Abstract
Keywords: ABA, abscisic acid, cytokinin signaling, desiccation tolerance, ethylene, green algae
The origin of phytohormones has been a puzzling question for decades; however, with the availability of large transcriptomic1 and genomic2 datasets of the early branching streptophytic green alga Klebsormidium this question can be addressed from a fresh perspective. Klebsormidium has recently been examined extensively for physiological and structural reactions to desiccation3-6 or cold temperatures,7 natural factors in soil crust living algae. Ecological influences have been made responsible for fine scaled structuring of genotypes and differentiation of cryptic species.8,9 But how do these organisms sense their changing environments?
In this addendum article, we explore our own data set from a transcriptomic study of severe desiccation stress in Klebsormidium crenluatum.1 The cells were desiccated for 2.5 h under monitored conditions over silicagel at ∼10% relative humidity. The relative water content of the desiccated cells was 6.54 ± 1.89%. For the molecular analysis we established a high-coverage reference transcriptome database which contained 24,183 contigs with a mean sequence length of 1,327b (N50 = 1,462). This database was used to evaluate which phytohormone pathways are present in Klebsormidium and might be involved for cellular response to desiccation stress. Desiccation is well studied in embryophytes, and the cytokinin, ethylene and abscisic acid (ABA) signaling pathways have been implicated in stress response. Given these facts we wondered whether ABA and/or ethylene and/or cytokinin signaling are involved in desiccation tolerance in K. crenulatum, and searched for the most similar transcripts to the K. flaccidum proteins reported to be putative orthologues of these 3 (ABA, cytokinin, ethylene) plant phytohormones signaling components. Here, we propose that at least 3 major signaling pathways for land plant hormone response are functional in Klebsormidiophyceae. Based on our transcriptomic data of severe desiccation stress auxin mediated signal transduction seem to be missing and in the case of jasmonic acid (JA) only the receptor JAR1 was found, but the further steps were absent in the analyzed K. crenulatum transcriptome.
Phytohormone Signaling in Klebsormidium and Other Streptophyte Green Algae
Using the KEGG pathway reconstruction tool we found almost complete pathways for cytokinin signaling, ABA signaling and ethylene response in K. crenulatum. Meanwhile the draft sequence of the K. flaccidum genome has become available and detected the genes for (nearly) complete signaling pathways for auxin, ABA, cytokinin, salicylic acid and JA.2 The physical presence of the auxin indole-3-acetic acid, ABA, the cytokinin isopentenyladenine, JA, and salicylic acid in K. flaccidum was also confirmed by mass spectrometry. While the ethylene signaling pathway was represented in the genome, no attempt was made to confirm its physical presence in K. flaccidum.2 identified counterparts for the hormone receptors ABP1, GTG, CRE1 and ETR for auxin, ABA, cytokinin and ethylene respectively. Interestingly,10 reported the conservation of the ethylene signaling pathway between conjugating green algae Spirogyra (representing the sister group to land plants) and embryophytes. No evidence for gibbelerinic acid signaling nor the recently described ABA receptor (PYR) and the EIN2 protein essential for the ethylene signaling pathway, were found in the Klebsormidium genome.2
ABA and Stress Response in Klebsormidium
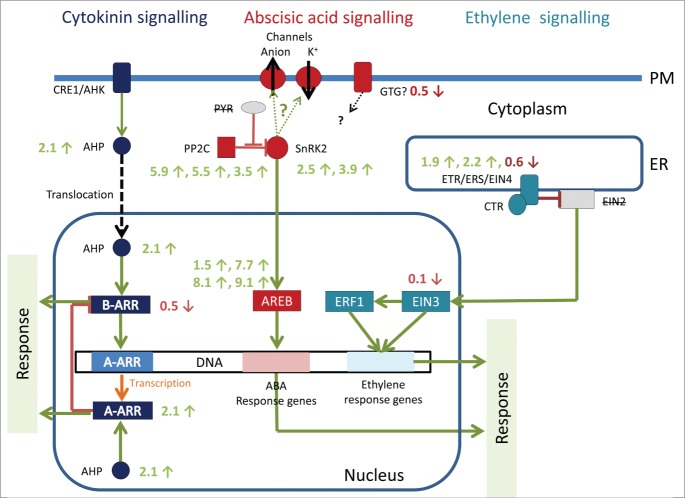
The ABA response is well known in abiotic stress reactions (e.g.11) often in interplay with cytokinins.12 The Klebsormidium genome contained a putative ortholog to the GTG protein,2 which has been proposed as ABA receptor. However recent work has cast doubts, whether this protein really serves as an ABA receptor. Upon desiccation stress the transcript level for the GTG ortholog was decreased by 0.5 times. In contrast the transcripts for the ABA signaling components PP2C as well as SnRK2 were significantly increased, as well as the nuclear AREB protein (Table 1, Fig. 1). However, no change in cold tolerance by ABA application (1–100 µm) was found experimentally in K. flaccidum by.7 The same discrepancy was earlier also observed in the chlorophyceae Stigeoclonium cf. tenue, where ABA had been detected, but exogenously applied ABA caused only a slight growth reduction and promoted senescence in some cases.13 Experimentally ABA was measured in numerous chlorophytes as well as in Chara foetida.13
Table 1.
summarizes our results. We detected transcripts for all reported proteins of the 3 signaling pathways. For a few proteins more than one transcript was observed (Table 1), the significance of these additional transcripts (that could be explained by alternative splicing or additional isoforms) remains unclear at the moment. Many Klebsormidium crenulatum transcripts for components of the plant signaling pathway show significant changes during desiccation with transcripts similar to components of the ABA signaling pathway upregulated up to 9-fold
| Pathway/Gene | K. flaccidum gene1 | Closest A.thaliana homologue1 | Most similar K. crenulatum Transcript | Differential expressed (control cells vs. desiccation: xfold change/padj) | Additional K. crenulatum transcripts |
|---|---|---|---|---|---|
| Cytokinin signaling | |||||
| CRE1,AHK | Kfl00234_0040 | AT1G27320 | — | — | |
| Kfl00990_0020 | — | — | — | ||
| Kfl00564_0090 | UN031179 | 0.7/0.08 | 0 | ||
| Kfl00331_0060 | — | — | — | ||
| AHP | Kfl00529_0020 | AT1G03430 | UN038987 | 2.1/0.0002 | 0 |
| A-ARR | Kfl00066_0050 | AT3G57040 | UN038317 | 2.1/5.0e-06 | 0 |
| B-ARR | Kfl00133_0040 | AT4G16110 | UN038143 | 0.5/6.2e-05 | 0 |
| Abscisic acid signaling | |||||
| GTG | Kfl00326_0060 | AT4G27630 | UN039964 | ||
| PYR | No Blast Hit | — | |||
| PP2C | Kfl00724_0050 | AT1G72770 | UN029018 | 5.9/1.7e-26 | 2 |
| AnRK2 | Kfl00097_0360 | AT4G33950 | UN034191 | 2.5/1.5e-09 | 1 |
| AREB | Kfl00015_0390 | AT3G56850 | UN032996 | 1.5/0.02 | 0 |
| Kfl00100_0080 | UN039695 | 7.8/9.8e-30 | 2 | ||
| Ethylene signaling | |||||
| ETR/ERS/EIN4 | Kfl00196_0020 | AT1G66340 | UN019655 | 0.6/0.001 | 1 |
| Kfl00385_0110 | UN001034 | 1.9/4.1e-05 | 1 | ||
| Kfl00524_0070 | UN023393 | 2.2/5.9e-06 | 1 | ||
| CTR1 | Kfl00622_0040 | AT5G03730 | UN037105 | 0.6/0.02 | 0 |
| EIN2 | No definite counterpart | ||||
| EBF | Kfl00213_0020 | AT2G25490 | UN037105 | 0.7/0.18 | 0 |
| EIN3 | Kfl00014_0150 | AT3G00770 | UN042380 | 0.1/6.7e-12 | 0 |
5.
Figure 1.
The typical plant signaling pathways for 3 phytohormones with the observed up- and down-regulation upon desiccation stress in K. crenulatum mapped onto the pathway. The observed up- and downregulation for the 3 pathways is different (up-or down-regulation, fold change in expression level). While these changes are interesting themselves, they do not represent direct evidence for phytohormone signaling in response to desiccation stress. Most interesting in this respect is the observed up-regulation of the single putative ortholog of type A ARR response regulators, which expression is under direct control of the cytokinin signaling pathway, suggesting that desiccation leads indeed to an activation of cytokinin signaling pathway in Klebsormidium crenulatum.
Recently, orhologues of the Arabidopis protein kinase OST1 (SnRK2 family protein, regulating stomata closure in guard cells) and the S-type anion channel gene SLAC1 (required for stomata function) were found in Klebsormidium nitens. Interestingly, KnOST1 was able to activate AtSlAC1,14 while neither algal nor embyrophyte OST1 were able to activate the SLAC1 protein from K. nitens. These data suggest that while the intracellular signaling pathway seems to be present early in plant evolution, the target as well as the receptor might have changed.
Cytokinin Signaling
Putative orthologues for cytokinin receptors CRE1/AHK in the plasma membrane, AHP in the cytoplasm as well as the transcription factor A-ARP were all up-regulated upon desiccation stress in K. cernulatum (Fig. 1, Table 1), while B-ARP were down-regulated. Most interesting is the up-regulation of A-ARP, which are under control of the cytokinin signaling pathway in plants (see Fig. 1), which provides direct evidence for the involvement of cytokinin signaling in the cellular response to desiccation stress.
In K. flaccidum the cytokinin isopentenyladenine was identified2 although at low concentrations.15 detected cytokinin at very low concentrations (0.29 nmol g1 DW) in K. flaccidum. Isopentenyladenine was previously chemically identified in the streptophyte green alga Chara globularis by combined GC/MS and appears to be the most common cytokinin in lower green plants including the moss Physcomitrella.16 No evidence for zeatin, the most common cytokinin of flowering plants has been found in green algae so far.
Highly Conserved Ethylene Response
Ethylene appears to be a highly conserved plant hormone for the last 450 mio years10 and the evolution of the ethylene receptor family in relation to land plant evolution has recently been summarized.17 EST sequences for subfamily I ethylene receptors of K. flaccidum are available18 and homologues to both plant ethylene receptors subfamilies are encoded in the Klebsormidium genome.18 However, it is currently not clear how the ethylene signal is transmitted in Klebsormidium as the EIN2 protein has not been found in the genome of Klebsormidium.2 In K. crenulatum an upregulation of ETR and ERS transcripts (ethylene receptor subfamily 1) were found upon desiccation stress (Fig. 1, Table 1). Interestingly, the EIN4 homolog (ethylene receptor subfamily 2) was downregulated, suggesting that in Klebsormidium the 2 receptor subfamilies serve different functions. All other components of the ethylene signaling machinery showed no or only slight changes in the expression level.
In plants the response to abiotic stress is modulated by changes in ethylene receptor transcript levels,19 suggesting that the observed changes in ethylene receptor transcripts levels serve similar function in K. crenulatum.
Conclusions and Outlook
In terrestrialization events, mechanisms to sense the external environmental situation that might fluctuate and allow cells to react immediately are crucial. Here, we demonstrate that the abiotic stress of severe desiccation regulates the expression of 3 classical phytohormone pathways in the early branching streptophyte algae K. crenulatum: Cytokinin, ABA and ethylene signaling. These data further support that Klebsormidiophyceae have the hormonal prerequisites for living on land. The interplay between the different pathways needs further examination, as well as possible roles of e.g., the jasmonic acid receptor JAR1, which is present and upregulated – whereas the further pathway is completely missing. Similar is the case of the salicylic pathway where only the transcription factor TGA is found upregulated in our transcripts. Taken together, we are convinced that plant phytohormone research in streptohyte algae will receive a renewed interest over the next years. Important signaling pathways were already established early in the evolution of plants, which might have been crucial for the colonization of land.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was supported by FWF project P24242-B16 to A.H.
References
- 1.Holzinger A, Kaplan F, Blaas K, Zechmann B, Komsic-Buchmann K, Becker B. Transcriptomics of desiccation tolerance in the streptophyte green alga Klebsormidium reveal a land plant-like defense reaction. PLoS One 2014; 9:e110630; PMID:25340847; http://dx.doi.org/ 10.1371/journal.pone.0110630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hori K, Maruyama F, Fujisawa T, Togashi T, Yamamoto N, Seo M, Sato S, Yamada T, Mori H, Tajima N, et al.. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nature Comm 2014; 5:3978; PMID:24865297; http://dx.doi.org/ 10.1038/ncomms4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsten U, Lütz C, Holzinger A. Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Charophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. J Phycol 2010. 46:1187-97; http://dx.doi.org/ 10.1111/j.1529-8817.2010.00921.x [DOI] [PubMed] [Google Scholar]
- 4.Holzinger A, Lütz C, Karsten U. Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. J Phycol 2011; 47:591-602; http://dx.doi.org/ 10.1111/j.1529-8817.2011.00980.x [DOI] [PubMed] [Google Scholar]
- 5.Karsten U, Holzinger A. Light, temperature and desiccation effects on photosynthetic activity and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microb Ecol 2012; 63:51-63; PMID:21811791; http://dx.doi.org/ 10.1007/s00248-011-9924-6 [DOI] [PubMed] [Google Scholar]
- 6.Holzinger A, Karsten U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front Plant Sci 2013; 4:327; PMID:23986769; http://dx.doi.org/ 10.3389/fpls.2013.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagao M, Matsui K, Uemura M. Klebsormidium flaccidum, a charophycean green alga, exhibits cold acclimation that is closely associated with compatible solute accumulation and ultrastructural changes. Plant Cell Environ 2008; 31:872-85; PMID:18315534; http://dx.doi.org/ 10.1111/j.1365-3040.2008.01804.x [DOI] [PubMed] [Google Scholar]
- 8.Škaloud P, Rindi F. Ecological differentiation of cryptic species within a asexual protist morphospecies: A case study of filamentous green alga Klebsormidium (Streptophyta). Eukar Microbiol 2013; 60:350-62; PMID:23648118; http://dx.doi.org/ 10.1111/jeu.12040 [DOI] [PubMed] [Google Scholar]
- 9.Ryšánek D, Hrčková K, Škaloud P. Global ubiquity and local endemism of free-living terrestrial protists: phylogeographic assessment of the streptophyte alga Klebsormidium. Environ Microbiol 2015; 17:689-98; PMID:24803402; http://dx.doi.org/ 10.1111/1462-2920.12501 [DOI] [PubMed] [Google Scholar]
- 10.Ju C, Van de Poel B, Cooper ED, Thierer JH, Gibbons TR, Delwiche CF, Chang C. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nature Plants 2015; 1:1-6; http://dx.doi.org/ 10.1038/nplants.2014.4 [DOI] [PubMed] [Google Scholar]
- 11.Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Gen Dev 2010; 24:1695-708; PMID:20713515; http://dx.doi.org/ 10.1101/gad.1953910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O´Brien JA, Benková E. Cytokinin cross-talking during biotic and abiotic responses. Front Plant Sci 2015; 4:451; PMID:24312105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tietz A, Ruttkowski U, Kohler R, Kasprik W. Further investigations on the occurrence and the effects of abscisic acid in algae. Biochem Physiol Pfl 1988; 184:259-66; http://dx.doi.org/ 10.1016/S0015-3796(89)80011-3 [DOI] [Google Scholar]
- 14.Lind C, Dreyer I, Lopez-Sanjurjo EJ, Mayer K, Ishizaki K, Kohchi T, Lang D, Zhao Y, Kreuzer I, Al-Rasheid KAS, et al.. Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Curr Biol 2015; 25:928-35; PMID:25802151; http://dx.doi.org/ 10.1016/j.cub.2015.01.067 [DOI] [PubMed] [Google Scholar]
- 15.Stirk WA, Ördög V, Novák O, Rolcik J, Strnad M, Bálint P, Staden J. Auxin and cytokinin relationships in 24 microalgal strains. J Phycol 2013; 49:459-67; http://dx.doi.org/ 10.1111/jpy.12061 [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Yamane H, Takahashi N, Chapman DJ, Phinney BO. Identification of a cytokinin in the green alga Chara globularis. Phytochemistry 1989; 28:337-8; http://dx.doi.org/ 10.1016/0031-9422(89)80007-X [DOI] [Google Scholar]
- 17.Gallie DR. Appearance and elaboration of the ethylene receptor during land plant evolution. Plant Mol Biol 2015; 87:521-39; PMID:25682121; http://dx.doi.org/ 10.1007/s11103-015-0296-z [DOI] [PubMed] [Google Scholar]
- 18.Yasumura Y, Pierik R, Fricker MD, Voesenek LACJ, Haberd P. Studies of Physcomitrella patens reveal that ethylene-mediated submergence response arose relatively early in land-plant evolution. Plant J 2012; 72:947-59; PMID:23046428 [DOI] [PubMed] [Google Scholar]
- 19.Zhao XC, Schaller GE. Effect of salt and osmotic stress upon expression of the ethylene receptor ETR1 in Arabidopsis thaliana. FEBS Lett 2004; 562:189-92; PMID:15044023; http://dx.doi.org/ 10.1016/S0014-5793(04)00238-8 [DOI] [PubMed] [Google Scholar]