Abstract
F-BAR domains form crescent-shaped dimers that bind to and deform lipid bilayers, and play a role in many cellular processes requiring membrane remodeling, including endocytosis and cell morphogenesis. Nervous Wreck (NWK) encodes an F-BAR/SH3 protein that regulates synapse growth in Drosophila. Unlike conventional F-BAR proteins that assemble tip-to-tip into filaments and helical arrays around membrane tubules, the Nwk F-BAR domain instead assembles into zigzags, creating ridges and periodic scallops on membranes in vitro. In cells, this membrane deforming activity generates small buds, which can lengthen into extensive protrusions upon actin cytoskeleton polymerization. Here, we show that Nwk-induced cellular protrusions contain dynamic microtubules, distinguishing them from conventional filopodia, and further do not depend on actin filaments or microtubules for their maintenance. Our results indicate new ways in which close cooperation between the membrane remodeling and cytoskeletal machinery underlies large-scale changes in cellular morphology.
Keywords: actin, F-BAR, microtubule, membrane, Nwk
Rapid membrane dynamics are crucial for many cellular processes, including endocytosis, intracellular trafficking, cell morphogenesis, and cell migration. These events are driven by membrane bending, budding, tubulation, fission, and fusion, all of which are energetically costly and require cooperation between lipid-remodeling protein machinery and the force-generating cytoskeleton.1 Fer/Cip4 homology Bin/amphiphysin/Rvs167 (F-BAR) proteins are one important class of lipid remodeling factors. These proteins form gently curved, crescent-shaped dimers that directly interact with negatively charged head groups of membrane lipids through their positively charged concave surfaces.2
Most F-BAR domain-containing proteins characterized to date induce positive curvature (i.e. curvature toward the protein-decorated leaflet of the membrane). Two such proteins, CIP4 and FBP17, have been shown to oligomerize on and form helical scaffolds around membranes, thereby inducing membrane tubulation in vitro, and membrane invaginations in cells.3 However, the mammalian F-BAR Slit-Robo GTPase activating proteins (SRGAPs) differ from canonical F-BAR proteins, as they induce negative curvature (i.e., curvature away from the protein-decorated leaflet of the membrane), forming invaginations into liposomes in vitro and filopodia-like protrusions in cultured cells.4,5 Interestingly, the F-BAR domain of Syndapin, which is generally thought to induce positive curvature in the form of membrane tubulation,6,7 can also form microspikes or protrusions in cells, potentially through an alternative organization of F-BAR dimers on the membrane (as proposed by Shimada et al. 20108). The mechanisms determining these different activities of individual BAR proteins are not well understood. Further, in addition to directly binding and shaping membranes through their membrane binding domains, F-BAR family proteins often interact with regulators of the actin and microtubule networks via additional protein binding domains, connecting the membrane remodeling machinery and the cytoskeleton.1
The actin and microtubule cytoskeletons are highly dynamic structures that can facilitate intracellular traffic, stabilization and rapid remodeling of cellular membranes. Both actin and microtubules are involved in the formation of cellular protrusions including lamellipodia and filopodia, and both are closely linked to membrane dynamics, with critical roles in endosomal traffic, receptor diffusion, and vesicle motility.9 Though clearly important to many events that require changes in membrane shape and curvature, how the cytoskeleton is mechanistically involved in these events is still unclear.9 Therefore, investigating the relationship between the actin and microtubule cytoskeletons and F-BAR domain-mediated membrane remodeling will be critical for understanding how membrane dynamics are regulated in the cell.
The Drosophila F-BAR/SH3 protein Nwk regulates growth factor signaling at the larval neuromuscular junction (NMJ) through interactions with the membrane, actin nucleation machinery, and other key endocytic proteins.10–13 nwk null mutant flies display synaptic overgrowth at the NMJ and experience temperature sensitive seizures, both phenotypes typical of endocytic mutants.13 We recently found that purified Nwk dimers exhibited the predicted F-BAR crescent shape. However, unlike the canonical F-BAR CIP4, which oligomerized tip-to-tip into linear filaments and helical arrays on membranes,14 Nwk unexpectedly associated tip-to-tip to form zigzag oligomers on lipid monolayers. As a result, the Nwk F-BAR domain created periodic membrane ridges and resultant inter-ridge scallops in vitro, instead of inducing membrane tubules like canonical F-BAR domains. These results suggest that distinct patterns of higher-order organization could determine the membrane remodeling activities of diverse F-BAR family members.
To investigate the membrane deforming activity of Nwk in a cellular context, we previously expressed the isolated F-BAR domain in Drosophila S2 cells, which do not express endogenous Nwk.15 Though this simplified cellular assay may not directly recapitulate the role of Nwk F-BAR-induced membrane deformation tied to endocytosis in synapses, it is an excellent model to test how F-BAR proteins manipulate membrane shape in cooperation with the cytoskeleton. We found that the Nwk F-BAR domain induced protrusions from the plasma membrane, in sharp contrast to the canonical F-BARs Syndapin and CIP4, which induced intracellular tubules. The membrane deforming activity of Nwk depended both on structural determinants at the tips of the Nwk F-BAR dimer (regions suggested to be important for oligomerization), and on electrostatic interactions between the membrane and the concave F-BAR surface.14
While our previous results indicate a novel form of membrane deformation arising from a canonical Nwk F-BAR membrane interacting surface together with a unique higher order zigzag assembly, they do not directly explain how a ridge and scallop-forming membrane-deforming protein generates protrusions in cells. Nwk-induced cellular protrusions are likely to be complex structures arising from a combination of membrane deformation and associated cytoskeletal remodeling. In control S2 cells, filamentous actin (F-actin) is present throughout the cell, and concentrated at the cell periphery. We previously showed that in Nwk-expressing cells, F-actin localizes to Nwk-induced cellular protrusions. We used live cell imaging to show that Nwk F-BAR-induced protrusions were initially formed from small cellular buds and that when actin assembly was blocked using the actin polymerization inhibitor latrunculin B (Lat B), protrusions were arrested at this small bud stage.14 Here, we found that once protrusions had already formed, they did not retract upon Lat B treatment and actin disassembly. This suggests that actin is required for the formation but not the maintenance of these structures (Fig. 1A). While the SH3 domains of full-length Nwk interact with the actin nucleation-promoting factor WASP,12 these sequences are not contained in our isolated F-BAR construct, and are thus not required for protrusion formation in cells. As no direct link between the Nwk F-BAR and the cytoskeletal machinery has yet been identified, one possible explanation is that by inducing membrane curvature, the Nwk F-BAR may alter membrane tension, elasticity or lipid organization, creating an environment more amenable to deformation by the cytoskeletal machinery.9
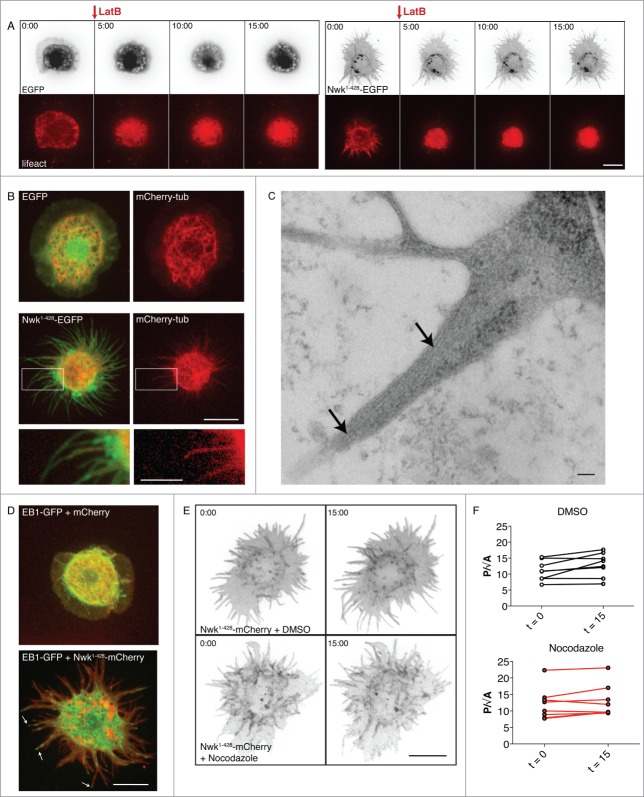
Figure 1 (See previous page).
Nwk-induced cellular protrusions do not depend on actin or microtubules for stability once formed. (A) Time-lapse imaging of S2 cells co-transfected with Lifeact-mCherry and either EGFP (left) or Nwk1-428-EGFP (right), and treated with Lat B after spreading on ConA. F-actin disassembles upon treatment, but the cellular protrusions remain. Images show 2D projections of confocal stacks. Scale bar, 10 µm. Time scale is in minutes. (B) Confocal microscopy of fixed S2 cells expressing EGFP or Nwk1-428-EGFP, and mCherry-tubulin. Nwk and tubulin localize to protrusions. Images show 2D projections of confocal stacks. Scale bar, 10 µm. Scale bar in magnified image is 5 µm. (C) Electron microscopy of Nwk1-428-EGFP–expressing S2 cells treated with Lat B to disrupt the actin cytoskeleton. Microtubules can be observed throughout the protrusion (arrows). Scale bar, 100 nm. (D) Live cell imaging of S2 cells expressing EB1-GFP with mCherry (top) or Nwk1-428-mCherry (bottom). EB1, a plus end microtubule binding protein, can be seen throughout protrusions (arrows, see also Movies 1 and 2). Scale bar, 10 µm. (E) Nwk-induced protrusions remain stable after microtubule depolymerization. Live cell imaging of S2 cells expressing Nwk1-428-mCherry and treated with Nocodazole, a microtubule-depolymerizing drug, or DMSO control. Images show 2D projections of confocal stacks taken before (t = 0), and 15 minutes after (t = 15) treatment. (F) Quantification of cell shape before and after treatment. Protrusion index is represented by cell perimeter divided by the square root of cell area (P/√A).
To determine if the protrusions induced by Nwk bear similarity to conventional filopodia, we investigated the organization of the microtubule cytoskeleton. Conventional filopodia are thin cellular protrusions filled with bundled actin, and are not deeply invaded by dynamic microtubules.16,17 In control S2 cells (which do not exhibit frequent filopodia), microtubules are concentrated in the central region of the cell, with occasional microtubules extending into the thin lamellipodium (Fig. 1B). In Nwk-expressing cells, mCherry-tubulin-labeled microtubules were present throughout Nwk-induced protrusions (Fig. 1B). Microtubules were also visible in protrusions in electron micrographs of Lat B-treated cells, in which the actin cytoskeleton was disrupted after protrusions had formed (Fig. 1C, arrows). Finally, the microtubule plus-end tracking protein EB1-GFP18 could be seen moving toward the tips of the protrusions (Fig. 1D and Movies 1 and 2), indicating the presence of growing microtubules. Treatment of these cells with the microtubule depolymerizing drug nocodazole did not abolish protrusions once they were formed, similar to the results obtained above for the Lat B-treated actin cytoskeleton (Fig. 1E and F). Taken together, our results suggest that Nwk F-BAR is inducing structures in these cells that are mechanistically distinct from filopodia.
These heterologous assays provide clues as to the nature of the interaction between the cytoskeletal machinery and the membrane deforming activity of Nwk, and also an exciting new model system for examining the interplay between the actin and microtubule cytoskeletons in cells. Membrane-deforming proteins and the force-generating actin and microtubule cytoskeletons must act in concert to create the complex membrane structures and shapes found in cells and tissues. Overall, our data suggest a model in which Nwk zigzag assembly induces plasma membrane ridges and inter-ridge scallops (in the form of membrane buds), which are then amplified by cytoskeletal forces into long protrusions (Fig. 2). While we still need to elucidate how this interplay between lipid deformation and the cytoskeleton contributes to membrane traffic at neuronal synapses, multiple studies have shown that both the actin and microtubule cytoskeletons play critical roles in the same pathways as Nwk in vivo.19–22
Figure 2.
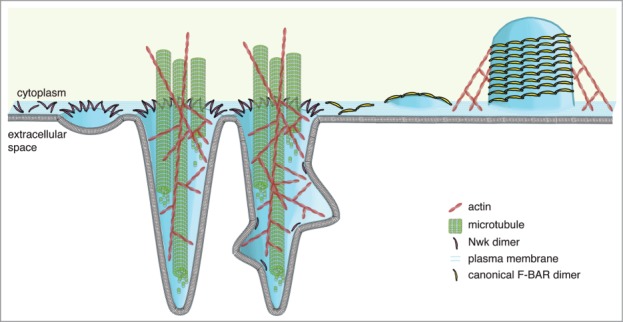
Model for formation of Nwk-induced protrusions at S2 cell plasma membranes. Higher-order assembly of Nwk in zigzags on the lipid bilayer results in membrane ridging and resultant inter-ridge buds or scallops (left), which are amplified by the cytoskeleton to form large-scale actin- and microtubule-filled cellular projections (center). In contrast, canonical F-BAR domains oligomerize into filaments and helical scaffolds to induce intracellular tubules (right).
In conclusion, the presence of cytoskeletal structures within Nwk-induced membrane protrusions suggests a complex interaction between the membrane, membrane deforming proteins, and the force-generating cytoskeleton. These results suggest that while it is capable of reshaping the membrane on a small scale, Nwk requires the involvement of the cytoskeleton to achieve large scale membrane remodeling. These results support the idea that cellular processes involving dynamic membrane remodeling require a tight partnership between the membrane remodeling and cytoskeletal machinery, and provide a specific mechanistic stepping point for future studies of their inter-related functions.
Movies 1 and 2. Live cell imaging of S2 cells expressing EB1-GFP and mCherry (Movie 1) or Nwk1–428-mCherry (Movie 2). EB1, a plus-end microtubule binding protein, can be seen throughout projections. Scale bar, 10 μm.
Methods
S2 cells were cultured according to standard protocols23 in Schneider's media supplemented with 10% fetal bovine serum and 0.1 mg/mL penicillin/streptomycin (Lonza), transfected using Effectene reagent (Qiagen) and incubated for 2 d at 25 °C. pBI-UAS-Nwk-F-BAR-EGFP,14 pEB1:EB1-GFP,18 and pMT-mCherry-tubulin24 have been described previously. Constructs were co-transfected with actin promoter-Gal4. To disrupt the actin cytoskeleton, Lat B (Millipore) was added at a final concentration of 125 μM. To disrupt microtubules, Nocodazole was added at a final concentration of 150 μM. To evaluate the effectiveness of Nocodazole treatment, cells were co-transfected with EB1-GFP and the disappearance of comets in Nocodazole but not in control DMSO-treated cells was confirmed by confocal imaging.
S2 cells were spread for 1 h on Concanavalin A (ConA, Sigma) for live cell imaging or before fixation. All confocal imaging was conducted on a Marianas spinning disk confocal system (3I, Inc.), consisting of a Zeiss Observer Z1 microscope equipped with a Yokagawa CSU-X1 spinning disk confocal head and a QuantEM 512SC EMCCD camera.
For correlative light and electron microscopy, S2 cells were spread on ConA-coated Aclar finder discs, and transfected cells were identified by fluorescence microscopy, as previously described.14 To disrupt the actin cytoskeleton, LatB (Millipore) was added at a final concentration of 50 μM for 10 minutes. Discs were then rapidly frozen using a Leica HPM-100 high-pressure freezer (Leica Microsystem) and fixed by freeze-substitution as previously described.14 Samples were then embedded in EMbed 812-Resin (EMS), trimmed and sectioned to contain the cells of interest. Sections were post-stained with uranyl acetate (2%) and Reynold's lead citrate, and inspected on a Morgagni transmission electron microscope (FEI) or a Tecnai F30 transmission electron microscope (FEI) operating at 300 kV and equipped with a 4 k × 4 k CCD camera (GATAN).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Emily Messelaar for input on the manuscript.
Funding
This work was supported in part by Basil O'Connor Starter Scholar Research Award Grant No. 5-FY12-99 from the March of Dimes Foundation and a Pew Scholar Award to A.A.R., and by the National Science Foundation (NSF-MRI-0722582 to D.N.).
References
- 1.Suetsugu S, Gautreau A. Synergistic BAR-NPF interactions in actin-driven membrane remodeling. Trends Cell Biol 2012; 22: 141–50; PMID:22306177; http://dx.doi.org/ 10.1016/j.tcb.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 2.Masuda M, Mochizuki N. Structural characteristics of BAR domain superfamily to sculpt the membrane. Semin Cell Dev Biol 2010; 21: 391–8; PMID:20083215; http://dx.doi.org/ 10.1016/j.semcdb.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 3.Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell 2008; 132: 807–17; PMID:18329367; http://dx.doi.org/ 10.1016/j.cell.2007.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson BR, Lloyd KE, Kruszewski A, Kim I-H, Rodriguiz RM, Heindel C, Faytell M, Dudek SM, Wetsel WC, Soderling SH. WRP/srGAP3 facilitates the initiation of spine development by an inverse F-BAR domain, and its loss impairs long-term memory. J Neurosci 2011; 31: 2447–60; PMID:21325512; http://dx.doi.org/ 10.1523/JNEUROSCI.4433-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin W-L, Frost A, Polleux F. The F-BAR Domain of srGAP2 Induces Membrane Protrusions Required for Neuronal Migration and Morphogenesis. Cell 2009; 138: 990–1004; PMID:19737524; http://dx.doi.org/ 10.1016/j.cell.2009.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Navarro MVaS, Peng G, Molinelli E, Goh SL, Judson BL, Rajashankar KR, Sondermann H. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc Natl Acad Sci U S A 2009; 106: 12700–5; PMID:19549836; http://dx.doi.org/ 10.1073/pnas.0902974106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessels MM, Qualmann B. Syndapin oligomers interconnect the machineries for endocytic vesicle formation and actin polymerization. J Biol Chem 2006; 281: 13285–99; PMID:16540475; http://dx.doi.org/ 10.1074/jbc.M510226200 [DOI] [PubMed] [Google Scholar]
- 8.Shimada A, Takano K, Shirouzu M, Hanawa-Suetsugu K, Terada T, Toyooka K, Umehara T, Yamamoto M, Yokoyama S, Suetsugu S. Mapping of the basic amino-acid residues responsible for tubulation and cellular protrusion by the EFC/F-BAR domain of pacsin2/Syndapin II. FEBS Lett 2010; 584: 1111–8; PMID:20188097; http://dx.doi.org/ 10.1016/j.febslet.2010.02.058 [DOI] [PubMed] [Google Scholar]
- 9.Kapus A, Janmey P. Plasma membrane–cortical cytoskeleton interactions: a cell biology approach with biophysical considerations. Compr Physiol 2013; 3: 1231–81; PMID:23897686 [DOI] [PubMed] [Google Scholar]
- 10.Rodal A, Blunk AD, Akbergenova Y, Jorquera Ra, Buhl LK, Littleton JT. A presynaptic endosomal trafficking pathway controls synaptic growth signaling. J Cell Biol 2011; 193: 201–17; PMID:21464232; http://dx.doi.org/ 10.1083/jcb.201009052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connor-Giles KM, Ganetzky B. Nervous Wreck Interacts with Thickveins and the Endocytic Machinery to Attenuate Retrograde BMP Signaling during Synaptic Growth. Neuron 2008; 58: 507–18; http://dx.doi.org/ 10.1016/j.neuron.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodal A, Motola-Barnes RN, Littleton JT. Nervous wreck and Cdc42 cooperate to regulate endocytic actin assembly during synaptic growth. J Neurosci 2008; 28: 8316–25; PMID:18701694; http://dx.doi.org/ 10.1523/JNEUROSCI.2304-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle IP, Koh Y-H, Lee W-CM, Slind J, Fergestad T, Littleton JT, Ganetzky B. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron 2004; 41: 521–34; PMID:14980202; http://dx.doi.org/ 10.1016/S0896-6273(04)00016-9 [DOI] [PubMed] [Google Scholar]
- 14.Becalska AN, Kelley CF, Berciu C, Stanishneva-Konovalova TB, Fu X, Wang S, Sokolova OS, Nicastro D, Rodal AA. Formation of membrane ridges and scallops by the F-BAR protein Nervous Wreck. Mol Biol Cell 2013; 24: 2406–18; PMID:23761074; http://dx.doi.org/ 10.1091/mbc.E13-05-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherbas L, Willingham A, Zhang D, Yang L, Zou Y, Eads BD, Carlson JW, Landolin JM, Kapranov P, Dumais J, et al.. The transcriptional diversity of 25 Drosophila cell lines. Genome Res 2011; 21: 301–14; PMID:21177962; http://dx.doi.org/ 10.1101/gr.112961.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 2008; 9: 446–54; PMID:18464790; http://dx.doi.org/ 10.1038/nrm2406 [DOI] [PubMed] [Google Scholar]
- 17.Schober JM, Komarova YA, Chaga OY, Akhmanova A, Borisy GG. Microtubule-targeting-dependent reorganization of filopodia. J Cell Sci 2007; 120: 1235–44; PMID:17356063; http://dx.doi.org/ 10.1242/jcs.003913 [DOI] [PubMed] [Google Scholar]
- 18.Currie JD, Stewman S, Schimizzi G, Slep KC, Ma A, Rogers SL. The microtubule lattice and plus-end association of Drosophila Mini spindles is spatially regulated to fine-tune microtubule dynamics. Mol Biol Cell 2011; 22: 4343–61; PMID:21965297; http://dx.doi.org/ 10.1091/mbc.E11-06-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwood NT, Sun Q, Xue M, Zhang B, Zinn K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol 2004; 2: e429; PMID:15562320; http://dx.doi.org/ 10.1371/journal.pbio.0020429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes P, Haines N, Kuppuswamy V, Fleet DJ, Stewart BA. Synaptic vesicle mobility and presynaptic F-actin are disrupted in a N-ethylmaleimide-sensitive factor allele of Drosophila. Mol Biol Cell 2006; 17: 4709–19; PMID:16914524; http://dx.doi.org/ 10.1091/mbc.E06-03-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khuong TM, Habets RLP, Slabbaert JR, Verstreken P. WASP is activated by phosphatidylinositol-4,5-bisphosphate to restrict synapse growth in a pathway parallel to bone morphogenetic protein signaling. Proc Natl Acad Sci U S A 2010; 107: 17379–84; PMID:20844206; http://dx.doi.org/ 10.1073/pnas.1001794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccioli ZD, Littleton JT. Retrograde BMP Signaling Modulates Rapid Activity-Dependent Synaptic Growth via Presynaptic LIM Kinase Regulation of Cofilin. J Neurosci 2014; 34: 4371–81; PMID:24647957; http://dx.doi.org/ 10.1523/JNEUROSCI.4943-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherbas L, Cherbas P. Drosophila cell culture and transformation. CSH Protoc 2007; 2007: pdb.top6; PMID:21357155 [DOI] [PubMed] [Google Scholar]
- 24.Jolly AL, Kim H, Srinivasan D, Lakonishok M, Larson AG, Gelfand VI. Kinesin-1 heavy chain mediates microtubule sliding to drive changes in cell shape. Proc Natl Acad Sci U S A 2010; 107: 12151–6; PMID:20566873; http://dx.doi.org/ 10.1073/pnas.1004736107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.