Abstract
Importance
Experts debate optimal 25(OH)D levels for musculoskeletal health.
Objective
To compare effects of placebo, low-dose and high-dose vitamin D on one-year changes in total fractional calcium absorption, bone mineral density, Timed-Up-and-Go and 5-sit-to-stand tests and muscle mass in postmenopausal women with vitamin D insufficiency.
Design
Randomized, double-blind, placebo-controlled, clinical trial conducted from May 2010 to August 2014.
Setting
Single-center trial conducted in Madison, Wisconsin.
Participants
230 postmenopausal women ≤75 years old with baseline 25(OH)D levels 14-27 ng/mL and no osteoporosis.
Intervention
Three arms included daily white and twice monthly yellow placebo (n=76), daily 800 IU vitamin D3 and twice monthly yellow placebo (n=76), and daily white placebo and twice monthly 50,000 IU vitamin D3 (n=79). The high-dose vitamin D regimen achieved and maintained 25(OH)D levels ≥30 ng/mL.
Main Outcome Measures
One year change in total fractional calcium absorption using two stable isotopes, bone mineral density and muscle mass using dual energy x-ray absorptiometry, Timed-Up-and-Go and 5-Sit-to-Stand tests, functional status (Health Assessment Questionnaire) and physical activity (Physical Activity Scale for the Elderly), with Benjamini-Hochberg correction of p-values to control the false discovery rate.
Results
After controlling for baseline absorption, calcium absorption increased 1% (10 mg/day) in the high-dose arm, but decreased by 2% in low-dose (p=0.005 vs. high-dose) and by 1.3% placebo (p=0.03 vs. high-dose) arms. We found no between-arm changes in spine, mean total hip, mean femoral neck or total body bone mineral density, trabecular bone score, muscle mass, 5-sit-to-stand or Timed-Up-and-Go test scores. Likewise, we found no between-arm differences for numbers of falls, number of fallers, physical activity or functional status.
Conclusion and Relevance
High-dose vitamin D therapy increased calcium absorption, but the effect was small and did not translate into beneficial effects on bone mineral density, muscle function, muscle mass or falls. We found no data to support experts’ recommendations to maintain serum 25(OH)D levels ≥30 ng/mL in postmenopausal women. Instead, we found that low and high-dose vitamin D were equivalent to placebo, in their effects on bone and muscle outcomes in this cohort of postmenopausal women with 25(OH)D levels <30 ng/mL.
ClinicalTrials.gov.Identifier:NCT00933244
Keywords: bone mineral density, calcium absorption, clinical trial, postmenopausal women, sarcopenia, vitamin D
INTRODUCTION
Nearly half of postmenopausal women sustain an osteoporotic fracture.1,2 Low vitamin D levels contribute to osteoporosis via decreased total fractional calcium absorption (TFCA), secondary hyperparathyroidism, increased bone resorption and decreased bone mineral density (BMD).3 Unfortunately, experts disagree on the optimal vitamin D level for skeletal health. Some4-6 contend that optimal serum 25(OH)D levels are ≥30 ng/mL and define vitamin D insufficiency (VDI) as <30 ng/mL. By contrast, the Institute of Medicine7 recommends levels ≥20 ng/mL. Disagreement continues, as many previous clinical trials did not recruit subjects based on initial 25(OH)D levels, failed to target or achieve 25(OH)D levels ≥30 ng/mL, and/or co-administered calcium supplements.
VDI, defined as a serum 25(OH)D <30 ng/mL, is widespread and affects ~75% of postmenopausal American women.8 Therefore, determining the ideal 25(OH)D level for optimal calcium homeostasis and bone health is of utmost clinical import. The aims of this double-blind, placebo-controlled trial were to evaluate the effects of high and low-dose vitamin D on one-year changes in TFCA (the fraction of ingested calcium absorbed in the intestine), BMD and muscle fitness in postmenopausal women with VDI. Women with osteoporosis were excluded. Based on our prior pilot study,9 we hypothesized that a high-dose vitamin D regimen, administered to achieve and maintain 25(OH)D >30 ng/mL for one year, would increase TFCA and BMD more than low-dose vitamin D or placebo.
METHODS
Study Design
With IRB approval, we conducted a single-center, randomized, double-blind, placebo-controlled trial involving postmenopausal women living around Madison, Wisconsin (latitude 43°N). Recruitment (Figure 1) occurred from May 2010 to July 2013 and the final visit was completed in August 2014. Individuals called in response to local advertisements, and were phone-screened for eligibility. After written consent, we measured eligible subjects’ serum 25(OH)D, calcium, albumin, creatinine and parathyroid hormone levels.
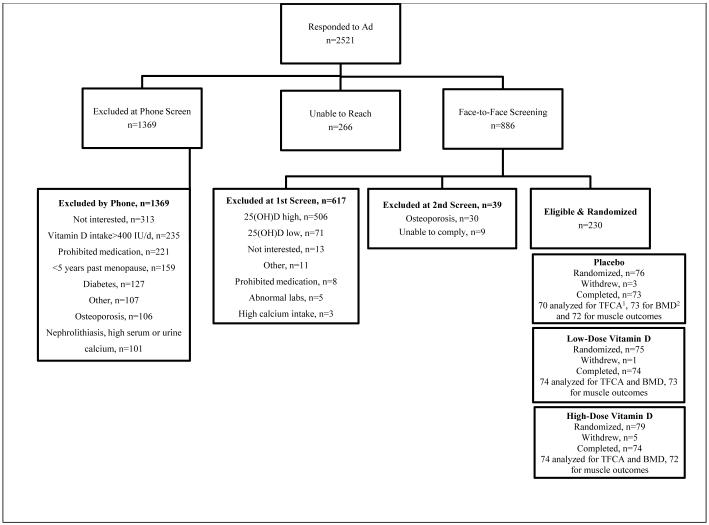
Figure 1. Participant Flow Diagram.
aTFCA denotes total fractional calcium absorption. bBMD denotes bone mineral density. The calcium isotope doses were not recorded in 2 subjects and a urine sample was mishandled in a 3rd. Muscle tests were not performed in 4 subjects due to pain and/or an injury.
We enrolled women with a 25(OH)D >14 ng/mL and ≤27 ng/mL, instead of <30 ng/mL, to allow for laboratory variability in measurements.10 Subjects were ≥5 years past menopause or oophorectomy, or ≥60 years old if prior hysterectomy without oophorectomy. Eligible subjects consuming <600 mg or >1400 mg calcium/day via questionnaire11 were counseled to consume 600-1400 mg/day by modifying their dietary and/or supplemental calcium intake. We targeted typical calcium intake of postmenopausal American women12 to ensure generalizability, minimize harms of high-dose vitamin D, and because passive calcium absorption lessens the import of vitamin D-mediated active absorption.13-15
We excluded women >75 years old as increasing age associates with intestinal resistance to vitamin D.16,17 We excluded women with hypercalcemia, nephrolithiasis, cancer within five years (excluding skin cancer), inflammatory bowel disease, malabsorption, celiac sprue, chronic diarrhea, glomerular filtration rate (GFR) <45 mL/minute,18 adult fragility fracture of the hip, spine or wrist and use of bone-active medications within the past 6 months including bisphosphonates, estrogens, calcitonin, teriparatide, oral corticosteroids, anticonvulsants, or vitamin D >400 IU/day.19 Women with diabetes were also excluded, as the disease and associated medications affect skeletal health.20 We measured subjects’ spine, bilateral hip and total body BMD and excluded those with T-scores ≤-2.5.
Subjects completed 4-7 day food diaries within one month of TFCA studies, using scales and household measuring tools to record intake. Food diaries were analyzed using Food Processor® Software (ESHA Research, Salem, OR) to calculate subjects’ customary intake of nutrients (Table 1), caffeine and alcohol. Dietary intake, except alcohol, was reproduced during TFCA studies.
Table 1.
Baseline Characteristics of Randomized Subjects
| All Subjects n=230 |
Placebo n= 76 |
Low-Dose Vitamin D n= 75 |
High-Dose Vitamin D n= 79 |
P
valuea |
|
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age, years | 61 ± 6 | 61 ± 6 | 60 ± 6 | 60 ± 5 | 0.78 |
| Weight, kg | 81 ± 18 | 81 ± 19 | 82 ± 18 | 80 ± 18 | 0.91 |
| Height, cm | 163 ± 6 | 163 ± 6 | 164 ± 6 | 162 ± 5 | 0.91 |
| Body Mass Index, kg/m2 | 30.8 ± 6.8 | 30.6 ± 6.6 | 31.2 ± 7.4 | 30.7 ± 6.5 | 0.91 |
| Race | |||||
| White | 207 (90%) | 68 (90%) | 67 (89%)b | 72 (91%) | |
| Black | 14 (6%) | 6 (8%) | 7 (9%) | 1 (1%) | |
| Asian | 5 (2%) | 1 (1%) | 1 (1%) | 3 (4%) | 0.74 |
| American Indian/Alaskan | 2 (1%) | 0 (0%) | 0 (0%) | 2 (3%) | |
| Hispanic/Latina | 2 (1%) | 1 (1%) | 0 (0%) | 1 (1%) | |
| Bone Mineral Density Measures | |||||
| Spine, g/cm2 | 1.155 (1.055, 1.286) | 1.143 (1.048, 1.228) |
1.145 (1.080, 1.275) |
1.163 (1.044, 1.280) |
0.91 |
| Spine T-Score | −0.2 (−1.1, +0.9) | −0.3 (−1.1, +0.9) | −0.3 (−0.8, +0.8) | −0.2 (−1.2, +0.9) | 0.91 |
| Hip, g/cm2 | 0.961 (0.900, 1.038) |
0.954 (0.882, 1.025) |
0.961 (0.905, 1.038) |
0.966 (0.911, 1.032) |
0.91 |
| Hip T-Score | −1.0 (−1.5, −0.5) | −1.0 (−1.7, −0.6) | −1.0 (−1.4, −0.4) | −1.1 (−1.6, −0.5) | 0.78 |
| Dietary Habits | |||||
| Kilocalories, kcal/day | 1842 (1539, 2198) | 1943 (1651, 2258) | 1782 (1558, 2045) |
1839 (1497, 2196) |
0.78 |
| Carbohydrates, g/day | 222 (175, 266) | 231 (194, 274) | 215 (171, 261) | 205 (171, 261) | 0.74 |
| Protein, g/day | 75 (62, 86) | 74 (59, 86) | 75 (65, 89) | 76 (64, 86) | 0.91 |
| Fat, g/day | 72 (60, 91) | 77 (58, 96) | 72 (60, 88) | 68 (61, 90) | 0.89 |
| Fiber, g/day | 19 (14, 25) | 21 (15, 28) | 19 (15, 24) | 17 (14, 24) | 0.74 |
| Dietary Calcium, mg/day | 905 (703, 1099) | 929 (777,1110) | 890 (678,1101) | 896 (706,1077) | 0.91 |
| Calcium Supplement, mg/day | 0 (0, 0) | 0 (0, 29) | 0 (0, 0) | 0 (0, 0) | 0.78 |
| All Calcium Intake, mg/day | 967 (752, 1215) | 1007 (808,1306) | 961 (699,1202) | 962 (739,1174) | 0.78 |
| Iron, mg/day | 13 (10, 16) | 14 (11, 16) | 12 (9, 16) | 13 (11, 16) | 0.78 |
| Magnesium, mg/day | 306 (247, 370) | 335 (261, 405) | 289 (244, 337) | 305 (247, 354) | 0.47 |
| Vitamin D, IU/day | 196 (115, 266) | 190 (138, 299) | 176 (115, 254) | 207 (107, 263) | 0.91 |
| Oxalate, servings/day | 0.9 (0.4, 1.8) | 1.1 (0.5, 2.1) | 0.9 (0.4, 1.9) | 0.6 (0.3, 1.4) | 0.46 |
| Serum Laboratory Measures | |||||
| Calcium, mg/dL | 9.1 ± 0.4 | 9.1 ± 0.3 | 9.2 ± 0.4 | 9.1 ± 0.4 | 0.91 |
| Albumin, g/dL | 3.9 ± 0.3 | 4.0 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.78 |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.78 |
| GFR, mL/minute | 79 ± 17 | 79 ± 17 | 77 ± 17 | 80 ± 16 | 0.78 |
| PTH, pg/dL | 41 (30, 54) | 40 (29, 53) | 42 (31, 56) | 42 (33, 52) | 0.78 |
| 25(OH)D, ng/mL | 21 ± 3 | 21 ± 3 | 21 ± 3 | 21 ± 3 | 0.91 |
| 1,25(OH)2D, pg/mLc | 41 (31, 54) | 41 (32, 51) | 42 (32, 55) | 40 (31, 53) | 0.91 |
| Estradiol, pg/mLc | 48 (40, 56) | 49 (40, 58) | 47 (42, 54) | 48 (39, 55) | 0.78 |
P-values were adjusted for multiple comparisons using the Benjamini and Hochberg method to control the false discovery rate.
Percentages do not equal 100 due to rounding.
Denotes measurement of laboratory studies at randomization rather than screening. Data with a normal distribution are summarized using mean and standard deviation, and analyzed using analysis of variance. Data with outliers are summarized using the median (1st, 3rd IQR) and analyzed using the Kruskal-Wallis test.
SI conversion factors: To convert calcium to mmol/L, multiply values by 025. To convert albumin to g/L, multiply values by 10. To convert creatinine to μmol/L, multiply values by 88.4. To convert PTH to ng/L, multiply values by 1. To convert 25(OH)D to nmol/L, multiply values by 2.496. To convert 1,25(OH)2D to pmol/L, multiply values by 2.6. To convert estradiol to pmol/L, multiply values by 3.671. At the UW Primate Center, serum 1,25(OH)2D was extracted, samples were evaporated and derivatized (Amplifex Diene, AB Sciex, Framington, MA) and 30 uL was injected for liquid chromatography-tandem mass spectrometry analysis using a Shimazdu LC system (Columbia, MD) coupled to a QTRAP 5500 equipped with a Turbo V Ion source (AB Sciex) as previously published with an intra and inter-assay variability of 9.3% and 14.1%, respectively. The UW Primate Center measured serum estradiol using an in-house assay. Samples were extracted with ethyl ether, the ether was evaporated and antibody (Holly Hill Biologicals) and trace (Perkin Elmer) were added with overnight incubation. The following day a charcoal solution was added, followed by incubation for 15 minutes, centrifugation, removal of the supernatant, addition of scintillation cocktail and then analysis using a beta counter. For lower estradiol levels typical of postmenopausal women, the intra and inter-assay variability for the assay is 4.5% and 5.8%, respectively. The UW Clinical Laboratory measured PTH using a chemiluminescent immunoassay performed on the Siemens ADIVA Centaur XP. The intra and inter-assay variability for the assay is 4.4% and 6.5%.
We purchased low-dose vitamin D (800 IU white capsules), high-dose vitamin D (50,000 IU yellow capsules) and identical placebo capsules (Tischon, Westbury, NY, USA) and independently verified capsule content prior to use. Subjects randomized to high-dose vitamin D received a loading dose (50,000 IU daily for 15 days) to quickly raise 25(OH)D >30 ng/mL,21 with sham loading of yellow placebo capsules in other arms to maintain blinding. After loading, subjects in the high-dose arm took one 50,000 IU capsule every 15th day for the next 11.5 months. Subjects in the low-dose arm took vitamin D 800 IU daily and yellow placebo capsules every 15th day. Subjects in the placebo arm ingested daily white placebo and every 15th day yellow placebo capsules (eFigure 1). We dispensed pre-filled 31-day pill boxes and counted remaining capsules at post-randomization visits to monitor adherence.
UW Pharmaceutical Research Center (PRC) personnel randomized eligible subjects into treatment arms in forced blocks of six (eFigure 1), stratifying by high PTH and calcium intake >1,000 mg/day. Stratification by PTH occurred because secondary hyperparathyroidism occurs in only 10% to 33% of people with VDI,22-25 and individuals without it might not benefit from vitamin D. Stratification by high calcium intake occurred because passive calcium absorption, facilitated by high calcium intake, lessens the import of vitamin D-mediated active absorption.13-15 Only PRC personnel, who had no direct contact with subjects, knew treatment allocation. We dispensed Total Block® sunscreen to subjects for use between April and October.26,21
Outcome Measures
The one-year change in TFCA was the primary outcome and change in BMD was the secondary outcome. Additional outcomes were the effect of placebo, low and high-dose vitamin D on muscle function, muscle mass, muscle mass, trabecular bone score and bone turnover. We also evaluated pain, functional status and physical activity during the study.
We measured TFCA using the gold-standard dual stable calcium isotope method, in which the intravenous isotope tracks renal re-absorption and endogenous fecal calcium excretion.27,28 Isotopes were purchased as calcium carbonate powder (Trace Sciences International, Wilmington, DE); purity and enrichment were confirmed by mass spectrometry. Waisman Clinical Biomanufacturing Facility personnel reconstituted isotopes25 and tested solutions for sterility and pyrogenicity.29 Solutions were stored and dispensed by the PRC.
For TFCA measurements, women fasted from midnight and attended the UW Clinical Research Unit (CRU) at ~0700. After phlebotomy, subjects consumed breakfast with ≤50 mL calcium-fortified orange juice containing ~8 mg of 44Ca, for a total oral calcium load of ~300 mg. The glass was rinsed with de-ionized water, which subjects also drank. Simultaneously, nurses infused ~3 mg of 42Ca over 5 minutes followed by ≤50 mL normal saline. Nurses weighed isotope syringes and recorded 42Ca and 44Ca doses. Subjects remained on the CRU during the 24-hour urine collection, consuming meals that replicated usual nutrient intake based on food diaries. Subjects continued outpatient medications and supplements, and began study capsules on discharge.
Wisconsin State Laboratory of Hygiene personnel quantified concentrations and ratios of calcium isotopes in 24-hour urine specimens by high-resolution inductively coupled plasma mass spectrometry as described.30,31,25 Subjects’ baseline and final urine samples were analyzed simultaneously. We calculated TFCA as the dose-corrected ratio of the two calcium isotopes in a 24-hour urine collection.25,32
Subjects returned for study visits ~30, 60, 120, 240 and 365 days following randomization. At each visit, we measured 25(OH)D, calcium, Timed Up and Go (TUG)33 and five sit-to-stand (STS)34 tests. Subjects reported pain over the prior week (10 cm scale), functional status (modified Stanford Health Assessment Questionnaire) and activity (Physical Activity for the Elderly Scale).35 Subjects reported all adverse events, specifically nephrolithiasis, fracture, fall, infection and hospitalization. At 0, 60, 120 and 365 days, subjects’ 24-hour urine calcium were measured.
PRC reviewed 25(OH)D levels at ~30, 60, 120 and 240 days. If a woman in the high-dose treatment arm had a 25(OH)D level <30 ng/mL, PRC adjusted her vitamin D dose. For example, a woman whose 25(OH)D level was 25 ng/mL received vitamin D3 50,000 IU/day for 7 days, then 50,000 IU once weekly to achieve and maintain repletion. To preserve blinding, ~8% of subjects in the other arms received sham adjustments of yellow placebo capsules.
One year after randomization, BMD was again measured using the same Lunar bone densitometry machine (GE Healthcare, Madison, WI). The trabecular bone score was determined using TBS iNsight version 2.1.0.0 (Medimaps Group, Switzerland). Muscle mass was calculated as the appendicular lean mass in kg, divided by height in m2.36 Serum 25(OH)D was measured at UW using an HPLC assay10 with between-run coefficients of variation (CV) of 3.2-13% for 25(OH)D2 and 2.6-4.9% for 25(OH)D3. Methods for other laboratory tests are described in Table 1.
Sample Size
The primary outcome was the effect of vitamin D on TFCA. With high-dose vitamin D,25 the standard deviation (SD) for absolute change in TFCA was 1%. With low-dose vitamin D,37 the SD for change in TFCA was 7%. Without intervention, the SD for monthly change in TFCA was 1%.38 Thus, recruitment of 70 women/arm (n=210) provided ~90% power to detect a 3% difference in the change in TFCA between high-dose and placebo arms, and ~80% power to detect a 3% difference between high-dose and low-dose vitamin D arms, with a two sided alpha of 0.05. To compensate for attrition, we planned to randomize up to 250 women.
Statistical Analysis
Data were graphed to determine distribution and outliers. Normal data were summarized using the mean ± standard deviation (SD) and analyzed by analysis of variance. Skewed data were summarized using the median (25th, 75th interquartile range) and analyzed using the Kruskal-Wallace rank sum test. To control the false discovery rate, we corrected p-values using the Benjamini and Hochberg method39 for subjects’ baseline characteristics (Table 1), subjects’ paired changes in dietary habits (eTable 2), between-arm changes in absolute and percent BMD (Figure 3, eTable 4), trabecular bone score (eTable 4), bone turnover (eTable 5) and adverse events (eTables 6, 7 and 9). Between-arm one-year changes in muscle outcomes were summarized using means and 95% confidence intervals corrected for multiple comparisons using the Tukey honest significant difference test (Table 2). All outcomes were analyzed by intent-to-treat principle, using R (The R Project for Statistical Computing, http://www.r-project.org). LASSO and StepAIC R programs were used for modeling.
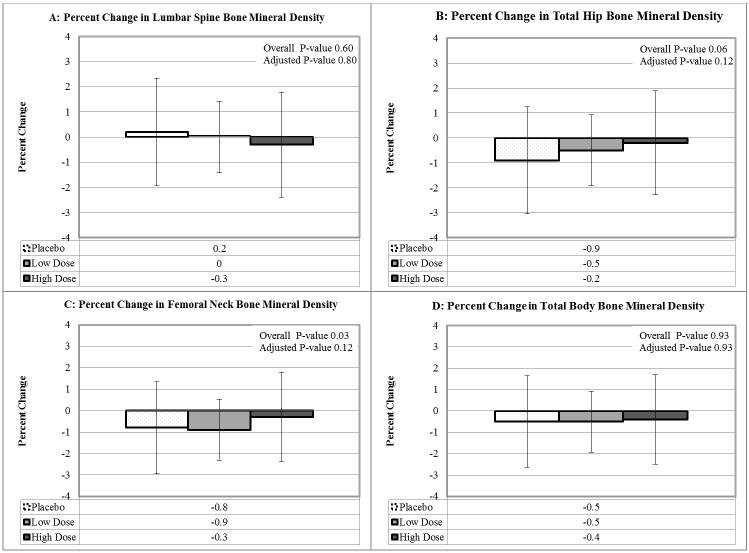
Figure 3. Annualized Percent Change in Bone Mineral Density by Treatment Assignment.
We found no significant between-arm differences for the change in spine, mean total hip, mean femoral neck or total body bone mineral density. Kruskal-Wallis tests were used to calculate the overall p-value, with correction of p-values to control the false discovery rate using the Benjamini and Hochberg method.
Table 2.
One-Year Changes in Muscle Outcomes
| Measure | Placebo n=73 of 76 |
Low-Dose Vitamin D n=73 of 75 |
High-Dose Vitamin D n=74 of 79 |
High vs. Lowc |
High vs. Placeboc |
Low vs. Placeboc |
|
|---|---|---|---|---|---|---|---|
| Timed Up and Go Test |
Baseline | 8.28 ± 1.69 | 8.04 ± 1.56 | 8.03 ± 1.70 | 0.05 (−0.42, 0.53) p=0.97 |
−0.03 (−0.50, 0.44) p=0.99 |
−0.08 (−0.56, 0.39) p=0.91 |
| 12 months | 7.92 ± 1.59 | 7.60 ± 1.55 | 7.65 ± 1.77 | ||||
| Changea | −0.35 (−0.70, −0.01) |
−0.44 (−0.66, −0.22) |
−0.38 (−0.66, −0.11) |
||||
| Five Sit- to-Stand Test |
Baseline | 10.32 ± 2.88 | 9.86 ± 2.50 | 9.83 ± 2.27 | −0.06 (−0.83, 0.72) p=0.98 |
−0.49 (−1.26, +0.29) p=0.3 |
−0.43 (−1.21, 0.34) p=0.39 |
| 12 months | 9.77 ± 3.02 | 8.88 ± 2.50 | 8.78 ± 2.09 | ||||
| Change | −0.55 (−1.02, −0.07) |
−0.98 (−1.49, −0.47) |
−1.04 (−1.44, −0.63) |
||||
| Health Assessment Questionnaire |
Baseline | 0.13 ± 0.25 | 0.14 ± 0.33 | 0.05 ± 0.14 | 0.04 (−0.04, 0.12) p=0.48 |
0.01 (−0.08, 0.09) p=0.99 |
−0.03 (−0.11, 0.05) p=0.58 |
| 12 months | 0.14 ± 0.33 | 0.12 ± 0.32 | 0.06 ± 0.21 | ||||
| Change | 0.01 (−0.03, 0.05) |
−0.02 (−0.09, 0.04) |
0.02 (−0.02, 0.05) |
||||
| Physical Activity Scale for the Elderly |
Baseline | 169 ± 96 | 167 ± 85 | 177 ± 83 | 17.6 (−13.4, 48.6) p=0.38 |
13.2 (−17.8, 44.3) p=0.57 |
−4.4 (−35.5, 26.8) p=0.94 |
| 12 months | 153 ± 86 | 146 ± 69 | 173 ± 74 | ||||
| Change | −17.25 (−39.08, 4.58) |
−21.64 (−37.66, −5.63) |
−4.04 (−21.40, 13.33) |
||||
| Muscle massb | Baseline | 7.24 ± 1.05 | 7.35 ± 1.24 | 7.29 ± 1.14 | −0.05 (−0.23,0.14) p=0.83 |
−0.1 (−0.29,0.08) p=0.39 |
−0.06 (−0.24,0.13) p=0.74 |
| 12 months | 7.35 ± 1.32 | 7.40 ± 1.40 | 7.30 ± 1.28 | ||||
| Change | 0.1 (−0.03, 0.24) |
0.05 (−0.05, 0.14) |
0.002 (−0.09, 0.10) |
||||
| Falls | n per Arm | 33 falls | 36 falls | 35 falls | p=0.92 | ||
| Fallers | subjects | 23 (30%) | 24 (32%) | 22 (32%) | p=0.92 | ||
We summarized within-arm one-year changes in continuous muscle outcomes using the mean (95% confidence interval).
Muscle mass was calculated as the appendicular lean mass (kg) divided by height in meters2.
To control the false discovery rate for multiple comparisons, we used Tukey’s method to adjust the confidence intervals and p-values.
A data safety monitoring board (DSMB) met every 18 months to monitor the trial’s progress and safety. Withdrawal occurred for three predefined events: nephrolithiasis, hypercalcemia (defined as a serum calcium ≥10.4 mg/dL twice over ~2 weeks) or fragility fracture (spine, wrist or hip). If subjects developed hypercalciuria (defined as >400 mg/24 hours), we repeated the test. For persistent hypercalciuria, we counseled subjects to reduce calcium intake. As hypercalciuria is common and often asymptomatic,34 its presence did not require withdrawal. All adverse events were categorized by system in Oncore.
We reported serious adverse events (death, hospitalization or predefined event) to the DSMB within 24 hours, and cumulative adverse events at DSMB meetings. To prepare reports, the team submitted subjects’ adverse events to the PRC, who entered treatment assignment and forwarded reports to the DSMB. We defined an excess harm Z value > −3.040,41 as an indication to prematurely stop the study.
RESULTS
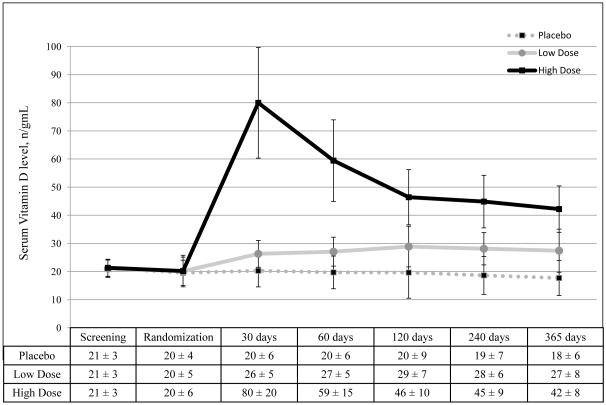
Figure 1 summarizes subject recruitment, randomization and completion. Nine women (4%) who withdrew from the study were similar to the remaining 221 subjects in age, race and 25(OH)D levels; all withdrew for personal reasons. Baseline demographics did not differ across treatment arms (Table 1). Serum 25(OH)D levels were significantly different between arms at all post-randomization visits (p<0.001, Figure 2). From 30 days to 365 days following randomization, average 25(OH)D levels were 19±5 ng/mL in placebo, 28±5 ng/mL in low-dose, and 56±12 ng/mL in high-dose treatment arms (p<0.001). Five subjects (7%) in the high-dose arm required additional vitamin D to maintain 25(OH)D ≥30 ng/mL. Adherence to therapy was ~100% across all arms (eTable 1, n=221). Subjects exhibited no significant pair-wise changes in dietary habits during the study (eTable 2).
Figure 2. Serum Vitamin D Levels by Treatment Assignment.
Serum vitamin D levels were summarized using the mean and standard deviation, and compared across treatment arms by analysis of variance, with correction of p-values to control the false discovery rate using the Benjamini and Hochberg method. Vitamin D levels were not significantly different across treatment groups at the screening (p=0.89) and randomization (p=0.89) visits. At all subsequent visits, serum vitamin D levels were significantly different (p<0.001) across all three treatment arms. Pairwise comparisons likewise showed p-values <0.001. To convert 25(OH)D from ng/mL to mmol/L, multiply values by 2.496.
Main Outcome Measures
TFCA is summarized in eFigure 2a. TFCA increased 0.6% in the high-dose arm and decreased by 4.5% in low-dose (p=0.009) and by 0.9% in the placebo arms (p=0.46 vs high-dose). By chance, the low-dose arm had higher baseline TFCA. In models controlling for baseline calcium absorption, TFCA increased 1% (10 mg/day) in the high-dose arm, but decreased 2% in low-dose (p=0.005 vs. high-dose) and 1.3% placebo (p=0.03 vs. high-dose) arms (eFigure 2b). In models (eTable 3), the one-year change in TFCA was inversely associated with baseline TFCA, baseline 25(OH)D and dietary sodium, and positively associated with body mass index, serum estradiol, GFR and 60-day 25(OH)D levels.
We found no between-arm differences for the absolute or annualized percent change in lumbar spine, mean total hip or total body BMD (Figure 3, eTable 4). Likewise, we found no significant between-arm differences for absolute or annualized percent changes in trabecular bone score (eTable 4). High-dose vitamin D had a small, beneficial effect on femoral neck BMD. The absolute change in mean femoral neck BMD with high-dose vitamin D was −0.003 g/cm2 (IQR −0.012, 0.005 g/cm2), with low-dose vitamin D was −0.009 g/cm2 (−0.02, 0.001 g/cm2) and with placebo was −0.008 g/cm2 (−0.016, −0.001 g/cm2). The overall p-value for between-arm changes was 0.03, but with adjustment to control the false discovery rate, the p-value was no longer significant (p=0.12). Annualized changes in hip BMD were associated with change in TFCA, but only in subjects randomized to high-dose vitamin D (rho 0.24, p=0.04).
The within-arm and between-arm one-year changes in muscle outcomes are summarized in Table 2. All treatment arms experienced slightly faster Timed-Up-and-Go and 5-Sit-to-Stand tests during the study. However, we found no between-arm differences for the degree of improvement in either of these tests. We likewise detected no between-arm differences in muscle mass, number of falls or number of fallers. Finally, we found no between-arm differences for the one-year change in Health Assessment Questionnaire score or Physical Activity for the Elderly score.
We measured bone turnover markers in subjects who attended all study visits before 10 am, fasting since midnight (n=149, 65%). We found no consistent between-arm differences in CTX or BSAP, when analyzed as changes from baseline (eTable 5 7) or in models (data not shown).
Predefined adverse events are summarized in eTable 6 8. Nephrolithiasis was incidentally detected in a woman in the low-dose arm who underwent abdominal imaging for other reasons; lack of prior imaging precluded ability to determine timing of the stone. Falls, fractures and hospitalizations were evenly distributed across arms. Two subjects in the low-dose vitamin D arm experienced transient asymptomatic hypercalcemia. Hypercalciuria occurred nine times, seven in the high-dose (4 subjects), once in low-dose, and once in the placebo arm (p=0.19). Serum calcium and phosphorus levels were similar between all arms (eTable 7). At 60 days, the high-dose arm had higher urine calcium levels than the low-dose (p=0.007) or placebo arms (p=0.001) arms (eTables 7 and 8). Likewise at 120 and 365 days, the high-dose arm experienced higher urine calcium levels than the placebo arm (eTables 7 and 8). We found no other differences in adverse effects across treatment arms (eTable 9).
COMMENT
Experts have heatedly debated optimal 25(OH)D levels needed to optimize musculoskeletal health. While some groups4-6,42 advocate levels ≥30 ng/mL, the Institute of Medicine7 defines vitamin D repletion as a level ≥20 ng/mL. We designed a clinical trial to directly address ongoing controversy about optimal vitamin D levels for musculoskeletal health. We found that compared to placebo, high-dose vitamin D had a very small effect on calcium absorption (1% or 10 mg/day) that did not translate into meaningful changes in lumbar spine, mean total hip, femoral neck or total body BMD, trabecular bone score, Timed Up and Go test score, 5-Sit-to-Stand score, muscle mass, falls or number of fallers. Study results do not support the recommendation to maintain serum 25(OH)D levels ≥30 ng/mL.
In a retrospective study43 of 316 postmenopausal women with serum 25(OH)D levels <17 ng/mL, women with levels ≤4 ng/mL had lower calcium absorption than those with higher 25(OH)D levels. Interestingly, 1,25(OH)2D levels were low only in women with 25(OH)D ≤4 ng/mL. Authors concluded that profound vitamin D deficiency must exist, in order to impair calcium absorption. However, the study did not test changes in calcium absorption with vitamin D therapy, limiting the ability to conclude that calcium absorption was “optimal” in women with 25(OH)D levels ≥5 ng/mL.
Two recent randomized clinical trials44,45 found that when controlling for baseline calcium absorption, high-dose vitamin D increased calcium absorption in postmenopausal women. In 163 women with 25(OH)D <20 ng/mL,44 calcium absorption increased in the 4800 IU/day arm compared to placebo. However, actual difference in calcium absorption between placebo and high-dose vitamin D was only 6 mg a day. In another trial, researchers45 randomized 67 women with 25(OH)D <30 ng/mL to 0, 800, 2000 or 4000 IU of vitamin D3 daily for 8 weeks. Calcium absorption decreased by 2.6% in the placebo arm and increased 6.7% in the 4000 IU arm. In both studies, baseline calcium absorption was a strong independent predictor of change in calcium absorption with vitamin D therapy.
Few studies have evaluated the relationship between calcium absorption and BMD. Most cross-sectional studies46-48 report no association. In the prospective “Study of Osteoporotic Fractures,” calcium absorption (measured by single serum radioisotope level) in 5,453 Caucasian postmenopausal women49 was weakly but significantly associated with femoral neck BMD (r = 0.06, p<0.001). Researchers subsequently recorded incident fractures for ~5 years. In models adjusting for age, each SD (7.7%) decrease in calcium absorption was associated with a 1.24 fold (95% CI, 1.05 to 1.48) increase in hip fracture, but not with fractures at other skeletal sites. The study, along with our own data, suggests that large increases in calcium absorption are needed to increase BMD and reduce fracture risk.
Even if high-dose vitamin D did not increase BMD, its use would be warranted if such therapy reduced falls, which almost always precede an osteoporotic fracture. A recent randomized clinical trial50 of 409 women ages 70-80 was specifically designed to evaluate the effect of vitamin D or placebo on falls risk. Authors detected no reduction in falls with vitamin D therapy, administered as 800 IU daily for two years.
Sanders and colleagues51 reported that vitamin D 500,000 IU intramuscular once yearly caused more fractures and falls than placebo. In a post-hoc analysis of a subset of subjects,52 those randomized to vitamin D had higher 1,25(OH)2D levels and bone resorption three months after randomization, potentially explaining the higher fracture rate. While we found no significant increase in bone resorption or declines in BMD associated with high-dose vitamin D, the benefits of high-dose vitamin D were too small to justify its routine use.
Our trial has several strengths. We recruited a large number of highly motivated subjects. Adherence to study medication was excellent, and attrition was low (4%). We replicated typical dietary habits during TFCA study visits. We used the gold-standard method to measure TFCA and subjects remained inpatients, permitting a complete 24-hour urine collection. Subjects received sunscreen to minimize sun-mediated increases in vitamin D levels. Vitamin D study capsule content was independently verified prior to study use. We measured covariates that could influence TFCA, BMD and/or muscle tests besides 25(OH)D, including subjects’ dietary habits, serum PTH, estradiol and 1,25(OH)2D levels, pain and activity. 25(OH)D levels were measured by HPLC, one of two gold-standard assays.53 Finally, PRC adjusted vitamin D doses to maintain 25(OH)D >30 ng/mL in the high-dose arm, with sham adjustments in other arms to maintain blinding.
We also note some study limitations. Few African American women participated, limiting our ability to detect differential responses to vitamin D based on race. Results cannot be used to guide vitamin D therapy for young adults, men or women >75 years old. Subjects participated for only one year; perhaps longer exposure to high-dose vitamin D through more remodeling cycles would yield greater effects on BMD.54
In conclusion, one year of high-dose vitamin D given to postmenopausal women with 25(OH)D levels <30 ng/mL (21 ± 3 ng/mL at baseline) had a trivial effect on calcium absorption, and no clinically meaningful beneficial effects on bone mineral density, muscle function or falls. Study results do not justify the common and frequently touted4-6,42 practice of administering high-dose vitamin D to older adults, in order to maintain serum 25(OH)D levels ≥30 ng/mL. Rather, study results support the Institute of Medicine’s conclusion that vitamin D repletion is a serum 25(OH)D level of ≥20 ng/mL.
Supplementary Material
Acknowledgments
Financial disclosures: none reported
Funding/Support: The study was supported by grants from the National Institute of Health, National Institute on Aging (R01 AG028739) and the Office of Dietary Supplements (R01 AG028739 supplement).
Role of the sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author contributions:
Study concept and design: Hansen
Acquisition of data: Hansen, Johnson, Lemon, Marvdashti, Chambers
Analysis and interpretation of data: Hansen, Marvdashti, Vo
Drafting of the manuscript: Hansen
Critical revision of the manuscript for important intellectual content: all authors
Statistical analysis: Hansen, Vo, Marvdashti
Obtained funding: Hansen
Administrative, technical, or material support: Johnson, Chambers, Marvdashti
Study supervision: Hansen supervised the study, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional contributions: We thank all participants, who devoted over one year of time to the trial. We thank the CRU and PRC staff for their excellent assistance in conducting the study. We are grateful for discussions with Dr. Alan J. Bridges (Professor of Medicine and Chief of Staff, William S. Middleton Memorial Veterans Affairs Hospital, Madison, WI) and Dr. Kevin McKown (Professor of Medicine and Chief, Rheumatology Division, University of Wisconsin School of Medicine & Public Health) about the manuscript. Finally, we thank DSMB members J. Christopher Gallagher (Professor of Medicine and Chief, Bone Metabolism Section, Creighton University, Omaha, NE), Kristine Ensrud (Professor of Medicine, and Director of Epidemiology, Clinical Research Center, U of MN), Yvette Schuster (Professor of Statistics, Rutgers University) and Judy Hannah (Professor and Head, Nutrition Office, National Institute on Aging). We thank UW Professor Hector DeLuca’s laboratory for independently verifying vitamin D content of study capsules.
REFERENCES
- 1.Melton LJ., 3rd Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res. 2003 Jun;18(6):1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002 May 18;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Garabedian M. Vitamin D: Photobiology, Metabolism, Mechanism of Action, and Clinical Applications. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th American Society for Bone and Mineral Research; Washington, D.C.: 2006. pp. 106–114. [Google Scholar]
- 4.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005 Jul;16(7):713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Dawson-Hughes B, Bischoff-Ferrari HA. Therapy of osteoporosis with calcium and vitamin D. J Bone Miner Res. 2007 Dec;22(Suppl 2):V59–63. doi: 10.1359/jbmr.07s209. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine . Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 8.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr. 2008 Dec;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 Jul;23(7):1052–1060. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006 Jun;52(6):1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 11.Schrager S, Girard M, Mundt M. Dietary calcium intake among women attending primary care clinics in Wisconsin. WMJ. 2005;104(6):47–50. [PubMed] [Google Scholar]
- 12.Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1988-91. Adv Data. 1994 Nov 14;(258):1–28. [PubMed] [Google Scholar]
- 13.Weaver CM, Fleet JC. Vitamin D requirements: current and future. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1735S–1739S. doi: 10.1093/ajcn/80.6.1735S. [DOI] [PubMed] [Google Scholar]
- 14.Deroisy R, Collette J, Albert A, Jupsin I, Reginster JY. Administration of a supplement containing both calcium and vitamin D is more effective than calcium alone to reduce secondary hyperparathyroidism in postmenopausal women with low 25(OH)vitamin D circulating levels. Aging Clin Exp Res. 2002 Feb;14(1):13–17. doi: 10.1007/BF03324412. [DOI] [PubMed] [Google Scholar]
- 15.McKane WR, Khosla S, Egan KS, Robins SP, Burritt MF, Riggs BL. Role of calcium intake in modulating age-related increases in parathyroid function and bone resorption. J Clin Endocrinol Metab. 1996 May;81(5):1699–1703. doi: 10.1210/jcem.81.5.8626819. [DOI] [PubMed] [Google Scholar]
- 16.Pattanaungkul S, Riggs BL, Yergey AL, Vieira NE, O'Fallon WM, Khosla S. Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: evidence for age-related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab. 2000 Nov;85(11):4023–4027. doi: 10.1210/jcem.85.11.6938. [DOI] [PubMed] [Google Scholar]
- 17.Marks HD, Fleet JC, Peleg S. Transgenic expression of the human Vitamin D receptor (hVDR) in the duodenum of VDR-null mice attenuates the age-dependent decline in calcium absorption. J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):513–516. doi: 10.1016/j.jsbmb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005 Jun;90(6):3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert MP, Pratley RE. The Impact of Diabetes Treatments On Bone Health in Patients with Type 2 Diabetes Mellitus. Endocrine reviews. 2015 Mar 4;:er20121042. doi: 10.1210/er.2012-1042. [DOI] [PubMed] [Google Scholar]
- 21.Hansen KE, Bartels CM, Gangnon RE, Jones AN, Gogineni J. An evaluation of high-dose vitamin D for rheumatoid arthritis. J Clin Rheumatol. 2014 Mar;20(2):112–114. doi: 10.1097/RHU.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001 Aug;86(Suppl 1):S97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 23.Souberbielle JC, Cormier C, Kindermans C, et al. Vitamin D status and redefining serum parathyroid hormone reference range in the elderly. J Clin Endocrinol Metab. 2001 Jul;86(7):3086–3090. doi: 10.1210/jcem.86.7.7689. [DOI] [PubMed] [Google Scholar]
- 24.Sahota O, Mundey MK, San P, Godber IM, Lawson N, Hosking DJ. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004 Jul;35(1):312–319. doi: 10.1016/j.bone.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? J Bone Miner Res. 2008 Jul;23(7):1052–1060. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988 Aug;67(2):373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 27.Abrams SA. Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr. 1999 Dec;70(6):955–964. doi: 10.1093/ajcn/70.6.955. [DOI] [PubMed] [Google Scholar]
- 28.Griffin IJ, Abrams SA. Methodological considerations in measuring human calcium absorption: relevance to study the effects of inulin-type fructans. Br J Nutr. 2005 Apr;93(Suppl 1):S105–110. doi: 10.1079/bjn20041344. [DOI] [PubMed] [Google Scholar]
- 29.Sterility Tests. Mack Printing Company; Easton, PA: 1989. Vol USP XXII, NF XVII. [Google Scholar]
- 30.Sturup S, Hansen M, Molgaard C. Measurement of 44Ca: 43Ca and 42Ca: 43Ca istopic ratios in urine using high resolution inductively coupled plasma mass spectrometry. Journal of Analytical Atomic Spectrometry. 1997;12:919–923. [Google Scholar]
- 31.Field M, Shapses S, Cifuentes M, Sherrell R. Precise and accurate determination of calcium isotope ratios in urine using HR-ICP-SFMS. Journal of Analytical Atomic Spectrometry. 2003;18:727–733. [Google Scholar]
- 32.Eastell R, Vieira NE, Yergey AL, Riggs BL. One-day test using stable isotopes to measure true fractional calcium absorption. J Bone Miner Res. 1989 Aug;4(4):463–468. doi: 10.1002/jbmr.5650040403. [DOI] [PubMed] [Google Scholar]
- 33.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991 Feb;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 34.Tiedemann A, Shimada H, Sherrington C, Murray S, Lord S. The comparative ability of eight functional mobility tests for predicting falls in community-dwelling older people. Age Ageing. 2008 Jul;37(4):430–435. doi: 10.1093/ageing/afn100. [DOI] [PubMed] [Google Scholar]
- 35.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 36.Beaudart C, Reginster JY, Slomian J, Buckinx F, Locquet M, Bruyere O. Prevalence of sarcopenia: the impact of different diagnostic cut-off limits. J Musculoskelet Neuronal Interact. 2014 Dec;14(4):425–431. [PubMed] [Google Scholar]
- 37.Kendler D, Robson R, Handel M, et al. A 4-week, double-blind, randomized, controlled multicenter clinical trial to examine the effect of once-weekly alendronate 70 mg and vitamin D3 2800 IU on fractional calcium absorption in postmenopausal osteoporotic women. Osteoporos Int. 2006;17(Suppl 2):S220. [Google Scholar]
- 38.Hansen KE, Jones AN, Lindstrom MJ, et al. Do proton pump inhibitors decrease calcium absorption? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010 Dec;25(12):2786–2795. doi: 10.1002/jbmr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 40.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971 Oct;44(526):793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 42.Roux C, Bischoff-Ferrari HA, Papapoulos SE, de Papp AE, West JA, Bouillon R. New insights into the role of vitamin D and calcium in osteoporosis management: an expert roundtable discussion. Curr Med Res Opin. 2008 May;24(5):1363–1370. doi: 10.1185/030079908x301857. [DOI] [PubMed] [Google Scholar]
- 43.Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 Nov;23(11):1859–1863. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D on calcium absorption in older women. The Journal of clinical endocrinology and metabolism. 2012 Oct;97(10):3550–3556. doi: 10.1210/jc.2012-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aloia JF, Dhaliwal R, Shieh A, et al. Vitamin D supplementation increases calcium absorption without a threshold effect. The American journal of clinical nutrition. 2014 Mar;99(3):624–631. doi: 10.3945/ajcn.113.067199. [DOI] [PubMed] [Google Scholar]
- 46.Chan EL, Lau E, Shek CC, et al. Age-related changes in bone density, serum parathyroid hormone, calcium absorption and other indices of bone metabolism in Chinese women. Clin Endocrinol (Oxf) 1992 Apr;36(4):375–381. doi: 10.1111/j.1365-2265.1992.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 47.Hoover PA, Webber CE, Beaumont LF, Blake JM. Postmenopausal bone mineral density: relationship to calcium intake, calcium absorption, residual estrogen, body composition, and physical activity. Can J Physiol Pharmacol. 1996 Aug;74(8):911–917. [PubMed] [Google Scholar]
- 48.Nordin BE, Robertson A, Seamark RF, et al. The relation between calcium absorption, serum dehydroepiandrosterone, and vertebral mineral density in postmenopausal women. J Clin Endocrinol Metab. 1985 Apr;60(4):651–657. doi: 10.1210/jcem-60-4-651. [DOI] [PubMed] [Google Scholar]
- 49.Ensrud KE, Duong T, Cauley JA, et al. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000 Mar 7;132(5):345–353. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 50.Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and Vitamin D in Fall Prevention Among Older Women: A Randomized Clinical Trial. JAMA Intern Med. 2015 Mar 23; doi: 10.1001/jamainternmed.2015.0225. [DOI] [PubMed] [Google Scholar]
- 51.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010 May 12;303(18):1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 52.Sanders KM, Ebeling P, McCorquodale T, Herrman M, Shore-Lorenti C, Nicholson G. The Efficacy of High-Dose Oral Vitamin D3 Administered Once a Year: Increased Fracture Risk Is Associated With 1,25 Vitamin D Level at 3-Months Post Dose. J Bone Miner Res. 2012;27(Supplement 1) [Google Scholar]
- 53.Cranney A, Horsley T, O'Donnell S, et al. Effectiveness and Safety of Vitamin D in Relation to Bone Health. Evidence Report/Technology Assessment No. 158. Agency for Healthcare Research and Quality; Rockville: 2007. AHRQ Publication No. 07-E013. [Google Scholar]
- 54.Brockstedt H, Kassem M, Eriksen EF, Mosekilde L, Melsen F. Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone. 1993 Jul-Aug;14(4):681–691. doi: 10.1016/8756-3282(93)90092-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.