant with DNA-based MSI testing with a good sensitivity (>90%) and a excellent specificity (100%) (4).
Only loss of hMLH1 protein expression has been described in sporadic CRCs (7). MLH1 and PMS2 proteins are often lost together, which indicates loss of MLH1 function generally due to epigenetic silencing or germline mutation. Isolated loss of PMS2 protein generally indicates an underlying germline PMS2 mutation.
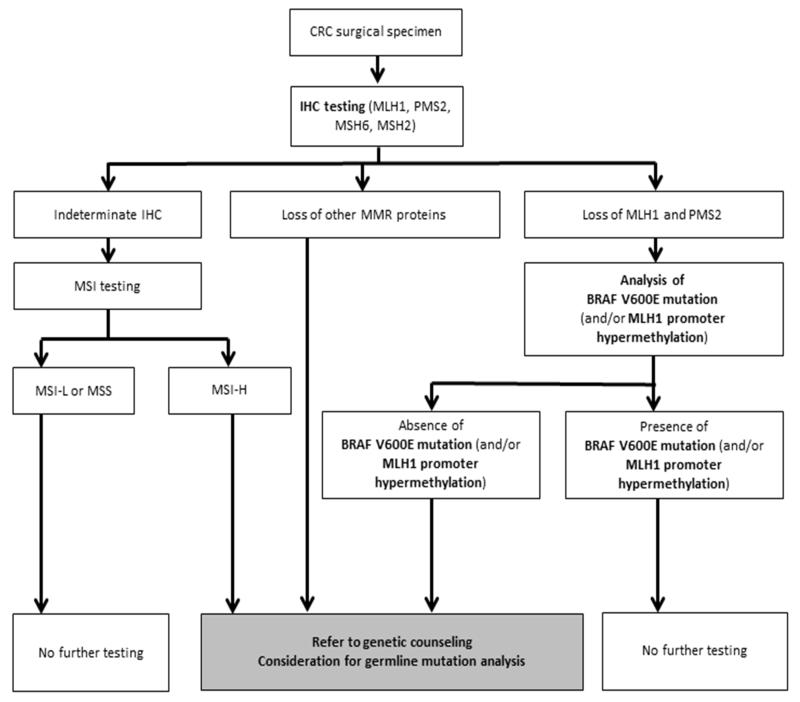
In CRCs with loss of MLH1 protein expression, testing for a mutation in the BRAF oncogene is the most cost-effective approach to confirm a sporadic case and generally exclude LS which support the use of this strategy for LS screening (8). Patients with non-mutated BRAF would then have germline testing for a mutation in the presumed altered MLH1 gene (Figure 1).
MSH2 and MSH6 proteins are often lost concurrently. Isolated loss of MSH2 or MSH6 proteins on IHC testing has high specificity for a germline mutation of these genes leading to the diagnosis of LS (Figure 1). Also, loss of the MSH2 protein can be caused by germline mutation in the EPCAM gene rather than MSH2 gene.
Tumors displaying loss of an MMR protein can be collectively referred to as dMMR and are considered to be MSI-H, whereas those with intact MMR proteins can be classified as pMMR are are expected to be microsatellite stable (MSS) or MSI-low (MSI-L).
Figure 1.
Algorithm for systematic evaluation for Lynch Syndrome in patients with colorectal cancer. CRC, colorectal cancer; IHC, immunohistochemistry.
-
➢Lynch Syndrome (LS)
- LS accounts for approximately 3-4% of all CRCs and one third of all dMMR/MSI-associated CRCs. LS is inherited in an autosomal dominant manner and results from a germline loss-of-function mutation (9) that occurs more commonly in MLH1 or MSH2, and infrequently in MSH6 or PMS2 (10). A germline mutation in an MMR gene followed by a second hit (somatic event) to the wild-type copy is needed to produce LS, and can occur due to point mutation, loss of heterozygosity or methylation.
- Patients with LS develop early age at onset of CRCs, and rates of synchronous CRCs increase with age.
- Patients with LS are at highest risk of developing CRC followed in frequency by endometrial carcinoma. Patients are also at increased risk of cancers of the stomach, ovary, urinary tract, small intestine, and prostate (11). The estimated cumulative risk of CRC by age 70 years for LS patients was approximately 50% in case of MLH1 or MSH2 mutations (12) (Figure 1).
- CRCs from LS patients are significantly less likely to carry KRAS mutations compared to pMMR/MSS cancers and importantly, BRAFV600E mutations are lacking in these patients. Among dMMR/MSI CRCs, BRAFV600E mutation testing can be performed to distinguish LS cases from sporadic dMMR tumors (13) (Figure 1).
- Bethesda criteria were revised in 2004 to guide selection of patients for LS testing (14). The guideline indicated that tumors should be tested for MSI in the following clinical situations:
- CRC diagnosed in a patient who is less than 50 years of age.
- Presence of synchronous, metachronous colorectal, or other LS-associated tumors, regardless of age.
- CRC with the MSI-H histology diagnosed in a patient who is less than 60 years of age.
- CRC diagnosed in one or more first-degree relatives with an LS-related tumor, with one of the cancers being diagnosed under age 50 years.
- CRC diagnosed in two or more first- or second-degree relatives with LS-related tumors, regardless of age.
- Patients with suspected hereditary CRC should be referred for genetic counseling, where the identification of germline mutations and evaluation/screening of family members can be appropriately addressed.
-
➢Sporadic dMMR CRC
- Approximately 12-15% of all CRCs have an MSI-H phenotype and about two-thirds of these MSI-H tumors are sporadics.
- Sporadic MSI-H CRCs show loss of MLH1 that generally occurs in a background of the CpG island methylator phenotype (CIMP) (17, 18). CIMP represents dense promoter hypermethylation of cancer-specific genes. CIMP-related silencing of the MLH1 gene is responsible for about 80% of cases in which MLH1/PMS2 protein expression are lost (7).
- Patients with MSI-H sporadic CRCs share most of the clinicopathological features with LS cases, yet have distinct epidemiological features including older age at diagnosis, predominance of female gender and increased rate of cigarette smoking (21).
Phenotypic features of deficient MMR CRCs
CRC patients with dMMR tumors have distinct clinical and pathologic features compared with their proficient MMR (pMMR) counterparts, including proximal colon predominance, poor differentiation and/or mucinous histology, increased numbers of tumor infiltrating lymphocytes, and diploid DNA content (2).
CRC with dMMR is more frequent in stage II (almost 20%) compared to stage III (12%), and are relatively uncommon among metastatic tumors (4%) (22). This highlights the importance of MSI testing in early stage disease where patients can be potentially cured by surgery alone or combined with adjuvant chemotherapy.
Prognostic value of MMR status
Multiple retrospective and population-based studies have shown that patients with dMMR CRCs have a more favorable stage-adjusted prognosis than those with pMMR tumors (23-27).
A meta-analysis from 32 studies with 1,277 MSI/dMMR cases included 7,642 patients with stage I-IV CRC. A better prognosis was found for patients with MSI/dMMR than those with MSS, MSI-L/pMMR tumors (28) among patients that were untreated or treated with 5-fluorouracil (5-FU)-based adjuvant chemotherapy. The hazard ratio (HR) for overall survival (OS) was 0.65 [95 % confidence interval (CI), 0.59-0.71] in favor of dMMR CRC patients. Results were confirmed when the analyses was restricted to patients with stage II or III CRC participating in clinical trials (28).
Important in the biology of CRC are somatic mutations in the KRAS and BRAF oncogenes and the status of the DNA MMR system. Recently, a couple of studies examined the utility of combining these molecular markers to subtype of CRC for prognosis (29, 30). One study analyzed stage III colon cancer patients from an adjuvant trial of FOLFOX-based chemotherapy (29). The study found patients with proficient (p) MMR tumors without BRAF or KRAS mutations had similar 5-year DFS rates as did dMMR sporadic or familial subtypes. In contrast, those patients whose tumors had mutated KRAS or BRAF and were pMMR exhibited poor 5-year DFS rates. Similar results were found in another study that investigated the association between CRC subtypes and survival in a large population-based registry cohort of patients that including analysis of the CpG island methylator phenotype (CIMP) (30).
Studies indicate that the better prognosis of dMMR CRCs is more apparent in earlier stage tumors (31).
Treatment
5-FU based adjuvant chemotherapy
A fluoropyrimidine (5-FU or capecitabine) combined with leucovorin is considered as standard care for patients with stage II CRC. Data indicate that 5-FU–based adjuvant chemotherapy is ineffective in stage II CRC patients with dMMR (32), consistent with the preclinical data showing that dMMR is associated with 5-FU resistance in CRC cells (33-38).
The prognostic/predictive value of dMMR was investigated in 457 stage II and stage III CRC patients from five randomized trials evaluating 5-FU + levamisole or leucovorin as adjuvant chemotherapy vs surgical treatment alone (39). Overall, patients with dMMR vs pMMR cancers had significantly better survival, yet dMMR patients with stage II and stage III tumors did not benefit from 5-FU-based adjuvant therapy. These findings were maintained in a pooled analysis (Table 1) that combined the cases above with those from a prior study from the same group (40). In the combined dataset of 1027 CRC patients, those with dMMR showed with more favorable outcome compared to pMMR cancers (DFS: HR, 0.51; 95% CI, 0.29 to 0.89; P = .009; OS: HR, 0.47; 95% CI, 0.26 to 0.83; P = .004)
In stage II CRC patients treated with 5-FU-based adjuvant chemotherapy vs surgery alone in the Quick and Simple and Reliable (QUASAR) trial, the recurrence rate for dMMR tumors was half that for pMMR tumors [11% (25/218) vs 26% (438/1,695); RR, 0.53; 95% CI, 0.40 to 0.70; P < .001) (41). Of note, the reduced risk of recurrence with chemotherapy did not differ significantly by MMR status (42).
In a study that included 2,141 stage II and stage III colon cancers from randomized trials of 5-FU-based adjuvant therapy, patients with dMMR tumors was associated with reduced rates of tumor recurrence, delayed TTR, and improved survival rates compared with patient with pMMR cancers (43).
A pooled data analysis from the ACCENT database involving 7,803 stage II and III CRC cases revealed that among stage II patients who received surgery alone, dMMR was strongly associated with delayed TTR (HR = 0.27; 95% CI, 0.10 to 0.75; P = 0.01) and improved OS (HR = 0.27; 95% CI, 0.10 to 0.74; P = 0.01) compared to pMMR, but not in stage III CRC patients (HR = 0.59; 95% CI, 0.28 – 1.23; P = 0.162) (44) (Table 1). In stage II CRCs treated with 5-FU adjuvant chemotherapy, TTR or OS did not differ between dMMR and pMMR (TTR, HR = 0.81, 95% CI, 0.55 to 1.19; P = 0.29; OS, HR = 0.87; 95% CI, 0.61 to 1.26; P = 0.47). In stage III CRC, however, patients with dMMR cancers treated with adjuvant 5-FU had better outcome compared to pMMR tumors (TTR, HR = 0.80, 95% CI, 0.66 to 0.97; P = 0.025; OS, HR = 0.79; 95% CI, 0.65 to 0.97; P = 0.023).
The favorable prognosis and the evidence of lack of benefit from 5-FU based adjuvant chemotherapy in stage II CRC patients with dMMR indicate that these patients should not receive adjuvant chemotherapy.
Table 1.
randomized trials evaluating the prognosis/predictive impact of the mismatch repair status in stage II and III colorectal cancer
| References | Tumor stage |
Treatment | MMR status |
Number of patients |
HR for DFS/TTR/RFS (95% CI) |
P value | HR for OS (95% CI) |
P value |
|---|---|---|---|---|---|---|---|---|
| 5-FU-based adjuvant chemotherapy | ||||||||
| Sargent et al. (39) (pooled analysis) |
II & III | Surgery alone 5-FU |
pMMR | 436 426 |
0.69 (0.55-0.86) DFS |
0.001 | 0.73 (0.58-0.91) | 0.006 |
| Surgery alone 5-FU |
dMMR | 79 86 |
1.61 (0.84-3.10) DFS |
0.15 | 1.58 (0.81-3.09) | 0.18 | ||
| Sargent et al. (44) (from ACCENT) |
II | Surgery alone | pMMR dMMR |
244 63 |
0.27 (0.10-0.75) RFS |
0.012 | 0.27 (0.10-0.74) | 0.011 |
| 5-FU | pMMR dMMR |
920 235 |
0.81 (0.55-1.19) RFS |
0.285 | 0.87 (0.61-1.26) | 0.469 | ||
| III | Surgery alone | pMMR dMMR |
277 37 |
0.59 (0.28-1.23) RFS |
0.162 | 0.69 (0.35-1.36) | 0.283 | |
| 5-FU | pMMR dMMR |
2333 390 |
0.80 (0.66-0.97) RFS |
0.025 | 0.79 (0.65-0.97) | 0.023 | ||
| Oxaliplatin-based adjuvant chemotherapy | ||||||||
| Gavin et al. (54) (from the NSABP C-07 study) |
II & III | 5-FU 5-FU+oxaliplatin |
pMMR | 635 675 |
0.82 (0.67-1.00) TTR |
0.054 | NA | |
| 5-FU 5-FU+oxaliplatin |
dMMR | 86 85 |
1.01 (0.45-2.25) TTR |
0.98 | NA | |||
| Gavin et al. (54) (from the NSABP C-07 and C-08 studies) |
II & III | 5-FU+oxaliplatin | pMMR dMMR |
806 102 |
0.58 (0.35-0.96) TTR |
0.03 | NA | |
| Flejou et al. (45) (from MOSAIC) |
II & III | 5-FU 5-FU+oxaliplatin |
dMMR | 50 40 |
0.52 (0.24-1.14) DFS |
NA | 0.45 (0.19-1.05) | NA |
| III | 5-FU 5-FU+oxaliplatin |
dMMR | 28 17 |
0.51 (0.18-1.41) DFS |
NA | 0.44 (0.15-1.34) | NA | |
| Sinicrope et al. (67) (from N0147) |
III | 5-FU+oxaiplatin | pMMR dMMR |
2575 329 |
0.82 (0.64-1.04) DFS |
0.106 | 0.83 (0.63-1.09) | 0.182 |
| 5-FU+oxaiplatin | pMMR dMMR |
2575 329 |
0.71 (0.55-0.93) TTR |
0.014 | ||||
DFS, disease free survival; RFS, recurrence free survival; TTR, time to recurrence; 5-FU, 5-fuluorouracil; HR, hazard ratio; CI, confidence interval; OS, overall survival; MMR, mismatch repair; pMMR, proficient MMR; dMMR, dMMR, deficient MMR; NA, not available
5-FU plus Oxaliplatin Adjuvant Therapy
In stage III colon cancer patients, oxaliplatin combined with 5-FU is the current standard of care for adjuvant chemotherapy (45-47).
In contrast to 5-FU, sensitivity to oxaliplatin was independent of the MMR system in CRC cell lines(48). Retrospective analyses of stage III colon cancer patients who received adjuvant FOLFOX suggest that dMMR CRCs maybe sensitive to oxaliplatin (49, 50); however, only limited data from prospective clinical trials evaluating oxaliplatin-based treatment are available (51, 50, 52).
An analysis of 2,299 stage II and stage III CRC patients from National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 [5-FU plus leucovolin (LV) ±oxaliplatin] and C-08 [FOLFOX ± bevacizumab] adjuvant studies revealed that dMMR was prognostic for recurrence in patients treated with FOLFOX (TTR, HR = 0.58; 95 % CI, 0.35 to 0.96; P =0.03) (53, 54), but not predictive of oxaliplatin efficacy since the interaction test between MMR status and treatment was not statistically significant (54) (Table 1).
In an analysis of the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) (45), MMR status was evaluated in 986 of the 2,240 patients enrolled. In a modest number of patients with dMMR colon cancers, a DFS benefit from FOLFOX compared with 5-FU alone was observed (55) (Table 1).
In stage III colon cancers, the addition of the anti-EGFR antibody cetuximab to FOLFOX as adjuvant chemotherapy did not improve outcome compared to FOLFOX alone in patients with wild-type KRAS tumors (North Central Cancer Treatment Group (NCCTG) N0147 trial) (56). In prospectively collected tumor samples (57-59), dMMR was detected in 314 (12 %) of 2,580 stage III tumors and was prognostic overall for TTR, but not for DFS or OS (57) (Updated data in Table 1, ref 60). However, dMMR vs pMMR was associated with significantly better outcome among tumors in the proximal colon (HR= 0.71; 95 % CI, 0.53-0.94; p=0.018), but not in the distal after adjustment for KRAS and BRAFV600E mutations and relevant covariates (57).
Available data in stage III CRC patients does not change the current approach of treating these patients, irrespective of MMR status, with adjuvant FOLFOX.
5-FU Plus Irinotecan-Based Adjuvant Therapy
Irinotecan is commonly used for the treatment of advanced CRC, however, it was ineffective in the adjuvant setting based on the several randomized phase III studies [Cancer and Leukemia Group B (CALGB) 89803 (60), FNCLCC Accord02/FFCD9802 (61), and Pan-European Trials in Alimentary Tract Cancers 3 (PETACC-3) trials (62)]. Accordingly, irinotecan is not used in the adjuvant setting in CRC patients.
In a retrospective analysis of the CALGB 89803 trial where patients with stage III colon cancer were randomly assigned to weekly bolus 5-FU/leucovorin (LV) or weekly bolus irinotecan, 5-FU, and LV (IFL), IFL-treated patients with dMMR/MSI-H tumors showed improved 5-year DFS as compared to pMMR tumors (HR=0.76; 95% CI, 0.64 to 0.88 vs. 0.59; 95% CI, 0.53 to 0.64; P = .03), which was not observed among patients treated with 5-FU/LV (63). However, data from the PETACC-3 study (64) failed to show a benefit for irinotecan in patients with dMMR colon cancers.
Bevacizumab in Adjuvant Setting
The National Surgical Adjuvant Breast and Bowel Project protocol C-08 (NSABP C-08) trial failed to show the benefit of adding 1 year of bevacizumab to standard FOLFOX in the treatment of stage II/III colon cancer (65). However, post hoc analyses found that patients with dMMR tumors derived a statistically significant survival benefit from the addition of bevacizumab (HR = 0.52; 95% CI = 0.29 to 0.94; P = .02), in contrast with no benefit in patients with pMMR tumors (HR = 1.03; 95% CI = 0.84 to 1.27; p = .78; P interaction = .04)(66). An explanation for this finding awaits further study and moreover, these preliminary results await confirmation.
Acknowledgement
FAS is supported by a National Cancer Institute Senior Scientist Award (Grant No. K05CA-142885). HK is supported by a fellowship grant from the Uehara Memorial Foundation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance, ** Of major importance
- **1.Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. This paper provides valuable data on the molecular heterogeniety of CRC.
- 2.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–87. e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–9. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 4.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 5.Buhard O, Cattaneo F, Wong YF, et al. Multipopulation analysis of polymorphisms in five mononucleotide repeats used to determine the microsatellite instability status of human tumors. J Clin Oncol. 2006;24:241–51. doi: 10.1200/JCO.2005.02.7227. [DOI] [PubMed] [Google Scholar]
- 6.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 7.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–5. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155:69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boland CR. Evolution of the nomenclature for the hereditary colorectal cancer syndromes. Fam Cancer. 2005;4:211–8. doi: 10.1007/s10689-004-4489-x. [DOI] [PubMed] [Google Scholar]
- 10.Peltomaki P. Lynch syndrome genes. Fam Cancer. 2005;4:227–32. doi: 10.1007/s10689-004-7993-0. [DOI] [PubMed] [Google Scholar]
- 11.Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444–9. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonadona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–10. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 13.Domingo E, Niessen RC, Oliveira C, et al. BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene. 2005;24:3995–8. doi: 10.1038/sj.onc.1208569. [DOI] [PubMed] [Google Scholar]
- 14.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasen HF, Mecklin JP, Khan PM, et al. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–5. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 16.Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA. 2005;293:1979–85. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyota M, Issa JP. CpG island methylator phenotypes in aging and cancer. Semin Cancer Biol. 1999;9:349–57. doi: 10.1006/scbi.1999.0135. [DOI] [PubMed] [Google Scholar]
- 18.Hughes LA, Khalid-de Bakker CA, Smits KM, et al. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta. 2012;1825:77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Cunningham JM, Winters JL, et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair. Cancer Res. 2003;63:5209–12. [PubMed] [Google Scholar]
- 20.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–9. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 21.Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–22. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74. doi: 10.1200/JCO.2009.23.3452. This study showed that BRAF mutation was not prognostic for recurrence-free survival but was for overall survival, particularly in patients with MSI-L and MSI-S tumors.
- 23.Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131:729–37. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Gafa R, Maestri I, Matteuzzi M, et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000;89:2025–37. [PubMed] [Google Scholar]
- 25.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 26.Lanza G, Gafa R, Santini A, et al. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol. 2006;24:2359–67. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 27.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917–23. [PubMed] [Google Scholar]
- 28.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- *29.Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. Stage-independent variability in clinical outcome and response to therapy is likely due to molecular heterogeneity. Using a combination of KRAS, BRAF and MMR status, useful subtyping of colon cancers for prognosis can be achieved as was shown in a large adjuvant chemotherapy trial.
- **30.Phipps AI, Limburg PJ, Baron JA, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87. e2. doi: 10.1053/j.gastro.2014.09.038. The findings suggest that the biologic distinctions between the subtypes based on combinations of tumor markers translate to important differences in survival.
- 31.Roth AD, Delorenzi M, Tejpar S, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104:1635–46. doi: 10.1093/jnci/djs427. [DOI] [PubMed] [Google Scholar]
- 32.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–62. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li LS, Morales JC, Veigl M, et al. DNA mismatch repair (MMR)-dependent 5-fluorouracil cytotoxicity and the potential for new therapeutic targets. Br J Pharmacol. 2009;158:679–92. doi: 10.1111/j.1476-5381.2009.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis TW, Wilson-Van Patten C, Meyers M, et al. Defective expression of the DNA mismatch repair protein, MLH1, alters G2-M cell cycle checkpoint arrest following ionizing radiation. Cancer Res. 1998;58:767–78. [PubMed] [Google Scholar]
- 35.Meyers M, Wagner MW, Hwang HS, et al. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61:5193–201. [PubMed] [Google Scholar]
- 36.Koi M, Umar A, Chauhan DP, et al. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N′-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994;54:4308–12. [PubMed] [Google Scholar]
- 37.Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003;106:66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- 38.Fischer F, Baerenfaller K, Jiricny J. 5-Fluorouracil is efficiently removed from DNA by the base excision and mismatch repair systems. Gastroenterology. 2007;133:1858–68. doi: 10.1053/j.gastro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- **39.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26. doi: 10.1200/JCO.2009.27.1825. The study examined the prognostic and predictive impact of DNA mismatch repair status for 5-fluorouracil-based adjvuant chemotherapy.
- 40.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–70. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 42.Group QC. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. The Lancet. 2007;370:2020–9. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 43.Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–75. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Sargent DJ, Shi Q, Yothers G, et al. Prognostic impact of deficient mismatch repair (dMMR) in 7,803 stage II/III colon cancer (CC) patients (pts): A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J Clin Oncol. 2014;32:5s. 2014 (suppl; abstr 3507) Study data confirm the lack of benefit of adjuvant 5-FU in stage II colon cancers with deficient mismatch repair.
- 45.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. The New England journal of medicine. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 46.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 47.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–71. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 48.Fink D, Nebel S, Aebi S, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6. [PubMed] [Google Scholar]
- 49.Zaanan A, Flejou JF, Emile JF, et al. Defective mismatch repair status as a prognostic biomarker of disease-free survival in stage III colon cancer patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer Res. 2011;17:7470–8. doi: 10.1158/1078-0432.CCR-11-1048. [DOI] [PubMed] [Google Scholar]
- 50.Zaanan A, Cuilliere-Dartigues P, Guilloux A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol. 2010;21:772–80. doi: 10.1093/annonc/mdp383. [DOI] [PubMed] [Google Scholar]
- 51.Des Guetz G, Lecaille C, Mariani P, et al. Prognostic impact of microsatellite instability in colorectal cancer patients treated with adjuvant FOLFOX. Anticancer Res. 2010;30:4297–301. [PubMed] [Google Scholar]
- 52.Kim ST, Lee J, Park SH, et al. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol. 2010;66:659–67. doi: 10.1007/s00280-009-1206-3. [DOI] [PubMed] [Google Scholar]
- **53.Gavin PG, Colangelo LH, Fumagalli D, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6531–41. doi: 10.1158/1078-0432.CCR-12-0605. Important manuscript that examined biomarkers with clinical outcome in a large cohort of stage II and III colon cancer patients from a phase III clinical trials. Study found that BRAF mutations were associated with poor survival post-recurrence.
- 54.Gavin PG, Paik S, Yothers G, et al. Colon cancer mutation: prognosis/prediction--response. Clin Cancer Res. 2013;19:1301. doi: 10.1158/1078-0432.CCR-13-0020. [DOI] [PubMed] [Google Scholar]
- 55.Flejou JF, Andre T, Chibaudel B, et al. Effect of adding oxaliplatin to adjuvant 5-fluorouracil/leucovorin (5FU/LV) in patients with defective mismatch repair (dMMR) colon cancer stage II and III included in the MOSIAC study. J Clin Oncol. 2013:31. [Google Scholar]
- 56.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383–93. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–72. doi: 10.1200/JCO.2013.48.9591. This paper revealed that the prognostic impact of dMMR on DFS was dependent on the primary tumor site in patients with stage III CRC treated with FOLFOX + cetuximab as adjuvant chemotherapy.
- 58.Gonsalves WI, Mahoney MR, Sargent DJ, et al. Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer, NCCTG/Alliance N0147. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sha D, Lee AM, Shi Q, et al. Association study of the let-7 miRNA-complementary site variant in the 3′ untranslated region of the KRAS gene in stage III colon cancer (NCCTG N0147 Clinical Trial) Clin Cancer Res. 2014;20:3319–27. doi: 10.1158/1078-0432.CCR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinicrope FA, Yoon HH, Mahoney MR, et al. Overall survival result and outcomes by KRAS, BRAF, and DNA mismatch repair in relation to primary tumor site in colon cancers from a randomized trial of adjuvant chemotherapy: NCCTG (Alliance) N0147. J Clin Oncol. 2014;32:5s. suppl; abstr 3525. [Google Scholar]
- 61.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456–61. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 62.Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Ann Oncol. 2009;20:674–80. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 63.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117–25. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 64.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol. 2009;27:1814–21. doi: 10.1200/JCO.2008.18.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *65.Klingbiel D, Saridaki Z, Roth AD, et al. Prognosis of stage II and III colon carcinoma treated with adjuvant 5-FU or FOLFIRI in relation to microsatellite status, results of the PETACC-3 trial. Ann Oncol. 2014 doi: 10.1093/annonc/mdu499. Adjuvant study found that MSI-H was significantly associated with RFS in stage II and III colon cancer patients treated with 5-FU/LV alone or combined with irinotecan. However, the relationship with OS was only significant for stage II patients.
- 66.Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–6. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Pogue-Geile K, Yothers G, Taniyama Y, et al. Defective mismatch repair and benefit from bevacizumab for colon cancer: findings from NSABP C-08. J Natl Cancer Inst. 2013;105:989–92. doi: 10.1093/jnci/djt140. Adjuvant chemotherapy study in stage III colon cancer patients suggested the potential survival benefit of the addition of bevacizumab to FOLFOX compared to FOLFOX alone among patients with dMMR tumors.