Potassium channel activity regulates vascular smooth muscle membrane potential, with increased channel activity resulting in membrane hyperpolarisation. This counteracts the activity of voltage dependent calcium channels, preventing calcium entry into the cell and subsequent contraction. Over the past decade the Kv7 family of voltage gated potassium channels have been identified as key players in the regulation of vascular tone. Activation of Kv7 channels produces relaxation in a range of precontracted vessels in murine, rat and human models while blockade of Kv7 channels produces contraction of various vessels at rest (see1 for recent review). Of the 5 Kv7 subtypes, Kv7.1 7.4 and 7.5 are consistently expressed in the vasculature where the predominant molecular architecture has been shown to be a Kv7.4/Kv7.5 heterotetramer.2,3 Recent research has focused on the role of Kv7 channels in vasodilations mediated by endogenous molecules, uncovering several vasodilators which act at least in part via activation of Kv7 channels. The seminal study by Chadha et al4 established that Kv7 channels contribute to cyclic AMP (cAMP) dependent isoproterenol mediated vasorelaxations in the rat renal artery. This work used a combination of pharmacological and molecular tools and showed that either blockade of Kv7 by linopirdine, or knockdown of Kv7.4 in vessels, attenuated the isoproterenol relaxation. Further studies have since shown that in other vascular beds Kv7 channels contribute to the cAMP-dependent relaxations. In the cerebral arteries calcitonin gene related peptide2 and forskolin relaxations5 are inhibited by linopirdine, while in coronary arteries adenosine relaxations are Kv7 dependent.6 These studies have established the importance of Kv7 channels in the mediation of cAMP dependent relaxations in the vasculature, but their role in other endogenous signaling pathways was not clear.
The recent findings published in Stott et al7 reveal that Kv7 channels are involved in mediating cyclic GMP (cGMP) dependent relaxations in the rat vasculature. The guanylate cyclase cGMP signaling pathway is a key vasodilator pathway involved in the regulation of vascular smooth muscle contractility. Activation of guanylate cyclase either by membrane bound natriuretic peptide receptor stimulation or by liberation of nitric oxide, results in an increase of cyclic GMP. By targeting these 2 mechanisms using the nitric oxide donor sodium nitroprusside (SNP) and either atrial or C-type natriuretic peptide (ANP and CNP, respectively), this study showed that the cGMP dependent relaxations produced by all of these agents were inhibited in the presence of linopirdine in the rat aorta. Interestingly, in the renal artery only the ANP relaxations were sensitive to linopirdine. Crucially these relaxations were not inhibited by the Kv7.1 specific blocker HMR 1556, or other potassium channel blockers such as other Kv channels (4-aminopyridine), KATP (Glibenclamide), BKCa(Paxilline) and KIR (Tertiapin Q). Moreover, in spontaneously hypertensive animals which show decreased vascular Kv7.4 expression, these responses were all compromised. This is consistent with previous findings that endogenous vasodilation that are Kv7 dependent are compromised in hypertensive animals (2,4,6 summarised in Fig. 1). Overall, this data shows for the first time the dependence of cGMP dependent vasorelaxations on Kv7 channels, and that these are compromised in hypertension.
Figure 1.
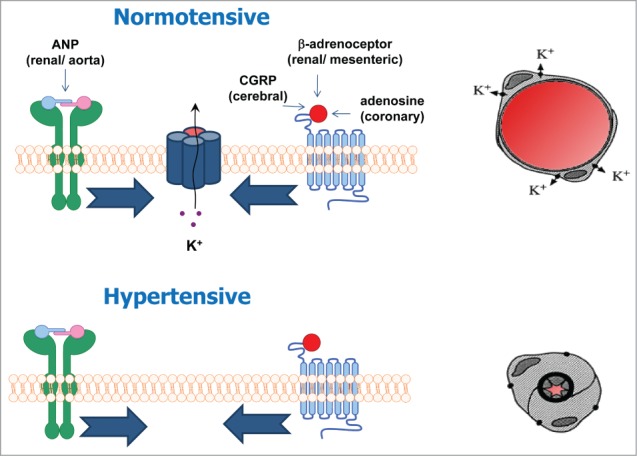
In normotensive vessels a number of endogenous vasodilators acting via either cyclic AMP (CGRP, adenosine, isoproterenol) or cyclic GMP (ANP) linked receptors, produce vasorelaxations that are dependent upon Kv7 channels. However, in hypertensive vessels where Kv7.4 protein levels are reduced, these agents are not able to produce relaxations.
A noteworthy aspect of this study was the revelation of a fascinating aspect of vascular physiology; that a vasorelaxant signaling pathway can be reliant upon different functional endpoints in different vascular beds. If cGMP relaxations can act via Kv7 channels in the aorta and renal artery and enhance Kv7.4 currents, why does this axis not come into play to mediate CNP or SNP relaxations in the renal artery? It is possible that the cellular architecture in aortic and renal myocyte differs in the relationship of Kv7 channels to natriuretic peptide receptors. There is a tendency to think of signaling events as comprising of definitive steps which must occur for an outcome to take place. However, perhaps they are actually much more opportunistic in reality - acting on the closest receptive effector that can get the job done. This would highlight how crucial subcellular localization can be to a physiological outcome of receptor mediated effects, and would go some way to explaining differences seen across the vascular tree. Nevertheless, the body of evidence bearing testimony to the crucial role of Kv7 channels in maintaining and regulating vascular tone continues to grow.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Stott JB, Jepps TA, Greenwood IA. Kv7 potassium channels: A new therapeutic target in smooth muscle disorders. Drug Discov Today 2014; 19(4):413-24; PMID:24333708; http://dx.doi.org/ 10.1016/j.drudis.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. Contribution of Kv7.4/Kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol 2014; 34(4):887-93; PMID:24558103; http://dx.doi.org/ 10.1161/ATVBAHA.114.303405 [DOI] [PubMed] [Google Scholar]
- 3.Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, Byron KL. Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem 2014; 289(4):2099-111; PMID:24297175; http://dx.doi.org/ 10.1074/jbc.M113.527820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension 2012; 59(4):877-84; PMID:22353613; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.111.187427 [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Yang Y, Tanner MA, Li M, Hill MA. Heterogenetiy in Kv7 channel function in the cerebral and coronary circulation. Microcirculation 2015; 22(2):109-21; PMID:25476662; http://dx.doi.org/ 10.1111/micc.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Greenwood IA, Olesen SP. Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension 2013; 62(6):1090-7; PMID:24082059; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.113.01244 [DOI] [PubMed] [Google Scholar]
- 7.Stott JB, Barrese V, Jepps TA, Leighton EV, Greenwood IA. Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension 2015; 65(3):376-82; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.114.04373 [DOI] [PubMed] [Google Scholar]


