Abstract
Background
The utility of estimated glomerular filtration rate (eGFR) and albuminuria for cardiovascular prediction is controversial.
Methods
We meta-analyzed individual-level data from 24 cohorts (with a median follow-up time longer than 4 years, varying from 4.2 to 19.0 years) in the Chronic Kidney Disease Prognosis Consortium (637,315 participants without a history of cardiovascular disease) and assessed C-statistic difference and reclassification improvement for cardiovascular mortality and fatal and non-fatal cases of coronary heart disease, stroke, and heart failure in 5-year timeframe, contrasting prediction models consisting of traditional risk factors with and without creatinine-based eGFR and/or albuminuria (either albumin-to-creatinine ratio [ACR] or semi-quantitative dipstick proteinuria).
Findings
The addition of eGFR and ACR significantly improved the discrimination of cardiovascular outcomes beyond traditional risk factors in general populations, but the improvement was greater with ACR than with eGFR and more evident for cardiovascular mortality (c-statistic difference 0.0139 [95%CI 0.0105–0.0174] and 0.0065 [0.0042–0.0088], respectively) and heart failure (0.0196 [0.0108–0.0284] and 0.0109 [0.0059–0.0159]) than for coronary disease (0.0048 [0.0029–0.0067] and 0.0036 [0.0019–0.0054]) and stroke (0.0105 [0.0058–0.0151] and 0.0036 [0.0004–0.0069]). Dipstick proteinuria demonstrated smaller improvement than ACR. The discrimination improvement with kidney measures was especially evident in individuals with diabetes or hypertension but remained significant with ACR for cardiovascular mortality and heart failure in those without either of these conditions. In participants with chronic kidney disease (CKD), the combination of eGFR and ACR for risk discrimination outperformed most single traditional predictors; the c-statistic for cardiovascular mortality declined by 0.023 [0.016–0.030] vs. <0.007 when omitting eGFR and ACR vs. any single modifiable traditional predictors, respectively.
Interpretation
Creatinine-based eGFR and albuminuria should be taken into account for cardiovascular prediction, especially when they are already assessed for clinical purpose and/or cardiovascular mortality and heart failure are the outcomes of interest (e.g., the European guidelines on cardiovascular prevention). ACR may have particularly broad implications for cardiovascular prediction. In CKD populations, the simultaneous assessment of eGFR and ACR will facilitate improved cardiovascular risk classification, supporting current CKD guidelines.
Funding
US National Kidney Foundation and NIDDK
Individuals with chronic kidney disease (CKD) are at high risk of cardiovascular disease,1 and approximately half die of cardiovascular disease without reaching end-stage renal disease.2 Two key kidney measures defining and staging CKD, glomerular filtration rate (GFR) and albuminuria, are consistently associated with high cardiovascular risk in a broad range of populations.3 However, previous studies examining whether these kidney disease measures improve cardiovascular risk prediction beyond traditional risk factors have demonstrated conflicting results,4–9 leading to controversy in primary prevention guidelines as to whether CKD status should be taken into account for cardiovascular risk classification.10,11
Significant associations do not necessarily result in risk prediction improvement,12 and prior studies varied substantially in terms of study population, cardiovascular outcomes or kidney disease measures of interest (often omitting albuminuria), and statistics for assessing prediction improvement,4–9 making it difficult to resolve the discrepancy between risk relationship vs. prediction in this context, and to achieve definitive conclusions. Therefore, we used the extensive database of the CKD Prognosis Consortium (CKD-PC) to examine the role of both measures of CKD in improving the prediction of various cardiovascular outcomes beyond traditional risk factors, using standard definitions and analytic approaches across contributing cohorts. We addressed these issues in primary prevention (i.e., persons without history of cardiovascular disease), where traditional risk factors are most relevant for cardiovascular risk prediction.5
Methods
Study Design
Details of the CKD-PC were previously described3,13 or can be found in the website: www.jhsph.edu/ckdpc. This analysis used data from 24 cohorts (19 general population cohorts, three high-risk cohorts of subjects with diabetes mellitus, and two CKD cohorts exclusively enrolling CKD patients), all with data on fatal and non-fatal cardiovascular outcomes and a median follow-up time longer than 4 years. This study was approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health.
Study Variables at Baseline
Estimated GFR (eGFR) was calculated by the CKD-EPI creatinine-based equation.13,14 We focused on creatinine-based eGFR, since this is widely used in clinical practice.14 We preferred urine albumin-to-creatinine ratio (ACR) (timed urine albumin excretion was considered equivalent) as the measure of albuminuria15 but also accepted urine protein-to-creatinine ratio (PCR) and semi-quantitative assessment of proteinuria using a dipstick test.
We defined the traditional risk factors to be race/ethnicity (white, black, Asian, Hispanic, and other) and those in the Framingham prediction model for general cardiovascular risk:16 age, sex, systolic blood pressure, antihypertensive drug use, total and high-density lipoprotein cholesterols, smoking status (current/not), and diabetes (defined as fasting glucose ≥7.0 mmol/L, non-fasting glucose ≥11.1 mmol/L, hemoglobin A1c ≥6.5%, use of glucose lowering drugs, or self-reported diabetes). Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or use of antihypertensive medication.
Cardiovascular Outcomes
Outcomes studied were cardiovascular mortality (death from myocardial infarction, stroke, heart failure, or sudden cardiac death), coronary heart disease (CHD) (myocardial infarction, fatal coronary heart disease, or coronary revascularization), stroke (ischemic and hemorrhagic stroke except subarachnoid hemorrhage), and heart failure (hospitalization or death due to heart failure) (appendix pp 6–12).
Statistical Analysis
Analyses were restricted to subjects aged 18 years or older without a history of CHD, stroke, or heart failure at baseline. Statistics were first obtained within each cohort and then pooled by a fixed-effect model, weighting by the number of events in each cohort.17 We first investigated the associations of eGFR and albuminuria with cardiovascular outcomes after adjusting for each other and traditional risk factors using Cox proportional hazards models in the general population and high-risk cohorts combined. We modeled eGFR and ACR using linear splines with knots at 30, 45, 60, 75, 90, and 105 ml/min/1.73m2 and 10, 30, and 300 mg/g, respectively. The reference points were eGFR 95 ml/min/1.73m2 and ACR 5 mg/g.3 ACR was log-transformed,3 as were all continuous traditional risk factors.11,16
We subsequently estimated the difference in Harrell’s c-statistics between prediction models that included or excluded kidney disease measures. To reduce the methodological advantage of having several spline terms, compared to the traditional risk factors which were, by convention, modeled linearly, in these models, eGFR was modeled with two linear terms (a single knot at 60 ml/min/1.73m2), based on the shape of its associations with cardiovascular outcomes. Log-ACR and log-PCR were linearly modeled, and dipstick proteinuria was categorized as negative (reference), trace, 1+, and ≥2+. In the general population and high-risk cohorts, we evaluated primarily whether the addition of kidney disease measures improves cardiovascular prediction beyond traditional risk factors.
We also meta-analyzed the subpopulation with CKD: participants in the CKD cohorts plus those from the other cohorts with low eGFR <60 ml/min/1.73m2 or high albuminuria [defined as ACR ≥30 mg/g, PCR ≥50 mg/g, or dipstick proteinuria ≥1+]13). As this setting inherently assumes existing data on kidney disease measures for identifying CKD,15 we assessed the omission of each of these kidney disease measures and modifiable traditional risk factors from the full models with all the predictors. This approach allows a fair comparison among every predictor independently of the order of predictors included in the models and thus gives unbiased evidence as to which predictors should be used in prediction.17
We also evaluated the categorical net reclassification improvement (NRI).18 Given the lack of internationally accepted risk thresholds for cardiovascular outcomes, conventional CHD risk categories that have been widely used in the literature (<10% [low], 10–19% [intermediate], and ≥20% [high] in 10 years - roughly equivalent to <5%, 5%-9%, and ≥10% in 5 years)17,19 were applied to each cardiovascular outcome, based on the relatively close annual incidence of new coronary attack, stroke, heart failure, and cardiovascular mortality in the US (600,000–800,000 cases for each).20 To provide a practical context for reclassification, we also estimated the number needed to screen (NNS).17 This is the required number of people to screen for preventing one event under the assumption that 20% of high risk individuals who developed cardiovascular events would have been prevented by an intervention (for example statins for CHD). To estimate NNS for the US, we assumed the population distribution data from NHANES III and risk estimates from all eligible US cohorts for each outcome (appendix pp 13–15).17
All models demonstrated good calibration according to visual inspection of observed vs. predicted risk and a modified Hosmer-Lemeshow statistic.16 Heterogeneity was quantified using the χ2 test and the I2 statistic. We conducted meta-regression analysis to explore sources of heterogeneity when we observed high heterogeneity (I2 statistic >75%21). Subgroup analyses were performed according to age, sex and race and by hypertension and diabetes status. Analyses were conducted using Stata/MP 13 (www.stata.com). A-priori a P-value below 0.05 was considered significant.
Role of the funding source
The sponsors had no role in the study design, data collection, analysis, data interpretation, or writing of the report. KM and JCo had full access to all analyses and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators.
Results
Overall, 637,315 individuals free of CVD history with a mean age of 47 (SD 16) years were followed up for a mean of 8.9 years (6 million person-years) (Table 1) after excluding 146,769 subjects with missing values for eGFR, albuminuria, or traditional risk factors at baseline was excluded (appendix pp 13–15).. Almost all blacks were from four US general population cohorts, and data for Asians were predominantly from cohorts with dipstick proteinuria. The prevalence of low eGFR and high albuminuria were 3.8% (n=23,076) and 2.9% (n=17,701) (0.6% [n=3,753] with both) in general population cohorts, 22.5% (n=7,909) and 13.4% (n=4,699) (4.3% [n=1,506]) in high-risk cohorts, and 56.5% (n=1,075) and 75.4% (N=1,434) (46.1% [n=877]) in CKD cohorts, respectively. During follow-up, 10,605 cardiovascular deaths were reported from 22 cohorts, 6,283 CHD events from 12 cohorts, 4,180 stroke events from 12 cohorts, and 2,066 HF events from 8 cohorts.
Table 1.
Characteristics of included cohorts
| Cohort | Country/ region |
Total N | Age, y |
% Female |
% Black |
% Current Smoker |
% DM |
% HTN drug |
SBP | Total Chol |
HDLC | % eGFR <60 |
% alb‡ |
CVM | CHD | Stroke | HF | F/U time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General Population |
||||||||||||||||||
| ARIC*a | US | 9540 | 63 (6) |
58% | 22% | 15% | 15% | 38% | 127 (19) |
5.2 (0.9) |
1.3 (0.4) |
5% | 7% | 277 | 1193 | 436 | 952 | 13 (12, 14) |
| AusDiab* a | Australia | 9933 | 50 (14) |
56% | 0% | 16% | 7% | 13% | 129 (18) |
5.7 (1.1) |
1.4 (0.4) |
5% | 6% | 104 | 154 | 8 (7, 8) | ||
| Beaver Dam a |
US | 4065 | 61 (11) |
58% | 0% | 21% | 8% | 27% | 132 (20) |
6.1 (1.1) |
1.4 (0.5) |
12% | 3% | 427 | 484 | 13 (12, 14) | ||
| CIRCS a | Japan | 4045 | 54 (9) |
61% | 0% | 26% | 4% | 13% | 131 (18) |
5.1 (0.9) |
1.5 (0.4) |
3% | 3% | 162 | 19 (15, 21) | |||
| COBRA* a | Pakistan | 2626 | 51 (11) |
52% | 0% | 39% | 20% | 14% | 136 (23) |
4.9 (1.0) |
1.0 (0.3) |
2% | 9% | 71 | 4 (4, 5) | |||
| ESTHER a | Germany | 4573 | 61 (7) |
57% | 0% | 16% | 16% | 37% | 139 (20) |
5.7 (1.3) |
1.4 (0.4) |
13% | 10% | 108 | 281 | 201 | 9 (9, 10) | |
| Framingham * a |
US | 2777 | 58 (10) |
55% | 0% | 15% | 8% | 26% | 128 (19) |
5.3 (1.0) |
1.3 (0.4) |
6% | 11% | 101 | 80 | 11 (10, 12) | ||
| Gubbio* a | Italy | 1592 | 54 (6) |
56% | 0% | 31% | 5% | 19% | 130 (18) |
5.9 (1.0) |
1.3 (0.3) |
1% | 4% | 70 | 11 (10, 12) | |||
| HUNT* a | Norway | 7317 | 60 (15) |
58% | 0% | 22% | 16% | 63% | 150 (24) |
6.3 (1.2) |
1.4 (0.4) |
8% | 10% | 586 | 13 (13, 14) | |||
| IPHS a | Japan | 78237 | 59 (10) |
75% | 0% | 8% | 5% | 20% | 133 (18) |
5.3 (0.9) |
1.4 (0.4) |
4% | 2% | 4165 | 17 (17, 17) | |||
| MESA* a | US | 6704 | 62 (10) |
53% | 28% | 13% | 13% | 37% | 127 (21) |
5.0 (0.9) |
1.3 (0.4) |
13% | 10% | 117 | 398 | 146 | 190 | 9 (8, 9) |
| NHANESIII * a |
US | 14388 | 44 (19) |
54% | 28% | 26% | 10% | 12% | 124 (19) |
5.2 (1.1) |
1.3 (0.4) |
5% | 10% | 601 | 9 (7, 10) | |||
| Ohasama a | Japan | 1428 | 63 (9) |
66% | 0% | 13% | 10% | 25% | 129 (17) |
5.1 (0.9) |
1.4 (0.4) |
5% | 7% | 58 | 83 | 11 (9, 12) | ||
| PREVEND* a |
Netherlands | 7433 | 48 (12) |
52% | 1% | 34% | 3% | 13% | 128 (20) |
5.6 (1.1) |
1.3 (0.4) |
5% | 10% | 117 | 376 | 145 | 10 (10, 11) | |
| Rancho Bernardo* a |
US | 1251 | 69 (12) |
62% | 0% | 8% | 11% | 34% | 134 (22) |
5.4 (0.9) |
1.5 (0.4) |
19% | 13% | 132 | 137 | 98 | 55 | 12 (9, 14) |
| REGARDS* a |
US | 22147 | 64 (9) |
58% | 41% | 14% | 18% | 47% | 127 (16) |
5.3 (1.0) |
1.4 (0.4) |
9% | 13% | 527 | 1041 | 586 | 5 (4, 6) | |
| Severance a | Korea | 45221 | 47 (12) |
64% | 0% | 22% | 6% | 6% | 123 (20) |
5.0 (0.9) |
1.4 (0.3) |
5% | 4% | 176 | 704 | 1566 | 189 | 11 (9, 14) |
| Taiwan a | Taiwan | 376104 | 40 (13) |
49% | 0% | 24% | 5% | 5% | 120 (19) |
5.0 (1.0) |
1.3 (0.4) |
3% | 1% | 1588 | 8 (4, 11) | |||
| ULSAM* a | Sweden | 957 | 71 (1) |
0% | 0% | 20% | 18% | 31% | 147 (18) |
5.8 (1.0) |
1.3 (0.3) |
7% | 15% | 158 | 124 | 149 | 13 (11, 14) | |
| High Risk | 3.8% | 2.9% | ||||||||||||||||
| ADVANCE *b |
Multiple | 7939 | 66 (6) |
46% | 0% | 16% | 100 % |
72% | 145 (21) |
5.3 (1.2) |
1.3 (0.4) |
14% | 30% | 261 | 456 | 243 | 168 | 5 (5, 5) |
| NZDCS* b | New Zealand |
26698 | 61 (14) |
49% | 0% | 15% | 100 % |
52% | 139 (19) |
5.3 (1.1) |
1.3 (0.4) |
25% | 8% | 794 | 989 | 390 | 283 | 8 (6, 9) |
| ZODIAC* b | Netherlands | 438 | 66 (12) |
63% | 0% | 20% | 100 % |
39% | 156 (26) |
5.7 (1.1) |
1.2 (0.4) |
30% | 35% | 51 | 10 (7, 10) | |||
| CKD | 22.5% | 13.4% | ||||||||||||||||
| MDRD†c | US | 748 | 51 (12) |
40% | 12% | 12% | 6% | 75% | 137 (19) |
5.6 (1.2) |
1.0 (0.4) |
90% | 85% | 136 | 17 (11, 18) | |||
| Sunnybrook *c |
Canada | 1154 | 55 (18) |
50% | 0% | 6% | 30% | 44% | 133 (22) |
5.0 (1.3) |
1.4 (0.5) |
43% | 71% | 50 | 6 (4, 9) | |||
| Total | 637315 | 10605 | 6283 | 4180 | 2066 | |||||||||||||
ARIC: Atherosclerosis Risk in Communities Study, AusDiab: Australian Diabetes, Obesity, and Lifestyle Study, Beaver Dam: Beaver Dam CKD Study, CIRCS: Circulatory Risk in Communities Study, COBRA: Control of Blood Pressure & Risk Attenuation Study, ESTHER: ESTHER Study, Framingham: Framingham Heart Study, Gubbio: Gubbio Study, HUNT: Nord Trøndelag Health Study, IPHS: Ibaraki Prefectural Health Study, MESA: Multi-Ethnic Study of Atherosclerosis, NHANES III: Third US National Health and Nutrition Examination Survey, Ohasama: Ohasama Study, PREVEND: Prevention of Renal and Vascular End-stage Disease Study, Rancho Bernardo: Rancho Bernardo Study, REGARDS: Reasons for Geographic And Racial Differences in Stroke Study, Severance: Severance Cohort Study, Taiwan: Taiwan MJ Cohort Study, ULSAM: Uppsala Longitudinal Study of Adult Men, ADVANCE: The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, NZDCS: New Zealand Diabetes Cohort Study, ZODIAC: Zwolle Outpatient Diabetes project Integrating Available Care, MDRD: Modification of Diet in Renal Disease Study, Sunnybrook: Sunnybrook Cohort;
DM: diabetes mellitus, HTN: hypertension, SBP: systolic blood pressure, HDLC: high density lipoprotein cholesterol, eGFR: estimated glomerular filtration rate, alb: albuminuria, CVM: cardiovascular mortality, CHD: coronary heart disease, HF: heart failure, F/U: median follow-up time (interquartile range);
Studies with urine albumin-to-creatinine ratio
Studies with urine protein-to-creatinine ratio
Proportion of participants with ACR ≥30 mg/g or PCR ≥50 mg/g or dipstick protein ≥1+.
General population cohorts,
High risk cohorts,
Chronic kidney disease cohorts
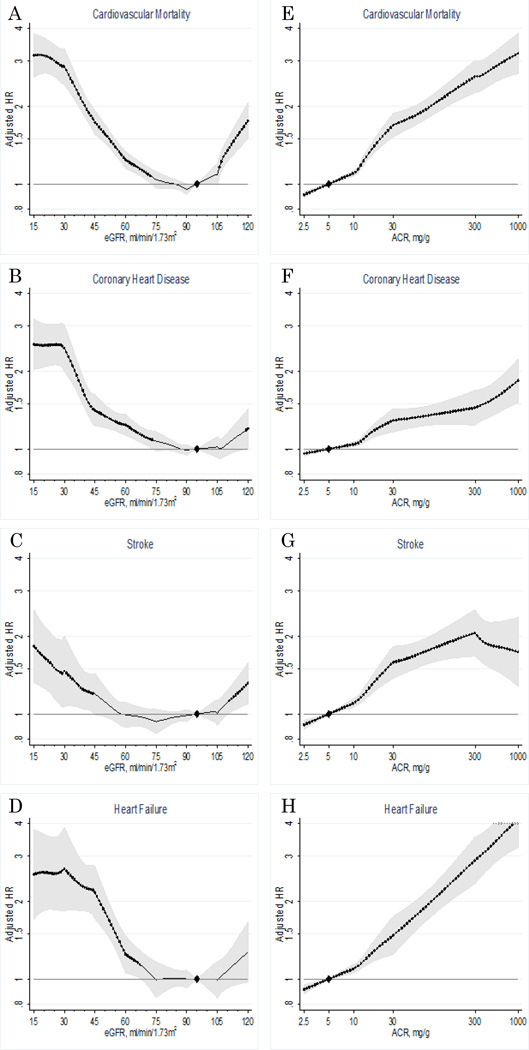
Adjusted cardiovascular risk was relatively constant at eGFR 75–105 ml/min/1.73m2 and increased steadily below this range (Figure 1A-1D). The risk gradient was steeper for cardiovascular mortality and heart failure compared to CHD and stroke. A J-shaped association with elevated risk at eGFR >105 ml/min/1.73m2 was observed for all outcomes but was most evident for cardiovascular mortality. Results for eGFR were largely similar between studies with data on ACR and dipstick proteinuria (appendix p 23). The relationships of ACR to cardiovascular outcomes were largely monotonic on the log-log scale (Figure 1E-1H). Similarly to eGFR, the risk gradient was sharper for cardiovascular mortality and heart failure compared to CHD and stroke. This pattern was consistent when we meta-analyzed only those studies with all four cardiovascular outcomes (data not shown).
Figure 1.
Adjusted hazard ratios and 95% CIs (shaded areas or whisker plots) of cardiovascular mortality (top row), coronary heart disease (second row), stroke (third row), and heart failure (bottom row) according to eGFR (left column) and ACR (right column) in the combined general population and high-risk cohorts. The reference is eGFR 95 ml/min/1.73m2 and ACR 5 mg/g (diamond). Dots represent statistical significance (P<0.05). *Adjustments were for age, sex, race/ethnicity, smoking, systolic blood pressure, antihypertensive drugs, diabetes, total and high-density lipoprotein cholesterol concentrations, and albuminuria (ACR or dipstick) or eGFR, as appropriate.
In the analyses of eGFR, there were 629,776 participants for cardiovascular mortality, 144,874 for coronary heart disease, 137,658 for stroke, and 105,127 for heart failure. In the analyses of ACR, there were 120,148 participants for cardiovascular mortality, 91,185 for coronary heart disease, 82,646 for stroke, and 55,855 for heart failure.
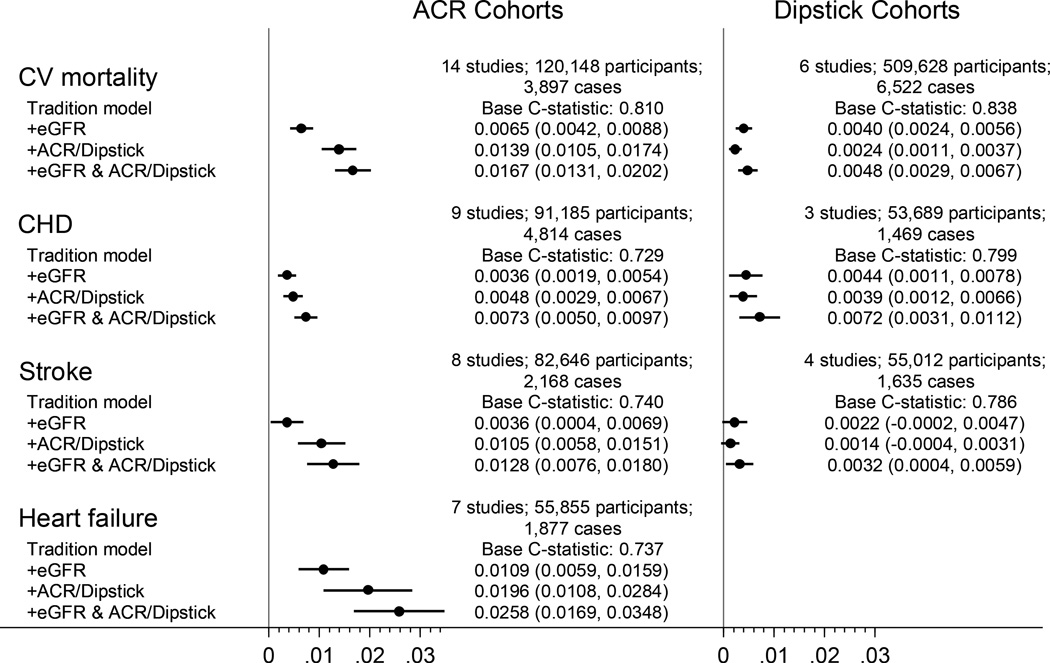
C-statistics for cardiovascular outcomes based on traditional risk factors ranged from 0.729–0.838 in the general and high-risk cohorts and were significantly improved with the addition of either or both measures of kidney disease (Figure 2). In line with the risk gradients in Figure 1, both kidney disease measures improved discrimination more evidently for cardiovascular mortality and heart failure than for CHD and stroke. There were some incremental improvements when eGFR and albuminuria were modeled simultaneously. Nevertheless, the discrimination improvement was greater with ACR than with eGFR or dipstick proteinuria for all cardiovascular outcomes. The results were qualitatively consistent across cohorts (appendix pp 24–25). When these kidney disease measures were contrasted with the modifiable traditional risk factors by omitting each from the full models, ACR contributed to better discrimination more than most of the traditional risk factors for all outcomes except CHD (appendix p 26). eGFR was at least as good as most of the traditional risk factors. Results were much the same in cohorts with dipstick proteinuria (appendix p 27) and for NRI (appendix pp 28–29).
Figure 2.
Difference in C-statistic for cardiovascular outcomes by adding kidney measure(s) to traditional models in the combined general population and high-risk cohorts. There was only one study with dipstick proteinuria and heart failure, and thus meta-analysis was not performed.
Improvements in discrimination with eGFR and ACR were more evident among individuals with diabetes or hypertension compared to those without either of these conditions (Figure 3). Nevertheless, ACR significantly improved the discrimination for cardiovascular mortality and heart failure among those without diabetes or without hypertension (Figure 3). The contribution of ACR and eGFR to better discrimination was generally consistent in subgroups defined by age, sex, and race (appendix p 30). One exception was considerably greater discrimination improvement, particularly for cardiovascular mortality and heart failure, with ACR in blacks than in whites (appendix p 30). Again, we observed consistent results for NRI (appendix pp 31–33).
Figure 3.
Change in c-statistics for cardiovascular outcomes by adding eGFR, ACR, and both to traditional risk factors in general population and high risk cohorts, according to the status of diabetes and hypertension.
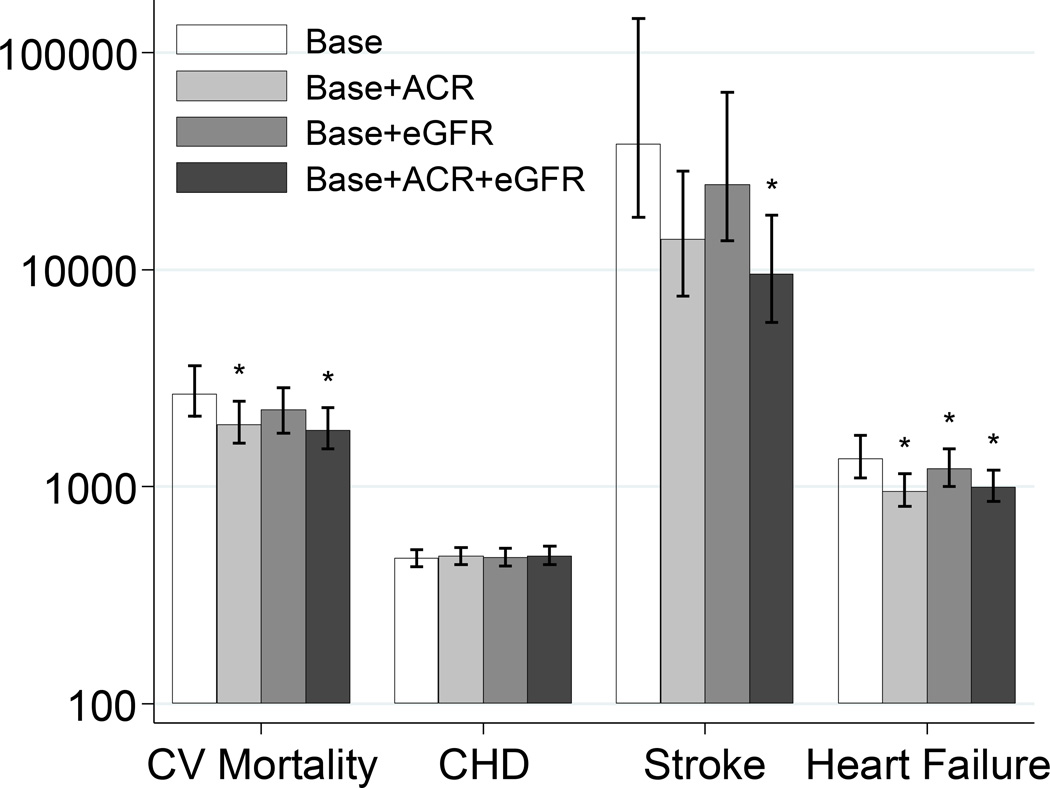
In line with the relatively small improvement with kidney disease measures for prediction of CHD and stroke, the estimated 5-year NNS of the models with eGFR and/or ACR for these outcomes for the US population was not significantly different from that for the model with only traditional risk factors (Figure 4). The results were consistent when we restricted to hard CHD (myocardial infarction or fatal events). Similar results were observed when we applied risk categories for the combination of hard CHD and stroke as recently proposed by the American Heart Association and American College of Cardiology (10-y risk <5%, 5–7.4%, and ≥7.5% rescaling to 5-y) (appendix pp 19–20).11 In contrast, the models with kidney disease measures, particularly ACR, significantly reduced the NNS for cardiovascular mortality and heart failure (Figure 4). These models estimated the 5-year NNS to be between 170 and 500 among those who were initially categorized as at intermediate risk with traditional risk factors (appendix p 21).17 Similar patterns were observed for cardiovascular mortality with risk categorization taken from the European guidelines10 (appendix p 22).
Figure 4.
Number needed to screen (NNS) for preventing one event among individuals at high risk of each CVD outcome. High risk was defined as 5-y risk ≥10%, and NNS is based on the assumption of 20% risk reduction by interventions. * indicates statistical significance (p <0.05) compared to NNS based on the base model with traditional predictors.
In these analyses there were 27,745 participants for cardiovascular mortality, 17,531 for coronary heart disease, 16,869 for stroke, and 19,265 for heart failure.
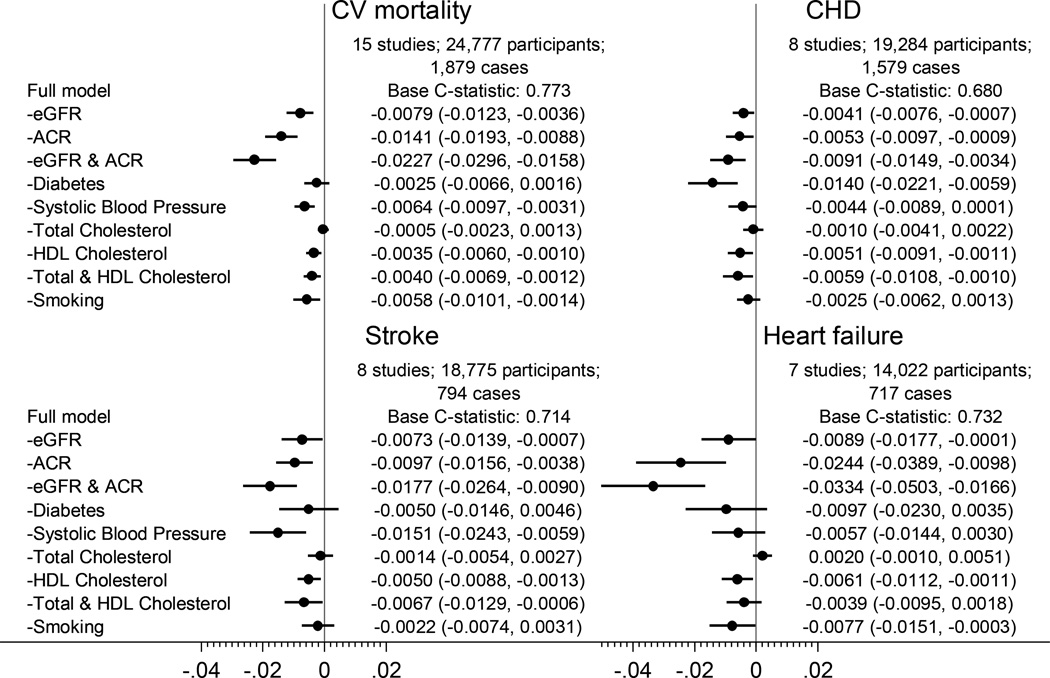
In the CKD population, ACR was again one of the strongest contributors for better discrimination for all cardiovascular outcomes (Figure 5). eGFR also significantly contributed to better discrimination for cardiovascular mortality and CHD in this population. Better discrimination of cardiovascular mortality with eGFR was confirmed in cohorts with dipstick proteinuria (appendix p 34). The combination of eGFR and ACR outperformed any single modifiable traditional risk factor, as well as the combination of total and high-density lipoprotein cholesterols, for all cardiovascular outcomes, except for diabetes in CHD prediction. Largely similar results were found across cohorts (appendix pp 35–38) and when NRI was tested (appendix p 39).
Figure 5.
C-statistic difference for four cardiovascular outcomes by omitting kidney disease measures and traditional risk factors from a model with all risk factors in a CKD population
Discussion
In this meta-analysis, eGFR and albuminuria independently improved the prediction of incident cardiovascular events beyond traditional risk factors. The improvement was greater with ACR than with eGFR or dipstick proteinuria and was more evident for cardiovascular mortality and heart failure than for CHD and stroke. ACR was superior to most of the modifiable traditional risk factors for predicting cardiovascular mortality, heart failure, and stroke in the general populations. The prediction improvement with kidney disease measures was more evident in those with diabetes or hypertension but was also significant with ACR for cardiovascular mortality and heart failure even among those without either of these conditions. In the CKD population, the combination of eGFR and ACR outperformed almost all single modifiable traditional risk factors and the combination of traditional lipid parameters for the prediction of all cardiovascular outcomes.
Several studies have investigated cardiovascular prediction improvement with eGFR and/or albuminuria in the primary prevention setting, with some reporting improvement4–6 and some not.7,8 With our meta-analysis, we may provide some explanation for the differences among them. Two studies with positive results focused on cardiovascular mortality,4,5 a cardiovascular outcome strongly related to kidney disease measures in our study, whereas a negative study dealt exclusively with CHD.8 The other positive study focused on a CKD population, a population in which kidney measures demonstrated superiority to traditional risk factors for cardiovascular prediction in our study. Most importantly, neither of two negative studies investigated ACR.7,8
ACR was one of the strongest predictors of cardiovascular outcomes other than CHD among general populations in our study. Our results also support ACR as a preferable measure of albuminuria over dipstick proteinuria, although dipstick has a cost advantage, particularly for mass screening.15 The pathophysiological mechanisms linking albuminuria to cardiovascular risk are not well understood. Albuminuria mainly results from damage to the glomerulus and thus is considered a marker of systemic vascular damage or microvascular disease in addition to kidney disease,5 which may explain its strong contribution to cardiovascular prediction. Indeed, the role of microvascular disease in the development of heart failure has recently attracted attention.22 Also, albumin in urine can directly damage the kidney,23 and thus whether these pathological changes in the kidney impact cardiovascular system would warrant investigations. Nevertheless, our results are in line with the observation that the reduction in albuminuria by renin angiotensin system inhibitors is associated with cardiovascular risk reduction.24
Although weaker than ACR, eGFR also contributed to better cardiovascular prediction in several circumstances. Again the pathophysiological mechanisms are not clear but the most robust prediction improvement with eGFR was for cardiovascular mortality, particularly in those with CKD. This may be because patients with lower eGFR manifest more severe cardiovascular disease compared to higher eGFR25 and tend to not receive optimal treatment for cardiovascular disease.26 Even for predicting the other cardiovascular outcomes, eGFR was not necessarily inferior to the modifiable traditional risk factors.
Even though the change in c-statistic of ~0.005 to ~0.03 by incorporating eGFR and/or ACR in prediction models may appear small to modest, it is similar or superior to the contributions of most of the individual traditional risk factors including blood pressure, lipids, and smoking. Furthermore these values are considerably higher than the increments in c-statistic gained by high-sensitivity C-reactive protein, a representative non-traditional predictor, in a previous meta-analysis.17 Of note, in contrast to many non-traditional predictors, GFR and albuminuria are already measured in several clinical scenarios. Indeed, their assessment is recommended among persons with diabetes and/or hypertension,27,28 and approximately 290 million tests of serum creatinine are carried out every year in the US.29 Thus, in these scenarios, their use for cardiovascular risk assessment is cost effective. This will be particularly the case for individuals identified as having CKD, and our results support the initial cardiovascular risk classification with both eGFR and ACR in CKD, as recommended in the Kidney Disease Improving Global Outcomes guidelines.15 Also, it is important to keep in mind that cardiovascular risk prediction may be beyond guiding drug therapy and may motivate lifestyle modification. Thus, any improvement with existing information may be valuable.
Whether the measurement of creatinine-based eGFR and albuminuria should be extended to the general population is under debate.30 ACR significantly improved the prediction of cardiovascular mortality and heart failure even among those without either diabetes or hypertension and reduced the overall NNS for these outcomes compared to models with traditional risk factors. These results suggest a potential benefit of expanding the groups for ACR assessment for the prediction of cardiovascular mortality and heart failure. The European prevention guidelines, indeed, use cardiovascular mortality to scale the risk but currently prioritize eGFR over ACR for cardiovascular risk classification and may benefit from greater emphasis on ACR.10 In terms of potential target population, ACR assessment particularly led to better cardiovascular prediction among blacks in our study, which confirms a recent report of stronger association of ACR with incident cardiovascular events in blacks than in whites.31 Nevertheless, the cost-effectiveness of screening and subsequent life-style/drug interventions should still be evaluated.
Our results should be interpreted in the context of certain limitations. The methods used to evaluate creatinine, albuminuria, and traditional risk factors varied across cohorts, despite our efforts to standardize definitions. However, this is unlikely to cause bias favoring kidney measures. Similarly, the ascertainment of cardiovascular outcomes was not necessarily consistent. Nevertheless, we observed qualitatively consistent prediction improvement in vast majority of cohorts (appendix pp 24–25). Our study was based on single assessments of creatinine-based eGFR and albuminuria.15 However, the misclassification due to their short-term variability, if any, would result in conservative estimates, and the traditional risk factors were assessed similarly. Direct measurement of GFR or other filtration markers, such as cystatin C, was not available in a majority of the cohorts and hence not evaluated. We anticipate more evident improvement with eGFR based on cystatin C than with creatinine-based eGFR.32 Also, confounding by low urine creatinine excretion may be an issue for the ACR-risk relationship.33 However, the prediction improvement was observed in studies with timed overnight urinary albumin excretion (appendix pp 13–15) and dipstick studies (Figure 2), which are not corrected for urine creatinine. Most of the blacks in our study were from US cohorts. Most Asian cohorts evaluated albuminuria using dipstick, and thus we cannot differentiate whether the difference between ACR and dipstick cohorts were confounded by racial or regional factors. Further investigation is needed for racial/ethnic groups other than Asians, whites, and blacks.
In conclusion, creatinine-based eGFR and albuminuria independently improved cardiovascular prediction, particularly for mortality and heart failure. ACR outperformed eGFR and most of the modifiable traditional risk factors for these two outcomes, as well as stroke, supporting its use for cardiovascular risk assessment in a broad range of settings. Among clinical populations, in which the assessment of eGFR and albuminuria is already recommended (e.g., individuals with CKD, diabetes, and hypertension), these kidney disease measures are especially useful for cardiovascular risk prediction.
Panel: Research in context
Evidence before this study
Electronic searches based on PubMed in addition to manual searches of reference lists of prior studies identified a few studies specifically assessing the improvement of cardiovascular risk prediction by incorporating either or both kidney measures (estimated GFR based on serum creatinine and/or cystatin C) and kidney damage (based on albuminuria or proteinuria), exclusively or predominantly in individuals without history of cardiovascular disease at baseline.4–9 However, these studies obtained conflicting results and varied substantially in terms of study population and method, making it difficult to achieve definitive conclusions and leading to inconsistent approaches about how to incorporate CKD in cardiovascular risk assessment across different clinical guidelines.10,11
Added value of this study
We meta-analyzed individual-level data from 24 cohorts (637,315 participants without a history of cardiovascular disease) and assessed risk prediction improvement with either or both of creatinine-based eGFR and albuminuria (ACR or dipstick proteinuria) for cardiovascular mortality, coronary disease, stroke, and heart failure. Although creatinine-based eGFR and albuminuria independently improved cardiovascular prediction in general, the improvement was particularly evident for cardiovascular mortality and heart failure. ACR outperformed eGFR and most of the modifiable traditional risk factors for these two outcomes, as well as stroke. The discrimination improvement with ACR was especially evident in individuals with diabetes or hypertension but remained significant for cardiovascular mortality and heart failure even in those without either of these conditions. When the analysis was restricted to persons with CKD, the combination of eGFR and ACR for risk discrimination outperformed most single traditional predictors, suggesting the value of their simultaneous assessment for cardiovascular risk classification.
Implications of all the available evidence
Creatinine-based eGFR and albuminuria should be taken into account for cardiovascular prediction, especially when they are already assessed for clinical purpose (e.g., individuals with CKD, diabetes, and hypertension), and/or cardiovascular mortality and heart failure are the outcomes of interest (e.g., the European guidelines on cardiovascular prevention).10 ACR may have particularly broad implications for cardiovascular prediction. In CKD populations, the simultaneous assessment of eGFR and ACR will facilitate improved cardiovascular risk classification, supporting current CKD guidelines.
Supplementary Material
Acknowledgements
The CKD-PC Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation (NKF funding sources include AbbVie, Amgen, and Merck) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK100446-01). A variety of sources have supported enrollment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies such as national institutes of health and medical research councils as well as foundations and industry sponsors listed in appendix pp 17–18. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dr. Matsushita reports grants from NIDDK during the conduct of the study; other from Mitsubishi Tanabe Pharma, other from Kyowa Hakko Kirin outside the submitted work; Dr. Coresh reports grants from NIH, NKF, and Amgen during the conduct of the study, in addition, Dr. Coresh has a patent regarding provisional patent submitted for glomerular filtration rate (GFR) estimation using a panel of biomarkers pending. Dr. Chalmers reports grants and personal fees from Servier outside the submitted work; Dr. Muntner reports grants from Amgen Inc. outside the submitted work; Dr. van Zuilen reports personal fees from Novartis, personal fees from Astellas, personal fees from Alexion outside the submitted work; Dr. Woodward reports personal fees from Novartis, personal fees from Amgen, personal fees from Sanofi outside the submitted work; Dr. Warnock reports grants from Amgen outside the submitted work;
Appendix
CKD-PC investigators/collaborators (study acronyms/abbreviations are listed in appendix p 16 and degrees and affiliations for collaborators listed in appendix pp 3–5): ADVANCE: Stephen MacMahon, John Chalmers, Hisatomi Arima, Mark Woodward; Aichi: Hiroshi Yatsuya, Kentaro Yamashita, Hideaki Toyoshima, Koji Tamakoshi; ARIC: Josef Coresh, Kunihiro Matsushita, Morgan Grams, Yingying Sang; AusDiab: Robert C. Atkins, Kevan R. Polkinghorne, Steven Chadban; Beaver Dam: Anoop Shankar, Ronald Klein, Barbara E.K. Klein, Kristine E. Lee; CARE: Marcello Tonelli, Frank M. Sacks, Gary C. Curhan; CHS: Michael Shlipak, Mark J Sarnak, Ronit Katz; CIRCS: Hiroyasu Iso, Akihiko Kitamura, Hironori Imano, Kazumasa Yamagishi; COBRA: Tazeen H. Jafar, Muhammad Islam, Juanita Hatcher, Neil Poulter, Nish Chaturvedi; CRIB: David C Wheeler, Jonathan Emberson, Jonathan N Townend, Martin J Landray; ESTHER: Hermann Brenner, Dietrich Rothenbacher, Heiko Müller, Ben Schöttker; Framingham: Caroline S. Fox, Shih-Jen Hwang, James B. Meigs, Ashish Upadhyay; Geisinger: Jamie Green, H Lester Kirchner, Robert Perkins, Alex R Chang; Gubbio: Massimo Cirillo; HUNT: Stein Hallan, Knut Aasarød, Cecilia M. Øien, Solfrid Romundstad; IPHS: Fujiko Irie, Hiroyasu Iso, Toshimi Sairenchi, Kazumasa Yamagishi; KSHS: Eliseo Guallar, Seungho Ryu, Yoosoo Chang, Juhee Cho, Hocheol Shin; Maccabi: Gabriel Chodick, Varda Shalev, Nachman Ash, Bracha Shainberg; MASTERPLAN: Jack F. M. Wetzels, Peter J Blankestijn, Arjan D van Zuilen; MDRD: Mark J Sarnak, Andrew S Levey, Lesley A Inker, Vandana Menon; MESA: Michael Shlipak, Mark Sarnak, Ronit Katz, Carmen Peralta; MRC Older: Paul Roderick, Dorothea Nitsch, Astrid Fletcher, Christopher Bulpitt; NZDCS: C Raina Elley, Timothy Kenealy, Simon A Moyes, John F Collins, Paul Drury; Ohasama: Takayoshi Ohkubo, Hirohito Metoki, Masaaki Nakayama, Masahiro Kikuya, Yutaka Imai; PREVEND: Ron T Gansevoort, Stephan JL Bakker, Hans L Hillege, Hiddo J Lambers Heerspink; Rancho Bernardo: Simerjot K Jassal, Jaclyn Bergstrom, Joachim H Ix, Elizabeth Barrett-Connor; REGARDS: David G Warnock, Paul Muntner, Suzanne Judd, William McClellan, Orlando Gutierrez; Severance: Sun Ha Jee, Heejin Kimm, Yejin Mok; Sunnybrook: Navdeep Tangri, Maneesh Sud, David Naimark; Taiwan: Chi-Pang Wen, Sung-Feng Wen, Chwen-Keng Tsao, Min-Kuang Tsai; ULSAM: Johan Ärnlöv, Lars Lannfelt, Anders Larsson; ZODIAC: Henk J Bilo, Nanne Kleefstra, Klaas H Groenier, Hanneke Joosten, Iefke Drion
CKD-PC Steering Committee: Josef Coresh (Chair), Ron T Gansevoort, Paul E de Jong, Kunitoshi Iseki, Andrew S Levey, Kunihiro Matsushita, Mark J Sarnak, Benedicte Stengel, David Warnock, Mark Woodward
CKD-PC Data Coordinating Center: Shoshana H Ballew (Coordinator), Josef Coresh (Principal investigator), Morgan Grams, Kunihiro Matsushita (Director), Yingying Sang (Lead programmer), Mark Woodward (Senior statistician)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: KM, JCo, RG, MS, DGW, MW and JA conceived of the study concept and design. KM, JCo, YS, MW, and the CKD-PC investigators/collaborators listed below acquired the data. YS and the Data Coordinating Center members listed below analyzed the data. KM and JCo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis and all authors had final responsibility for the decision to submit for publication, informed by discussions with collaborators. KM, JCo, YS, JCh, CF, EG, TJ, SKJ, GWDL, PM, PR, TS, BS, AS, MSh, MT, JT, AvZ, KaY, KeY, RG, MSa, DGW, MW, JA took part in the interpretation of the data. KM, JCo, YS, MW, and JA drafted the manuscript, and KM, JCo, YS, JCh, CF, EG, TJ, SKJ, GWDL, PM, PR, TS, BS, AS, MSh, MT, JT, AvZ, KaY, KeY, RG, MSa, DGW, MW, JA provided critical revisions of the manuscript for important intellectual content. All collaborators shared data and were given the opportunity to comment on the manuscript. JCo obtained funding for CKD-PC and individual cohort and collaborator support is listed in appendix pp 17–18.
Conflict of Interest Disclosures: All other coauthors have nothing to disclose.
References
- 1.Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(6):572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 2.Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72(1):92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallan SI, Astor BC, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older versus younger individuals; the HUNT II study. Arch Intern Med. 2007;167(22):2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 5.Nerpin E, Ingelsson E, Riserus U, Sundstrom J, Larsson A, Jobs E, et al. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant. 2011;26(9):2820–2827. doi: 10.1093/ndt/gfq848. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, Su HM, Tsai YC, Huang JC, Chang JM, Hwang SJ, et al. Framingham risk score with cardiovascular events in chronic kidney disease. PLoS ONE. 2013;8(3):e60008. doi: 10.1371/journal.pone.0060008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito H, Pacold IV, Durazo-Arvizu R, Liu K, Shilipak MG, Goff DC, Jr, et al. The effect of including cystatin C or creatinine in a cardiovascular risk model for asymptomatic individuals: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174(8):949–957. doi: 10.1093/aje/kwr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner DE, Tighiouart H, Griffith JL, Elsayed E, Levey AS, Salem DN, et al. Kidney disease, Framingham risk scores, and cardiac and mortality outcomes. Am J Med. 2007;120(6):552 e1–552 e8. doi: 10.1016/j.amjmed.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 9.Clase CM, Gao P, Tobe SW, McQueen MJ, Grosshennig A, Teo KK, et al. Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med. 2011;154(5):310–318. doi: 10.7326/0003-4819-154-5-201103010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) * Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 11.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 12.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 17.Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net Reclassification Indices for Evaluating Risk Prediction Instruments. Epidemiology. 2014;25(1):114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Expert Panel on Detection Evaluation Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart Disease and Stroke Statistics--2014 Update: A Report From the American Heart Association. Circulation. 2013;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges L, JPT H, HR R. Introduction to meta-analsys. West Sussex: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 22.Paulus WJ, Tschöpe C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 23.Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, et al. Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol. 2002;13(5):1179–189. [PubMed] [Google Scholar]
- 24.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 25.Garimella PS, Hart PD, O’Hare A, DeLoach S, Herzog CA, Hirsch AT. Peripheral artery disease and CKD: a focus on peripheral artery disease as a critical component of CKD care. Am J Kidney Dis. 2012;60(4):641–654. doi: 10.1053/j.ajkd.2012.02.340. [DOI] [PubMed] [Google Scholar]
- 26.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Executive summary: Standards of medical care in diabetes--2012. Diabetes Care. 2012;(35 Suppl 1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Inker LA, Levey AS. Pro: Estimating GFR using the chronic kidney disease epidemiology collaboration (CKD-EPI) 2009 creatinine equation: the time for change is now. Nephrol Dial Transplant. 2013;28(6):1390–1396. doi: 10.1093/ndt/gft003. [DOI] [PubMed] [Google Scholar]
- 30.Moyer VA. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(8):567–570. doi: 10.7326/0003-4819-157-8-201210160-00533. [DOI] [PubMed] [Google Scholar]
- 31.Gutiérrez OM, Khodneva YA, Muntner P, Rizk DV, McClellan WM, Cushman M, et al. Association Between Urinary Albumin Excretion and Coronary Heart Disease in Black vs White Adults. JAMA. 2013;310(7):706–713. doi: 10.1001/jama.2013.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: the Heart and Soul Study. Circulation. 2010;121(11):1295–1303. doi: 10.1161/CIRCULATIONAHA.109.924266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.