Abstract
Background and Purpose
The HIV-envelope glycoprotein Gp120 is involved in neuronal injury and is associated with neuro-AIDS pathogenesis in the brain. Endocannabinoids are important lipid ligands in the CNS regulating neural functions, and their degeneration is controlled by hydrolysing enzymes such as the fatty acid amide hydrolase (FAAH). Here, we examined whether in vivo genetic deletion of Faah gene prevents HIV-1 Gp120-mediated effects on neurogenesis.
Experimental Approach
We generated new GFAP/Gp120 transgenic (Tg) mice that have genetic deletion of Faah gene by mating glial fribillary acidic protein (GFAP)/Gp120 Tg mice with Faah−/− mice. Neurogenesis and cell death were assessed by immunocytochemical analysis.
Key Results
Endocannabinoid levels in the brain of the double GFAP/Gp120//Faah−/− mice were similar to those observed in Faah−/− mice. However, unlike the impaired neurogenesis observed in GFAP/Gp120 Tg mice and Faah−/− mice, these GFAP/Gp120//Faah-/ mice showed significantly improved neurogenesis in the hippocampus, indicated by a significant increase in neuroblasts and neuronal cells, an increase in BrdU+ cells and doublecortin positive cells (DCX+), and an increase in the number of PCNA. Furthermore, a significant decrease in astrogliosis and gliogenesis was observed in GFAP/Gp120//Faah−/−mice and neurogenesis was stimulated by neural progenitor cells (NPCs) and/or the newly formed NPC niches characterized by increased COX-2 expression and elevated levels of PGE2.
Conclusions and Implications
In vivo genetic ablation of Faah, resulted in enhanced neurogenesis through modulation of the newly generated NPC niches in GFAP/Gp120//Faah−/− mice. This suggests a novel approach of using FAAH inhibitors to enhance neurogenesis in HIV-1 infected brain.
Introduction
Infection of the CNS by HIV-1 is associated with a spectrum of neurological disorders, ranging from HIV-associated dementia (HAD; Fauci, 1988; Portegies and Brew, 1991) to milder HIV-associated neurocognitive disorders (Ellis et al., 2007). The cognitive deficits in patients with HIV are probably related to damage to the synapto-dendritic apparatus of the neurons (Masliah et al., 1992; Mucke et al., 1995) and injury of neural progenitors (Lawrence et al., 2004; Schwartz and Major, 2006; Schwartz et al., 2007).
Neurogenesis occurs in the subventricular zone (SVZ) and in the subgranular zone (SGZ) of the dentate gyrus (DG) of hippocampus (Abrous et al., 2005). HIV-1 infection negatively affects the process of neurogenesis in the adult brain (Tran and Miller, 2005). Diminished adult neurogenesis is considered a potential mechanism in the pathogenesis of HAD (Peng et al., 2001). Human brain-derived progenitor cells (hNPCs) are permissive for infection by HIV-1 and support a latent infection that can be re-activated by differentiation or cytokine stimulation. HIV-1 as well as its viral Gp120 inhibits NPC proliferation (Krathwohl and Kaiser, 2004; Muboh et al., 2006; Okamoto et al., 2007), induces neuronal death and prevents potential repair mechanisms in the CNS through NPCs (Krathwohl and Kaiser, 2004; Muboh et al., 2006; Okamoto et al., 2007). HIV-1 Gp120 was also reported to decrease adult NPC proliferation via checkpoint kinase-mediated cell cycle withdrawal and G1 arrest (Okamoto et al., 2007). Further, exposure of hNPCs to HIV-1 causes quiescence of NPCs, through engagement of the chemokine receptor CXCR4 (for receptor nomenclature see Alexander et al., 2013; Krathwohl and Kaiser, 2004). These neurocognitive deficits in HAD may be attributed to a loss of NPC function.
The endogenous cannabinoid system is a ubiquitous lipid signalling system that has important regulatory functions in a variety of physiological and pathological conditions (Fernández-Ruiz et al., 2011; Fernández-Ruiz, 2012; Han et al., 2012). Endocannabinoids mediate their effects via binding to CB1 and CB2 receptors. CB1 receptors are expressed at high levels in the CNS, whereas CB2 receptors are concentrated predominantly in cells of the immune system (Panikashvili et al., 2006; Fernández-Ruiz, 2012; Han et al., 2012). Cannabinoids have potential neuroprotective effects in some neurodegenerative disorders in adults (Lu et al., 2008; De Lago et al., 2009; Fernández-Ruiz et al., 2010a,b,; Sagredo et al., 2011; Fernández-Ruiz, 2012). In vivo, cannabinoids decrease hippocampal neuronal loss and the volume of brain damage after cerebral ischaemia (Nagayama et al., 1999) and acute brain trauma (Panikashvili et al., 2006). Inhibition of fatty acid amide hydrolase (FAAH) enzyme results in accumulation of the endocannabinoid anandamide (AEA) and the subsequent activation of the endocannabinoid signalling pathway, promoting neuronal maintenance and function (Aguado et al., 2005; Karanian et al., 2007; De Lago et al., 2009; Fernández-Ruiz et al., 2010a,b,; Vinod et al., 2012). Interestingly, enhanced AEA degradation was reported to be associated with neuronal apoptosis induced by the HIV-1 Gp120 in the rat neo-cortex (Maccorone et al., 2004). Here, by exposing newly created glial fribillary acidic protein (GFAP)/Gp120//Faah−/− mice to a Gp120 insult, we aimed to examine whether genetic ablation of the Faah gene, as an important component of the endocannabinoid system, exerts neuroprotective effects on Gp120-mediated insults on neurogenesis.
Methods
Please see Supporting Information Figure S1, for some detailed information and Methods.
Ethics statement
The protocol used in the paper was approved by the Institutional Animal Care & Use Committee (IACUC) of Beth Israel Deaconess Medical Center. This IACUC is affiliated with Beth Israel Deaconess Medical Center and Harvard Medical School. The protocol number is #047-2-1226. Our IACUC protocol adhered to the guidelines from the National Institutes of Health and the Association for Assessment and Accreditation of Laboratory Animal Care. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010; http://onlinelibrary.wiley.com/doi/10.111/J.1476–5381.2010.00822.x.9pdf) and the BJP Editorial guidelines. A total of 260 animals were used in the screening of the GFAP/Gp120//Faah−/− mice and in the experiments as detailed here. These animals were housed under a 12 h/12 h light/dark cycle (lights on 07:00–19:00 h) with controlled room temperature (20–26°C) and humidity (35–75%) and were allowed ad libitum access to a diet of standard laboratory chow and water.
Generation of [GFAP/Gp120//Faah−/−] mice
The GFAP/Gp120 transgenic (Tg) mouse model develops a spectrum of neuronal and glial changes and has learning and memory deficits, which replicate some of the neurological deficits seen in patients with HIV-infection because of high levels of Gp120 expression (Toggas et al., 1994; 1996,). Dr Lennart Mucke kindly provided us with these mice (Toggas et al., 1994; 1996,). In the brains of Gp120 Tg mice, astrogliosis appears around 5–6 months and degeneration of neurons appears at 7–9 months (D'hooge et al., 1999). The severity of damage in the brains of GFAP/Gp120 Tg mice is correlated positively with the level of Gp120 expression in various brain regions (Krucker et al., 1998). Moreover, Gp120 Tg mice display deficits in neurogenesis in the hippocampus (Okamoto et al., 2007). In addition to synapto-dendritic injury, the changes include reactive astrogliosis, increased number and activation of microglia, and loss of large pyramidal neurons.
We mated GFAP/Gp120 Tg mice (Toggas et al., 1994) with Faah−/− mice (Cravatt et al., 2001) (Figure 1A). Dr B. Cravatt (The Scripps Research Institute, San Diego, CA, USA) kindly provided us with the Faah−/− mice. These mice have an accumulation of AEA because of Faah deficiency (Cravatt et al., 2001). Faah−/− mice possess 15-fold augmented endogenous brain levels of AEA (Cravatt et al., 2001). The brain extracts from Faah−/− mice hydrolysed AEA more slowly than brain extracts from Faah+/+ mice by 50–100-fold (Cravatt et al., 2001).
Figure 1.
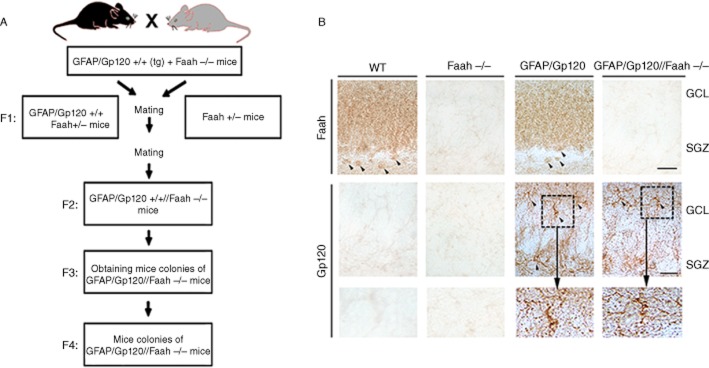
Generation and characterization of GFAP/Gp120//Faah−/− mice. (A) For this purpose, GFAP/Gp120 Tg mice containing both Faah copies were crossed with homozygous Faah KO (Faah−/−) mice resulting in the F1 mice of GFAP/Gp120 Tg containing one copy of Faah (+/−). These mice were in turn crossed with (Faah +/−) mice to obtain in F2 GFAP/Gp120 Tg that contain homozygous (Faah−/−). These F2 mice were then crossed for two more generations (F3 and F4) to obtain the colonies of [GFAP/Gp120//Faah−/−] mice utilized for subsequent experiments. (B) Immunocytochemical characterization of expression of Gp120 and Faah in the hippocampus DG of mice from the four genotype groups. In the hippocampus, Faah immunostaining was noted in the GCL of the DG as well as in the neuronal cells in the SGZ (arrow heads). No labelling was observed in Faah−/− mice. With an antibody against Gp120, in the GFAP/Gp120 mice there was immunoreactivity associated with astroglial cells (dotted box) in the GCL and SGZ. Bar = 40 μm.
Faah gene and GFAP/Gp120 are located on different chromosomes. For the generation of [GFAP/Gp120//Faah−/−] mice, we employed the following PCR primers for screening:
For Gp120 mouse genotyping, the PCR primers were:
Gp120-F: GCGGGAGAATGATAATGGAG
Gp120-R: TATGGGAATTGGCTCAAAGG
For Faah mouse genotyping, the PCR primers were:
Faah-F: TAACTAGGCATGCTGACTCTAG
Faah-G-R1: ACTCAAGGTCAGCCTGAAACC (wild type)
Faah-R2: TTTGTCACGTCCTGCACGACG (knockout, KO)
The mouse strain background of the Gp120 Tg mice was B6 X SJL (Toggas et al., 1996). The mouse strain background of Faah−/− mice was derived from breeders back-crossed onto a C57B1/6J background for 13 generations (Cravatt et al., 2001). There was no genetic heterogeneity among the different groups, as analysed by genomic screening of the pups.
For the purpose of generating GFAP/Gp120//Faah−/− mice, GFAP/Gp120//Faah+/+ mice were crossed with homozygous Faah KO [Faah−/−] mice resulting in the F1 GFAP/Gp120 Tg containing one copy of Faah (+/−) [GFAP/Gp120//Faah+/−], and heterozygous Faah KO mice [Faah +/−]. The GFAP/Gp120//Faah+/− mice were in turn crossed with Faah +/− mice to obtain F2 GFAP/Gp120 Tg that were homozygous for Faah−/− [GFAP/Gp120//Faah−/−]. The GFAP/Gp120+/+//Faah−/− mice were then crossed for two more generations (F3 and F4) to obtain the colonies of GFAP/Gp120//Faah−/− mice utilized for subsequent experiments, (see Supporting Information Figure S1 in supplemental methods for genotyping of these mice).
Further characterization of these new mice was performed as previously described (Toggas et al., 1994; 1996,) with vibratome sections by immunocytochemistry with antibodies against Faah (Cayman Chemicals, Ann Arbor, MI, USA) and Gp120 (AIDS reagents). Neurogenesis in these mice was analysed as previously described (Rockenstein et al., 2007) and as detailed later. In order to label newly generated cells, all groups received i.p. injections of bromodeoxyuridine (BrdU, 50 mg·kg−1) for 5 consecutive days at the age of 5–6 months and were killed 28 days after the first BrdU injection as previously described (Winner et al., 2007).
Analysis of neurogenesis
These assays were performed as previously described (Rockenstein et al., 2007)
TUNEL assay
Apoptotic NPCs were examined by in situ TUNEL as described previously (Rockenstein et al., 2007).
Immunocytochemical analysis of markers of neurogenesis and cell death
Neurogenesis analysis was performed as described previously (Rockenstein et al., 2007). Briefly, for detection of markers of neurogenesis, vibratome sections oriented in the sagittal plane were pretreated with 50% formamide/2 X SSC (2 X SSC: 0.3 M NaCl, 0.03 M sodium citrate) at 65°C, rinsed for 5 min in 2 X SSC, and incubated for 30 min in 2 M HCl at 37°C, followed by a 10 min rinse in 0.1 M boric acid, pH 8.5. The sections were incubated with antibodies against BrdU (marker of dividing cells; rat monoclonal, 1:100, Oxford Biotechnology, Oxford, UK), proliferating cell nuclear antigen (PCNA, marker of proliferation; mouse monoclonal, 1:250, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or doublecortin (DCX, marker of migrating neuroblasts; goat polyclonal, 1:500, Santa Cruz Biotechnology, Inc.) overnight at 4°C. Sections were then incubated with biotinylated secondary antibodies directed against rat, mouse or goat. After intermittent rinses in TBS, avidin–biotin–peroxidase complex was applied (ABC Elite kit, Vector Labs, Burlingame, CA, USA) followed by peroxidase detection with diaminobenzidine in 0.01% H2O2, 0.04% NiCl in TBS. For analysis of the proportion of BrdU+ cells converting into neurons or astroglial cells, double immunoflorescence labelling was performed with antibodies against BrdU and NeuN, and BrdU and GFAP. All sections were processed under the same standardized conditions. The immunolabelled blind-coded sections were imaged with laser scanning confocal microscopy (LSCM; MRC1024, Bio-Rad, Philadelphia, PA, USA).
For detection of NPC, the TUNEL detection method using the ApopTag in situ Apoptosis Detection Kit (Chemicon, Billerica, MA, USA) was used with slight modifications. Detection was performed with Avidin-FITC and sections were mounted under glass coverslips with anti-fading media (Vector, Burlingame, CA, USA) for confocal microscopy analysis. To verify that NPC underwent apoptosis, sections were double-labelled with a monoclonal antibody against activated caspase-3 (1:200, Stressgen Bioreagents, Ann Arbor, MI, USA) and the polyclonal antibody against DCX (1:500, Santa Cruz Biotechnology, Inc.), followed by incubation with fluorochrome-labelled secondary antibodies and imaging on the LSCM.
Quantification of COX-2 and PGE2 immunostaining levels using Volocity Software
Fluorescence intensity measurements were performed by using Volocity Software (Perkin Elmer, Waltham, MA, USA). The mean COX-2 or PGE2 fluorescence intensity of tested areas in brain from GFAP/Gp120, Faah−/− and GFAP/Gp120//Faah−/− mice were background corrected and normalized to that of wild-type (WT) mice.
Statistical analysis
Analyses were carried out with the StatView 5.0 programme (SAS Institute Inc., Cary, NC, USA). Differences among means were assessed by one-way anova with post hoc Dunnett's (when comparing to the non-Tg control group) or Tukey–Kramer (when comparing between treatment groups). Comparisons between two groups were done by Student's two-tailed unpaired t-test. Correlation studies were carried out by simple regression analysis and the null hypothesis was rejected at P = 0.05 level. All values are expressed as mean ± SEM.
Results
Generation and characterization of GFAP/Gp120//Faah−/− mice
Neurogenesis in the hippocampal DG is an active process in mature CNS and plays a key role in synaptic plasticity, memory and learning (Abrous et al., 2005). It is shown that HIV-1 Gp120 inhibits adult hippocampal neurogenesis in mouse model of HIV neurologic disease (Toggas et al., 1994; 1996,). To examine whether genetic ablation of Faah, which leads to increase of the endocannabinoid AEA, can promote neurogenesis in GFAP/Gp120 Tg mice, we generated GFAP/Gp120 Tg//Faah−/− mice by mating GFAP/Gp120 Tg mice (Toggas et al., 1994) with Faah−/− mice (Cravatt et al., 2001) (Figure 1A). Gp120 was shown to be expressed throughout various brain regions in GFAP/Gp120 mice (Toggas et al., 1994) as well as in GFAP/Gp120//Faah−/− mice. In the hippocampus, Faah immunostaining was noted in the granular cell layer (GCL) of the DG as well as in the neuronal cells in the SGZ (arrow heads). No labelling was observed in Faah−/− mice. With an antibody against Gp120 in the GFAP/Gp120 mice, there was immunoreactivity associated with astroglial cells (doted box) in the GCL and SGZ. Genetic ablation of Faah had no effect on Gp120 distribution or Gp120 levels, as determined by immunostaining in brain sections of these mice (Figure 1B).
Endocannabinoid levels in GFAP/GP120//Faah−/− mice
To determine the levels of endocannabinoids in the brains of GFAP/Gp120//Faah−/− mice as compared with GFAP/Gp120 Tg, Faah−/− and WT mice, we analysed the levels of AEA (Figure 2A), palmitoylethanolamide (PEA) (Figure 2B), oleoy lethanolamide (OEA) (Figure 2C) and 2-arachidonoyl glycerol (2-AG) (Figure 2D) in the brains of these mice as described previously (Karanian et al., 2007; Williams et al., 2007). As shown in Figure 2, the expression levels of AEA in GFAP/GP120//Faah−/− mice were statistically higher when compared with WT or GFAP/GP120 Tg mice, but close to those of Faah−/− mice. The expression levels of PEA and OEA in GFAP/GP120//Faah−/− mice were statistically similar to those of Faah−/− mice, but higher than those of WT or GFAP/GP120 Tg mice. Thus, the profile of endocannabinoid expression levels is similar between Faah−/− and GFAP/Gp120//Faah−/− mice. There were no differences in the 2-AG levels between the groups of mice.
Figure 2.
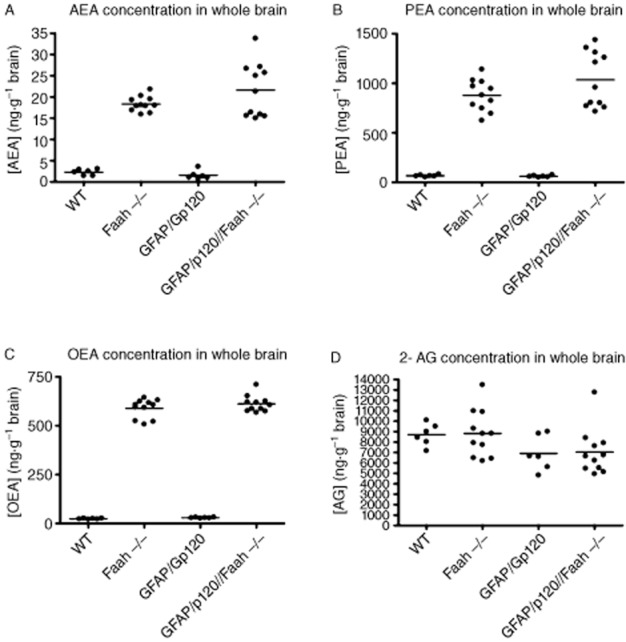
Analysis of endocannabinoid levels in brain tissues from WT, Faah−/−, GFAP/Gp120 Tg, and GFAP/Gp120//Faah−/− mice. Whole brain samples from mice in each group (10 mice per group) were used for quantification of (A) AEA; (B) PEA; (C) OEA; and (D) 2-AG to determine the effects of Faah KO or excess of Gp120 on brain endocannabinoid levels. Brain tissue samples were analysed using LC-MS/MS analysis. Statistical significance (P < 0.05) was determined using one-way anova and Tukey's multiple comparison-post test.
Analysis of neurogenesis in GFAP/Gp120//Faah−/− mice
We then analysed neurogenesis in the GFAP/Gp120//Faah−/− mice, as compared with GFAP/GP120 Tg mice and its WT littermate control, and Faah−/− mice and its WT littermate control. Six-month-old mice received series of 10 BrdU injections, and then the levels of markers of neurogenesis were analysed in the hippocampal SGZ, as reported previously (Rockenstein et al., 2007).
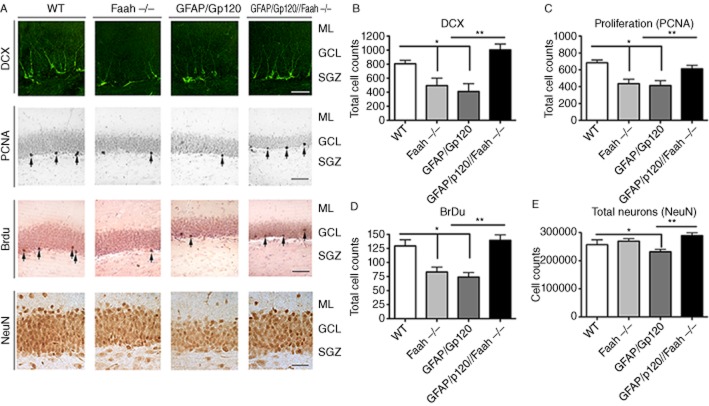
Analysis of the hippocampus molecular layer, GCL and SGZ showed presence of abundant DCX, BrdU+, PCNA positive (PCNA+) and NeuN+ cells in the WT mice and GFAP/Gp120//Faah−/−, but not in GFAP/Gp120, and Faah−/− mice (Figure 3A).
Figure 3.
Analysis of neurogenesis in the crosses between GFAP/Gp120 Tg and Faah−/− mice. (A) Immunohistochemical analysis of markers of neurogenesis in the hippocampus. All panels are representative images displaying the hippocampal molecular layer (ML), GCL and SGZ of mice at 400× magnification showing DCX+, PCNA+, BrdU+ and NeuN positive cells in WT, Faah−/−, GFAP/Gp120 tg and GFAP/Gp120//Faah−/− mice respectively. (B) Immunocytochemical analysis of DCX+ cells. Quantitative analysis using the dissector method in the SGZ shows increased numbers of DCX+ neurons in GFAP/Gp120//Faah−/− mice compared with GFAP/Gp120 Tg, Faah−/−, and WT mice. *P < 0.001 and **P < 0.005 to the mice groups as indicated compared with GFAP/Gp120 Tg mice and ***P < 0.001 to Faah−/− mice compared with WT by one way anova with post hoc Dunnett's (n = 8). (C) Immunocytochemical analysis of PCNA+ cells in GFAP/Gp120//Faah−/−, WT, Faah−/−, and GFAP/Gp120 Tg mice. Quantitative analysis using the dissector method in the SGZ showing the numbers of PCNA+ NPCs. *P < 0.001 to the mice groups as indicated compared with GFAP/Gp120 Tg mice and **P < 0.005 to Faah−/− mice compared with WT by using one way anova with post hoc Dunnett's. (D) Quantitative analysis in the SGZ of BrdU+ cells. *P < 0.001 and **P < 0.005 to the mice groups as indicated compared with GFAP/Gp120 Tg mice by Student's t-test. (n = 8 mice per group). (E) Estimates of the numbers of NeuN+ neurons in the hippocampal DG using the dissector method. *P < 0.001 and **P < 0.005 to the mice groups as indicated compared with GFAP/Gp120 Tg mice by one way anova with post hoc Tukey–Kramer (n = 8).
Analysis of the DCX, PCNA, BrdU and NeuN cells in the DG of the hippocampus was performed by the disector method (Rockenstein et al., 2007) (Figure 3B, C, D, E). This study showed that compared with the WT animals, the GFAP/Gp120 and Faah−/− mice displayed a significant reduction in the numbers of DCX (Figure 3B), PCNA (Figure 3C) and BrdU (Figure 3D) cells. (one-way anova, post hoc Dunnet's, P < 0.05). In addition and compared with the WT, the GFAP/Gp120 mice displayed reduced NeuN-neuronal cells in the DG (Figure 3E) (One way anova, post hoc Dunnet's, P < 0.05). In contrast, the GFAP/Gp120/Faah−/− mice displayed levels of DCX (Figure 3B), PCNA (Figure 3C), BrdU (Figure 3D) and NeuN comparable with the WT control group.
Next, we determined the effects of Faah on conversion of BrdU positive cells to astroglial (GFAP) cells (Masliah et al., 2011). Compared with the WT mice, GFAP/Gp120 Tg and Faah−/− mice displayed increased levels of GFAP immunoreactivity in the hippocampus (Figure 4A and C). In contrast, the GFAP/Gp120//Faah−/− displayed levels of astroglial cell immunoreactivity similar to the WT control (Figure 4A and C), indicating that neuroinflammation in GFAP/Gp120//Faah−/− mice was reduced. The reduction in astrogliosis levels in the GFAP/Gp120//Faah−/− mice DG (Figure 4) was detected by the GFAP known marker of glial cells (P < 0.001) (Ramírez et al., 2005, 53; Masliah et al., 2011).
Figure 4.
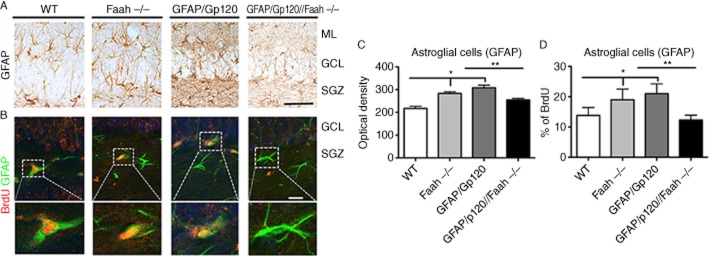
Analysis of astrogliosis and neuroblasts converting into glial cells in GFAP/Gp120//Faah−/− mice. (A) Representative images of sections from WT, Faah−/−, GFAP/Gp120 Tg, and GFAP/Gp120//Faah−/− mice immunostained with an antibody against GFAP showing astrogliosis in the DG of the Faah−/− and GFAP/Gp120 Tg mice. (B) Representative images of the double immunolabelling and laser scanning confocal microscopy analysis or sections reacted with antibodies against BrdU (red channel) and GFAP (FITC channel). The dotted box represents individual cells at higher magnification. (C) Image analysis of levels of GFAP immunoreactivity in the hippocampal DG of crosses between GFAP/Gp120 Tg and Faah−/− mice. (D) Image analysis of the % of BrdU cells that displayed GFAP immunoreactivity in the DG of crosses between GFAP/Gp120 tg and Faah−/− mice *P < 0.001 to the mice groups as indicated compared with GFAP/Gp120 Tg mice, *P < 0.001 to Faah−/− mice compared with WT by one way anova with post hoc Tukey–Kramer (n = 8). (B) The number of neuroblasts converting into glial cells. Double-immunocytochemical analysis was performed to determine the proportion of BrdU+ cells that are GFAP+ glial cells. *P < 0.001 and **P < 0.005 to the mice groups as indicated compared with GFAP/Gp120 Tg mice by one-way anova with post hoc Tukey–Kramer (n = 8).
To determine if the effects in the Faah−/− mice were related to a correction in the baseline alterations in the proportion of NPCs within the SGZ converting into neurons or astroglia in the GFAP/Gp120//Faah−/− mice, we performed analysis on brain sections double labeled for BrdU and GFAP (Figure 4B and D). The % of BrdU cells that were GFAP, which indicate the % of neuroblasts converting into glial cells was increased in the GFAP/Gp120 Tg and Faah−/− mice (P < 0.001) (Figure 4B and D). In contrast, the GFAP/Gp120//Faah−/− mice displayed % of BrdU cells that were GFAP positive similar to the WT controls (Figure 4B and D). Taken together, these findings indicate that in the Faah−/− background, the Gp120 neurogenesis phenotype is significantly rescued.
Expression of COX-2 and PGE-2 in the hippocampus of GFAP/Gp120//Faah−/− mice
It was reported that these Faah−/− mice are in a pro-convulsive state (Cravatt et al., 2001; Clement et al., 2003). In addition to Faah−/− mice having highly elevated levels of AEA, we observed impaired neurogenesis in these mice (Figures 3 and 4). Interestingly, it was reported that HIV-1 Gp120 reduced the levels of AEA in addition to its known cytotoxic effects by involving in several pathways such as activating inflammation and impairing neuron functions (Bari et al., 2010). Therefore, we focused on investigating the mechanism(s) that may be involved in enhancing neurogenesis in the GFAP/Gp120//Faah−/− mice. Several factors were reported to regulate in vivo cell proliferation and neurogenesis in the SVZ and the DG of the hippocampus (Abrous et al., 2005). To investigate potential mechanisms that regulate neurogenesis in GFAP/Gp120//Faah−/− mice, we first examined which factors in the newly generated NPC niches in the double mice GFAP/Gp120//Faah−/− mice could improve neurogenesis and affect the newly formed neurons. We observed that COX-2, the enzyme catalysing the first committed step in PGE-2 synthesis, was enhanced in the hippocampus in these GFAP/Gp120//FAAH−/− mice, by immunostaining hippocampal brain sections using COX–2-specific antibodies (Figure 5A). Quantification of immunofluorescence signal intensity of COX-2 in the hippocampus of the brain section areas using the Volocity software confirmed the abundant expression of COX-2 in the brains of these mice, as compared with WT, Faah−/−, and GFAP/Gp120 mice (Figure 5B). To determine the relationship of the COX–2-positive cells in the DG of the hippocampus with markers of neurogenesis, double labelling was performed with antibodies against Nestin, NeuN and GFAP. Confocal microscopy studies of the GFAP/Gp120//Faah−/− mice showed that the COX–2-positive cells in the SGZ co-localized with Nestin and NeuN and to a lesser extent with GFAP (Figure 6A and B). Similar results were observed with the WT, Faah−/− and GFAP/Gp120 mice (not shown).
Figure 5.
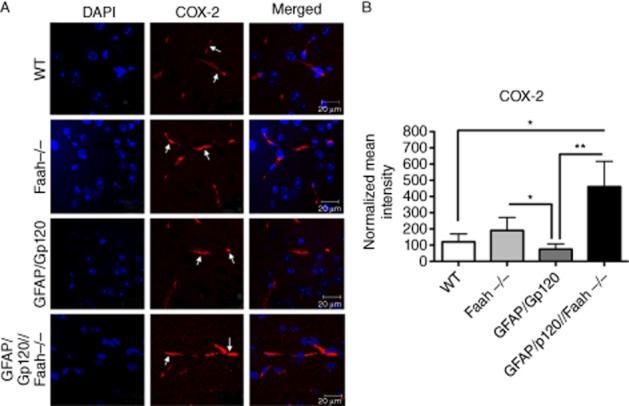
Expression of COX-2 in vivo in hippocampal brain sections. (A) COX-2 expression by immunostaining analysis of hippocampal brain sections in WT, GFAP/Gp120 Tg, Faah−/− and GFAP/Gp120//Faah−/− mice. COX-2 antibody was used as primary antibody and fluorescence Texas Red was used as a secondary antibody to detect the expression of COX-2 in the hippocampus brain sections as indicated (red colour). Nuclei in brain were counterstained with DAPI (blue colour). Images shown are from a representative experiment and are representative of over 50 fields examined in three independent experiments. (B) Quantification of COX-2 expression intensity. Normalized (to WT) COX-2 immunofluorescence intensity of tested brain sections from WT, GFAP/Gp120 Tg, Faah−/− and GFAP/Gp120//Faah−/− mice. Results are expressed as mean ± SEM; n = 3.
Figure 6.
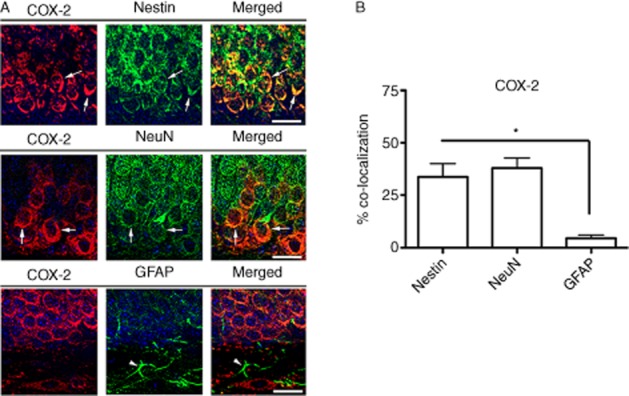
Double immunolabelling and confocal microscopy analysis of the COX–2-positive cells in the DG of the GFAP/Gp120//Faah−/− mice. Double labelling was performed with antibodies against COX-2 (red channel) and Nestin, NeuN or GFAP (FITC channel). (A) Representative confocal microscopy images of the GFAP/Gp120//Faah−/− mice showing the presence of COX–2-positive cells in the SGZ co-localizing with Nestin and NeuN, but not GFAP. (B) Analysis of % of Nestin, NeuN and GFAP cells that displayed COX-2 immunostaining (*P < 0.05, one way anova with post hoc Tukey–Kramer).
As PGE-2 is a downstream target of COX-2 and contributes to neurogenesis, we examined PGE-2 involvement in this process. Our analysis showed elevated expression levels of PGE-2 in the GFAP/Gp120//Faah−/− brains, as shown by immunostaining (Figure 7A) and quantitative analysis of immunostaining for PGE-2 (Figure 7B). Taken together, these results strongly suggest that enhanced neurogenesis observed in GFAP/Gp120//Faah−/− are due to enhanced expression of both COX-2 and PGE-2 in the new NPC niches generated in these mouse brains.
Figure 7.
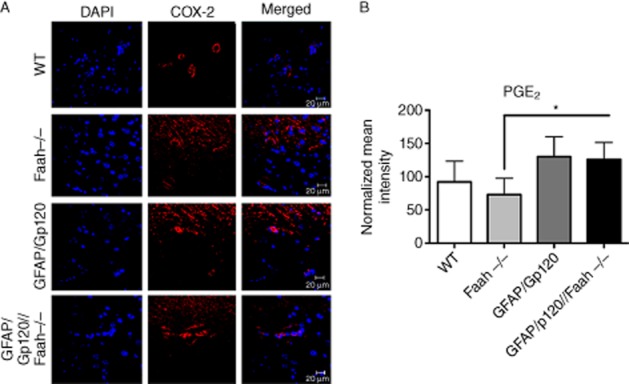
Expression in vivo of PGE2 in hippocampal brain sections. (A) PGE2 expression by immunostaining analysis of the hippocampal brain sections in WT, GFAP/Gp120 Tg, Faah−/− and GFAP/Gp120//Faah−/− mice. PGE2 antibody was used as primary antibody and FITC-conjugated secondary antibody was used to detect the expression of PGE2 in the hippocampus brain sections as indicated. Brain nuclei were counterstained with DAPI (blue colour). Images from three independent representative experiments are shown. In each experiment, over 50 fields were examined. Scale bar = 20 μM. (B) Quantification of PGE2 expression intensity. Immunofluorescence signal intensity per pixel of PGE2 in tested areas of the hippocampal brain sections from WT, GFAP/Gp120 Tg, Faah−/−, and GFAP/Gp120//Faah−/− mice were quantified using the Volocity software. The data are presented as average % change in mean signal intensity per pixel. Data were normalized to values in WT mice.
Discussion
It is well established that neurogenesis occurs in the hippocampal SGZ and the SVZ (Abrous et al., 2005). Our in vivo data showed that genetic ablation of Faah can prevent the impaired neurogenesis that occurs in the GFAP/Gp120 mice (Figure 3). Interestingly, while GFAP/Gp120 Tg and Faah−/− mice showed decreased neurogenesis, double GFAP/Gp120//Faah−/− mice showed significantly improved neurogenesis in the hippocampus (Figure 3A), as indicated by a significant increase in neuroblasts and neuronal cells, BrdU+ cells and DCX+ cells as well as increase in the number of PCNA (Figure 3B). Furthermore, a significant decrease in astrogliosis and gliogenesis was observed in GFAP/Gp120//Faah−/− mice as compared with GFAP/Gp120 Tg mice (Figure 4). Neurogenesis is stimulated in these GFAP/Gp120//Faah−/− mice by the molecular characteristics of newly formed NPC niches. These results suggest that in vivo genetic deletion of Faah restores HIV-1 Gp120-induced impaired neurogenesis.
The mechanisms that control neurogenesis are not well defined (for review, see Abrous et al., 2005). Both cell intrinsic progress and extracellular/environmental factors are important in regulating neurogenesis (Abrous et al., 2005). NPC proliferation and differentiation are dependent on the cellular composition and molecular characteristics of the niche in which NPCs reside (Seri and Alvarez-Buylla, 2002; Abrous et al., 2005). Regulation of adult neurogenesis by glial cells is also documented (Seri and Alvarez-Buylla, 2002). Astrocytes and other glial cells, which play an important role as sensors of changes in their extracellular microenvironment, may regulate neurogenesis by secreting local signals such as growth factors and cytokines involved in neurogenesis (Spranger et al., 1990; Yoshida et al., 1992; Kamiguchi et al., 1996; Junier, 2000; Laming et al., 2000; Scemes, 2000). As recent data suggest, the COX-2 pathway is also required for neurogenesis (Goncalves et al., 2010). COX-2 catalyses the first committed step in the synthesis of prostanoids, a large family of arachidonic acid metabolites comprising PGs including PGE2, prostacyclin acid thromboxanes (Cruz Duarte et al., 2012). Interestingly, in mammalian brain, COX-2 is expressed in neuronal populations under normal conditions (Abrous et al., 2005; Goncalves et al., 2010; Cruz Duarte et al., 2012). COX-2 inhibitors suppress neurogenesis in the hippocampus in the brain of adult mice (Goncalves et al., 2010), and COX-2 KO mice have impaired neurogenesis (Cruz Duarte et al., 2012). Furthermore, in the adult mammalian CNS, COX-2 and PGE2 play an important role in maintaining neurogenesis (Abrous et al., 2005; Cruz Duarte et al., 2012). One possibility is that COX-2 affects neurogenesis through the production of PGE2 (Abrous et al., 2005; Cruz Duarte et al., 2012), which may act directly via prostanoid EP3 receptors expressed in the SGZ at the DG of the hippocampus. Further, COX-2 inhibitors prevent the increase in expression levels of PGE2 and brain-derived growth factor (BDNF; Cruz Duarte et al., 2012). In addition, pharmacological inhibition of FAAH enzyme results in elevated BDNF levels in the rat brain (Vinod et al., 2012) and PGE2 contributes to the synthesis of BDNF in the primary sensory neuron in ganglion explants (Bath et al., 2012). The findings mentioned earlier indicate that the COX-2/PGE2 axis enhances neurogenesis.
However, as Faah deletion resulted in increased AEA, the enhanced neurogenesis observed in the GFAP/Gp120 Faah−/− mice could also be due to the synthesis of prostamides in the brain of these mice. AEA may be converted by COX-2 and PGF synthases, such as prostamides/PGF synthase, to PGF22 ethanolamide (PGF22EA). The prostamides are neutral lipids and are COX-2 derived oxidation products of the endocannabimoid/endovanniboid AEA (Woodward et al., 2008). As COX-2 was elevated in the double mice, it also could result in increased prostamides, which could affect neurogenesis.
Many constituents of the endogenous cannabinoid system like the CB1 and CB2 receptors and their endogenous ligands AEA and 2-AG as well as the AEA-degrading enzyme FAAH and the 2–AG-synthesizing enzyme DAG lipases are found in neuronal development and adult neurogenesis (Harkany et al., 2007; Goncalves et al., 2008). Several studies investigating the role of the cannabinoid system in adult neurogenesis found that stimulation of CB1 receptors seemed to either increase or decrease adult neurogenesis (Aguado et al., 2006; 2007,; Harkany et al., 2007). In other studies, CB1 receptor activation promoted precursor cell proliferation and the generation of neurospheres ex vivo, which was attenuated in CB1-deficient precursor cells, and proliferation of hippocampal precursor cells was increased in Faah-deficient mice (Aguado et al., 2006; 2007,; Harkany et al., 2007). In adult CB1-deficient mice, neural progenitor proliferation also decreased. In addition, endocannabinoid signalling controlled neural progenitor differentiation of newly born cells (Aguado et al., 2006). Furthermore, the endocannabinoid AEA inhibited neuronal progenitor cell differentiation through attenuation of the extracellular signal regulated kinase pathway in vitro (Rueda et al., 2002). Adult neurogenesis in the DG was reported to be significantly decreased by the AEA analogue methanandamide and increased by the CB1 antagonist SR141716 (Rueda et al., 2002). Cannabinoids also rescued structural progenitor cells in chronic Borna disease viral encephalitis in rats (Solbrig and Hermanowicz, 2008). In this context, it is important to note that pharmacological manipulation of the cannabinoid signalling system reduced neuroinflammation associated with normal ageing (Bardou et al., 2012). Therefore, cannabinoids may have benefits in enhancing neurogenesis and reducing neuroinflammation.
Under pathogenic conditions, cannabinoids have shown to affect the following processes: (i) normalize glutamate homeostasis; (ii) reduce excitotoxicity; (iii) inhibit calcium influx; (iv) lower intracellular levels and subsequently activate Ca2+-dependent destructive pathways; (v) reduce generation of reactive oxygen intermediates to limit their toxicity; and (vi) decrease oxidative injury (Fernández-Ruiz et al., 2010a,b,; Sagredo et al., 2011; Fernández-Ruiz, 2012). Cannabinoids also decrease local inflammation events by acting on glial processes that regulate neuronal survival (Ramírez et al., 2005; De Lago et al., 2009). Neuroprotective effects have been demonstrated for cannabinoids in several in vitro and in vivo neurotoxicity models (Fernández-Ruiz et al., 2008; Vardeh et al., 2009).
Although Faah KO (Faah−/−) mice display highly elevated (>15-fold) levels of N-acylethanolamines and N-acyltaurines in various tissues (Cravatt et al., 2001; see also Figure 2), their neurogenesis is impaired, similar to that of GFAP/Gp120 Tg (Figure 3). The impaired neurogenesis in Faah−/− mice was evident by a significant decrease in the number of BrdU+ cells and DCX neural progenitor cells, a decrease in the proliferation of NPCs as indicated by the decrease in PCNA+ cells, as compared with WT mice (Figure 3B). Furthermore, there was a decrease in the neuroblasts and neuronal cells as shown by the analysis of NeuN+ cells (Figure 4B) and significant enhancement of astrogliosis as detected by the marker of glial cells (Figure 4A) in Faah−/− mice. These results are in agreement with the report that Faah−/− mice display a pro-convulsive state because of high levels of AEA, which is also found in reduced pain sensation (Clement et al., 2003). In addition, the increased seizure susceptibility and pro-convulsive activity that is observed in Faah−/− mice are correlated with the greatly elevated endogenous levels of AEA in the hippocampus of these mice (Clement et al., 2003). Thus, our results of impaired neurogenesis in Faah−/− (Figure 3A) support the notion that high levels of AEA might be neurotoxic to NPC functions. However, our results differed from the results published by Aguado et al. (2005), where they showed, by BrdU staining, that the proliferation of hippocampal NPCs is increased in Faah−/− mice Aguado et al. (2005). The Galve-Roperh report is also in contrast with the finding that these mice are in a pro-convulsive state, as described and detailed by Cravatt's group (Clement et al., 2003). Furthermore, our in vivo neurogenesis analysis in Faah−/− mice, as shown in Figures 3 and 4, presents a decrease in the following markers: BrdU+ cells, NeuN+ cells, DCX+ cells as well as a decrease in the number of PCNA and an increase in astrogliosis, which further support the conclusion that there is a decrease in NPC proliferation in Faah−/− mice.
Interestingly, the GFAP/Gp120//Faah−/− mice showed similar profiles of endocannabinoid expression levels in brain, to those observed in Faah−/− mice (Figure 2). As Faah−/− mice have impaired neurogenesis, this suggested that the protection of neurogenesis in GFAP/Gp120//Faah−/− mice must be due to a different mechanism. Interestingly, we observed that neurogenesis may be stimulated in GFAP/Gp120//Faah−/− mice because of molecular characteristics of the newly formed NPC niches, which include induction of COX-2 (Figure 5) and PGE2 (Figure 6), both of these factors were reported to stimulate neurogenesis (Bath et al., 2012). Neurogenesis is under the control of both autocrine and paracrine signalling pathways (Abrous et al., 2005), as well as intrinsic progress and extrinsic factors. It is noteworthy to add that administration of arachidonic acid (AA) successfully and dramatically increased neurogenesis in Pax6 (+/−) rats (Maekawa et al., 2009) and both PGE2 and endocannabinoids are metabolites of AA. Further, it is reported that PGE2 plays an important role in neurogenesis (Cruz Duarte et al., 2012), stimulates CB2 receptors, and promotes mouse neural stem cell proliferation (Molina-Holgado et al., 2006). COX-2 inhibitors were shown to suppress neurogenesis in the adult mouse brain (Goncalves et al., 2010). Interestingly, pharmacological inhibition of FAAH led to elevated BDNF levels in Wistar Kyoto rats and improved neurogenesis (Vinod et al., 2012). Hence, the regulation of the level of FAAH enzyme activity might be a mechanism by which neurogenesis is regulated via the COX-2/PGE2 axis.
We propose that cleavage of AA from phospholipids and PLA2 and its oxidation by elevated levels of COX-2 are likely to occur in NPC niches, leading to PGE2 synthesis and an up-regulation of the COX-2/PGE2 axis. Furthermore, the up-regulation of COX-2/PGE2 may lead to enhanced neurogenesis in GFAP/Gp120//Faah−/− mice. Future studies will focus on elucidating the molecular mechanism of this proposed pathway. In fact, several FAAH inhibitors are in clinical trials for treatment of pain, cannabis dependence and other conditions (http://www.clinicaltrials.gov).
Based on the results presented here, FAAH inhibitors may protect NPCs from the neurotoxic effects of HIV proteins such as Gp120, and may therefore represent a potentially promising neuroprotective treatment for patients with HIV-associated neurocognitive disorders.
Acknowledgments
We thank Benjamin Chen for his help and support in the typing, proofreading and editing of the paper. We also thank Kyle Whitten and Subramanian K Vadivel for making the endocannabinoid standards and deuterated standards. Supported by the National Institutes of Heatlh grants AG043384 and MH062962 to EM and by departmental grants for Hava Avraham and Shalom Avraham.
Glossary
- AEA
anandamide
- BrdU
bromodeoxyuridine
- DCX
doublecortin
- DG
dentate gyrus
- FAAH
fatty acid amide hydrolase
- GFAP
glial fibrillary acidic protein
- HAD
HIV-associated dementia
- hNPC
human brain-derived progenitor cell
- LSCM
laser scanning confocal microscopy
- NeuN
neuronal nuclei
- PCNA
proliferating cell nuclear antigen
- SGZ
subgranular zone
- SVZ
subventricular zone
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 (A) Generation of ‘double gene change’ mice; (B) double gene change genotyping by PCR.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguado T, Romero E, Monory K, Palazuelos J, Sendtner M, Marsicano G, et al. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J Biol Chem. 2007;282:23892–23898. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou I, DiPatrizio N, Brothers HM, Kaercher RM, Baranger K, Mitchem M, et al. Pharmacological manipulation of cannabinoid neurotransmission reduces neuroinflammation associated with normal aging. Health. 2012;4(9A):679–684. [Google Scholar]
- Bari M, Rapino C, Mozetic P, Maccarrone M. The endocannabinoid system in gp120-mediated insults and HIV-associated dementia. Exp Neurol. 2010;224:74–84. doi: 10.1016/j.expneurol.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Bath KG, Akins MR, Lee FS. BDNF control of adult SVZ neurogenesis. Dev Psychobiol. 2012;54:578–589. doi: 10.1002/dev.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AB, Hawkins EG, Lichtman AH, Cravatt BF. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J Neurosci. 2003;23:3916–3923. doi: 10.1523/JNEUROSCI.23-09-03916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz Duarte P, St-Jacques B, Ma W. Prostaglandin E2 contributes to the synthesis of brain-derived neurotrophic factor in primary sensory neuron in ganglion explant cultures and in a neuropathic pain model. Exp Neurol. 2012;234:466–481. doi: 10.1016/j.expneurol.2012.01.021. [DOI] [PubMed] [Google Scholar]
- De Lago E, Gómez-Ruiz M, Moreno-Martet M, Fernández-Ruiz J. Cannabinoids, multiple sclerosis and neuroprotection. Expert Rev Clin Pharmacol. 2009;2:645–660. doi: 10.1586/ecp.09.42. [DOI] [PubMed] [Google Scholar]
- D'hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J. Cannabinoid drugs for neurological diseases: what is behind? Rev Neurol. 2012;54:613–628. [PubMed] [Google Scholar]
- Fernández-Ruiz J, Pazos MR, Garciá-Arencibia M, Sagredo O, Ramos JA. Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol Cell Endocrinol. 2008;286:S91–S96. doi: 10.1016/j.mce.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets. 2010a;14:387–404. doi: 10.1517/14728221003709792. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Hernández M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010b;16:e72–e91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Moreno-Martet M, Rodríguez-Cueto C, Palomo-Garo C, Gómez-Cañas M, Valdeolivas S, et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br J Pharmacol. 2011;163:1365–1378. doi: 10.1111/j.1476-5381.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves MB, Suetterlin P, Yip P, Molina-Holgado F, Walker DJ, Oudin MJ, et al. A diacylglycerol lipase-CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age-dependent manner. Mol Cell Neurosci. 2008;38:526–536. doi: 10.1016/j.mcn.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Goncalves MB, Williams EJ, Yip P, Yáñez-Muñoz RJ, Williams G, Doherty P. The COX-2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br J Pharmacol. 2010;159:1118–1125. doi: 10.1111/j.1476-5381.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Junier MP. What role(s) for TGFalpha in the central nervous system? Prog Neurobiol. 2000;62:443–473. doi: 10.1016/s0301-0082(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshida K, Wakamoto H, Inaba M, Sasaki H, Otani M, et al. Cytokine-induced selective increase of high-molecular-weight bFGF isoforms and their subcellular kinetics in cultured rat hippocampal astrocytes. Neurochem Res. 1996;21:701–706. doi: 10.1007/BF02527728. [DOI] [PubMed] [Google Scholar]
- Karanian DA, Karim SL, Wood JT, Williams JS, Lin S, Makriyannis A, et al. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther. 2007;322:1059–1066. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- Laming PR, Kimelberg H, Robinson S, Salm A, Hawrylak N, Müller C, et al. Neuronal-glial interactions and behaviour. Neurosci Biobehav Rev. 2000;24:295–340. doi: 10.1016/s0149-7634(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TS, Avraham HK, Seng S, Tachado SD, Makriyannis A, Avraham S. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvasculature endothelial cells. J Immunol. 2008;181:6406–6416. doi: 10.4049/jimmunol.181.9.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccorone M, Piccirill S, Battista N, Del Duca C, Nappi G, Corasaniti TM, et al. Enhanced anandamide degradation is associated with neuronal apoptosis induced by the HIV-1 coat glycoprotein gp120 in the rat neocortex. J Neurochem. 2004;89:1293–1300. doi: 10.1111/j.1471-4159.2004.02430.x. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Takashima N, Matsumata M, Ikegami S, Kontani M, Hara Y, et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS ONE. 2009;4:e5085. doi: 10.1371/journal.pone.0005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Rubio-Araiz A, García-Ovejero D, Williams RJ, Moore JD, Arévalo-Martín A, et al. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2006;25:629–634. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]
- Muboh MK, Johson HF, Farenkia GN. HIV-1 infection of human brain-derived neural progenitor cells. Retrovirology. 2006;3:43. [Google Scholar]
- Mucke L, Abraham CR, Ruppe MD, Rockenstein EM, Toggas SM, Mallory M. Protection against HIV-1 gp120-induced brain damage by neuronal expression of human amyloid precursor protein. J Exp Med. 1995;181:1551–1556. doi: 10.1084/jem.181.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin K, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, et al. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Shein NA, Mechoulam R, Thembovler V, Kohen R, Alexandrovich A, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Peng D, Hu TL, Jiang A, Washington MK, Moskaluk CA, Schneider-Stock R, et al. Location-specific epigenetic regulation of the metallothionein 3 gene in esophageal adenocarcinomas. PLoS ONE. 2001;6:e22009. doi: 10.1371/journal.pone.0022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portegies P, Brew BJ. Update on HIV-related neurological illness. AIDS. 1991;5(Suppl. 2):S211–S217. doi: 10.1097/00002030-199101001-00029. [DOI] [PubMed] [Google Scholar]
- Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer's disease by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mante M, Adame A, Crews L, Moessler H, Masliah E. Effects of cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer's disease. Acta Neuropathol. 2007;113:265–275. doi: 10.1007/s00401-006-0166-5. [DOI] [PubMed] [Google Scholar]
- Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- Sagredo O, Pazos MR, Satta V, Ramos JA, Pertwee RG, Fernández-Ruiz J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J Neurosci Res. 2011;89:1509–1518. doi: 10.1002/jnr.22682. [DOI] [PubMed] [Google Scholar]
- Scemes E. Components of astrocytic intercellular calcium signaling. Mol Neurobiol. 2000;22:167–179. doi: 10.1385/MN:22:1-3:167. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, et al. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13:274–283. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- Seri B, Alvarez-Buylla A. Neural stem cells and the regulation of neurogenesis in the adult hippocampus. Clin Neurosci Res. 2002;2:11–16. doi: 10.1016/S1566-2772(02)00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbrig MV, Hermanowicz N. Cannabinoid rescue of striatal progenitor cells in chronic Borna disease viral encephalitis in rats. J Neurovirol. 2008;14:252–260. doi: 10.1080/13550280802074521. [DOI] [PubMed] [Google Scholar]
- Spranger M, Lindholm D, Bandtlow C, Heumann R, Gnahn H, Näher-Noé M, et al. Regulation of nerve growth factor (NGF) synthesis in the rat central nervous system: comparison between the effects of interleukin-1 and various growth factors in astrocyte cultures and in vivo. Eur J Neurosci. 1990;2:69–76. doi: 10.1111/j.1460-9568.1990.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. HIV-1, chemokines and neurogenesis. Neurotox Res. 2005;8:149–158. doi: 10.1007/BF03033826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardeh D, Wang D, Costigan M, Lazarus M, Saper CB, Woolf CJ, et al. COX-2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest. 2009;119:287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Xie S, Psychoyos D, Hungund BL, Cooper TB, Tejani-Butt SM. Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS ONE. 2012;7:e36743. doi: 10.1371/journal.pone.0036743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Wood J, Pandarinathan L, Karanian DA, Bahr BA, Vouros P, et al. Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmospheric pressure chemical ionization-MS. Anal Chem. 2007;79:5582–5593. doi: 10.1021/ac0624086. [DOI] [PubMed] [Google Scholar]
- Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, et al. Mutant alpha-synuclein esacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2007;29:913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Liang Y, Krauss AH-P. Prostamides (prostaglandin-ethanolamides) and their pharmacology. Br J Pharmacol. 2008;153:410–419. doi: 10.1038/sj.bjp.0707434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Kakihana M, Chen LS, Ong M, Baird A, Gage FH. Cytokine regulation of nerve growth factor-mediated cholinergic neurotrophic activity synthesized by astrocytes and fibroblasts. J Neurochem. 1992;59:919–931. doi: 10.1111/j.1471-4159.1992.tb08331.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A) Generation of ‘double gene change’ mice; (B) double gene change genotyping by PCR.