Abstract
Filopodia are active tubular structures protruding from the cell surface which allow the cell to sense and interact with the surrounding environment through repetitive elongation-retraction cycles. The mechanical behavior of filopodia has been studied by measuring the traction forces exerted on external substrates.1 These studies have revealed that internal actin flow can transduce a force across the cell surface through transmembrane linkers like integrins. In addition to the elongation-retraction behavior filopodia also exhibit a buckling and rotational behavior. Filopodial buckling in conjunction with rotation enables the cell to explore a much larger 3-dimensional space and allows for more complex, and possibly stronger, interactions with the external environment.2 Here we focus on how bending of the filopodial actin dynamically correlates with pulling on an optically trapped microsphere which acts like an external substrate attached to the filopodial tip. There is a clear correlation between presence of actin near the tip and exertion of a traction force, thus demonstrating that the traction force is transduced along the actin shaft inside the filopodium. By extending a filopodium and holding it while measuring the cellular response, we also monitor and analyze the waiting times for the first buckle observed in the fluorescently labeled actin shaft.
Keywords: filopodia, actin dynamics, helical buckling, optical trapping, rotation, traction
Actin rich tubular structures like invadopodia, filopodia and podosomes are frequently observed on the cell surface of a number of different cell types. Common to these structures is that they facilitate direct intercellular communication as well as cellular interactions with the extracellular matrix. Filopodia arepresent ina large number of cell types and are highly dynamic. They are found at the tip of neurons and are responsible for steering the neuronal growth by sensing and responding to chemical cues from the environment.3,4 Embryonic cells use filopodia for long range intercellular communication5as well as in morphogenetic processes like compaction of the early embryo which is an essential step in mammalian development.6 Filopodia also play essential roles in fibroblasts and endothelial cells during intercellular interactions and cellular migration.7 Cancer cells employ filopodia or filopodia-like structures during migration to facilitate efficient invasion of external tissue.8,9 Finally, an interesting example of filopodia function can be found in macrophages which have been shown to exhibit complex interactions with foreign objects like bacteria adherent on flat substrates10 or 3 dimensionally trapped dielectric particles.11
Filopodial rotation and bending are frequently observed,2,3,12 but these phenomena are poorly studied and their biological functions are not well understood. Rotational behavior has been detected in macrophages, neuronal cells and HEK293 cells, but it is unclear whether this rotational behavior is a generic property of filopodia in all cell types. Detection of rotational behavior is easier when the filopodia exhibit a kink large enough to be visible, e.g., in a confocal microscope and the studies which have reported rotational behavior of filopodia have also reported simultaneous buckling. Therefore, rotation of the actin shaft (the visible part of the actin) within the filopodial membrane tube could well be a generic property of all filopodia which first becomes visible by microscopy during buckling. However, buckling and rotation were shown to be coupled, in HEK293 cells, since rotation of the internal actin shaft induced buckling through a friction mechanism between the actin shaft and the filopodial membrane.2 By extending filopodia a few micrometers, and simultaneously visualizing the actin, we show here that frequent buckles appear on the actin shaft as shown in Figure 1A. Initially, the actin appears straight, but after a certain waiting time (see Fig. 1B) buckles start to appear as shown in Fig. 1A. Extension of the filopodial membrane is accompanied with extension and retraction of the visible part of the actin.2,13 Extension of the actin to the tip region results in transient pulling events which are frequently observed in such experiments.2,13,14 We visualized the fluorescently labeled actin inside filopodia while simultaneously measuring the force exerted by the cell on the optically trapped bead attached to the filopodial membrane. The combined optical tweezers and confocal microscope used for this study is described in.15 Quantification of the intensity of actin at the tip region revealed a clear correlation between the presence of actin and the generation of a force on the trapped particle. By calibrating the optical trap2 we were able to quantify the force while also visualizing and quantifying the actin signal in a region which includes the filopodial tip. As shown in Figure 1C, the force (blue curve) correlates strongly with the actin intensity (green curve) at the times when the force exhibits abrupt changes (see arrows). We also note that occasional non-correlative behavior can be seen, this seems mainly to occur when the actin is slowly polymerizing toward the tip.
Figure 1.
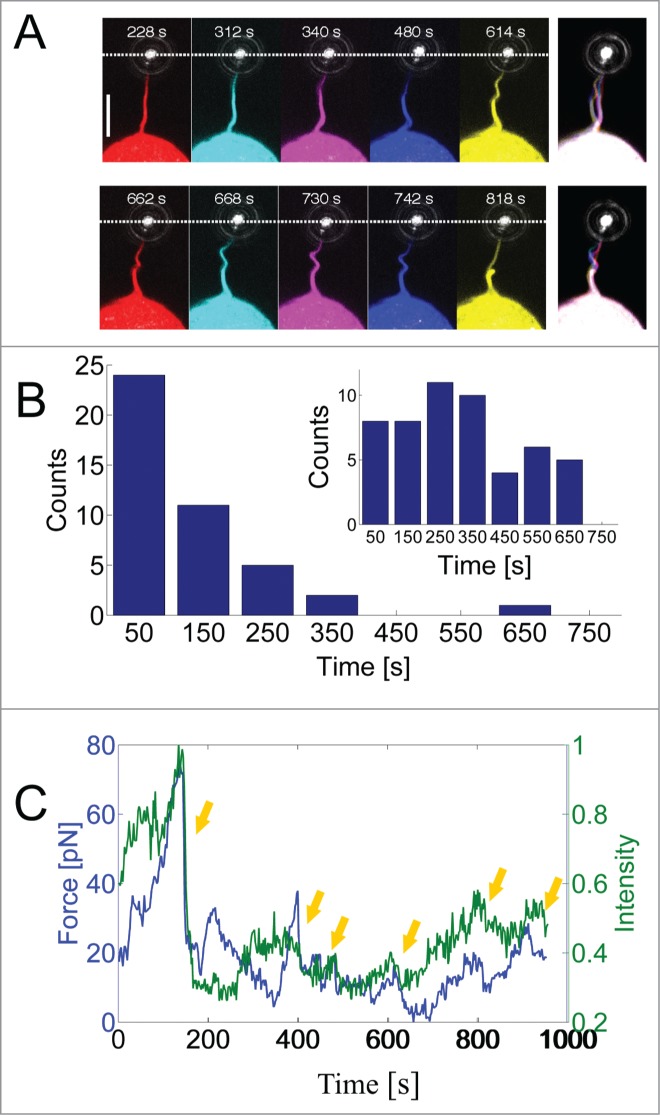
Dynamics of actin inside a filopodium extended from a HEK293 cell. (A) Fluorescent images of actin within a filopodium extended and held by an optically trapped bead. The snap shots are taken at different times during one experiment where the actin exhibits different degrees of bending and pulling. Pulling was detected as a downward movement of the bead (toward the cell and away from the optical trap). The last image to the right is an overlay of all the other images. Scale bar, 5 μm. (B) Statistics of the waiting times for the first buckle to occur on filopodia that are extended by the optical trap, N = 43 buckles. The inset shows the waiting times after which additional buckles occurred (second, third, …), N = 52 buckles. Up to 6 buckles could be observed in a single filopodium during a measurement period of ∼10 min. Only clearly resolved and isolated buckles were counted and the uncertainty in determining the buckling time from the image series was ∼3 s. (C) Correlation between the force on the trapped bead and the actin intensity at the tip region. Rapid drops in the force (blue curve) were associated with rapid decreases in the actin intensity (green curve). Yellow arrows denote correlative events at which the force drops rapidly with associated decrease in actin signal.
Interestingly, there is a correlation between buckling and pulling, this is visible in Figure 1A. At t = 340 s the cell pulled on the trapped bead which consequently moved toward the cell and at t = 480 s we observe a relaxation of the force (bead moves away from the cell) with a concomitant increase of the curvature of the actin shaft (increased buckling). This strongly suggests that bending is not caused by compressive buckling which has been proposed as a mechanism for helical buckling in free filopodia experiencing an axial compressive tension from the membrane.16,17 We held the membrane with the optically trapped bead and thereby exerted axial tension to the actin shaft just prior to an increase in buckling. This intrinsic tendency of the filopodium to buckle against an external load indicates that the structure of the filopodium is not just a straight rod composed of a parallel bundle of actin filaments. Interestingly, and in accordance with this, recent cryogenic electron microscopy images have revealed discontinuous structures of actin in filopodia18,19 which could more easily favor a bent structure and lower the bending energy. Actin filaments in HEK293 cells are cross-linked by fascin which provides rigidity to the structure, but also flexibility through its dynamic association with the actin filaments. The off-rate of fascin has been measured to be koff = 0.12 s−1and has been shown to allow for a gradual and slow deformation of the structure to occur by dynamic cross-linking of the actin filaments.20 Moreover, actin severing proteins like cofilin5,18 and gelsolin21 have been associated with destabilization of retracting filopodia and might play a role in the bending of actin. In Ref. 2 it was shown how a physical mechanism could contribute to buckling of the actin. The mechanism is based on torsional twist accumulated within the actin structure during rotation of the actin within the filopodial membrane. Friction between the membrane and the actin (from, e.g., transmembrane proteins linked to actin) causes actin to twist which subsequently is converted into actin buckling. Moreover, this mechanism can explain the traction which can occur simultaneously with actin buckling.2
The waiting time before onset of the first buckle and the periodicity of the buckles in the actin shaft showed great variations as presented in Figure 1B. We observed the first buckle on a single shaft typically within ca. 100 s after extending the filopodium to ∼10 μm, only about 15% of the first buckles appeared later. Additional buckles on a single shaft were also frequently observed as shown in the inset of Fig. 1B. Up to 6 buckles could be detected on the same shaft while keeping the filopodium extended for ∼10 min. Filopodia which are extended by an optically trapped bead at their tip are prevented from natural retraction and integration into the lamellipodium as seen in free filopodia. Since free filopodia are frequently observed to buckle just prior to integration into the lamellipodium we anticipate that holding the filopodium extended will force the cell to perform several protrusion-retraction cycles for the same filopodium and hence several buckles occur at multiple times during the experiment.
We note that buckling and rotation were observed regardless of the labeling method; rotation and buckling were observed both when the actin was labeled with GFP-Utrophin or Lifeact-GFP. Also, rotation and buckling could be observed with unlabeled actin in which the membrane was fluorescently labeled (via membrane fluorophores or transmembrane proteins tagged with GFP).
Filopodia exhibit extremely diverse mechanical behavior by combining physical and biochemical mechanisms to facilitate complex interactions with external objects. However, much is still to learn about how physical forces like pulling, rotation and bending act in concert while being controlled or affected by molecular factors like actin binding proteins, transmembrane signaling proteins, or myosin motors. Direct visualization of cellular proteins like actin with parallel detection of the force, possibly in connection to genetic knock-out of essential components, will provide an important strategy for understanding the biomechanical processes underlying filopodial dynamics. Such future efforts will result in a deeper insight into the interplay between essential molecular components that are important for filopodial dynamics.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We acknowledge financial support from the Lundbeck Foundation, the Villum Kann Rasmussen Foundation, and the Danish National Research Councils (grant DFF – 4002-00099).
References
- 1. Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science 2008; 322:1687-91; PMID:19074349 [DOI] [PubMed] [Google Scholar]
- 2. Leijnse N, Oddershede LB, Bendix PM. Helical buckling of actin inside filopodia generates traction. Proc Natl Acad Sci USA 2015; 112:136-41; PMID:25535347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamada A, Kawase S, Murakami F, Kamiguchi H. Autonomous right-screw rotation of growth cone filopodia drives neurite turning. J Cell Biol 2010; 188:429-41; PMID:20123994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Bio 2009; 10:332-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 2013; 497:628-32; PMID:23624372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fierro-Gonzalez JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol 2013; 15:1424-33; PMID:24270889 [DOI] [PubMed] [Google Scholar]
- 7. Bornschlogl T. How filopodia pull: what we know about the mechanics and dynamics of filopodia. Cytoskeleton 2013; 70:590-603; PMID:23959922 [DOI] [PubMed] [Google Scholar]
- 8. Arjonen A, Kaukonen R, Ivaska J. Filopodia and adhesion in cancer cell motility. Cell Adh Migr 2011; 5:421-30; PMID:21975551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stylli SS, Kaye AH, Lock P. Invadopodia: At the cutting edge of tumour invasion. J Clin Neurosci 2008; 15:725-37; PMID:18468901 [DOI] [PubMed] [Google Scholar]
- 10. Moller J, Luhmann T, Chabria M, Hall H, Vogel V. Macrophages lift off surface-bound bacteria using a filopodium-lamellipodium hook-and-shovel mechanism. Sci Rep 2013; 3: 2884; PMID:24097079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kress H, Stelzer EHK, Holzer D, Buss F, Griffiths G, Rohrbach A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc Natl Acad Sci USA 2007; 104:11633-8; PMID:17620618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zidovska A, Sackmann E. On the mechanical stabilization of filopodia. Biophys J 2011; 100:1428-37; PMID:21402024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bornschlogl T, Romero S, Vestergaard CL, Joanny JF, Nhieu GTV, Bassereau P. Filopodial retraction force is generated by cortical actin dynamics and controlled by reversible tethering at the tip. Proc Natl Acad Sci USA 2013; 110:18928-33; PMID:24198333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farrell B, Qian F, Kolomeisky A, Anvari B, Brownell WE. Measuring forces at the leading edge: a force assay for cell motility. Integr Biol-Uk 2013; 5:204-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson AC, Reihani N, Oddershede LB. Combining confocal microscopy with precise force-measuring optical tweezers. SPIE Proc 2006; 6326:28-38 [Google Scholar]
- 16. Daniels DR, Turner MS. Islands of conformational stability for filopodia. PloS ONE 2013; 8:e59010; PMID:23555612; http://dx.doi.org/ 10.1371/journal.pone.0059010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pronk S, Geissler PL, Fletcher DA. Limits of filopodium stability. Phys Rev Letts 2008; 100:25; http://dx.doi.org/ 10.1103/PhysRevLett.100.258102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Breitsprecher D, Koestler SA, Chizhov I, Nemethova M, Mueller J, Goode BL, Small JV, Rottner K, Faix J. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J Cell Sci 2011; 124:3305-18; PMID:21940796; http://dx.doi.org/ 10.1242/jcs.086934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben-Harush K, Maimon T, Patla I, Villa E, Medalia O. Visualizing cellular processes at the molecular level by cryo-electron tomography. J Cell Sci 2010; 123:7-12; PMID:20016061; http://dx.doi.org/ 10.1242/jcs.060111 [DOI] [PubMed] [Google Scholar]
- 20. Aratyn YS, Schaus TE, Taylor EW, Borisy GG. Intrinsic dynamic behavior of fascin in filopodia. Mol Biol Cell 2007; 18:3928-40; PMID:17671164; http://dx.doi.org/ 10.1091/mbc.E07-04-0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu M, Witke W, Kwiatkowski DJ, Kosik KS. Delayed retraction of filopodia in gelsolin null mice. J Cell Biol 1997; 138:1279-87; PMID:9298983; http://dx.doi.org/ 10.1083/jcb.138.6.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]


