Abstract
Manganese serves as a cofactor to a variety of proteins necessary for proper bodily development and function. However, an overabundance of Mn in the brain can result in manganism, a neurological condition resembling Parkinson’s disease (PD). Bulk sample measurement techniques have identified the globus pallidus and thalamus as targets of Mn accumulation in the brain, however smaller structures/cells cannot be measured. Here, X-ray fluorescence microscopy determined the metal content and distribution in the substantia nigra (SN) of the rodent brain. In vivo retrograde labeling of dopaminergic cells (via FluoroGold™) of the SN pars compacta (SNc) subsequently allowed for XRF imaging of dopaminergic cells in situ at subcellular resolution. Chronic Mn exposure resulted in a significant Mn increase in both the SN pars reticulata (>163%) and the SNc (>170%) as compared to control; no other metal concentrations were significantly changed. Subcellular imaging of dopaminergic cells demonstrated that Mn is located adjacent to the nucleus. Measured intracellular manganese concentrations range between 40–200 μM; concentrations as low as 100 μM have been observed to cause cell death in cell cultures. Direct observation of Mn accumulation in the SNc could establish a biological basis for movement disorders associated with manganism, specifically Mn caused insult to the SNc. Accumulation of Mn in dopaminergic cells of the SNc may help clarify the relationship between Mn and the loss of motor skills associated with manganism.
Introduction
Neurodegenerative diseases often present a spectrum of symptoms ranging from mood variations to motor impairments. Parkinson’s disease (PD) is the most prevalent disorder in which loss of dopaminergic (DA) neurons within the substantia nigra pars compacta (SNc) results in motor dysfunction. The exact cause of PD is unknown, however environmental pollutants may play a role; one such pollutant is manganese (Mn). Manganese is required for normal body development and functions and its dietary intake is regulated by limited absorption in gut. Exposure to Mn via inhalation or injection, routes which bypass the gut, can increase Mn accumulation in the brain resulting in Mn-induced motor dysfunction (i.e. manganism). Cases of manganism are reported in miners,1,2 welders, smelters,3,4 and abusers of ephedrine/methcathinone.5,6 Additionally, patients suffering from liver dysfunction demonstrate Mn poisoning due to decreased removal of Mn from the blood.7,8 The commonality of symptoms shared between PD and advanced manganism suggests a potential relationship between the diseases; see review articles.9–11 For this reason, various epidemiological studies have examined the correlation between increased occupational and/or environmental Mn exposure and incidence of PD.12–19 While it has been found that key proteins in the PD etiology exhibit differential expression under Mn exposure,20–22 a recent critical review on epidemiology of PD concludes that the evidence for Mn as environmental factor remains inadequate.23
Studies using cell cultures consistently demonstrate that DA neurons are susceptible to Mn exposure.20,24–29 However, these experiments fail to model the effects of the blood-brain and blood-cerebrospinal fluid barriers on Mn access to the brain and distribution of Mn amongst different brain structures. Magnetic resonance imaging of Mn distribution in brain is a powerful technique for medical diagnosis of Mn exposure in vivo which has reported increased T1 weighted signal intensity in the SN of primates and humans.9,30–32 Current resolutions fall short of single cell imaging. Conversely, techniques which can provide subcellular resolution require ultra-thin samples and cannot be used for large-area imaging (e.g. electron energy loss spectroscopy) or have limited sampling volumes and therefore have not been used for Mn quantification (e.g. secondary ion mass spectroscopy). X-ray fluorescence (XRF) microscopy presents an alternative approach, permitting the use of thick (≥10 μm), unfixed samples for Mn quantification with a range of possible resolutions (0.03–100 μm).24,25,33–35
Here, we report quantitative XRF imaging of Mn in SNc and SNr of rat model of Mn induced neurotoxicity. Identification of DA neurons of the SNc while maintaining physiological metal concentrations presents an additional difficultly for in situ measurements. Immunohistochemical (IHC) staining for tyrosine hydroxylase (TH) would identify DA cells, however Mn leaching from the tissue has been observed following staining of tissue.34 Here, we addressed the difficulty of in situ cell identification by administering FluoroGold™ (FG) retrograde tracer during survival surgery to label DA cells of the SNc in vivo. Only a sub-population of cells of the SNc accumulate FG, however of these cells, a high percentage (>98%) are immunopositive for TH.36,37 We show that DA neurons are preferential targets of Mn accumulation in the rodent brain. The spatial distribution of Mn in these cells is permissive to reactivity with dopamine which could be a cause of toxicity.
Experimental procedures
Animals
Adult male Sprague Dawley® outbred rats were purchased from Harlan (Indianapolis, IN) at the age of 8 weeks (225–249 g). Rats were maintained on Purina rodent chow 5001 containing 70 ug g−1 Mn content. Upon receipt, rats were housed in a 12 h/12 h light/dark room and allowed access to rat chow and distilled, de-ionized water ad libitum. A 3 day acclimation period followed receipt of the animals before initiating treatment protocol.
Treatment
Animals were randomly divided into 2 experimental groups for treatment; one group underwent chronic Mn exposure via intraperitoneal (i.p.) injections of MnCl2 in sterile saline (6 mg of Mn per kilogram body weight, 109 mM) while the control group received saline injections of the same volume. Injections were performed weekdays for a period of 4 weeks. At the conclusion of the treatment protocol a 24 hour waiting period was observed prior to animal sacrifice. Animals that underwent FluoroGold™ (Fluorochrome, LLC) retrograde tracer injections underwent a Mn/saline treatment protocol with survival surgery being performed at the end of 4 weeks followed by an additional 2 weeks of Mn/saline treatment prior to sacrifice. FluoroGold™ was dissolved in 0.9% saline solution to a final concentration of 2% w/v. Prior to administration rats were anesthetized by inhalation of 2–5% isoflurane which was then maintained at 1–3% during the surgery. The scalp was shaved and disinfected with betadine for 1 minute after which an incision was made to reveal the bregma point on the skull. A burr hole was drilled and a microsyringe was inserted to the depth of the caudate putamen (A/P: 1.0; R/L: 3.0; V: −4.5 mm to bregma). The FG solution (0.2 μL) was injected over the course of five minutes. The microsyringe was then removed, the burr hole sealed, and the scalp closed with stitches. Ketoprofen (5 mg kg−1 subcutaneous) was administered as an analgesic before surgery and every 24 h afterwards for up to 72 h. All experiments complied with animal rights regulations and were approved by the Institutional Committee on Animal Use at Purdue University.
Sacrifice and tissue sectioning
Prior to sacrifice, animals were anesthetized with a solution containing ketamine (75 mg kg−1) and xylazine (10 mg kg−1) via i.p. injection. Unconsciousness occurs within several minutes of injection; an additional injection was administered as necessary in the event that the initial injection was insufficient. Unconsciousness was assessed by performing a tail pinch or eye tap. Sacrifice was performed by severing the iliac artery and draining the blood from the animal; the brain stem was then severed resulting in death. After removing the skin, the skull was peeled away to reveal the brain, which was removed using spatula onto a clean Kim-wipe. The brain was blotted to remove excess blood from the surface and snap frozen in liquid nitrogen. Once completely frozen, as verified by a change in sample color, the sample was wrapped in foil and placed in −80 °C freezer for long term storage. No chemical fixation was used to avoid modifications of metal distribution. Sectioning was performed using a cryotome at an ambient cutting temperature of −12 °C. Coronal sections 10–30 μm thick were collected from bregma −5.20 mm on X-ray compatible supports and on glass slides. Samples were returned to −80 °C until measured.
Data collection
Samples from FG treated animals were imaged using a Leica DM6000 fluorescence microscope at 10× magnification with a DAPI filter cube set (BP360/40, 400, BP470/40) at room temperature to identify FG accumulating cells. A XRF scan of the same area was then performed with 1 μm × 1 μm resolution to obtain K and Zn images to identify individual cells. Comparison of the XRF image with the optical fluorescence image confirmed the location of FG accumulating cells. Samples from non-FG treated animals were XRF imaged at low resolution (10 μm × 10 μm) and the area of high Mn signal was taken as the SNc (Fig. S1A, ESI‡). Higher resolution images (2 μm × 2 μm) were taken and elevated intracellular Zn content within the SNc was assumed to represent the nuclei of DA cells based on preliminary data (Fig. S1B, ESI‡). Single cell images taken at 0.3 μm × 0.3 μm were then taken and used for analysis (Fig. S1C, ESI‡) Tissue level scans of the SN were performed at the Advanced Photon Source (APS) Sector 8 BM and Sector 18 ID (BioCAT) beamlines and cell level scans were performed at APS Sector 2ID-D and ID-E beamlines. All scans were carried out at room temperature in a helium environment with the exception of BioCAT measurements which were performed in atmosphere. Parameters used for XRF imaging are presented in Table 1. Fitting of XRF imaging spectra was performed using the MAPS program38 and quantification of areal content performed using either NBS1832/1833 or an AXO thin film XRF reference standard. Standards were measured under the same experimental conditions as the samples. To convert from areal content to concentration, the sample is assumed to be of uniform thickness with an average density of 1 g cm−3.
Table 1.
XRF imaging parameters
| Beamline | Pixel size (μm × μm) |
Dwell (s) |
Flux (photons s−1) |
Figure |
|---|---|---|---|---|
| 8 BM-B | 25 × 25 | 2–4 | 3.5 × 1010 | |
| 18 ID-D | 20 × 20 | 0.5–1 | 1.8 × 1011 | Fig. 1 and Fig. S2A (ESI) |
| 2 ID-D | 10 × 10 | 0.5 | 4 × 109 | Fig. S1A (ESI) |
| 2 × 2 | 0.5 | Fig. S1B (ESI) | ||
| 0.3 × 0.3 | 0.5–2 | Fig. S1C (ESI) | ||
| 2 ID-E | 1 × 1 | 0.5–4 | 5 × 108 | Fig. 3B |
| 0.5 × 0.5 | Fig. 3C, Fig. S3A and B (ESI) |
To verify that counting statistics were above the minimum analyzable limit, a Kolmogorov-Smirnov test for similarity was employed to test Kα peaks obtained through two different fitting methods. Specifically, one Kα peak was obtained by first summing the pixel spectra followed by fitting whereas the second Kα peak was obtained by first fitting the pixel spectra and summing the fitted results. A null result for the Kolmogorov–Smirnov test indicated that the two Kα peaks were statistically identical and therefore each individual spectrum had sufficient counting statistics. Several control samples demonstrated a significant difference between the compared peaks for Mn Kα and therefore Mn values represent an upper limit of the actual Mn concentration.
Image analysis
For tissue level scans, the boundary of the SN was delineated by hand based on the Fe and Mn signals (Fig. S2A and B, ESI‡). K-means cluster analysis (n = 2 clusters) of the Fe and Mn signals at equal weighs was then employed to identify the SNc and SNr for Mn treated samples. The resulting clusters were then filled to display a continuous region. In control samples, although the increased Mn content of the SNc is visible, it is too low to reliably identify the SNc using a clustering algorithm. Therefore, the SNr and SNc were delineated by hand based on Mn and Fe content, respectively (Fig. S2A and B, ESI‡). A similar approach using clustering was used to delineate the cell nucleus using the Zn signal (n = 3 clusters) whereas S, Cl, and K signals were used at equal weights to identify the cell body (n = 2 clusters; Fig. S3A, ESI‡).
Individual pixel data were used to create scatter plots of Mn versus each of the other metals examined. Three divisions were considered; the entire cell, the nucleus, and the cell body. For a given division, the average metal content of the region was subtracted from each of the pixel values to remove the cell-to-cell variability. A robust linear regression was then applied to the mean-corrected data to obtain the slope and the standard error. Analysis of covariance was used to evaluate changed slopes between Mn treated and control samples in FG and non-FG treated experimental groups (α = 0.05).
When noted, pixels demonstrating the highest Mn accumulation were identified by applying an iterative threshold selection algorithm.39 The average Mn content was then determined using the pixels above the threshold (Fig. S3B, ESI‡).
Results
XRF imaging of the SN
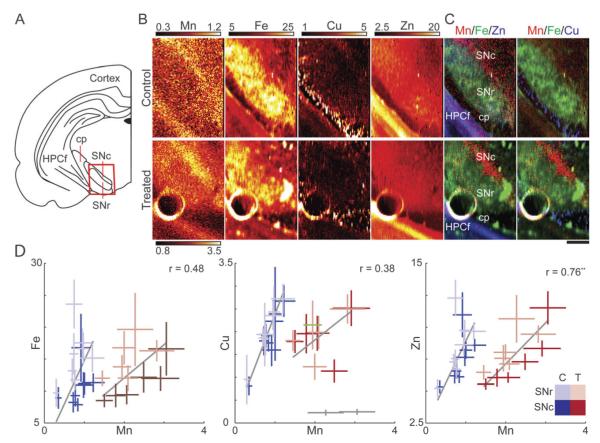
XRF imaging of a rat model of Mn exposure has revealed the SNc as one of the targets of Mn accumulation in the brain.34 Here we expand on the previous study by collecting a larger data set of the SNc and substantia nigra pars reticulata (SNr) with increased spatial resolution (Fig. 1). XRF images allow identification of the SNr based on its Fe content, the cerebral peduncle based on Cu content, and the cornu ammonis 3 (CA3) layer of the hippocampal formation (HPCf) due to strong Zn signal. The SNc, a small area several hundred microns wide, is located medially to the SNr (Fig. 1A and B) based on immunohistochemical staining of adjunct sections for tyrosine hydroxylase. This area displayed an increased Mn accumulation.
Fig. 1.
Tissue level XRF analysis of the SN. (A) Schematic of the coronal plane illustrating the substantia nigra (SN). The substantia nigra pars reticulata (SNr) separates the hippocampal formation (HPCf) and the substantia nigra pars compacta (SNc), which accumulates Mn. The red box corresponds to the regions featured in B. For clarity, the cerebral peduncle (cp) is also labeled. (B) XRF images of the SNr and the SNc, identifiable by the strong Fe and Mn signals respectively. The high Zn content to the lower left is due to the CA3 layer of the HPCf. Maximum scale for Mn in control is one-third that of the Mn treated sample for viewing purposes. (C) Tri-colored plots demonstrate the highest Mn accumulation adjacent to the SNr in both control and treated animals. Brain areas of SNr, SNc, caudal peduncle (cp), and HPCf are indicated on the plot. (D) Scatter plots of average metal concentration versus Mn for the SNc (dark tint) and SNr (light tint) for control (blue, n = 16 points) and Mn treated animals (red, n = 12 points). Gray lines indicate the best fit of the data weighted according to standard deviation of the individual data points (bars). Grayed data points identified as outliers and not considered for fitting purposes. Pearson correlation coefficient (r) and significance level (**p < 0.01) are displayed for metal/Mn of treated samples (Table S1, ESI‡). All numbers given are in μg g−1.
Quantitative analysis of metal concentrations at the tissue level showed no statistical difference for the two different Mn exposure durations (4 or 6 weeks) for any considered metal (Fig. S4, ESI‡) and therefore FG/Non-FG (see below for explanation) tissue level data were combined.
A significant change in Mn content was observed in Mn treated groups as compared to saline injected control animals in both the SNr (>163%) and SNc (>170%). However, Fe, Cu, or Zn concentrations in the SN did not change as a result of Mn treatment. Comparison of the SNc and SNr within an experimental group demonstrated less than a 10% difference in average Mn concentration (Table 2). The SNc and SNr had statistically different Fe content. Scatter plots of the average metal concentration of the SNc and SNr for Mn treated samples (Fig. 1C) revealed the strongest correlation between Zn and Mn (r = 0.76, p < 0.01; Table S1, ESI‡).
Table 2.
Metal concentrations measured at the tissue level
| Region | Group | Mn | Fe | Cu | Zn |
|---|---|---|---|---|---|
| SNc | C | 1.70a | 21.12 ± 5.68 | 3.79 ± 1.13 | 14.90 ± 3.62 |
| T | 4.60 ± 1.25** | 22.64 ± 5.96 | 3.74 ± 0.99 | 14.93 ± 4.46 | |
| SNr | C | 1.59a | 30.83 ± 8.44++ | 4.01 ± 1.07 | 16.77 ± 4.38 |
| T | 4.19 ± 0.89** | 30.15 ± 6.05++ | 3.86 ± 0.94 | 16.43 ± 3.13 |
Given values are in μg g−1 presented as mean ± std; n = 16/12 points control (C)/treated (T).
Represents an upper limit of the actual concentration.
p < 0.01 as compared to control.
p < 0.01 as compared to SNc of same experimental group.
Retrograde tracing of DAergic neurons
Survival surgery coupled with unilateral FG tracer injection permitted optical identification of cells in the SNc for subsequent XRF imaging. A schematic diagram demonstrates the approximate location of FG tracer injection (Fig. 2A). Injection of FG into the caudate putamen (CPu) resulted in uptake by axonal terminals (Fig. 2B) leading to DA cell body labeling in the SNc (Fig. 2C and D); minimal labeling of DA cells was observed for the contralateral side. This approach resulted in the labeling of DA cells in vivo thereby maintaining physiological metal concentrations in the targeted cells (Fig. 3).
Fig. 2.
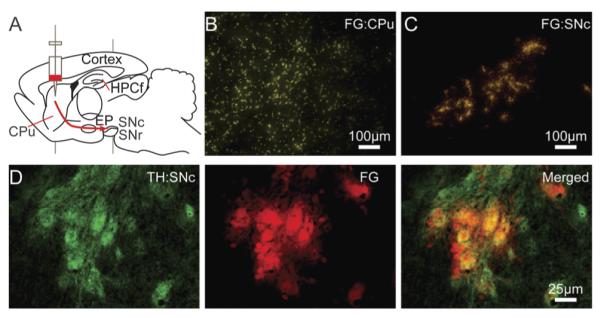
FG labeling of DA cells. (A) Diagram of a sagittal plane of the rodent brain with the approximate location of FG injection. The red arrow gives an approximate route of the nigra-neostriatum pathway. Injection into the CPu (B) results in uptake into axonal terminals and transport to DA cell bodies of the SNc (C). Scale bars represent a length of 100 μm (10 × objective). (D) Tyrosine hydroxylase IHC staining (green) reveals a more dense population of DA cells in the SNc than FG tracer (red), however all FG accumulating cells are positive for TH verified by two color image. Scale bars represent a length of 25 μm (40 × objective).
Fig. 3.
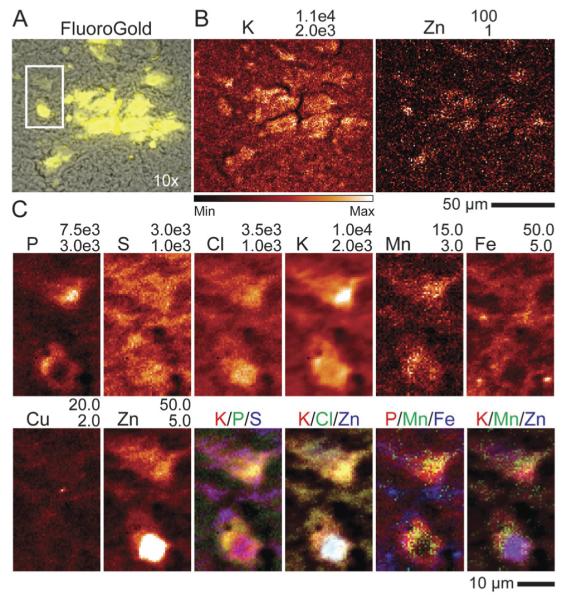
Optical and XRF imaging of FG, Mn treated rat brain. (A) Brightfield (BF) image of the SNc (gray) with FG fluorescence image overlayed (yellow). Not all dopaminergic cells of the SNc are FG positive. (B) Fly scan XRF image of K and Zn that assist in co-localizing XRF imaging with BF/FG optical images. Dashed white box indicates an area measured at high resolution which contains two cells (C). Example of XRF image recorded at subcellular resolution (0.5 μm × 0.5 μm). A high Zn content was taken to indicate the location of the nucleus. All numbers given are in μg g−1.
Cells labeled by FG were identified in XRF images by comparison of optical fluorescence image with K and Zn distributions (Fig. 3A and B). The strong Zn signal indicates the location of the nucleus40,41 whereas the S, Cl, and K signals help to identify the extent of the soma (Fig. 3C). Manganese content in both the cell nucleus and cell body of treated cells was significantly increased as compared to control samples (Table 3). Tri-colored images show that Mn does not co-localize with either Fe or Cu hotspots but is heterogeneously distributed in the cell body, primarily localizing along the boundary of the strong Zn signal. To account for this heterogeneous distribution, auto-thresholding of Mn content in the cell body (excluding nucleus) was applied and resulted in increased average Mn values by 21–35% as compared to the unfiltered average (Table 3). The average metal concentration in FG positive cells was consistently higher than that of non-FG treated animals except in Mn content. We attribute this difference to better localization of intact cells in FG samples. Increased phosphorous signals support such possibility and some statistically significant differences in Fe and Cu are removed if concentration of these elements is normalized by phosphorus. Overall cellular metal content imaged in situ from brain tissue is highly variable and in some cases the standard deviation within an experimental group was as large as 35% (Table 3, Fig. S5, ESI‡).
Table 3.
Metal concentration measured from cells
| Nucleus |
Control |
Treated |
||
|---|---|---|---|---|
| Element | Non-FG | FG | Non-FG | FG |
| P(×103) | 2.42 ± 0.12 | 3.94 ± 0.65+ | 2.84 ± 0.17 | 4.01 ± 0.82+ |
| K(×103) | 3.69 ± 0.19 | 5.34 ± 0.38 | 4.19 ± 0.11 | 6.08 ± 1.56+ |
| Mn | 3.15 ± 0.28 | 2.45 ± 0.17 | 9.53 ± 0.93** | 7.62 ± 1.06** |
| Fe | 11.36 ± 1.11 | 26.53 ± 7.39++ | 17.57 ± 5.72 | 21.22 ± 5.84 |
| Cu | 2.53 ± 0.25 | 4.79 ± 0.68++ | 3.01 ± 0.50 | 4.89 ± 0.81++ |
| Zn | 26.92 ± 4.49 | 36.78 ± 8.59 | 30.15 ± 7.63 | 40.64 ± 14.07 |
| Cell body |
Control |
Treated |
||
|---|---|---|---|---|
| Element | Non-FG | FG | Non-FG | FG |
| P(×103) | 2.58 ± 0.16 | 3.90 ± 0.57 | 2.89 ± 0.32 | 4.06 ± 0.95 |
| K(×103) | 3.57 ± 0.23 | 4.82 ± 0.43 | 3.85 ± 0.38 | 5.50 ± 1.60 |
| Mn | 3.00 ± 0.22 | 2.37 ± 0.19 | 8.42 ± 1.50** | 7.03 ± 1.23** |
| Mnthresh | 3.62 ± 0.30 | 3.21 ± 0.22 | 10.95 ± 1.87** | 9.08 ± 1.5** |
| Fe | 13.58 ± 0.48 | 28.66 ± 6.90++ | 18.10 ± 2.93 | 23.52 ± 6.31 |
| Cu | 3.05 ± 0.13 | 6.01 ± 1.30 | 3.66 ± 0.56 | 5.95 ± 2.13 |
| Zn | 18.23 ± 3.40 | 21.98 ± 4.29 | 18.79 ± 3.18 | 22.65 ± 6.31 |
Given values are in μg g−1 presented as mean ± std; n = 4/5/4/12 control/FluoroGold™ (FG) control/Mn/Mn FG.
p < 0.01,
p < 0.05 as compared to control of the same non-FG/FG treatment group
p < 0.01,
p < 0.05 as compared to non-FG sample of the same control/Mn treated group
For additional analysis of Mn distribution in cells, scatter plots of individual pixel concentrations were quantified (Table S3, ESI‡). Fe/Mn and Cu/Mn pairs were in general lacking correlation ( p < 0.30) which reinforces visual lack of co-localization of Mn with Cu or Fe hot spots in the cell. Strong K/Mn and moderate P/Mn correlations were observed for Mn treated groups. A moderate to strong Zn/Mn correlation was observed in the cell body whereas the two metals were uncorrelated in the nucleus. Observed trends are very similar to these reported for single cell imaging in the hippocampal formation on Mn treated rats35 suggesting similar mechanisms of Mn accumulation inside the cell.
Discussion
Animal model
The plasma Mn concentrations in treated animals at day 30 following the dose regimen were between 11–36 μg L−1 while Mn concentrations in untreated animals were between 3.5-5.6 μg L−1.42,43 For reference, human studies indicate that Mn-poisoned workers usually have blood Mn concentrations in the 4–15 μg L−1 range and a human study of 39 Mn-poisoned welders in Beijing revealed that the welders with distinct manganism had blood Mn levels between 8.2–36 μg L−3. A case study involving 3 methcathinone patients reported plasma Mn content was reported as 33–82 μg L−1 assuming 55% plasma/blood ratio (v/v; blood Mn content of 18–45 μg L−1).44 The rodent model of chronic Mn exposure used in this study also demonstrates significantly increased Mn level in brain tissue.34,35
Manganese accumulation in tissue
Accumulation of Mn in the SNr is expected as gabaergic cells of the SNr are functionally and morphologically similar to those of the globus pallidus, which preferentially accumulate Mn upon exposure.10,34,45 Previous work by this lab has suggested that the SNc as well as SNr accumulates Mn under normal and chronic exposure conditions in the rodent brain.34 Manganese concentration in the SNc and the SNr differed by less than 10% (with greater Mn content in the SNc). Tissue concentrations of Mn fall within the predicted range of Mn levels for normal (1.1–2.9 μg g−1) and aberrant function (3.2–8.6 μg g−1) in humans.46 Manganese accumulation in Mn treated rodents demonstrated no difference between FG and non-FG treated animals despite a longer treatment time (4 vs. 6 weeks). This result indicates that the total cumulative dose does not accurately reflect the total accumulation of Mn in the brain but rather that a steady state has been achieved with regards to Mn influx/efflux. Although the specific mechanisms are unknown, Mn efflux from the brain has been shown to consist of fast (1–3 d) and slow (1–3 months) clearance mechanisms.47–49 It is possible that the Mn load presented to the SN is sufficiently addressed by these mechanism(s).
The combined approach of retrograde tracer labeling and XRF imaging in situ quantitatively established Mn accumulation in DA cells. Measurement of DA cell Mn concentration in tissue is particularly novel as cell culture models omit the action of brain barriers. Cellular Mn concentrations from control samples reported here are in agreement with XRF imaging study of pyramidal cells of the CA3 layer of the hippocampal formation35 and the results of Carmona et al.,24 which observed <3 μg g−1 Mn content for untreated cells. These concentrations probably reflects baseline cellular needs in Mn for normal brain cell function.
Average cellular Mn content above the applied threshold for chronic exposure animals is as high as 10.95 μg g−1 (200 μM) which high enough to affect cell viability in dopaminergic neuronal cell cultures20,24,29 although less than the half-maximal inhibitory concentration of 300–1000 μM.24,25,28 Note, however, that duration of exposure can have a profound effect. Exposures in cell cultures are usually limited to short periods of time from 3–48 h. Comparison of Mn treated pyramidal and DA cells demonstrate an increased Mn concentration in DA cells (29–59%) highlighting the ability of DA cells to accumulate more Mn. This is in agreement with results reported for PC12 cell culture exposed to 100 μM Mn where similar Mn content of 10 ± 3 μg g−1 was determined using a combined approach of proton induced X-ray emission and backscattering spectrometry.24
Subcellular targets of Mn accumulation
In studies of Mn induced neurotoxicity, attention was foremost given to the subcellular target of Mn accumulation, including the mitochondrion, the nucleus, and recently the Golgi apparatus. The highest Mn concentrations from fractionalized liver cells following acute Mn exposure (i.p. injection of 56Mn) were reported in mitochondria (40.6% of total content), followed by the supernatant (28.4%) and the nucleus (16.1%).50 However, measurements were taken following suspension of the homogenate in fresh buffer (performed 5 ×) which removed Mn from the organelles to the supernatant. Another study of fractionalized liver cells (4.5 mg kg−1 intravenous injection) observed no statistical difference between nucleus, cytoplasm, and mitochondria considering Mn content normalized by protein concentration.51 However, that study perfused the liver prior to dissection and re-suspended homogenate only 2×, suggest ing that Mn may be more tightly bound to the mitochondrial fraction as compared to the cytoplasm and nucleus. A study using electron energy loss spectroscopy observed dense Mn bodies in mitochondria in brain tissue of rats exposed through drinking water (20 mg mL−1; 100 d) although perfusion removed 50% of the Mn.52 Similarly, a study using chronic Mn exposure through drinking water (10 mg mL−1; 120 d) noted the highest absolute Mn concentration in fractionalized brain cells was in mitochondria followed by the cytosol and then the nucleus (3–5 washes).53
In contrast to the above reports, a cell culture study of four immortalized cell lines found that mitochondria contained less than 0.5% of the total Mn content following 24 hour incubation in 100 μM solution and separation involving 4 washes.27 Rather, the aforementioned study found that mesencephalic DA neuronal cells (N27) and PC12 cells accumulate more Mn in the soma (35% and 69% of total Mn content respectively) as opposed to brain barrier cell models RBE4 and Z310 which demonstrated substantially lower percentages (5% and 7%). Here we suggest that if Mn was mainly confined to mitochondria, we would observe small (0.5–1 μm in diameter), localized Mn hotspots in the cytoplasm; no such spots were observed. Thus, our results combined with prior studies show that Mn observed in the cell is not preferentially accumulated in mitochondria, is highly mobile, and may be easily modified during cell fractionation, cell fixation and any other manipulations. Our result agree well with other XRF studies of PC12 cell culture which observed Mn in the Golgi apparatus at 100 μM MnCl2 with an altered distribution at 300 μM.24 In that study, accumulation in the Golgi apparatus was confirmed by disrupting the organelle with brefeldin A and observing the subsequent redistribution of Mn in the cell. A recent study further confirmed this assessment via correlative microscopy using Golgi-GFP and XRF imaging of Mn.25 XRF imaging studies have reported Mn primarily in the cytoplasm and perinuclear space for GFP-TH positive neurons in mixed neuronal culture exposed to 500 μM MnCl2 for 3 hours followed by cryofixation.26 Distribution in the soma was also observed in pancreatic ß-cells following a 20 minute incubation in 50 μM MnCl2, 20 minute incubation in 4% paraformaldehyde, several washes, and air drying.54
Our study shows sufficiently delocalized (with preferred peri-nuclear localization) Mn distributions in the DA cells of SNc which have been shown to be susceptible to Mn toxicity upon Mn exposure in culture.20,24–29 Thus, Mn is available to interact with dopamine and with systems of its production, regulation and release. Multiple studies shown that dopamine release in striatum is decreased or impaired under both acute and chronic Mn exposure.55–59 One possible explanation is Mn facilitated auto-oxidation of excess DA.60–62 Transgenic Caenorhabditis elegans expressing DAT loss of function, resulting in increased extracellular DA, are more sensitive to Mn-induced toxicity (9 mM LD50) than wild type controls (47 mM LD50).62 Although this has been studied in the context of extra-cellular DA, DAT is also present on the surface of the endoplasmic reticulum and Golgi apparatus.63 Tangentially, DAT inhibition or knockout has been shown decrease Mn content in the rodent globus pallidus and CPu;45,64 it is unclear whether this is directly due to DAT loss of function or to an effect on a Mn transporter (e.g. DMT-1).
Conclusions
In this study, we found that the SNc is an additional target of Mn accumulation in rats following chronic Mn exposure and demonstrates higher accumulations than previously reported structures, such as the globus pallidus. Furthermore, in vivo retrograde tracer administration permitted identification of DA neurons of the SNc using optical fluorescence and XRF imaging allowed for quantification of Mn of these cells in situ. The highest Mn content was extranuclear, ranging between 40–200 μM; concentrations above 100 μM have shown to cause cell death in cell culture models.20,29 Together, these findings highlight a possibility of Mn induced malfunction of dopaminergic neurons.
Supplementary Material
Acknowledgements
We thank Dr Lan Hong, Dr Wendy Jiang, and Dr Wei Zheng of the School of Health Sciences at Purdue University for providing the rodent brains used in this study. We also would like to acknowledge Dr Lydia Finney, Dr Charlotte Gleber, Dr Barry Lai, and Evan Maxey for their assistance in operating their respective beamline facilities. We also greatly appreciate the help of Dr Stefan Vogt in fitting with the MAPS program. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. BioCAT is a National Institutes of Health-supported Research Center RR-08630.
Abbreviations
- APS
Advanced photon source
- cp
Caudal peduncle
- CPu
Caudate putamen
- CA3
Cornus ammonis 3
- DA
Dopaminergic
- FG
FluoroGold™
- HPCf
Hippocampal formation
- i.p.
Intraperitoneal
- PD
Parkinson’s disease
- SN
Substantia nigra
- SNc/r
Substantia nigra pars compacta/reticulata
- XRF
X-ray fluorescence
Footnotes
This work was supported by NIH/National Institute of Environmental Health Sciences grants R01 ES008146-14 (Y.P.) and R00 ES019879 (J.R.C.) and by the Purdue University Research Foundation.
Electronic supplementary information (ESI) available: Fig. S1: identification of non-FG labeled cells in SNc. Fig. S2: line plots of Mn and Fe in the SN; Fig. S3: representative example of cell clustering and Mn thresholding results; Fig. S4: metal content for SNr/c for 4 and 6 week treatment times; Fig S5: normalized metal content for measured cells. Table S1: metal/Mn correlations for concentrations measured from tissue. Table S2: cell metal concentrations (combined nucleus and cell body); Table S3: linear fit parameters for entire cell, nucleus, and cell body. See DOI: 10.1039/c5mt00023h
Notes and references
- 1.Couper J. Brit. Ann. Med. Pharm. 1837;1:41–42. [Google Scholar]
- 2.Rodier J. Br. J. Ind. Med. 1955;12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crossgrove J, Zheng W. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, Cowl CT. Neurology. 2005;64:2033–2039. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- 5.Stepens A, Logina I, Liguts V, Aldins P, Eksteina I, Platkajis A, Martinsone I, Terauds E, Rozentale B, Donaghy M. N. Engl. J. Med. 2008;358:1009–1017. doi: 10.1056/NEJMoa072488. [DOI] [PubMed] [Google Scholar]
- 6.Sikk K, Haldre S, Aquilonius SM, Asser A, Paris M, Roose Ä, Petterson J, Eriksson SL, Bergquist J, Taba P. Eur. J. Neurol. 2013;20:915–920. doi: 10.1111/ene.12088. [DOI] [PubMed] [Google Scholar]
- 7.Burkhard PR, Delavelle J, Du Pasquier R, Spahr L. Arch. Neurol. 2003;60:521–528. doi: 10.1001/archneur.60.4.521. [DOI] [PubMed] [Google Scholar]
- 8.Long LL, Li XR, Huang ZK, Jiang YM, Fu SX, Zheng W. Exp. Biol. Med. 2009;234:1075–1085. doi: 10.3181/0903-RM-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilarte TR. Environ. Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olanow CW. Ann. N. Y. Acad. Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- 11.Roth J. NeuroMol. Med. 2009;11:281–296. doi: 10.1007/s12017-009-8088-8. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein MM, Jerrett M. Environ. Res. 2007;104:420–432. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Neurology. 1997;48:650–658. doi: 10.1212/wnl.48.3.650. [DOI] [PubMed] [Google Scholar]
- 14.Gorell JM, Peterson EL, Rybicki BA, Johnson CC. J. Neurol. Sci. 2004;217:169–174. doi: 10.1016/j.jns.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Lucchini RG, Albini E, Benedetti L, Borghesi S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. Am. J. Ind. Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- 16.Marsh GM, Gula MJ. J. Occup. Environ. Med. 2006;48:1031–1046. doi: 10.1097/01.jom.0000232547.74802.d8. [DOI] [PubMed] [Google Scholar]
- 17.Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- 18.Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Neurology. 2005;64:230–235. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- 19.Santamaria AB, Cushing CA, Antonini JM, Finley BL, Mowat FS. J. Toxicol. Environ. Health, Part B. 2007;10:417–465. doi: 10.1080/15287390600975004. [DOI] [PubMed] [Google Scholar]
- 20.Higashi Y, Asanuma M, Miyazaki I, Hattori N, Mizuno Y, Ogawa N. J. Neurochem. 2004;89:1490–1497. doi: 10.1111/j.1471-4159.2004.02445.x. [DOI] [PubMed] [Google Scholar]
- 21.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet J-C, Lindquist S. Nat. Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sriram K, Lin GX, Jefferson AM, Roberts JR, Wirth O, Hayashi Y, Krajnak KM, Soukup JM, Ghio AJ, Reynolds SH, Castranova V, Munson AE, Antonini JM. FASEB J. 2010;24:4989–5002. doi: 10.1096/fj.10-163964. [DOI] [PubMed] [Google Scholar]
- 23.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Eur. J. Epidemiol. 2011;26:S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 24.Carmona A, Deves G, Roudeau S, Cloetens P, Bohic S, Ortega R. ACS Chem. Neurosci. 2010;1:194–203. doi: 10.1021/cn900021z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmona A, Roudeau S, Perrin L, Veronesi G, Ortega R. Metallomics. 2014;6:822–832. doi: 10.1039/c4mt00012a. [DOI] [PubMed] [Google Scholar]
- 26.Dučić T, Barski E, Salome M, Koch JC, Bähr M, Lingor P. J. Neurochem. 2013;124:250–261. doi: 10.1111/jnc.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalia K, Jiang W, Zheng W. Neurotoxicology. 2008;29:466–470. doi: 10.1016/j.neuro.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B. J. Neurochem. 2009;110:378–389. doi: 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata Y. Neurotoxicol. Teratol. 2002;24:639–653. doi: 10.1016/s0892-0362(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 30.Levin OS. Zhurnal Nevrologii I Psikhiatrii Imeni S. S. Korsakova. 2005;105:12–20. [PubMed] [Google Scholar]
- 31.Newland MC, Ceckler TL, Kordower JH, Weiss B. Exp. Neurol. 1989;106:251–258. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- 32.Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, Perl DP, Olanow W, Calne DB. Neurology. 1995;45:1199–1204. doi: 10.1212/wnl.45.6.1199. [DOI] [PubMed] [Google Scholar]
- 33.Daoust A, Barbier EL, Bohic S. NeuroImage. 2013;64:10–18. doi: 10.1016/j.neuroimage.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 34.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, Barrea R, Marcus MA, Zheng W, Pushkar Y. PLoS One. 2012;7:e48899. doi: 10.1371/journal.pone.0048899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robison G, Zakharova T, Fu S, Jiang W, Fulper R, Barrea R, Zheng W, Pushkar Y. Metallomics. 2013;5:1554–1565. doi: 10.1039/c3mt00133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seroogy KB, Dangaran K, Lim S, Haycock JW, Fallon JH. J. Comp. Neurol. 1989;279:397–414. doi: 10.1002/cne.902790306. [DOI] [PubMed] [Google Scholar]
- 37.Yao F, Yu F, Gong L, Taube D, Rao DD, MacKenzie RG. J. Neurosci. Methods. 2005;143:95–106. doi: 10.1016/j.jneumeth.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 38.Vogt S. J. Phys. IV. 2003;104:635–638. [Google Scholar]
- 39.Ridler TW, Calvard S. IEEE Trans. System, Man and Cybernetics. 1978;SMC–8:630–632. [Google Scholar]
- 40.MacDonald RS. J. Nutr. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 41.Wu FYH, Wu CW. Annu. Rev. Nutr. 1987;7:251–272. doi: 10.1146/annurev.nu.07.070187.001343. [DOI] [PubMed] [Google Scholar]
- 42.Zheng W, Zhao QQ, Slavkovich V, Aschner M, Graziano JH. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng W, Jiang YM, Zhang YS, Jiang WD, Wang XQ, Cowan DM. Neurotoxicology. 2009;30:240–248. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Ann. Neurol. 1994;36:871–875. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- 45.Anderson JG, Cooney PT, Erikson KM. Environ. Toxicol. Pharmacol. 2007;23:179–184. doi: 10.1016/j.etap.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowman AB, Aschner M. Neurotoxicology. 2014;41:141–142. doi: 10.1016/j.neuro.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newland MC, Cox C, Hamada R, Oberdörster G, Weiss B. Fundam. Appl. Toxicol. 1987;9:314–328. doi: 10.1016/0272-0590(87)90054-6. [DOI] [PubMed] [Google Scholar]
- 48.Takeda A, Sawashita J, Okada S. Brain Res. 1995;695:53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- 49.Vitarella D, Wong BA, Moss OR, Dorman DC. Toxicol. Appl. Pharmacol. 2000;163:279–285. doi: 10.1006/taap.1999.8874. [DOI] [PubMed] [Google Scholar]
- 50.Maynard LS, Cotzias GC. J. Biol. Chem. 1955;214:489–495. [PubMed] [Google Scholar]
- 51.Ayotte P, Plaa GL. Biochem. Pharmacol. 1985;34:3857–3865. doi: 10.1016/0006-2952(85)90435-6. [DOI] [PubMed] [Google Scholar]
- 52.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, Forte G, Martorana A, Bemardi G, Sancesario G. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Lai JCK, Minski MJ, Chan AWK, Leung TKC, Lim L. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- 54.Leoni L, Dhyani A, La Riviere P, Vogt S, Lai B, Roman BB. Contrast Media Mol. Imaging. 2011;6:474–481. doi: 10.1002/cmmi.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vidal L, Alfonso M, Campos F, Faro LRF, Cervantes RC, Durán R. Neurochem. Res. 2005;30:1147–1154. doi: 10.1007/s11064-005-7775-6. [DOI] [PubMed] [Google Scholar]
- 56.Guilarte TR, Burton NC, McGlothan JL, Verina T, Yun Z, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. J. Neurochem. 2008;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Exp. Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 58.McDougall SA, Reichel CM, Farley CM, Flesher MM, Der-Ghazarian T, Cortez AM, Wacan JJ, Martinez CE, Varela FA, Butt AE, Crawford CA. Neuroscience. 2008;154:848–860. doi: 10.1016/j.neuroscience.2008.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez-Betancourt J, Anaya-Martínez V, Gutierrez-Valdez AL, Ordoñez-Librado JL, Montiel-Flores E, Espinosa-Villanueva J, Reynoso-Erazo L, Avila-Costa MR. Neurotoxicology. 2012;33:1346–1355. doi: 10.1016/j.neuro.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Migheli R, Godani C, Sciola L, Delogu MR, Serra PA, Zangani D, De Natale G, Miele E, Desole MS. J. Neurochem. 1999;73:1155–1163. doi: 10.1046/j.1471-4159.1999.0731155.x. [DOI] [PubMed] [Google Scholar]
- 61.Serra PA, Esposito G, Enrico P, Mura MA, Migheli R, Delogu MR, Miele M, Desole MS, Grella G, Miele E. Br. J. Pharmacol. 2000;130:937–945. doi: 10.1038/sj.bjp.0703379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. PLoS Genet. 2010;6:e1001084. doi: 10.1371/journal.pgen.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. J. Comp. Neurol. 1997;388:211–227. [PubMed] [Google Scholar]
- 64.Erikson KM, John CE, Jones SR, Aschner M. Environ. Toxicol. Pharmacol. 2005;20:390–394. doi: 10.1016/j.etap.2005.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.