Abstract
Objective
To determine the effects of estrogen therapy (ET) on carotid artery inflammation when initiated early and late relative to surgical menopause.
Methods and Results
Female cynomolgus macaques consuming atherogenic diets were ovariectomized and randomized to control or oral estradiol (E2, human equivalent dose of 1 mg/d micronized E2) initiated at 1 month (Early menopause, n=24) or 54 months, (Late menopause, n=40) post-ovariectomy. Treatment period was 8 months. Carotid artery expression of markers of monocyte/macrophages (CD68, CD163), dendritic cells (CD83), NK cells (NCAM-1), and IFN-γ was significantly lower in the E2-treated animals in the early but not late menopause group (p<0.05). In contrast, carotid artery transcripts for T cell markers (CD3, CD4, CD8, and CD25), interleukin (IL)-10, type I collagen, MCP-1, MMP-9, and TNF-α were lower in E2-treated monkeys regardless of menopausal stage (p<0.05).
Conclusions
ET initiated soon after menopause inhibited macrophage accumulation in the carotid artery, an effect not observed when E2 was administered after several years of estrogen deficiency. No evidence for pro-inflammatory effects of late ET was observed. The results provide support for the timing hypothesis of postmenopausal ET with implications for interpretation of outcomes in the Women’s Health Initiative.
Keywords: Estrogen, menopause, cynomolgus macaque, atherosclerosis, macrophage, inflammation, timing hypothesis
Introduction
The presence of immune cells, particularly macrophages and T cells, is a common characteristic of developing atherosclerotic lesions1. Activated macrophages produce inflammatory cytokines that mediate pro-inflammatory effects through both innate and adaptive immunity. Most of the activated T cells in atheromas are CD4+ T cells2, which contribute to the progression of atherosclerosis in atherosclerosis-prone mice3. CD8+ T cells are less common in atheromas, and their role in atherogenesis is less well understood. Various types of activated T cells influence adaptive immunity by producing pro- and/or anti-inflammatory cytokines. T helper-1 (Th-1) cells, major activated T cells in lesions, produce pro-atherogenic cytokines such as interferon-γ (IFN-γ), TNF-α, and IL-2, whereas T helper-2 (Th-2) cells may oppose Th-1 effects by secreting the anti-inflammatory cytokines IL-4 and IL-101. During early T cell development, under certain circumstances, Th-1 and Th-2 cells can switch their cytokine expression patterns4.
Estrogen appears to influence atherogenesis and cardiovascular disease through multiple mechanisms, since it alters various vasoactive molecules, lipid concentrations, and antioxidant, coagulation, and fibrinolytic systems5. Although ET may produce beneficial effects on plasma lipoprotein concentrations, the changes explain less than half of the atheroprotective effects of estrogen6, suggesting a large lipid-independent effect. Estrogen has anti-inflammatory properties, reducing circulating levels of monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion molecule-1 (VCAM-1)7, 8, likely in part through transcription factor cross-talk involving estrogen receptor (ER) antagonism of NF-kB activity9. ER-α and ER-β are present within the arterial wall, and there is evidence for direct effects of estrogens on arteries and arterial cells10–12. ERs are also expressed by immune cell populations13.
Data from observational studies in women and experimental studies in animals provide support that estrogen protects against coronary heart disease (CHD) and atherosclerosis6, 14. Some of the earliest evidence that the atheroprotective effects of estrogens may be attenuated in late menopause once atherosclerosis was well established came from our own work in the ovariectomized non-human primate model15,16. Subsequently, data from the Women’s Health Initiative (WHI), a randomized controlled trial, demonstrated a lack of a beneficial effect of estrogen plus progestogen therapy (EPT) overall and adverse effects on clinical CHD outcomes in the first year of treatment of older postmenopausal women 17,18. Secondary analyses of the WHI found a significant graded relationship between the years since menopause and the relative hazard for CHD among women who received ET or EPT compared to placebo; the hazard ratio for women assigned to hormone therapy (HT) within 10 years of menopause was 0.76 (95% CI 0.50, 1.16) compared to 1.28 (95% CI 1.03, 1.58) for women assigned to HT 20 years or later after menopause19. The “timing hypothesis” emerged from these and other data, which suggested that the stage of atherosclerosis and/or timing of initiation of ET relative to menopause might be important determinants of estrogen’s ability to favorably affect atherosclerosis20.
The present study was designed to test the “timing hypothesis” in a prospective manner in cynomolgus monkeys, a well-established nonhuman primate model of women’s health and atherosclerosis20. Cynomolgus monkeys are similar to women in reproductive system function, including a regular 28-day menstrual cycle and similar gonadotropin and sex hormone variations across the cycle. Also like women, female cynomolgus monkeys are protected against the development of CHD premenopausally and lose that protection postmenopausally21. We hypothesized that estrogen treatment would inhibit atherogenesis associated arterial inflammatory responses when administered early after ovariectomy, but not after an extended estrogen-deficient period.
Methods
Animals and experimental design
The purpose of the present study was to assess the atheroprotective effects of estrogen when administered early (1 month) and late (54 months or 4.5 years) after surgical menopause in ovariectomized female cynomolgus monkeys (Macaca fascicularis). The animals were obtained through the Institut Pertanian Bogor, Indonesia. Age was estimated by dentition at the time of importation. For the early menopausal cohort, 24 monkeys were fed an atherogenic diet containing 35% of calories from fat (predominantly saturated fat) with 0.20 mg cholesterol per calorie, 46% of calories from carbohydrate, and 19% of calories from protein (casein and lactalbumin) for 6 months before ovariectomy and randomization to control or estradiol (E2) at one month after ovariectomy. The late menopausal cohort consisted of 34 monkeys which were ovariectomized and consumed an atherogenic diet for 4.5 years prior to randomization to receive control or E2. E2-treated monkeys received a human equivalent dose of 1 mg/day oral micronized E2 for 8 months before necropsy. Blood samples were obtained at baseline, before E2 administration, and periodically throughout the study, and carotid arteries and other tissues were obtained at necropsy. Animals were housed in social groups of 4–6 monkeys each. One animal in early menopausal cohort died 4 months after ovariectomy due to unknown causes. All procedures were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and regulations and guidelines established by the Wake Forest University Animal Care and Use Committee. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Necropsy and arterial processing
At the end of the treatment period, monkeys were deeply sedated with pentobarbital (30 mg/kg i.v.) and euthanized. The carotid arteries were removed, cleaned of adherent connective tissues, opened longitudinally, and sectioned. The common carotid arteries were subdivided into individual segments and processed as previously described for iliac arteries22. Briefly, adjacent segments were flash frozen in liquid nitrogen for RNA isolation and fixed in 4% paraformaldehyde for histologic examination. Proximal sections were cryopreserved in OCT embedding medium for immunohistochemical analyses.
Morphologic and Immunohistochemical Analyses of Arterial Segments
Atherosclerosis was determined as previously described (Walker et al., 2008). Briefly, paraffin blocks were sectioned at 5 μm and stained with Verhoeff Van Gieson as well as hematoxylin and eosin for assessment of tissue histology. Plaque area (mm2) of the carotid artery sections were determined by computer assisted histomorphometric methods using Image Pro Plus software22 (Media Cybernetics, Inc. Silver Springs, MD).
Immunohistochemical staining was performed on paraffin and OCT-embedded carotid artery biopsy sections. Sections were cut at 5 μm thickness, dried overnight at room temperature, and stored at −20°C. Serial sections were stained for 1) hematoxylin and eosin, 2) CD4 (T-cells), 3) CD8 (T-cells), 4) Ham56 (macrophages), and 6) CD68 (macrophages). OCT-embedded sections of colon (CD4, CD8) and liver (Ham56, CD68) were used as positive control tissues. For immunohistochemical staining, sections were brought to room temperature and fixed in cold acetone for 10 minutes. Epitopes were then detected using the following commercially available primary monoclonal antibodies: mouse anti-human CD4 (1:100 L200 clone, BD Pharmingen, San Jose, CA), mouse anti-human CD8 (1:10 RPA-T8 clone, BD Pharmingen, San Jose, CA), mouse anti-human Ham56 (1:1 Ham56, DAKO, Carpinteria, CA), and mouse anti-human CD68 (1:100 Y1/82A clone, BD Pharmingen, San Jose, CA). Primary antibodies were localized with appropriate biotinylated secondary antibodies (BioGenex, Inc, San Ramon, CA), streptavidin-alkaline phosphatase (BioGenex, Inc, San Ramon, CA), and Vector Red substrate (Vector Laboratories, Burlingame, CA). Tissue sections were incubated in primary antibody for 1.5 hours at room temperature (RT), secondary antibody for 20 min at RT, and streptavidin-alkaline phosphatase for 20 minutes at RT, followed by Vector Red chromagen for 3 minutes. Sections were counterstained with Mayer’s Hematoxylin and examined by light microscopy. Immunohistochemical cell staining was quantified using computer assisted morphometry (Image Pro Plus 5.1). A grid filter (8.5 μm2) was applied to digitize images and each cross-hatch within the intima was evaluated for positive or negative staining. Staining density was expressed as the percentage of intima occupied by positive cell staining. Tissue paraffin blocks were cut at 5 μm and deparaffinized. Sections were stained with Verhoeff and Van Gieson or hematoxylin and eosin for assessment of tissue histology. Plaque area (mm2) of the carotid artery sections were determined by computer assisted histomorphometric methods using Image Pro Plus software22 (Media Cybernetics, Inc. Silver Springs, MD). All measures were made by a trained observer blind to both treatment groups.
Plasma lipid measurements
TPC, HDLc, and plasma triglycerides (TGs) were determined in the Wake Forest Primate Center Clinical Chemistry Laboratory using reagents (ACE cholesterol, ACE HDL-C, and ACE Triglycerides) and instrumentation (ACE ALERA autoanalyzer) from Alfa Wasserman Diagnostic Technologies (West Caldwell, NJ). Cholesterol and HDLc were standardized to calibrated controls from the Centers for Disease Control and Prevention-National Institutes of Health Lipid Standardization Program. Intra- and inter-assay coefficients of variation were less than 5% for all analytes. Non-HDLc, which approximates the sum of low-density lipoprotein (LDL) cholesterol and very-low-density lipoprotein cholesterol, was calculated by subtracting HDLc from TPC.
Serum estradiol measurements
Serum E2 was determined in ether-extracted serum samples from fasted animals using a radioimmunoassay from Diagnostic Systems Laboratories, as previously described8.
RNA isolation and quantification
RNA was isolated from carotid artery sections as described previously23 followed by a cleanup step on QIAGEN RNeasy columns. Samples were assessed for total RNA concentration and purity using ND-1000 Spectrophotometer (NanoDrop, Fisher Thermo, Wilmington, DE). Total RNA (2 μg) was reverse transcribed to generate cDNA archive using a high-capacity cDNA archive kit (Applied Biosystems). Quantitative real-time reverse transcriptase-polymerase chain reaction (PCR) assays were performed on an ABI Prism 7500 FAST system using macaque-specific TaqMan FAM-MGB primer-probe assays for CD3, CD4, CD8, CD25, CD56 or neural cell adhesion molecule-1 (NCAM-1), CD83, CD163, forkhead box P3 (FoxP3), interleukin-2 (IL-2), interleukin-4 (IL-4), IL-10, tumor necrotic factor-alpha (TNF-α), interferon-γ (IFN-γ), matrix metalloproteinase-9 (MMP-9), MCP-1, chemokine (C-C motif) receptor 6 (CCR6), ER-α and ER-β or human-specific CD68, collagen type I (Col1), and smooth muscle actin (ACTA2) (Applied Biosystems)8, 22. Individual PCR reactions were performed using cDNA generated from 27 ng total RNA. All quantitative reverse transcriptase-PCR data were normalized to the mean of endogenous control genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and B-actin using the 2−ΔCTprocedure24. Target amplifications not reaching threshold by 40 cycles were designated as zero expression.
Statistical analysis
All data were assessed for normality and transformed if necessary. Data are presented as means along with 95% confidence interval limits in parentheses. Baseline characteristics immediately before E2 administration were analyzed using Student’s t test. Post-treatment lipid profiles and body weight were analyzed using analysis of covariance (ANCOVA) controlling for baseline lipid measures. Relationships between baseline lipid measures and gene expression data or carotid intima area were assessed within individual cohorts by Pearson correlations. ANCOVAs were used to assess the main effects of treatment and menopause on gene expression data and carotid intima area; tests for interactions were used to examine the consistency of treatment effects between menopausal cohorts. In accordance with a priori strategies for analysis developed because the early and late menopausal cohorts represented unique experimental conditions, differences between control and E2 treatment groups in gene expression data were investigated within individual cohorts using Student’s t test. Pearson correlations between molecular endpoints and post-treatment lipids were also determined. The level of significance was set at p ≤ 0.05. All analyses were performed using SPSS ver 16.0 (SPSS Inc., Chicago, IL). Correlations between immunohistochemical and gene expression data were determined using Spearman rank order correlation analysis.
Results
Baseline measurements
There were no significant differences at baseline between control and E2-treated animals in body weight or in plasma TPC, TG, HDLc and non HDLc concentrations in either menopausal cohort (Table 1).
Table 1.
Baseline characteristics of monkeys by treatment and timing of treatment initiation
| Early Menopause | Late Menopause | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=12) | E2 (n=11) | P | Control (n= 18) | E2 (n= 16) | P | |
| Body weight, kg | 2.88 (2.59, 3.21) | 3.07 (2.68, 3.51) | 0.432 | 3.21 (2.90, 3.55) | 3.30 (3.01, 3.62) | 0.676 |
| TPC mmol/L | 6.59 (5.35, 8.12) | 7.23 (5.90, 8.86) | 0.492 | 8.21 (6.72, 10.04) | 9.71 (8.49, 11.10) | 0.155 |
| HDLc mmol/L | 0.94 (0.82, 1.09) | 0.98 (0.78, 1.24) | 0.722 | 1.37 (1.12, 1.69) | 1.14 (0.87, 1.50) | 0.256 |
| Non-HDLc mmol/L | 5.54 (4.31, 7.13) | 6.00 (4.49, 8.02) | 0.649 | 6.41 (4.83, 8.51) | 8.19 (6.65, 10.09) | 0.149 |
| TGs mmol/L | 0.30 (0.22, 0.42) | 0.26 (0.21, 0.32) | 0.398 | 0.47 (0.39, 0.57) | 0.57 (0.44, 0.74) | 0.192 |
| TPC/HDLc ratio | 7.00 (5.19, 9.45) | 7.35 (4.88, 11.07) | 0.833 | 5.98 (4.19, 8.54) | 8.50 (6.05, 11.95) | 0.143 |
Data are back-transformed means and (95% confidence intervals) in log scale. E2 = estradiol
The differences between the geometric mean of the control and estradiol groups were assessed with Student t-test.
Post-treatment measurements
The mean body weights of E2-treated animals were less than those of controls in the late menopausal cohort. However, the correlations between body weights and lipid profile and gene expression data were statistically similar between cohorts (all p>0.05, data not shown). In the early menopausal group, plasma TPC and non-HDLc concentrations were significantly decreased in the E2-treated group compared to controls. In the late menopausal cohort, plasma HDLc concentrations were significantly decreased and plasma TG concentrations were significantly increased in the E2-treated group compared to controls (Table 2).
Table 2.
Post-treatment lipid profiles of monkeys by treatment and timing of treatment initiation
| Early Menopause | Late Menopause | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (n=12) | E2 (n=11) | P | Control (n= 18) | E2 (n= 16) | P | |
| Body weight, kg | 2.94 (2.84, 3.05) | 2.94 (2.82, 3.05) | 0.934 | 3.31 (3.16, 3.46) | 3.10 (2.95, 3.24) | 0.046 |
| TPC mmol/L | 8.75 (7.73, 9.89) | 7.00 (6.15, 7.96) | 0.017 | 6.28 (5.41, 7.29) | 5.62 (4.80, 6.59) | 0.315 |
| HDLc mmol/L | 0.80 (0.71, 0.90) | 0.76 (0.67, 0.85) | 0.476 | 1.05 (0.91, 1.22) | 0.77 (0.66, 0.91) | 0.008 |
| Non-HDLc mmol/L | 7.83 (6.73, 9.12) | 6.10 (5.21, 7.14) | 0.027 | 4.81 (3.89, 5.94) | 4.47 (3.56, 5.60) | 0.635 |
| TGs mmol/L | 0.43 (0.33, 0.56) | 0.43 (0.33, 0.58) | 0.917 | 0.33 (0.27, 0.42) | 0.50 (0.38, 0.70) | 0.019 |
| TPC/HDLc ratio | 10.96 (8.97, 13.40) | 9.23 (7.48, 11.38) | 0.233 | 6.04 (4.61, 7.89) | 7.16 (5.38, 9.53) | 0.389 |
Data are ANCOVA adjusted back-transformed means and 95% confidence intervals. E2 = estradiol
The differences between the geometric means of the control and estradiol groups were assessed with ANCOVA, analysis of covariance, controlling for pre-treatment lipid profiles.
Gene expression measures
Expression of inflammatory markers in the carotid artery is presented in Table 3. The two-factor analysis of variance revealed significant main effects of treatment of all inflammatory markers, but not ACTA2 and ER-α (Table 4). There was no significant interaction between treatment and time since menopause, except for the macrophage marker CD163 (P = 0.046). We also examined mean expression ratio of E2/control of each inflammatory marker, using ANCOVAs to adjust for pretreatment plasma lipids. Carotid artery expression levels of the macrophage markers CD68 and CD163, the dendritic cell marker CD83, and the NK cell marker NCAM-1 were all significantly lower in E2-treated monkeys compared to controls in the early menopausal (but not late menopausal) cohort (Table 4). Transcription levels of markers for T cells (CD3), T-effector cells (CD4), and cytotoxic T cells (CD8), as well as CD25 (Tregs, others) were significantly lower in the E2 group in both early and late menopausal cohorts. No significant effect of E2 was observed for expression of FoxP3, a key marker for T-regulatory cells. Transcription levels of CCR6, a marker for both Th-1 and Th-17 cells, were decreased in the E2 group in the late menopausal cohort only.
Table 3.
Geometric means of carotid artery gene expression relative to the endogenous control genes, GADPH and B-actina
| Early Menopause
|
Late Menopause
|
|||
|---|---|---|---|---|
| Control (n=12) | E2 (n=11) | Control (n= 18) | E2 (n= 16) | |
| CD68 b,c | 1.86 (0.75, 4.60) | 0.31 (0.15, 0.63) | 0.41 (0.22, 0.77) | 0.20 (0.10, 0.40) |
| CD163 d,e | 2.21 (1.27, 3.82) | 0.88 (0.51, 1.51) | 1.85 (1.15, 2.99) | 1.79 (1.38, 2.33) |
| CD83 d,e | 2.48 (0.85, 7.21) | 0.44 (0.19, 1.03) | 0.34 (0.14, 0.83) | 0.19 (0.08, 0.46) |
| CD3 f,g | 1.37 (0.66, 2.84) | 0.30 (0.18, 0.51) | 1.11 (0.72, 1.71) | 0.40 (0.24, 0.66) |
| CD4 b,c | 1.28 (0.65, 2.49) | 0.49 (0.31, 0.78) | 0.74 (0.44, 1.22) | 0.25 (0.17, 0.37) |
| CD8d,e | 1.10 (0.54, 2.26) | 0.24 (0.16, 0.38) | 0.68 (0.41, 1.12) | 0.22 (0.13, 0.40) |
| CD25 d,e | 1.39 (0.43, 4.47) | 0.19 (0.06, 0.59) | 0.23 (0.09, 0.61) | 0.04 (0.01, 0.12) |
| FoxP3f,g | 0.60 (0.30, 0.12) | 0.30 (0.19, 0.48) | 0.24 (0.17, 0.35) | 0.15 (0.10, 0.23) |
| CCR6 f,g | 2.60 (1.46, 4.60) | 1.76 (0.83, 3.75) | 0.51 (0.29, 0.91) | 0.19 (0.13, 0.27) |
| NCAM-1 d,e | 1.54 (0.96, 2.49) | 0.73 (0.46, 1.16) | 1.37 (0.98, 1.91) | 1.25 (0.89, 1.75) |
| ACTA2* | 0.98 (0.58, 1.66) | 1.35 (0.79, 2.31) | 1.30 (0.86, 1.98) | 1.36 (0.86, 2.15) |
| ColI b,c | 2.07 (1.36, 3.14) | 0.61 (0.38, 0.97) | 0.35 (0.23, 0.52) | 0.21 (0.13, 0.33) |
| MCP-1† d,e | 6.70 (3.29, 13.65) | 1.72 (0.82, 3.61) | 3.18 (1.77, 5.74) | 1.17 (0.63, 2.18) |
| IFN-γj,k‡ | 5.22 (2.52, 9.90) | 0.93 (0.03, 2.61) | 2.25 (1.07, 4.12) | 1.33 (0.39, 2.89) |
| TNFα b,c | 1.32 (0.63, 2.74) | 0.26 (0.15, 0.44) | 0.30 (0.18, 0.53) | 0.14 (0.09, 0.23) |
| IL-2h,i‡ | 1.87 (0.96, 3.58) | 0.93 (0.42, 1.95) | 0.32 (0.15, 0.58) | 0.16 (0.05, 0.35) |
| IL-10 f,g | 1.44 (0.65, 3.20) | 0.44 (0.23, 0.87) | 1.22 (0.76, 1.96) | 0.54 (0.32, 0.93) |
| IL-4j,k | 1.72 (0.22, 5.06) | 0.34 (0.14, 1.07) | 0.68 (0.15, 1.46) | 0.23 (0.06, 0.60) |
| MMP-9 d,e†§ | 5.42 (2.11, 13.93) | 1.44 (0.57, 3.64) | 2.67 (1.28, 5.57) | 0.66 (0.30, 1.45) |
| ERᇠb,c | 0.23 (0.14, 0.36) | 0.20 (0.12, 0.33) | 0.45 (0.31, 0.65) | 0.28 (0.19, 0.42) |
| ERβ f,g‡ | 0.51 (0.29, 0.89) | 0.29 (0.16, 0.53) | 0.65 (0.42, 0.10) | 0.34 (0.21, 0.57) |
Data are back-transformed means and 95% confidence intervals in log scale.
Mean × 10−1.
C.I. × 10−1.
Mean × 10−2.
C.I. × 10−2.
Mean × 10−3.
C.I. × 10−3.
Mean × 10−4.
C.I. × 10−4.
Mean × 10−5.
C.I. × 10−5.
adjusted mean for nonHDLc,
adjusted mean for TPC/HDLc,
adjusted mean for TG,
adjusted mean for TPC
Table 4.
Main effects of timing of treatment initiation and treatment on carotid gene expression and mean expression ratio of E2/control in carotid gene expression.
|
P Values
|
Ratio (E2 /control) of Early Menopause cohort
|
Ratio (E2/control) of Late Menopause cohort
|
|||||
|---|---|---|---|---|---|---|---|
| Menopause | Treatment | Interaction | Mean Ratio | P | Mean Ratio | P | |
| CD68 | 0.007 | 0.001 | 0.130 | 0.16 | 0.002 | 0.48 | 0.106 |
| CD163 | 0.223 | 0.032 | 0.046 | 0.40 | 0.008 | 0.97 | 0.900 |
| CD83 | 0.002 | 0.012 | 0.206 | 0.18 | 0.015 | 0.56 | 0.307 |
| CD3 | 0.884 | <.001 | 0.339 | 0.22 | <.001 | 0.36 | 0.003 |
| CD4 | 0.015 | <.001 | 0.812 | 0.38 | 0.014 | 0.34 | 0.001 |
| CD8 | 0.300 | <.001 | 0.460 | 0.22 | 0.001 | 0.33 | 0.002 |
| CD25 | 0.002 | 0.001 | 0.877 | 0.14 | 0.017 | 0.16 | 0.008 |
| FoxP3 | 0.002 | 0.028 | 0.492 | 0.50 | 0.061 | 0.70 | 0.222 |
| CCR6 | <.001 | 0.016 | 0.306 | 0.68 | 0.351 | 0.39 | 0.008 |
| NCAM-1 | 0.262 | 0.026 | 0.079 | 0.47 | 0.010 | 0.91 | 0.696 |
| ACTA2* | 0.568 | 0.467 | 0.574 | 1.37 | 0.400 | 1.04 | 0.892 |
| ColI | <.001 | <.001 | 0.092 | 0.29 | <.001 | 0.59 | 0.047 |
| MCP-1† | 0.095 | 0.001 | 0.593 | 0.26 | 0.011 | 0.37 | 0.025 |
| IFN-γ‡ | 0.465 | 0.004 | 0.112 | 0.31 | 0.004 | 0.71 | 0.308 |
| TNFα | 0.001 | <.001 | 0.090 | 0.19 | <.001 | 0.50 | 0.050 |
| IL-2‡ | 0.000 | 0.048 | 0.760 | 0.52 | 0.138 | 0.62 | 0.190 |
| IL-10 | 0.862 | 0.001 | 0.448 | 0.31 | 0.009 | 0.47 | 0.040 |
| IL-4 | 0.286 | 0.041 | 0.295 | 0.49 | 0.046 | 0.79 | 0.418 |
| MMP-9†§ | 0.131 | 0.001 | 0.926 | 0.27 | 0.037 | 0.25 | 0.009 |
| ERᇠ| 0.045 | 0.149 | 0.389 | 0.89 | 0.711 | 0.62 | 0.074 |
| ER⇠| 0.492 | 0.018 | 0.891 | 0.56 | 0.145 | 0.53 | 0.051 |
p <0.05 are in boldface.
Controlling for * nonHDLc, † TPC/HDLc, ‡ TG, § TPC. Main effect of menopause status and treatment on carotid gene expression was analyzed by factorial ANCOVA controlling for correlated lipid profile.
Cytokines and other markers
Transcript levels for the pro-inflammatory cytokine IFN-γ and anti-inflammatory IL-4 were significantly lower in E2-treated monkeys in the early, but not the late, menopausal cohort. Transcript levels for the chemokine MCP-1, the pro-inflammatory cytokine TNF-α, and the anti-inflammatory mediator IL-10 was significantly lower in E2-treated monkeys in both cohorts.
MMP-9 was significantly lower in E2-treated monkeys compared to controls in both cohorts. Collagen type I expression was significantly lower in the E2-treated group in both menopausal cohorts. Expression of ER-α, ER-β, and smooth muscle cell α-actin did not significantly differ in E2- treated monkeys compared to controls in either cohort.
Relationships Between Immune Cell Staining Densities and Arterial Gene Expression Measures
Intimal IHC staining of markers for macrophages and T-cells was significantly correlated with corresponding measures of expression of macrophage and T-cell markers measured in a distal segment of the carotid artery. Intimal CD68+ staining was correlated with mRNA levels for CD68 (r=0.45, p<0.001), which was used as a molecular marker for macrophage content. Intimal CD4+ staining density was correlated with CD4 mRNA levels (r=0.33, p<0.05). Intimal CD8+ staining density was highly correlated with CD8 mRNA expression (r=0.40, p<0.01).
Discussion
In this study, the effects of estrogen on expression of genes relevant to atherosclerosis in the carotid artery depended on whether treatment was initiated at an early stage or after many years of estrogen deficiency. Arterial macrophages, dendritic and NK cell markers, and pro-inflammatory cytokines were significantly decreased in monkeys treated with estradiol early after menopause, an effect that was not observed or was dampened in the late menopausal cohort. In contrast, estrogen treatment in both early and late menopausal cohorts resulted in lower expression of T-cell markers, including T helper cells and cytotoxic T cells. The current results support the hypothesis that the timing of estrogen treatment relative to menopause has a profound impact on the vascular effects of estrogens, which may be mediated in part by differential immune modulation of events within the arterial wall. The mechanism by which estradiol preferentially reduced arterial macrophage content in the early but not late menopausal group is unclear, but may relate to the differential nature of the lesions. In the late menopausal monkeys, lesions had developed and evolved over many years of estrogen deficiency and may have been relatively resistant to treatment.
The diversity of immune cells in the plaque, reflecting both innate and adaptive immune responses, is a distinctive feature of atherosclerosis. Macrophages are critical components of the initiating and ongoing inflammatory processes in atherogenesis and progression from early fatty streaks to vulnerable atherosclerotic plaques. Macrophages and dendritic cells accumulate in the atherosclerotic plaque and in rupture-prone lesions25, and contribute to innate and adaptive immune responses. Macrophages take up modified LDL through scavenger receptors, and may become foam cells. Activated macrophages and dendritic cells generate pro-inflammatory cytokines, which recognize and present foreign antigens to naive T cells. Activated T cells in atherosclerotic plaques have predominantly Th-1 properties and generate cytokines IFN-γ, IL-2, and TNF-α26. IFN-γ, also elaborated by NK cells and macrophages, appears to promote atherogenesis. In one report, apolipoprotein E- and LDL receptor-deficient mice lacking IFN-γ exhibited attenuated atherosclerosis27. IFN-γ contributes to the recruitment of T cells and macrophages to the plaques, augments class II histocompatibility expression, and enhances secretion of Th-1-promoting cytokines. Th-2 cells secrete IL-4, IL-5, IL-10, and IL-13 and generally oppose Th-1 responses, although their role in atherogenesis is controversial28. Regulatory T cells (CD4+ and CD25+ cells) modulate Th-1 effects by secreting anti-inflammatory cytokines. The roles of cytotoxic T cells (CD8+) and NK cells in atherosclerosis are not well understood1.
Arterial intimal lesion size (cross-sectional area) is a classic measurement of atherosclerosis in our nonhuman primate model which develops over relatively long periods of time. As we expected, there was no significant difference in carotid intima area between control and treated animals in either cohort of animals after 8 months, a short duration of treatment. However, this metric provides little information about metabolic characteristics of the lesion. Exploration of arterial gene expression is a sensitive means to evaluate intra-arterial cellular and molecular events, and provides data which may elucidate mechanisms of pathogenesis and improve prediction of treatment efficacy and disease progression, with a prospect of fostering new clinical strategies. We assessed the scavenger receptors CD68 and CD163 as macrophage markers. While CD68 is also expressed to some extent by other cell types (such as dendritic cells), CD163 expression is relatively restrictedly to the monocyte/macrophage lineage in general, and to resident tissue macrophages with an M2-like anti-inflammatory phenotype29. CD83 is a marker for mature dendritic cells but also is expressed on other cell types, such as CD8+ T cells and B cells30. CD3, CD4, and CD8 are T-cell-specific markers, with CD3 being present on most T cells, whereas CD4 and CD8 are found on T-helper cells and cytotoxic T cells, respectively. Expression of both CD3 and CD25 is used as a marker of regulatory T cells.
Our studies suggest that early administration of estrogen relative to menopausal onset reduced arterial macrophages (CD68, CD163), dendritic (CD83), and NK cells (CD56). In contrast, estradiol treatment resulted in reduced arterial expression of markers for T cells (CD3), T helper cells (CD4), cytotoxic T cells (CD8), and regulatory T cells (CD4+ and CD25+) in both early and late menopausal cohorts. Notably, expression of chemokine receptor 6, a CC chemokine receptor expressed on Th-17, Th-1 and unactivated memory T cells, was reduced in the late menopausal cohort alone. Estradiol had no effect on FoxP3, a marker for adaptive and natural regulatory T cells also transiently expressed in some other activated CD4+ cells31. Importantly, levels of expression of the genes used as markers for individual cell types were significantly correlated with immunohistochemical staining for that corresponding marker in adjacent arterial sections. Gene expression analyses provides a useful method for measurement of molecular events occurring in a larger representative section of the artery than feasible with immunohistochemical analyses, which generally relies on a single 5–7 um section of artery per specific antibody. The intima-media segments of artery used for gene expression in this experiment corresponded to approximately 1000 microtome sections of 5 um each. In this context, gene expression assays represents a useful tool for characterizing more globally the presence of specific cell types within atherosclerotic lesions, as this method allows for a more complete characterization of the segment examined. The composition of atherosclerotic lesions changes dynamically as the disease progresses. Early lesions are characterized by accumulation of intra- or extracellular lipid, presence of macrophage foam cells, and possibly vascular smooth muscle cells (VSMCs) containing lipid droplets32. Accumulation of lipids, cells, and matrix components often associated with structural disorganization, repair, intimal thickening, and deformity of the arterial wall is a common feature of advanced lesions33. The proliferation and accumulation of VSMCs is often seen in such lesions. VSMCs may switch their phenotypes from a contractile to synthetic state and produce significant amounts of extracellular matrix (ECM) components, such as collagen type I and type III, which are abundant in plaques34. VSMCs may also produce pro-inflammatory mediators, such as MCP-1 and MMPs. MMPs and other proteases are also secreted by inflammatory cells, ultimately degrading the ECM. This process results in tissue remodeling in developing atheromas and plaque rupture in vulnerable plaques35. Vulnerable plaques and unstable lesions often contain numerous inflammatory cells at the lesion edges, and are often rich in lipids, with fewer VSMCs than stable lesions36. Although E2 did not influence SMC α-actin expression, it was associated with significantly lower expression of type I collagen in the early menopausal cohort. Therefore, early administration of E2 seems to attenuate both inflammation and extracellular matrix deposition, significant features of plaque progression, while apparently not influencing VSMC content.
A balance between arterial pro- and anti-inflammatory cytokine expression is a likely determinant of the ultimate fate of the atherosclerotic lesion. Pro-inflammatory cytokines such as IFN-γ, TNF-α, and others may promote atherogenesis and increase lesion complexity, while anti-inflammatory cytokines such as IL-4 and IL-10 may limit these processes and inhibit disease progression. Estrogen appeared to reduce expression of both pro- and anti-inflammatory mediators examined here. In addition, IL-10, a pleiotropic anti-inflammatory cytokine expressed by many cell types within the adaptive immune system37, was decreased in the E2 groups in both the early and late menopausal cohorts.
The anti-inflammatory response of E2 observed here could occur through modulation of the activity of the nuclear transcription factor NF-κB, which regulates the expression of adhesion molecules, cytokines, and chemokines within developing atheromas38. Estrogen can inhibit NF-κB signaling via ERs through a variety of mechanisms39. ERs are expressed in the arteries in cynomolgus macaques, and are inversely correlated with atherosclerosis extent23. Nevertheless, in this study, ER-α and ERβ gene expression were not diminished in the late menopausal group compared to the early menopausal group.
Conclusion
This study directly addresses, for the first time, the timing hypothesis in an animal model by exploring arterial responses to estrogen treatment in nonhuman primates with short (1 month) and long (4.5 years) postmenopausal periods of estrogen deficiency before initiation of estrogen treatment. These periods correspond to about 3 months and 12 to 15 years, respectively, in women. The exact ages of the animals were unknown which limited our ability to assess the impact of age on the outcomes. Nevertheless, all animals were premenopausal at ovariectomy and thus would be representative of women under the age of 50. Clinically, ET generally begins with the onset of menopause to treat hot flushes and other menopausal symptoms, and would rarely be delayed and introduced later except in the context of a randomized trial. The findings from this unique experimental design support the idea that estrogen inhibits inflammation in arteries of animals following a short but not long menopausal interval. This effect appeared to be limited to selected targets and cell populations; E2-treated monkeys had reduced expression of IFN-γ, arterial macrophages, and dendritic cell markers in the early but not late menopausal group, while arterial T cell marker expression was lower in the E2-treated groups in both early and late menopause. Arterial MMP-9 was reduced by estrogen treatment in both groups, arguing against the theory that estrogen’s adverse effects on cardiovascular events (i.e. increased plaque rupture) in late menopause are caused by higher levels of MMP-9 within arterial plaques. These findings bring new information to the clinically important field of hormone replacement and cardiovascular disease, and may help to explain the relative lack of benefit in the older women in the WHI.
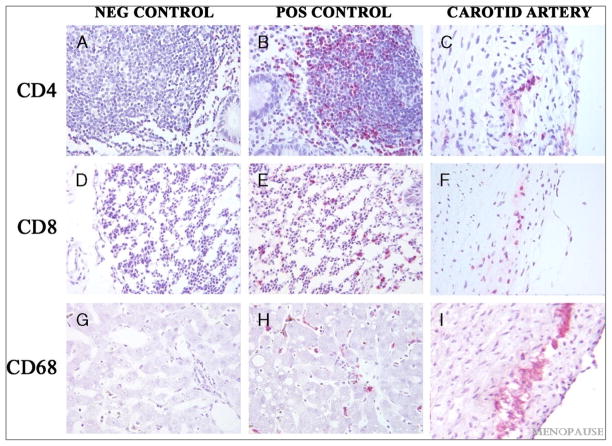
FIG. 1.
Immunohistochemical staining of CD4, CD8, and CD68 in sections of monkey carotid artery along with positive and negative controls: Negative Controls (A, D, G); Positive Controls (B, E, H); Arterial tissues (C, F, I). A–C, Immunostaining for CD-4; D–F, Immunostaininig for CD8; G–I, Immunostaining for CD68. Mucosa associated lymphoid tissue from cynomolgus macaque colon was used as the control tissue for CD4 and CD8, liver was used as the control tissue for CD68. Sections depicted in the first column (A, D, G) were incubated with normal mouse serum instead of primary antibody. All figures at 40X magnification.
Acknowledgments
SOURCE OF FUNDING
This project was supported by NIH grants AG18170 (TCR), AG 28641 (TCR), and HL 45666 (TBC), and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
The authors wish to thank J.D. Bottoms, Lisa O’Donnell, Maryanne Post, Debbie Golden, and Hermina Borgerink for their excellent technical assistance.
Footnotes
DISCLOSURES: None
DISCLAIMERS: None
References
- 1.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 2.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134(1):33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102(24):2919–22. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20(1):4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89(12A):12E–7E. doi: 10.1016/s0002-9149(02)02405-0. discussion 7E–8E. [DOI] [PubMed] [Google Scholar]
- 6.Adams MR, Kaplan JR, Manuck SB, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10(6):1051–7. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 7.Register TC, Cann JA, Kaplan JR, et al. Effects of soy isoflavones and conjugated equine estrogens on inflammatory markers in atherosclerotic, ovariectomized monkeys. J Clin Endocrinol Metab. 2005;90(3):1734–40. doi: 10.1210/jc.2004-0939. [DOI] [PubMed] [Google Scholar]
- 8.Register TC, Wagner JD, Zhang L, Hall J, Clarkson TB. Effects of tibolone and conventional hormone replacement therapies on arterial and hepatic cholesterol accumulation and on circulating endothelin-1, vascular cell adhesion molecule-1, and E-selectin in surgically menopausal monkeys. Menopause. 2002;9(6):411–21. doi: 10.1097/00042192-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89(9):823–30. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- 10.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94(9):3513–20. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J Steroid Biochem Mol Biol. 1998;64(3–4):187–91. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 12.Register TC. Primate models in women’s health: inflammation and atherogenesis in female cynomolgus macaques (Macaca fascicularis) Am J Primatol. 2009;71(9):766–75. doi: 10.1002/ajp.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shim GJ, Gherman D, Kim HJ, et al. Differential expression of oestrogen receptors in human secondary lymphoid tissues. J Pathol. 2006;208(3):408–14. doi: 10.1002/path.1883. [DOI] [PubMed] [Google Scholar]
- 14.Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335(7):453–61. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 15.Williams JK, Anthony MS, Honore EK, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15(7):827–36. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- 16.Register TC, Adams MR, Golden DL, Clarkson TB. Conjugated equine estrogens alone, but not in combination with medroxyprogesterone acetate, inhibit aortic connective tissue remodeling after plasma lipid lowering in female monkeys. Arterioscler Thromb Vasc Biol. 1998;18:1164–1171. doi: 10.1161/01.atv.18.7.1164. [DOI] [PubMed] [Google Scholar]
- 17.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 18.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson TB, Mehaffey MH. Coronary heart disease of females: lessons learned from nonhuman primates. Am J Primatol. 2009;71(9):785–93. doi: 10.1002/ajp.20693. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson TB, Hughes CL, Klein KP. The nonhuman primate model of the relationship between gonadal steroids and coronary heart disease. Prog Cardiovasc Dis. 1995;38(3):189–98. doi: 10.1016/s0033-0620(95)80011-5. [DOI] [PubMed] [Google Scholar]
- 22.Walker SE, Adams MR, Franke AA, Register TC. Effects of dietary soy protein on iliac and carotid artery atherosclerosis and gene expression in male monkeys. Atherosclerosis. 2008;196(1):106–13. doi: 10.1016/j.atherosclerosis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker SE, Register TC, Appt SE, et al. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15(5):950–7. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Niessner A, Weyand CM. Dendritic cells in atherosclerotic disease. Clin Immunol. 2010;134(1):25–32. doi: 10.1016/j.clim.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145(1):33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 27.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23(3):454–60. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 28.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr Opin Pharmacol. 2010;10(2):197–202. doi: 10.1016/j.coph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47(7–8):1650–60. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Prazma CM, Tedder TF. Dendritic cell CD83: a therapeutic target or innocent bystander? Immunol Lett. 2008;115(1):1–8. doi: 10.1016/j.imlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagoo P, Lombardi G, Lechler RI. Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant. 2008;13(6):645–53. doi: 10.1097/MOT.0b013e328317a476. [DOI] [PubMed] [Google Scholar]
- 32.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89(5):2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 33.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 34.Skjot-Arkil H, Barascuk N, Register T, Karsdal MA. Macrophage-mediated proteolytic remodeling of the extracellular matrix in atherosclerosis results in neoepitopes: a potential new class of biochemical markers. Assay Drug Dev Technol. 2010;8(5):542–52. doi: 10.1089/adt.2009.0258. [DOI] [PubMed] [Google Scholar]
- 35.Pyle AL, Young PP. Atheromas feel the pressure: biomechanical stress and atherosclerosis. Am J Pathol. 2010;177(1):4–9. doi: 10.2353/ajpath.2010.090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke M, Bennett M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am J Nephrol. 2006;26(6):531–5. doi: 10.1159/000097815. [DOI] [PubMed] [Google Scholar]
- 37.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 38.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 39.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29(3):289–95. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]