Abstract
Plasma membrane proteins play pivotal roles in mediating responses to endogenous and environmental cues. Regulation of membrane protein levels and establishment of polarity are fundamental for many cellular processes. In plants, IRON-REGULATED TRANSPORTER 1 (IRT1) is the major root iron transporter but is also responsible for the absorption of other divalent metals such as manganese, zinc and cobalt. We recently uncovered that IRT1 is polarly localized to the outer plasma membrane domain of plant root epidermal cells upon depletion of its secondary metal substrates. The endosome-recruited FYVE1 protein interacts with IRT1 in the endocytic pathway and plays a crucial role in the establishment of IRT1 polarity, likely through its recycling to the cell surface. Our work sheds light on the mechanisms of radial transport of nutrients across the different cell types of plant roots toward the vascular tissues and raises interesting parallel with iron transport in mammals.
Keywords: endocytosis, iron, metals, plant nutrition, polarity
Discussion
Because plants are fixed to a specific location, they have to constantly monitor and quickly respond to environmental changes. In particular, nutrient uptake must be tightly controlled to optimize plant growth and development to nutrient availability in soils. This is achieved in part by a tight regulation of transporter levels at the cell surface. In addition, many nutrient transporters driving the uptake of nutrient from the soil are polarly localized in root epidermal cells and enriched in the outer plasma membrane domain facing the soil. Lateral polarity of plasma membrane transporters has been previously demonstrated in rice for the Low silicon rice 1 (Lsi1) silicon influx channel and the Low silicon rice 2 (Lsi2) silicon exporter localized at the outer and the inner polar domain of the plasma membrane, respectively.1,2 The radial transport of boron across the root of the model plant Arabidopsis thaliana involves the boric acid channel NIP5;1, which is laterally polarized at the outer domain of the plasma membrane of root epidermal cells, and the borate efflux transporter BOR1 localized at the inner polar domain of root cells.3 Such lateral polarity is believed to be important for the radial transport of nutrients across cells found between the root epidermis and the vascular tissues, although no clear demonstration currently exists in the literature.
Recently, we identified the mechanisms driving the dynamics and the polarity of IRON-REGULATED TRANSPORTER 1 (IRT1), a metal iron transporter involved in iron acquisition from the soil and that also transports highly reactive metal substrates such as Zinc (Zn), Manganese (Mn), Colbalt (Co) and Cadmium (Cd).4-6 IRT1 is found at the outer polar plasma membrane domain of root epidermal cells, but exclusively when plants are grown in the absence of its secondary metal substrates7 (Fig. 1A). In the presence of such metals, IRT1 undergoes internalization from the plasma membrane into early endosomes/trans-Golgi network (EE/TGN)7,8 (Fig. 1B). IRT1 endocytosis requires monoubiquitination of 2 cytosolic lysine residues and the IRT1 DEGRADATION FACTOR 1 (IDF1) RING-type E3 ubiquitin ligase.8,9 This mechanism is crucial to limit the overaccumulation of noxious heavy metals through IRT1 and for the control of metal homeostasis,7,8,10 as plants expressing the non-ubiquitinatable IRT1K154RK179R form rapidly die of metal overload. In parallel, we identified the endosomal FYVE1 protein as an IRT1 partner in vivo. Interestingly, Arabidopsis plants overexpressing FYVE1 accumulate IRT1 at the cell surface in an apolar fashion, making of FYVE1 an important regulator of IRT1 polarity and a great tool to investigate the role of transporter polarity in nutrient distribution11 (Fig. 1C). FYVE1-overexpressing plants show hypersensitivity to low iron content and decreased metal content, although harboring wild-type levels of IRT1 protein. Altogether, these observations point to a defect in the radial transport of metals associated with the loss of IRT1 polarity.
Figure 1.
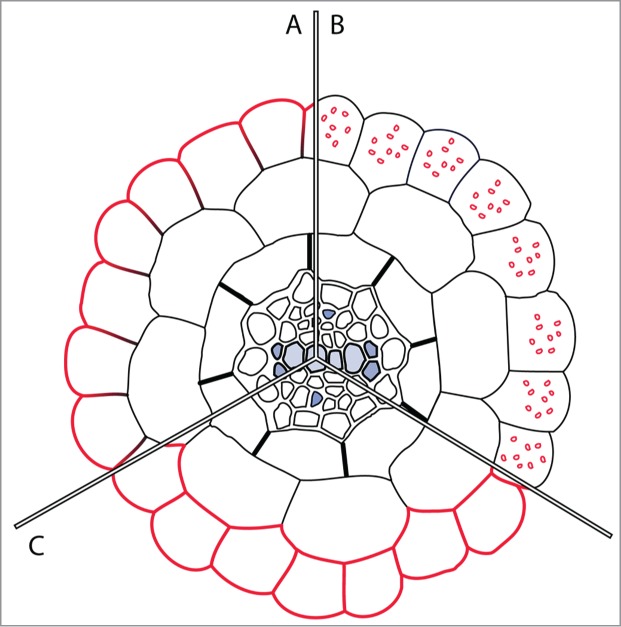
Trafficking and lateral polarity of IRT1 in plant root epidermal cells. (A) IRT1 (red) is expressed in root epidermal cells and localized at the outer polar domain of the plasma membrane when grown in the absence of its non-iron metal substrates, i.e. zinc, manganese and colbalt, or in standard conditions for the non-ubiquitinatable IRT1K154K179R mutant form. (B) IRT1 localizes to the early endosome/trans-Golgi network in standard growth conditions, i.e., in the presence of its secondary metal substrates Zn, Mn and Co. (C) IRT1 accumulates at the cell surface in an apolar fashion upon overexpression of endosomal FYVE1 protein.
Once taken up by root epidermal cells through IRT1, iron has to travel through the root cortex, the endodermis - including the Casparian strip, a physical barrier blocking the apoplastic flow - and the pericycle where it is released in the vasculature through the FERROPORTIN 1 (FPN1).12 The fact that the loss of IRT1 polarity impairs the radial transport of iron and metals suggests that metals don't travel through the plant cell-cell communications called plasmodesmata to exit epidermal cells. This idea is further supported by the loss of symplasmic connection between differentiated root epidermal cells, where IRT1 is expressed, and underlying cortical cells.13 We can therefore hypothesize that efflux transporters polarized to the inner plasma membrane domain of root epidermal cells are required for the exit of iron from epidermal cells (Fig. 2). How iron and metals are then transported into underlying cortical cells remains an open question. Several multigenic families of metal transporters able to transport metal ions or metal-chelates are found in the Arabidopsis genome.14 Deciphering the identity of the metal transporters driving the radial movement of metals between the different root cell types represents a major challenge in our understanding of the physiology of plant nutrition. This will notably require a careful investigation of the expression territories and subcellular localization of these different metal transporters.
Figure 2.
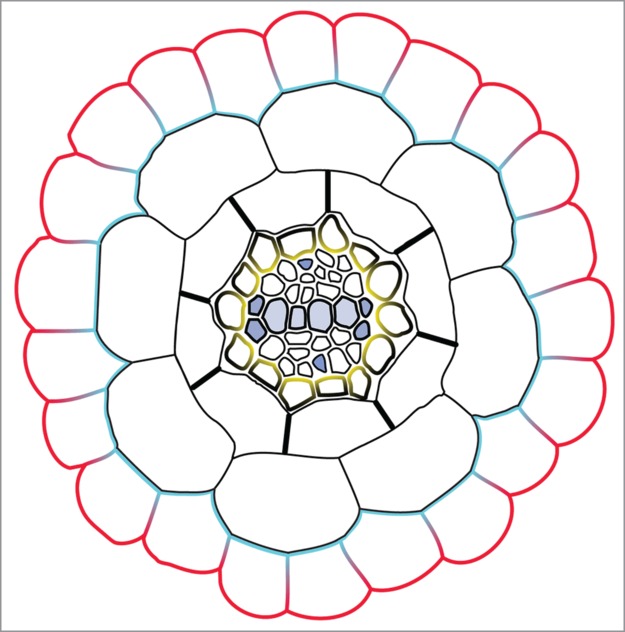
Model of radial transport of iron in plant roots. In plants, iron absorption from the rhizosphere is mediated by IRT1 (red). A putative efflux transporter (blue) is likely located at the inner polar domain of the plasma membrane to allow iron exit from root epidermal cells.
The mechanisms of iron nutrition in plants share similarities with the well-known absorption and transport of iron in mammals. In enterocytes, DIVALENT METAL TRANSPORTER 1 (DMT1) is the major iron influx transporter driving dietary iron absorption from the lumen of the duodenum.15 DMT1 is polarized at the apical domain of the enterocyte.15 Working in concert with DMT1, the efflux transporter FPN1 is localized to the basal plasma membrane domain of enterocytes and is responsible for iron secretion in the bloodstream.16 Interestingly, DMT1 is also regulated by an ubiquitin-dependent mechanism. DMT1 ubiquitination involves WWP2, a HECT-type ubiquitin ligase of the Nedd4 family and Ndfip1/2, that constitute adaptors of this E3 ligase.17,18 In contrast to animals that possess a single cell type to perform the influx of iron from the duodenum and efflux into the bloodstream, plants have several cell layers and probably many transporters at stake between the root epidermis and the vascular tissues. This greatly increases the complexity of iron transport and has greatly hampered our ability to decipher the precise molecular and physiological mechanisms of plant iron nutrition.
Our work opens new field of investigation on the establishment of lateral polarity of plasma membrane proteins and on the physiology of radial transport of nutrients in plant roots. Taking animals as a source of inspiration may be motivating but plant specificities, including numerous cell types to cross before reaching the vasculature and a polarized epithelia located deeper inside the root, must be obviously considered to fully grasp the mechanisms involved in plant nutrition.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by grants from Marie Curie Action (PCIG-GA-2012–334021) and Agence Nationale de la Recherche (ANR-13-JSV2–0004–01) to G.V.
References
- 1.Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature 2006; 440:688-91; PMID:16572174; http://dx.doi.org/ 10.1038/nature04590 [DOI] [PubMed] [Google Scholar]
- 2.Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. An efflux transporter of silicon in rice. Nature 2007; 448:209-U12; PMID:17625566; http://dx.doi.org/ 10.1038/nature05964 [DOI] [PubMed] [Google Scholar]
- 3.Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci 2010; 107:5220-5; PMID:20194745; http://dx.doi.org/ 10.1073/pnas.0910744107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci U S A 2000; 97:12356-60; PMID:11035780; http://dx.doi.org/ 10.1073/pnas.210214197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vert G, Briat JF, Curie C. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J 2001; 26:181-9; PMID:11389759; http://dx.doi.org/ 10.1046/j.1365-313x.2001.01018.x [DOI] [PubMed] [Google Scholar]
- 6.Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002; 14:1223-33; PMID:12084823; http://dx.doi.org/ 10.1105/tpc.001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barberon M, Dubeaux G, Kolb C, Isono E, Zelazny E, Vert G. Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc Natl Acad Sci U S A 2014; 111:8293-8; PMID:24843126; http://dx.doi.org/ 10.1073/pnas.1402262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barberon M, Zelazny E, Robert S, Conejero G, Curie C, Friml J, Vert G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci U S A 2011; 108:E450-8; PMID:21628566; http://dx.doi.org/ 10.1073/pnas.1100659108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin LJ, Lo JC, Chen GH, Callis J, Fu H, Yeh KC. IRT1 degradation factor1, a ring E3 ubiquitin ligase, regulates the degradation of iron-regulated transporter1 in Arabidopsis. Plant Cell 2013; 25:3039-51; PMID:23995086; http://dx.doi.org/ 10.1105/tpc.113.115212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelazny E, Barberon M, Curie C, Vert G. Ubiquitination of transporters at the forefront of plant nutrition. Plant Signal Behav 2011; 6:1597-9; PMID:21918375; http://dx.doi.org/ 10.4161/psb.6.10.17134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberon M, Dubeaux G, Kolb C, Isono E, Zelazny E, Vert G. Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis. Proc Natl Acad Sci U S A 2014; 111(22):8293-8; PMID:24843126; http://dx.doi.org/ 10.1073/pnas.1402262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrissey J, Baxter IR, Lee J, Li L, Lahner B, Grotz N, Kaplan J, Salt DE, Guerinot ML. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 2009; 21:3326-38; PMID:19861554; http://dx.doi.org/ 10.1105/tpc.109.069401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duckett C, Oparka K, Prior D, Dolam L, Roberts K. Dye-coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development 1994; 120:3247-55 [Google Scholar]
- 14.Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 2012; 63:131-52; PMID:22404471; http://dx.doi.org/ 10.1146/annurev-arplant-042811-105522 [DOI] [PubMed] [Google Scholar]
- 15.Anderson GJ, Vulpe CD. Mammalian iron transport. Cell Mol Life Sci 2009; 66:3241-61; PMID:19484405; http://dx.doi.org/ 10.1007/s00018-009-0051-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, et al.. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000; 403:776-81; PMID:10693807; http://dx.doi.org/ 10.1038/35001596 [DOI] [PubMed] [Google Scholar]
- 17.Foot NJ, Dalton HE, Shearwin-Whyatt LM, Dorstyn L, Tan SS, Yang B, Kumar S. Regulation of the divalent metal ion transporter DMT1 and iron homeostasis by a ubiquitin-dependent mechanism involving Ndfips and WWP2. Blood 2008; 112:4268-75; PMID:18776082; http://dx.doi.org/ 10.1182/blood-2008-04-150953 [DOI] [PubMed] [Google Scholar]
- 18.Howitt J, Putz U, Lackovic J, Doan A, Dorstyn L, Cheng H, Yang B, Chan-Ling T, Silke J, Kumar S, et al.. Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons. Proc Natl Acad Sci U S A 2009; 106:15489-94; PMID:19706893; http://dx.doi.org/ 10.1073/pnas.0904880106 [DOI] [PMC free article] [PubMed] [Google Scholar]


