Abstract
Organisms exposed to environmental stressors respond by rapidly synthesising a suite of highly conserved proteins called heat shock proteins (HSPs). Environmental stress can also enhance and/or block memory formation, with long-term memory formation requiring gene activation and protein synthesis. Thermal stress in the pond snail Lymnaea stagnalis can enhance memory formation, and, in this study, the effect of thermal stress on HSP gene expression in the nervous system was investigated. Time-related expression profiles for HSP40 and HSP70 indicated rapid (<30 min) induction for both transcripts. For HSP40, induction was <20 fold relative to control and expression returned to control levels within 8 h, whereas HSP70 induction was >100 fold and expression did not return to control levels within 8 h.
Keywords: environmental stress, gene expression, learning, long-term memory, pond snail
Abbreviations
- LTM
long-term memory
- HSP
heat shock protein
- STM
short-term memory
Organisms exposed to different environmental stressors, including temperature changes, trace metals and ultraviolet light, respond by rapidly synthesising a suite of highly conserved proteins called heat shock or stress proteins.1,2 Under stressful conditions, such as extreme temperature, some proteins lose their higher order structures and related functions, and HSPs are synthesized to promote refolding of these denatured proteins.3,4 Heat shock proteins (HSPs) work as molecular chaperones to protect the organism under stressful conditions and repair stress-damaged proteins to enable normal functions to continue.4-6 HSP40 and HSP70 function as co-chaperones and are typically involved in folding, assembly and transport of proteins, working together to minimize protein aggregation.7,8
Temperature stress can influence many biological processes, including the formation of memory. Memory formation is a vital process that enables an individual to adapt its behavior to current or future conditions.9 Short-term memory (STM), lasting seconds to minutes, is a result of functional changes in pre-existing synapses.10 Whereas, long-term memory (LTM), lasting hours to days, involves gene activation and the synthesis of new proteins.10-12 Memory formation is dynamic,9 and environmental stressors can change the way an animal is able to learn and form memory, either by enhancing or blocking memory formation, depending on the nature of the stress and timing relative to the learning period.13
The great pond snail Lymnaea stagnalis is widely used as a model system to study learning and memory because of its relatively simple neuronal system and easily recordable set of behaviors that can be altered through training.13,14 A range of environmental stressors have been shown to both enhance and reduce the ability of L. stagnalis to learn and form memories.15 In particular, stress associated with thermal stimulus has previously been shown to enhance memory formation in L. stagnalis.9 L. stagnalis lives in shallow, often stagnant, bodies of water that can be exposed to a range of temperature fluctuations depending on weather conditions.9 The stress associated with sudden 1 h duration exposures to 30°C pond water was found to enhance memory formation.9 Here, we investigate time-course gene expression profiles of HSP40 and HSP70 in the central nervous system of L. stagnalis. Activation of these heat shock proteins may be involved in the enhancement of LTM formation.
To examine whether thermal stress induces expression of HSP40 and HSP70 in L. stagnalis, we subjected a group of snails to a thermally stressful condition (30°C for 1 h) and sampled central nervous system tissue for gene expression analysis at 6 time intervals following the termination of thermal stress (0 h, 0.5 h, 1 h, 2 h, 4 h, 8 h).
Both HSP40 and HSP70 were rapidly induced in the central nervous system within 30 min of the end of thermal stress (Fig. 1). HSP40 reached maximum induction before HSP70, between 1 and 2 h following termination of thermal stress (Fig. 1A). The induction of HSP70 was greater than that of HSP40, with over 4 times the relative fold change (Fig. 1). HSP70 expression began to decline approximately 4 h following the end of thermal stress (Fig. 1B). A critical exponential curve applied to the change in gene expression over time was a good fit to the data for HSP40 (R2 = 0.99, p = 0.0202; Fig. 1A) but fit less well for HSP70 (R2 = 0.88, p = 0.1767; Fig. 1B).
Figure 1.
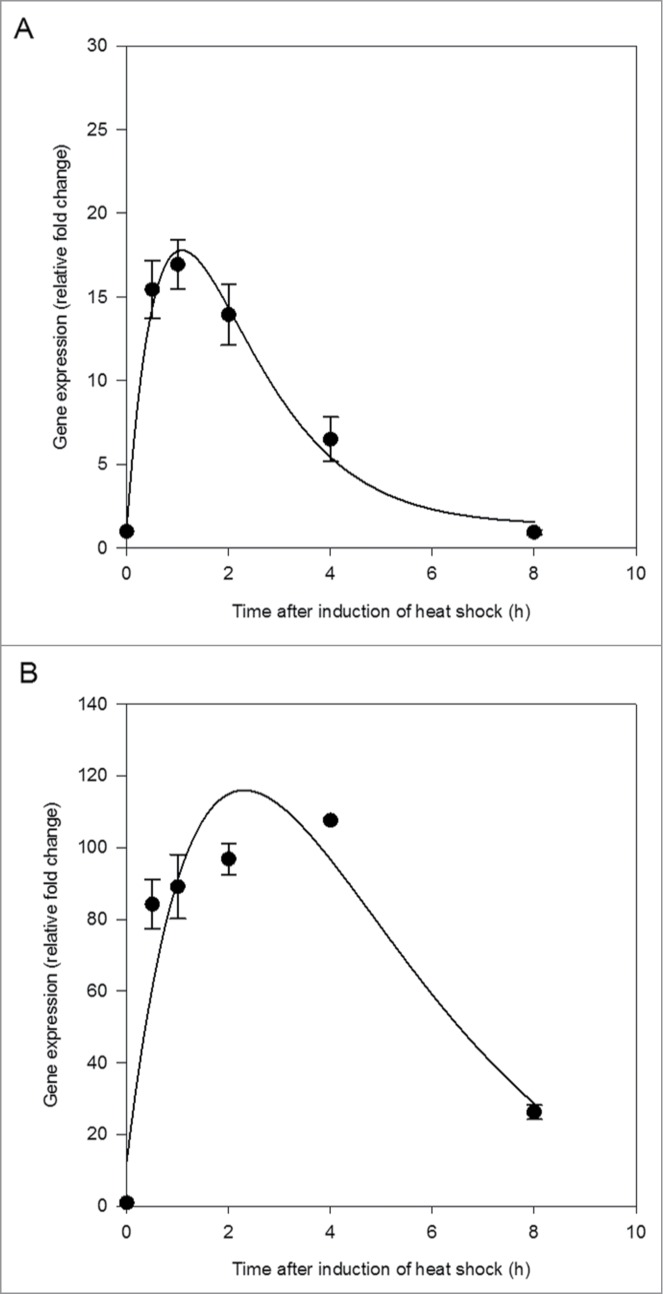
Heat shock protein 40 (HSP40) expression (A) and heat shock protein 70 (HSP70) expression (B) in the central nervous system of Lymnaea stagnalis following exposure to thermal stress. Data are means ± SE from replicate samples at each time point. Critical exponential curve fitted to the data (HSP40, R2 = 0.99, p = 0.0202; HSP70, R2 = 0.88, p = 0.1767).
These results demonstrate that exposure to an acute thermal stress of 30°C was sufficient to increase the synthesis of HSPs above constitutive levels in L. stagnalis. The temporal changes in expression of HSPs observed in the current study were similar to those recorded previously in other organisms. Bahrndorff, et al.16 observed a peak in the expression of HSP70 in Orchesella cincta within 2 h of exposure to thermal stress followed by a sharp decrease after 6 h. Here, expression of HSP70 peaked approximately 4 h after the end of thermal stress, followed by a sharp decline (Fig. 1B).
Altered gene activity and protein synthesis are known to be required for LTM formation in L. stagnalis.10,11 Specifically, molluscan insulin-related peptide II and protein kinase C are thought to play a role in the LTM formation of conditioned taste aversion.12,17 The observed increase in the synthesis of HSPs in the central nervous system of L. stagnalis, following exposure to thermal stress, suggests that these proteins may play a role in LTM formation in relation to a thermal stimulus. Thus, further investigation of the role of HSPs in memory formation is warranted.
Materials and Methods
L. stagnalis were subjected to a thermally stressful condition (30°C for 1 h) and central nervous system tissue was sampled at 6 time intervals following the end of the thermal stress (0 h, 0.5 h, 1 h, 2 h, 4 h, 8 h). Immediately upon sampling, snails were euthanized by placing in ice water for 15 min, the central nervous system was dissected and then completely submerged in RNAlater (Qiagen) in a 1.5 mL micro-centrifuge tube. Central nervous system tissue was pooled from 2 snails for each replicate at each time point, thus, there were 3 replicates per time point. Samples were stored at 4°C prior to analysis.
RNA was extracted from the samples using the RNeasy mini kit (Qiagen) following the manufacturer's protocol, with initial sonication (5–10 s), additional tissue disruption with a QiaShredder column (Qiagen), and a 15 min DNase treatment. RNA was eluted in 30 µL of RNase-free water and the concentration and quality of total RNA was determined using a NanoDrop 1000 spectrophotometer (NanoDrop Technologies). Only samples that met quality criteria (260/280 ratio >2.0) were used for further analyses. Samples were stored at −80°C until required. All samples were diluted to 50 ng µL−1 and 400 ng of RNA was used to synthesize cDNA following the manufacturer's protocol for the ImProm-II™ Reverse Transcription System (Promega), with hexanucleotide primers and deoxynucleotide mix (Sigma-Aldrich). cDNA was synthesized under the following conditions: annealing at 25°C for 5 min, extending at 42°C for 60 min, and heat-inactivating transcriptase at 70°C for 15 min (GeneAmp® PCR System, 9700, Applied Biosystems). cDNA was stored at −80°C until q-RT-PCR gene expression analysis.
Heat shock protein primers (Table 1) were selected using Primer Blast (NCBI). The amplicons were designed to span 1 intron junction and were checked to avoid secondary structure, self-annealing sites, complementarity, and potential hairpins using OligoCalc (Northwestern University, USA). Amplicon size was verified on a 2% agarose gel after PCR amplification. Elongation factor 1-alpha primers (Table 1) were taken from van Nierop, et al.18
Table 1.
Lymnaea stagnalis gene specific primers for heat shock protein genes (HSP40 and HSP70) and a housekeeping gene (elongation factor 1-α, ELF-1α). Reference numbers from NCBI, product length in base pairs (bp)
| Gene | Ref. Num. | Forward (5′-3′) | Reverse (5′-3′) | Product (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| HSP40 | DQ278442.1 | ATGTTAAACCTGGATGGAAGGCAGG | GCAGGCACGTTTTGCGGTGTTT | 79 | 58 |
| HSP70 | DQ206432.1 | TGCTGGCCGAAGCGGAGAAG | CCTCAAGCTGGTTCCTGGCCG | 78 | 60 |
| ELF-1α | DQ278441.1 | ACCACAACTGGCCACTTGATC | CCATCTCTTGGGCCTCTTTCT | 85 | 54 |
To conduct quantitative reverse transcriptase PCR (qRT-PCR), lyophilized primers (Eurofins MWG Operon) were reconstituted to 100 µmol with RNase-free water and mixed with SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich) to give a final reaction concentration of 375 nmol in a 20 µL total volume. Fluorescence was detected (StepOne Real-Time PCR System, Applied Biosystems) over 40 cycles with cycling conditions of denaturing at 94°C for 15 s, annealing at 55°C for 1 min and extension at 72°C for 1 min. For analysis, the cycle threshold was set to 25,000 for all qPCR runs. A standard curve of cDNA template (from a known sample) was run on each plate for each gene to allow for within experiment plate normalization. The efficiency of qRT-PCR was calculated (e = 10−1/slope) − 1) from the standard curve for each plate. Only efficiencies between 0.85 and 1.2 were used for further analysis, and comparative quantification, using the efficiency corrected method,19 was used to determine fold-changes in the genes of interest normalized to ELF-1α. The expression of ELF-1α in the central nervous system of L. stagnalis was not affected by thermal stress, thus, the use of ELF-1α as a housekeeping gene was justified.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded in part by the Faculty of Science and Technology, Plymouth University. KL received support from NSERC. We would like to thank Dr. Helena Reinardy for support in the laboratory.
References
- 1.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet 1988; 22:631-677; PMID:2853609; http://dx.doi.org/ 10.1146/annurev.ge.22.120188.003215 [DOI] [PubMed] [Google Scholar]
- 2.Sanders BM. Stress proteins in aquatic organisms - an environmental perspective. Critic Rev Toxicol 1993; 23(1):49-75; PMID:8471160; http://dx.doi.org/ 10.3109/10408449309104074 [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (Hsp70) expression in four limpets of the genus lottia: Interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull 2008; 215(2):173-181; PMID:18840778; http://dx.doi.org/ 10.2307/25470698 [DOI] [PubMed] [Google Scholar]
- 4.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu Rev Physiol 1999; 61:243-282; PMID:10099689; http://dx.doi.org/ 10.1146/annurev.physiol.61.1.243 [DOI] [PubMed] [Google Scholar]
- 5.Sagarin RD, Somero GN. Complex patterns of expression of heat-shock protein 70 across the southern biogeographical ranges of the intertidal mussel Mytilus californianus and snail Nucella ostrina. J Biogeograp 2006; 33(4):622-630; http://dx.doi.org/ 10.1111/j.1365-2699.2005.01403.x [DOI] [Google Scholar]
- 6.Brun NT, Bricelj VM, MacRae TH, Ross NW. Heat shock protein responses in thermally stressed bay scallops, Argopecten irradians, and sea scallops, Placopecten magellanicus. J Exp Marine Biol Ecol 2008; 358(2):151-162; http://dx.doi.org/ 10.1016/j.jembe.2008.02.006 [DOI] [Google Scholar]
- 7.Fink AL. Chaperone-mediated protein folding. Physiol Rev 1999; 79(2):425-449; PMID:10221986 [DOI] [PubMed] [Google Scholar]
- 8.Li CH, Li LY, Liu F, Ning XX, Chen AQ, Zhang LB, Wu HF, Zhao JM. Alternation of Venerupis philippinarum Hsp40 gene expression in response to pathogen challenge and heavy metal exposure. Fish Shellfish Immunol 2011; 30(1):447-450; PMID:21056105; http://dx.doi.org/ 10.1016/j.fsi.2010.10.023 [DOI] [PubMed] [Google Scholar]
- 9.Teskey ML, Lukowiak KS, Riaz H, Dalesman S, Lukowiak K. What's hot: the enhancing effects of thermal stress on long-term memory formation in Lymnaea stagnalis. J Exp Biol 2012; 215(24):4322-4329; PMID:22972889; http://dx.doi.org/ 10.1242/jeb.075960 [DOI] [PubMed] [Google Scholar]
- 10.Sangha S, Morrow R, Smyth K, Cooke R, Lukowiak K. Cooling blocks ITM and LTM formation and preserves memory. Neurobiol Lear Memory 2003; 80(2):130-139; PMID:12932428; http://dx.doi.org/ 10.1016/S1074-7427(03)00065-0 [DOI] [PubMed] [Google Scholar]
- 11.Kandel ER, Pittenger C. The past, the future and the biology of memory storage. Philosophical transactions of the royal society of London. Series B: Biol Sci 1999; 354(1392):2027-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azami S, Wagatsuma A, Sadamoto H, Hatakeyama D, Usami T, Fujie M, Koyanagi R, Azumi K, Fujito Y, Lukowiak K et al.. Altered gene activity correlated with long-term memory formation of conditioned taste aversion in Lymnaea. J Neurosci Res 2006; 84(7):1610-1620; PMID:16941636; http://dx.doi.org/ 10.1002/jnr.21045 [DOI] [PubMed] [Google Scholar]
- 13.Dalesman S, Karnik V, Lukowiak K. Sensory mediation of memory blocking stressors in the pond snail Lymnaea stagnalis. J Exp Biol 2011; 214(15):2528-2533; PMID:21753046; http://dx.doi.org/ 10.1242/jeb.058024 [DOI] [PubMed] [Google Scholar]
- 14.Lukowiak K, Sangha S, Scheibenstock A, Parvez K, McComb C, Rosenegger D, Varshney N, Sadamoto H. A molluscan model system in the search for the engram. J Physiol-Paris 2003; 97(1):69-76; PMID:14706692; http://dx.doi.org/ 10.1016/j.jphysparis.2003.10.008 [DOI] [PubMed] [Google Scholar]
- 15.Lukowiak K, Orr M, de Caigny P, Lukowiak KS, Rosenegger D, Il Han J, Dalesman S. Ecologically relevant stressors modify long-term memory formation in a model system. Behavioural Brain Res 2010; 214(1):18-24; PMID:20478338; http://dx.doi.org/ 10.1016/j.bbr.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 16.Bahrndorff S, Mariën J, Loeschcke V, Ellers J. Dynamics of heat-induced thermal stress resistance and hsp70 expression in the springtail, Orchesella cincta. Funct Eco 2009; 23(2):233-239; http://dx.doi.org/ 10.1111/j.1365-2435.2009.01541.x [DOI] [Google Scholar]
- 17.Takigami S, Sunada H, Lukowiak K, Kuzirian AM, Alkon DL, Sakakibara M. Protein kinase C mediates memory consolidation of taste avoidance conditioning in Lymnaea stagnalis. Neurobiol Lear Memory 2014; 111(0):9-18; PMID:24613854; http://dx.doi.org/ 10.1016/j.nlm.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 18.van Nierop P, Bertrand S, Munno DW, Gouwenberg Y, van Minnen J, Spafford JD, Syed NI, Bertrand D, Smit AB. Identification and functional expression of a family of nicotinic acetylcholine receptor subunits in the central nervous system of the mollusc Lymnaea stagnalis. J Biol Chem 2006; 281(3):1680-1691; PMID:16286458; http://dx.doi.org/ 10.1074/jbc.M508571200 [DOI] [PubMed] [Google Scholar]
- 19.Pfaffl MW. Quantification strategies in real-time PCR In: Bustin SA, editor. A-Z of quantitative PCR. La Jolla, CA, USA: International University Line; 2004. p 26. [Google Scholar]


