Abstract
Background and Purpose
The function of the endocannabinoid system (ECS) in renal tissue is not completely understood. Kidney function is closely related to ion reabsorption in the proximal tubule, the nephron segment responsible for the re-absorption of 70–80% of the filtrate. We studied the effect of compounds modulating the activity of cannabinoid (CB) receptors on the active re-absorption of Na+ in LLC-PK1 cells.
Experimental Approach
Changes in Na+/K+-ATPase activity were assessed after treatment with WIN55,212-2 (WIN), a non-selective lipid agonist, and haemopressin (HP), an inverse peptide agonist at CB1 receptors. Pharmacological tools were used to investigate the signalling pathways involved in the modulation of Na+ transport.
Key Results
In addition to CB1 and CB2 receptors and TRPV1 channels, the mRNAs encoding for enzymes of the ECS were also expressed in LLC-PK1. WIN (10−7 M) and HP (10−6 M) altered Na+ re-absorption in LLC-PK1 in a dual manner. They both acutely (after 1 min) increased Na+/K+-ATPase activity in a TRPV1 antagonist-sensitive way. WIN's stimulating effect persisted for 30 min, and this effect was partially blocked by a CB1 antagonist or a PKC inhibitor. In contrast, HP inhibited Na+/K+-ATPase after 30 min incubation, and this effect was attenuated by a CB1 antagonist or a PKA inhibitor.
Conclusion and Implications
The ECS is expressed in LLC-PK1 cells. Both CB1 receptors and TRPV1 channels regulate Na+/K+-ATPase activity in these cells, and are modulated by lipid and peptide CB1 receptor ligands, which act via different signalling pathways.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Enzymesd |
| CB1 receptor | DGL |
| CB2 receptor | ERK1 |
| Ion channelsb | ERK2 |
| TRPV1 | FAAH |
| Transportersc | PKA |
| Na+/K+-ATPase | PKC |
| LIGANDS | |
|---|---|
| 2-AG | Capsazepine |
| AM251 | H89 |
| Anandamide | IBMX |
| Calphostin C | Ouabain |
| cAMP | WIN55,212-2 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,dAlexander et al., 2013a,b,c,d).
Introduction
In mammals, the endocannabinoid system (ECS) is classically represented by (i) several endogenous lipids, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG); (ii) cannabinoid receptors; and (iii) enzymes responsible for the synthesis and degradation of these lipids (Howlett, 2002). CB1 and CB2 receptors are members of the GPCR family, both of which are primarily coupled to the Gi protein leading to adenylate cyclase inhibition, thereby reducing cAMP production (Devane et al., 1988; Howlett et al., 1990). AEA can act on other receptors, such as TRPV1, an ion channel of the endovanilloid system (Zygmunt et al., 1999). Despite the well-known lipidic endogenous cannabinoids, Heimann and co-workers (2007) showed that haemopressin (HP), a nonapeptide produced by the in vitro cleavage of the α1chain of haemoglobin, acts as an inverse agonist of the CB1 receptor. In vivo, the major endogenous peptides that interact with the ECS are RVD-HP (RVDPVNFKLLSH) and VD-HP (VDPVNFKLLSH), which behave as agonists (Gomes et al., 2010). Although the ECS is abundantly expressed in the nervous system, it is also present in the immune, reproductive and renal systems (Devane et al., 1988; Howlett et al., 1990; Matsuda et al., 1990; Di Marzo et al., 1998). In the latter system, the ECS maintains renal function by its ability to promote the relaxation of the kidney endothelium (Deutsch et al., 1997). Cannabinoids also dilate afferent and efferent arteries, decrease the glomerular filtration rate (Koura et al., 2004), reduce blood pressure via the CB1 receptor, and increase urine volume due to their interaction with TRPV1 receptors (Li and Wang, 2006). However, the mechanisms related to its effects on renal tissue are not fully understood.
We investigated the presence of the ECS in LLC-PK1 (immortalized epithelial cells derived from pig kidney proximal tubule), a renal proximal tubule cell line, by measuring the expression of CB1 and CB2 receptors and the main enzymes involved in the synthesis and degradation of AEA and 2-AG [N-acylphosphatidylethanolamine-PLD (NAPE-PLD), fatty acid amide hydrolase (FAAH), diacylglycerol lipase (DGL) and monoacylglycerol lipase (MGL)], by biochemical, pharmacological and morphological analysis. We also hypothesized that lipidic and peptide cannabinoids might regulate Na+/K+-ATPase activity, a Na+ pump present in the renal proximal tubular epithelium that is important for maintaining the volume and composition of the extracellular fluid through different signalling pathways.
Methods
Nomenclature
All the drug/molecular target nomenclature conforms to BJP's Concise Guide to Pharmacology (Alexander et al., 2013a).
Kidney epithelial cell culture
LLC-PK1 cells (immortalized epithelial cells derived from pig kidney proximal tubule; ATCC – LLC-PK1: ATCC® CL-101™) were grown in DMEM supplemented with 10% FBS for 48 h at 37°C in an air with 5% CO2. Experiments involved cultures that were 90% confluent; the cells had adhered to the flask and their polarity was preserved, an important characteristic of this cell line. After treatment with the various drugs, the cells were washed with PBS, scraped from the flasks and homogenized. Total protein concentration was measured by the method of Lowry et al. (1951).
Quantitative real-time qRT-PCR
Total RNA was isolated from LLC-PK1 cells with TRIzol-Reagent (Sigma-Aldrich, Milan, Italy), reacted with DNase-I (1 U·mL−1; Sigma-Aldrich) for 15 min at room temperature, and followed by spectrophotometric quantification. The final preparation of RNA was essentially DNA-and protein-free when the ratio of readings at 260 and 280 nm were ≥1.7. Isolated mRNA was reverse-transcribed with iScript kit Reverse Transcriptase (Bio-Rad, Milan, Italy). Quantitative real-time PCR was carried out in a CFX384 real-time PCR detection system (Bio-Rad, Milan, Italy), with specific primers for the main enzymes involved in the synthesis and degradation of AEA or 2-AG (Table 1); SYBR Green was used as the detection agent (Bio-Rad, Segrate MI, Italy). Samples were amplified simultaneously in quadruplicate in one-assay run with a non-template blank for each primer pair as a control for contamination or the formation of primer-dimers; the ct (cycle threshold) value for each experimental group was determined. The housekeeping gene, ribosomal protein S16, an internal control to normalize the ct values using the formula 2−ΔCt. Differences in mRNA content between groups are as expressed as 2−ΔΔct (Iannotti et al., 2013).
Table 1.
Primers used for qRT-PCR in LLC-PK1 cells
| Forward primer | Reverse primer | |
|---|---|---|
| NAPE-PLD | 5′ACCGGCCTCTGAGAAAATGG3′ | 5′AGGGTTAACTGGGGAGACCT3′ |
| DGL-α | 5′GAAACCAAACACGCCTCCAC3′ | 5′CAACCCAGCAGCAAAGGAAC3′ |
| DGL-β | 5′TTTGTAATCCCGGACCACGG3′ | 5′ATTCTCGTTTCCCACACGCT3′ |
| FAAH | 5′TGCCACCGTGCAAGAAAATG3′ | 5′CCACTGCCCTAACAACGACT3′ |
| MGL | 5′CACTTCTCCGGCATGGTTCT3′ | 5′CGTGAAACGGCGTTGAGC3′ |
| CB1 | 5′CGCCAATGACATTCAGGGAAG3′ | 5′CGGGAATGGAGGATAACGCA3′ |
| CB2 | 5′TTTATAGCCTGGCCTCCCCT3′ | 5′TTTTCCCGTCTGCCTCTGTC3′ |
| TRPV1 | 5′TAAGACCAGCTCCAGCCAAG3′ | 5′AACTCGCTGTCCACCAGATG3′ |
| S16 | 5′TCATCAAAGTGAACGGGCGA3′ | 5′GACGAGGATCAGCTACCAGC3′ |
Electrophoresis and immunodetection
Proteins in the cell homogenate were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Laemmli, 1970). Immunodetection was performed by incubating the samples with a specific primary antibody (anti-ERK1/2 or anti-pERK1/2, 1:1000 dilutions) from Cell Signaling, for at least 24 h, whereas incubation with the secondary antibody (Peroxidase labelled ECL™; GE Healthcare, Buckinghamshire, UK) was for only 2 h. The proteins of interest were finally detected using the ECL system and a Hyperfilm (Hyperfilm-ECL – GE Healthcare).
Immunofluorescence
LLC-PK1 cells were grown on 13 mm coverslips for 48 h, after which they were washed with PBS (pH 7.4) and fixed for 15 min in paraformaldehyde (4%) in 0.1 M PBS. The coverslips were washed three times with PBS and incubated for 15 min with 0.5%Triton X-100 in PBS (v/v) before the samples were incubated for 2 h at room temperature with a blocking buffer (3% donkey serum in PBS). This buffer was removed and the primary antibodies of interest added [for Na+/K+-ATPase (1:100), CB1 (1:100), and CB2 (1:100) receptors and TRPV1(1:100)] before the samples were incubated for 24 h at 4°C. The coverslips were washed with PBS and fluorochrome-conjugated secondary antibody added for 3 h at room temperature in the dark. Finally, coverslips were examined by use of a fluorescence microscope (ApoTome 2® – Zeiss, Oberkochen, Germany).
Na+/K+-ATPase activity
Na+/K+-ATPase activity was measured colorimetrically by quantifying the difference in inorganic phosphate (Pi) released from ATP (Taussky and Shorr, 1953) in the absence and presence of ouabain (2 mM), a specific inhibitor of Na+/K+-ATPase. LLC-PK1 homogenates were prepared after treatment with the drugs in each experimental set.
cAMP quantification assay
cAMP content was measured by the competitive binding assay (De Mello, 1978). To promote the accumulation of cAMP, the LLC-PK1 cells were treated with IBMX (500 μM), an inhibitor of PDEs, for 15 min before the drugs were added. The reaction was stopped by the addition of TCA 100% (w/v) and the supernatant was transferred to plastic tubes for storage at −20°C for 24 h. After centrifugation (1000× g for 30 min), the supernatant was eluted through an ion-exchange resin column (Dowex AG50W-X8 200–400 mesh; Sigma-Aldrich, St. Louis, MO, USA) to remove TCA and nucleotides. Samples containing cAMP were incubated in the presence of the regulatory subunit of PKA and a trace amount of [3H]-cAMP to compete for the PKA site, using 50 mM acetate buffer at pH 4.0 for 90 min at 4°C. The reaction was stopped by adding 200 mM phosphate buffer at pH 6.0. The samples were filtered through Millipore (Merck, Darmstadt, Germany) filters (0.22 μm) and the bound radioactivity measured in a liquid scintillation analyser (Tri-Carb 2810 TR, Perkin Elmer, Hopkinton, MA, USA).
Statistical analysis
Assays were often done in quadruplicate to ensure reliability of individual independent values. Results are presented as mean ± SEM. Graphpad Prism®5 (La Jolla, CA, USA) software was used for analysing the results by one-way anova, followed by Tukey's post-test. P < 0.05 was taken as the significance level, and the number of experiments is shown in each figure legend.
Materials
All the materials used were of the highest analytical grade. Antibodies against cannabinoid receptors, CB1 and CB2 were from Proteimax (São Paulo, Brazil), Na+/ K+-ATPase from Sigma-Aldrich and TRPV1 from Santa Cruz (Dallas, TX, USA). Anti-ERK1/2 and phospho-ERK1/2 (pERK1/2) were from Cell Signaling (Beverly, MA, USA). WIN55,212-2 and AM251 were purchased from Cayman Chemical (Ann Arbor, MI, USA). HP, RVD-HP and VD-HP (RVD-HP and VD-HP) were obtained from Proteimax. H-89, Calphostin C and pertussis toxin (PTX) were from Sigma-Aldrich. Reagents for RT-PCR were from BioRad (Hercules, CA, USA), and primers were from Eurofins MWG (Ebersberg, Germany) operon (Table 1).
Results
The ability of LLC-PK1 cells to express the main components of the ECS was analysed first by qRT-PCR. Figure 1A shows that the main enzymes of the ECS, for AEA (NAPE-PLD) and 2-AG (DGLα and DGLβ) biosynthesis, and AEA (FAAH) and 2-AG (MGL) degradation are expressed in LLC-PK1 cells. Also, cannabinoid receptors (CB1, CB2) and TRPV1 were shown by qRT-PCR (Figure 1B) and immunofluorescence (Figure 2). The expression of Na+/K+-ATPase (red) was also obvious, and it was exclusively present in the basolateral membranes of these cells (third column in Figure 2). The expression of CB1 and CB2 receptors and TRPV1 appear as green, in rows A, B and C of Figure 2 respectively. The merged images show that the location of the CB1, CB2 and TRPV1 receptors is different from that of Na+/K+-ATPase, suggesting an apical distribution (fourth column, Figure 2). Negative controls for each marker are depicted in the first column (Figure 2).
Figure 1.
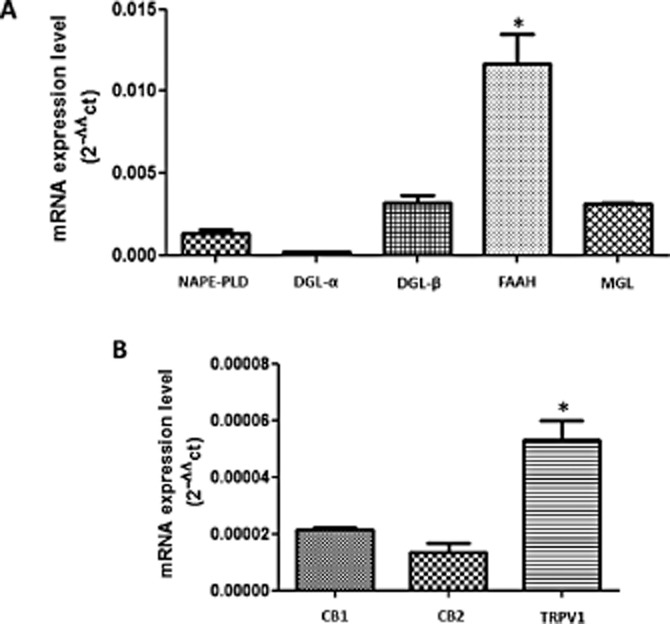
qRT-PCR for the main enzymes and receptors of ECS in LLC-PK1 cells. (A) Detection of mRNA for the enzymes responsible for AEA (NAPE-PLD) and 2A-G (DGL-α and β) synthesis, respectively. The main enzymes for the degradation of AEA and 2-AG, FAAH and MGL, respectively, were also expressed. (B) LLC-PK1 cells also possess the mRNA for CB1, CB2 and TRPV1 receptors. Data are means ± SEM of n = 3; *Indicates different from control (P < 0.05). We also calculated the Power Test (Error Type II β), which was 99.9%.
Figure 2.
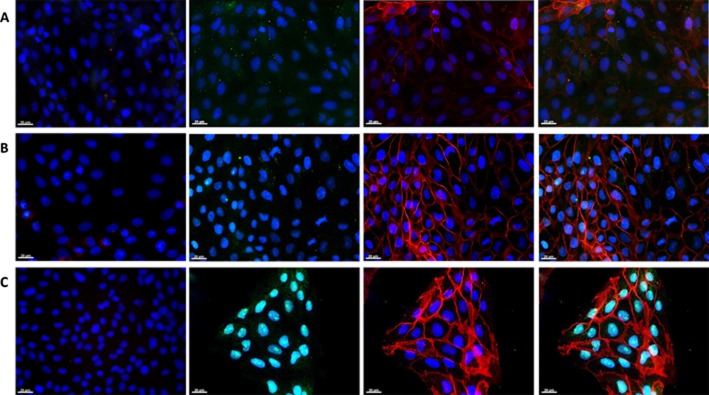
Immunofluorescence of CB1 and CB2 receptors, and TRPV1 channels. In all the panels, cell nuclei are marked in blue (DAPI), cannabinoid receptors are in green (Alexa 488) and Na+/K+-ATPase in red (Alexa 594). Rows are as follows (A) CB1, (B) CB2 and (C) TRPV1. Columns are as follows: first column is the negative control (no primary antibody incubation); the second shows the expression of each studied receptor; the third presents the Na+/K+-ATPase distribution; and the fourth is the merged image of each receptor (green) and Na+/K+-ATPase (red). Representative images from three different immunofluorescence assays for each receptor. Magnification 40×.
To test whether the Na+/K+-ATPase activity is modulated by cannabinoids, LLC-PK1 cells were treated with the non-selective CB1/CB2 agonist, WIN at different concentrations (10−9, 10−8 and 10−7 M). Figure 3A shows that only 10−7 M WIN lead to a significant increase in Na+/K+-ATPase activity after 30 min. Figure 3B shows that this effect initially occurs after 1 min and is sustained until 30 min of treatment. We also tested different concentrations (10−7 and 10−6 M) of HP, an inverse agonist of the CB1 receptor, and Figure 4A clearly shows that after 30 min of incubation both concentrations lead to a significant inhibition of Na+/K+-ATPase activity. Surprisingly, 10−6 M HP displayed a biphasic effect (Figure 4B), leading to a significant increase in the Na+/ K+-ATPase activity after 1 min, whereas it acted as a potent inhibitor of the pump after 15 min; this effect was sustained for 30 min. Thus HP behaved in an opposite manner to that observed for WIN at longer incubation times.
Figure 3.
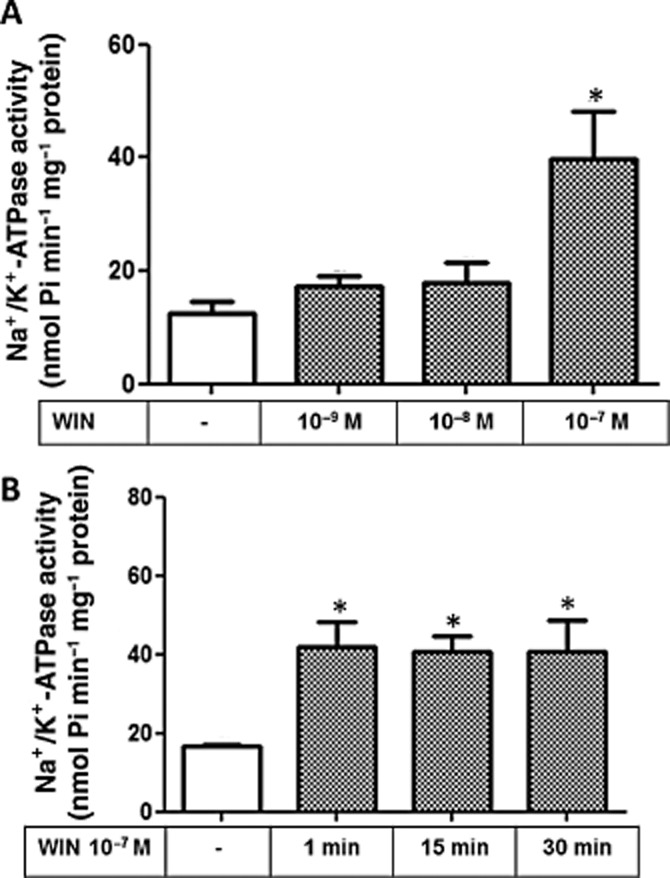
Effect of WIN on the Na+/K+-ATPase activity in LLC-PK1 cells. The Na+ /K+-ATPase activity was determined according to Methods. (A) The different concentrations of WIN used are depicted below the bars. (B) time-course of modulation of Na+/K+-ATPase activity of LLC-PK1 cells treated with 10−7 M WIN. Data are means ± SEM from six different experiments (n = 6); *Indicates different from control (P < 0.05).
Figure 4.
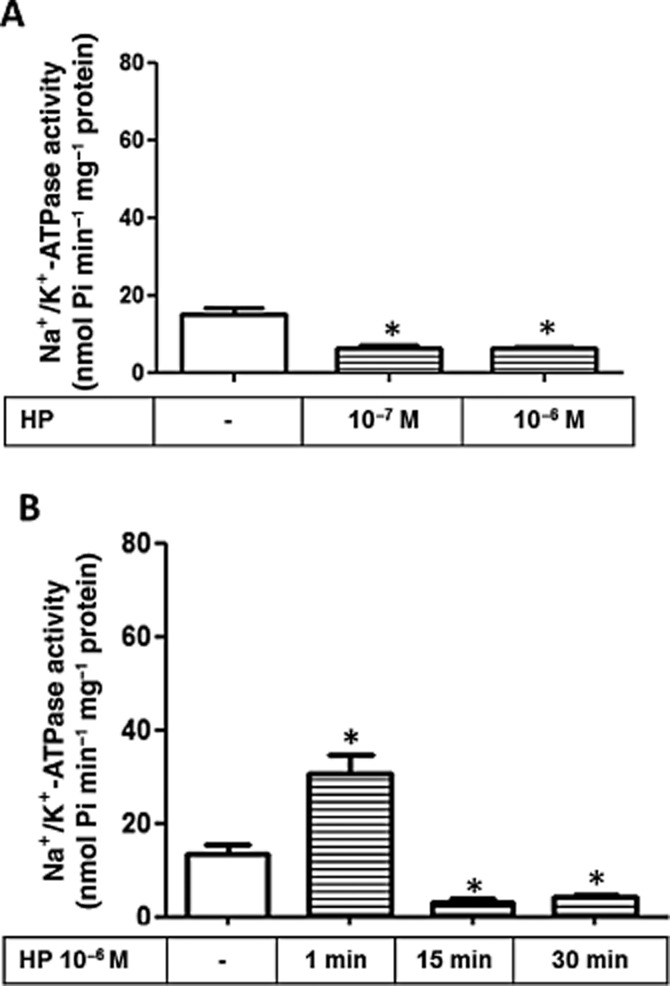
Effect of HP on Na+/K+-ATPase activity in LLC-PK1 cells. The Na+/K+-ATPase activity was determined according to Methods. (A) Cells were incubated for 30 min with two different concentrations of HP (10−7 M and 10−6 M), a CB1 inverse agonist, as depicted. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05). (B) Time-course analysis for effect of HP 10−6 M treatment on LLC-PK1 Na+/K+-ATPase activity. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05).
Since WIN is a non-selective CB1/CB2 receptor agonist, we tested whether the addition of AM251, a selective CB1 receptor antagonist, prevented the activation of Na+/K+-ATPase induced by WIN. AM251 at 10−7 M completely abolished the effect of WIN on Na+/K+-ATPase activity after 30 min, but had no effect at 1 min (Figure 5A). Similarly, 10−7 M AM251 had no effect on Na+/K+-ATPase activity stimulated by HP when pre-incubated for 1 min, but abolished the inhibitory effect of WIN observed after 30 min incubation (Figure 5B). These results suggest that Na+/K+-ATPase can be modulated by, at least, two different regulatory mechanisms induced by cannabinoids: (i) a fast one (1 min) that is independent of CB1 receptors; and (ii) a slow one (30 min) that is dependent on CB1 receptors.
Figure 5.
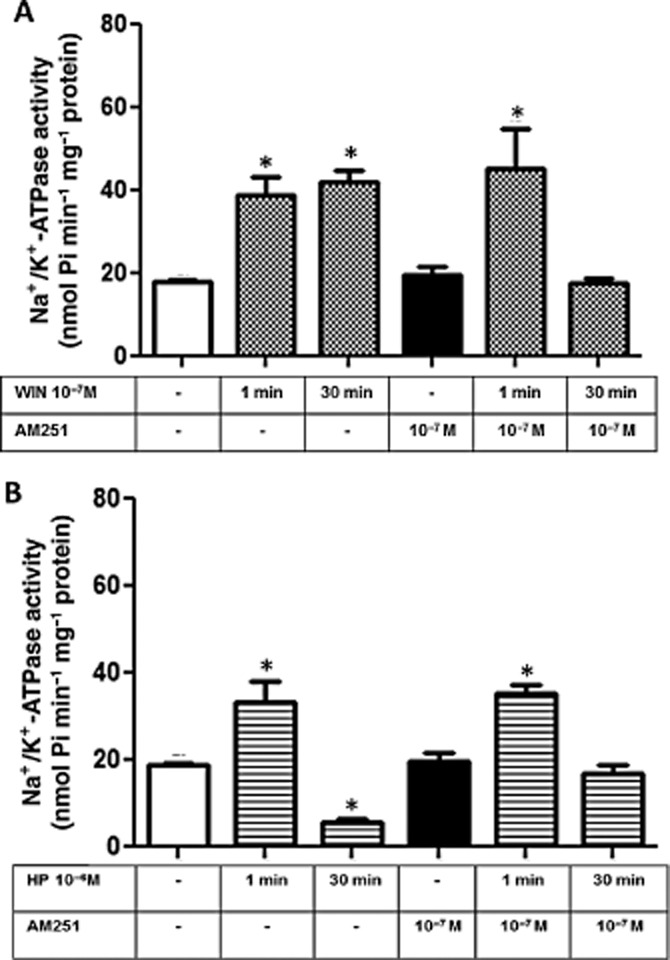
CB1 receptor is involved in the WIN and HP effects on Na+/K+-ATPase activity from LLC-PK1 cells. (A) Cells were pretreated for 15 min with the CB1 antagonist, AM251 (10−7 M), and then stimulated with 10−7 M WIN for 1 or 30 min. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05). (B) Cells were pretreated with AM251 (10−7 M) for 15 min before the incubation with HP at 10−6 M. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05).
Some cannabinoid drugs can trigger TRPV1 receptors in different cell lines (Jeske et al., 2006; Patwardhan et al., 2006). To explore a possible involvement of TRPV1 receptors in the modulation of Na+/K+-ATPase activity induced by cannabinoids, we first confirmed by immunofluorescence that the LLC-PK1 cells express TRPV1 (Figure 2C). Pre-incubation (10 min) of LLC-PK1 cells with 10−7 M capsazepine (CPZ), a TRPV1 antagonist, completely abolished the fast stimulating effect of either WIN or HP on Na+/K+-ATPase activity (Figure 6).
Figure 6.
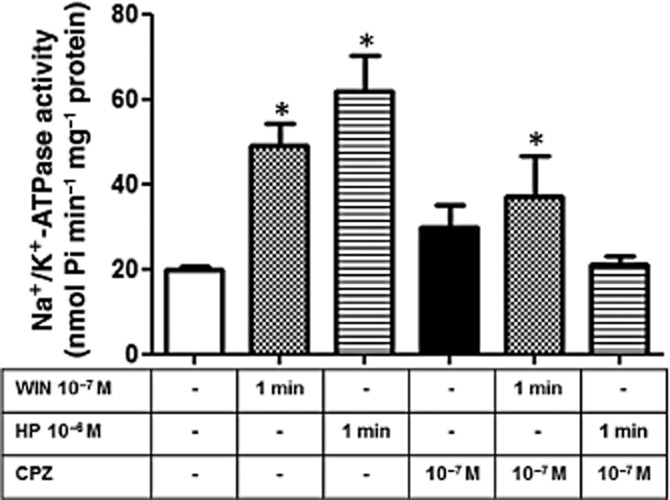
The TRPV1 channel participates in the modulation of Na+/K+-ATPase activity induced by WIN and HP in LLC-PK1 cells. Cells were pretreated for 15 min with CPZ (10−7 M), a TRPV antagonist, before the incubation with WIN (10−7 M) and HP (10−6 M). Na+/K+-ATPase activity was determined 1 min after agonist stimulation. Data are means ± SEM (n = 6); *Indicates different from control (P < 0.05).
To show that endogenous cannabinoid peptides are also able to trigger CB1 receptors in LLC-PK1 cells, we used RVD-HP and VD-HP. Figure 7 shows that both increased Na+/K+-ATPase activity.
Figure 7.
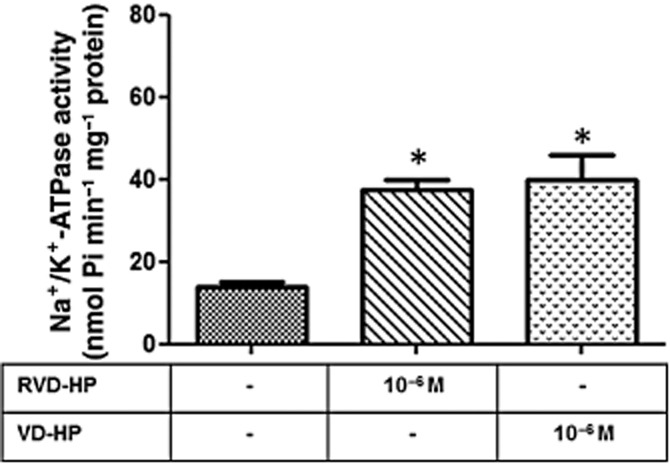
Effect of endogenous peptides on Na+/K+-ATPase activity in LLC-PK1 cells. Cells were incubated with RVD-HP and VD-HP (10−6 M), and Na+/K+-ATPase activity was determined after 30 min. Data are means ± SEM (n = 6); *Indicates different from control (P < 0.05).
The next step was to investigate the cell signalling pathway downstream of the activation of CB1 receptors. LLC-PK1 cells were pretreated with 100 ng·mL−1 PTX, an inhibitor of the αi subunit of G protein (Skorecki et al., 1987) and we did not observe any alteration in the regulatory effects of WIN or HP on the Na+/K+-ATPase activity (Figure 8A), which allowed us to rule out the participation of a Gi in the response to CB1 receptor activation. Furthermore, as the activation of CB1 receptors can both decrease or, less frequently, increase adenylate cyclase activity (Glass and Felder, 1997), we decided to measure the cAMP levels in LLC-PK1 cells upon stimulation by the cannabinoids. We observed that the addition of 10−7 M WIN for 30 min had no effect on the cytosolic cAMP levels. However, 10−6 M HP significant increased the cAMP levels after 30 min, an effect that was completely abolished by the pre-incubation of the cells with AM251 (Figure 8B). Due to the changes in cAMP levels, we also evaluated the possible role of PKA. After a 10 min pretreatment with 10−5 M H89, a permeable PKA inhibitor (Chijiwa et al., 1990), the stimulating effect induced by 10−7 M WIN on Na+/K+-ATPase activity was not impaired (Figure 9A). However, inhibition of PKA abolished the inhibitory effect induced by 10−6 M HP (Figure 9A).
Figure 8.
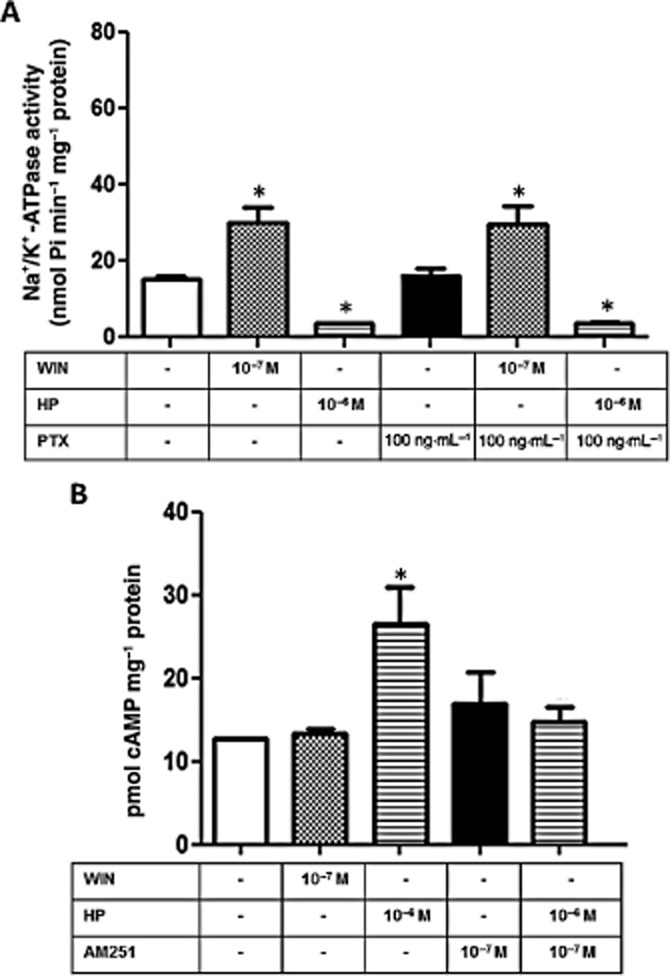
HP effect on Na+/K+-ATPase activity in LLC-PK1 cells involves an increase in cAMP levels. (A) Cells were pretreated for 24 h with PTX (100 ng·mL−1) before the incubation with WIN (10−7 M) or HP (10−6 M). Na+/K+-ATPase activity was determined after 30 min incubation. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05). (B) cAMP quantification was performed as described under Methods after 30 min incubation with WIN (10−7) or HP (10−6 M). Where indicated, cells were pretreated with the CB1 antagonist AM251 (10−7 M) for 15 min before the addition of WIN or HP. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05).
Figure 9.
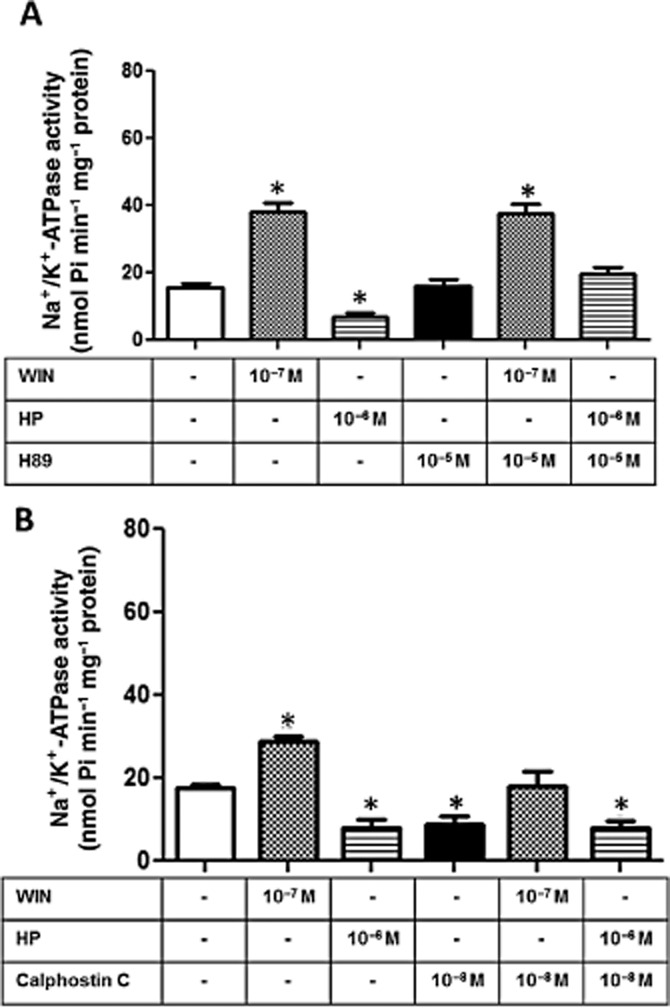
Involvement of PKA and PKC in the modulation of Na+/K+ -ATPase activity induced byHP and WIN. (A) When indicated, cells were pretreated for 15 min with H89 (10−5 M), a PKA cell-permeable inhibitor, before the incubation with WIN (10−7 M) or HP (10−6 M). Na+/K+-ATPase activity was determined after 30 min incubation. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05). (B) When indicated, cells were pretreated for 15 min with, calphostin C (10−8 M), a PKC inhibitor, before the incubation with WIN (10−7 M) or HP (10−6 M). Na+/K+-ATPase activity was determined after 30 min incubation. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05).
PKC-dependent events are very important and frequent in LLC-PK1 cells (Peruchetti et al., 2011). Therefore, we decided to investigate whether PKC was involved in the modulation of Na+/K+-ATPase by WIN or HP. Pre-incubation of the cells with 10−8 M calphostin C, a selective PKC inhibitor for classic and novel PKC isoforms at this concentration (Kinoshita et al., 1990), completely inhibited the stimulating effect of WIN on the Na+/K+-ATPase activity for 30 min, whereas it had no effect on the inhibitory action of HP (Figure 9B).
Finally, the activation of cannabinoid receptors is commonly associated with the activation of different signalling cascades that culminate in the activation of protein kinases involved in cell proliferation, survival and apoptosis (Bouaboula et al., 1995). In this scenario, MAPKs, such as the ERKs could be involved in the effects of cannabinoids in LLC-PK1 cells. Figure 10 shows that 10−6 M HP decreased ERK1/2 phosphorylation after 30 min, an effect that was abolished by the pretreatment with AM251. However, WIN had no effect on the phosphorylation of ERK1/2.
Figure 10.
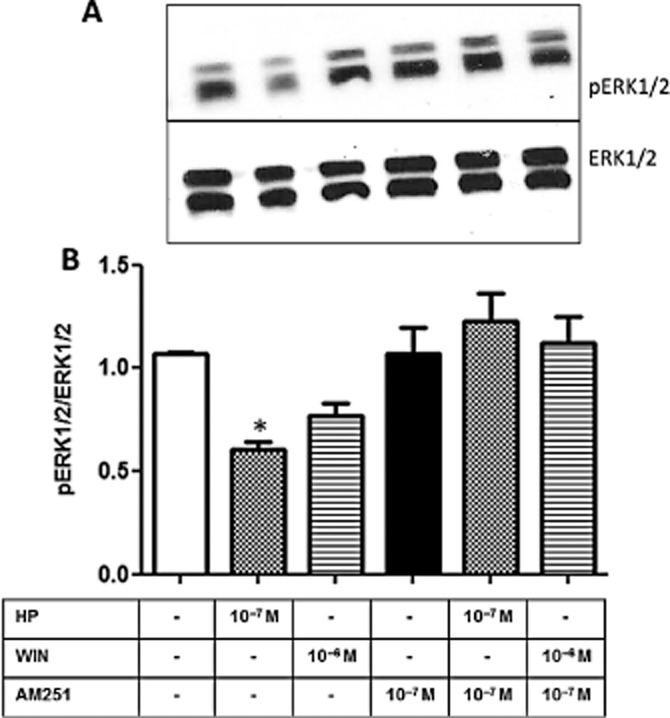
HP decreases ERK1/2 phosphorylation in LLC-PK1 cells. Whole cell homogenates were obtained from cultures treated with WIN (10−7 M), HP (10−6 M), in the absence or presence of the CB1 antagonist AM251 (10−7 M) for 30 min, as depicted. SDS-PAGE and immunoblotting were performed according to Methods. (A) Representative image of pERK1/2 and ERK 1/2 immunodetection. (B) Graph shows the ratio between pERK/ERK after densitometric analysis of the chosen bands in the different experimental situations. Data are means ± SEM from different experiments (n = 6); *Indicates different from control (P < 0.05).
Discussion
Since it was first described in the CNS, the ECS is now thought to be a regulator of different physiological mechanisms. More information is now available about this signalling system, but, as yet, there is little evidence for its role outside the CNS. In the present study, we have shown that the ECS can affect renal Na+ reabsorption by directly stimulating Na+/K+-ATPase in LLC-PK1 cells (kidney epithelia).
Na+/K+-ATPase, located in the basolateral membrane of tubular cells, is the main system responsible for Na+ reabsorption and the generation of a Na+ gradient across the epithelium, and is an important target for hormones, autacoids and bioactive molecules in general within the nephron segments (Schwartz et al., 1974). Recently, cannabinoids were shown to be modulators of Na+/K+-ATPase in different tissues; Araya and co-workers demonstrated that they can modulate synaptosomal Na+/K+-ATPase via stimulation of CB1 receptors (Araya et al., 2007).
Here we showed that renal epithelial cells express the mRNA for the main ECS enzymes and receptors, which were also detected by immunoblotting (Figures 1 and 2). When LLC-PK1 cells were stimulated by the CB agonist, WIN, there was a clear increase on Na+/K+-ATPase activity that was completely inhibited by the CB1 antagonist, AM251 (Figure 5). Conversely, the addition of HP, a haemoglobin-derived peptide, and CB1 inverse agonist (Heimann et al., 2007), led to a significant decrease in Na+/K+-ATPase activity, an effect that was also completely blocked by AM251 (Figure 5). These initial observations allowed us to demonstrate a potential role for the ECS in renal tissue, as it was able to regulate Na+/K+-ATPase through the activation of the CB1 receptor leading to effects on Na+ homeostasis. In spite of the presence of CB2 receptors, we did not investigate their participation in this phenomenon as the treatment with the specific CB1 antagonist (AM251) was enough to completely abolish the effects of WIN and HP on Na+/K+-ATPase activity (Figures 5 and 8). We do not rule out the involvement of CB2 receptors in other renal functions, and are currently studying its participation in lesion/repair processes.
Various studies have concluded that GPCR-dependent events are important controllers of different renal outcomes and mainly act on proximal tubule cells. These signalling cascades can start either from the apical and/or basolateral cell membranes, leading to a variety of biological effects including the modulation of Na+/K+-ATPase (Gomes and Soares-Da-Silva, 2002; Efendiev et al., 2003; Peruchetti et al., 2011). Among the different signalling systems already described for kidney tubular cells, we have now shown that the cAMP/PKA, PKC and ERK pathways are involved in the regulation of Na+/K+-ATPase activity by the ECS. While the incubation of LLC-PK1 cells with HP (30 min) increased the cAMP levels thus suggesting the involvement of a Gαs, the same was not true when the cells were stimulated by WIN (Figure 8B). These combined observations allowed us to affirm that lipidic and peptide endogenous cannabinoids could trigger different pools of CB1 receptors, thus stimulating different signalling pathways resulting in different effects on the Na+/K+-ATPase. Little is known about how HP interacts with CB1 receptors (Scrima et al., 2010) and how lipidic and peptides from the ECS differentially activate CB receptors. One explanation could be that endogenous lipids (AEA, 2-AG, etc) and RVD-HP or VD-HP (the endogenous peptides that function as selective agonists for CB1 receptors in vivo; Gomes et al., 2010), interact with different binding sites on the CB1 receptor. Our results support this view, as we observed that H89 abolished the inhibitory effect of HP on Na+/K+-ATPase activity, but had no effect on WIN treated cells. It was also demonstrated that while HP decreased the phosphorylation levels of ERK on LLC-PK1 cells, the same was not observed for WIN. Conversely, we observed that the PKC pathway also plays a role in the modulation of Na+/K+-ATPase activity induced by the ECS, as 10−8 M calphostin C blocked the stimulation of Na+/K+-ATPase induced by WIN, but had no effect on the inhibitory effect of HP (Figure 9B).
It is worth mentioning that activation of CB1 receptors has been shown to be involved in inflammation and cell death in different experimental models of disease, including kidney diseases. Feizi et al. (2008) showed that endocannabinoid analogues could prevent the appearance of characteristic lesions present in kidneys due to ischaemia and reperfusion injury. In addition, Mukhopadhyay et al. (2010) elegantly demonstrated that genetic deletion or pharmacological inhibition of CB1 receptors, either by AM281 or SR141716, significantly prevented some of the harmful effects induced by the chemotherapeutic drug cisplatin on renal remodelling, dysfunction, oxidative/nitrosative stress and cell death pathways, which are very common in this well studied model of kidney injury. Importantly, Barutta and co-workers (2010) showed that CB1 blockers are able to protect the kidney in diabetic rats. In this regard, our data indicate that the ECS is an important target for the prevention of kidney injuries and provide further evidence that HP, an inverse agonist of the CB1 receptor, might play an important role in kidney diseases, probably activating cAMP/PKA pathways, as shown for the LLC-PK1 cells.
Cannabinoids interact with other proteins (Jenkin et al., 2010), such as TRPV1, a member of the TRPV group of cation channels, stimulated by noxious and inflammatory signals such as capsaicin (Di Marzo and De Petrocellis, 2012). Therefore, it is not difficult to imagine that the rapid effects of cannabinoids on the Na+/K+-ATPase from LLC-PK1 cells could be due to their interaction with a non-selective cation channel. Indeed, these cells express TRPV1 on their membranes (Figures 1 and 2), and CPZ treatment completely abolished the effects of WIN or HP when incubated for 1 min (Figure 6). However, intramedullary infusion of methanandamide in the kidney has been shown to increase the urinary volume and consequently reduce blood pressure by activating CB1 receptors in a TRPV1-independent manner (Li and Wang, 2006). Also, Jenkin et al. (2010) demonstrated that the mRNA and protein for CB1, CB2 and TRPV1 receptors are expressed in human proximal tubular cells (HK2), and that CB1 receptor activation induced hypertrophy and apoptosis. In contrast, the treatment of HK2 cells with CB2 (AM630) and TRPV1 (SB366791) antagonists increased hypertrophy (Jenkin et al., 2010). Our data on pig proximal tubular cells corroborate these results obtained with human proximal tubule HK2 cells, where different receptors regulate cellular functions by activating distinct signalling pathways.
A notable regulatory role for PKA and PKC on Na+ active transporters has already been demonstrated in kidney tubular cells. PKC promotes the translocation of the Na+/K+-ATPase into the basolateral membrane and modulates the Na+ influx (Budu et al., 2002), whereas PKA phosphorylates the Na+/K+-ATPase leading to a decrease in its activity (Cheng et al., 1999). In the present study, we showed that both the PKA and PKC cascades are also triggered by the cannabinoid receptor ligands in a different manner: whereas WIN seems to increase Na+/K+-ATPase activity through the PKC pathway, whereas the inhibitory effect of HP seems to be mediated by PKA (Figure 9). It was previously shown that the activation of ERK 1/2 can be correlated to an increase in kidney Na+/K+-ATPase activity (Gildea et al., 2012). Here, we showed that HP promoted a significant decrease in ERK phosphorylation, which was completely abolished by pretreatment of the cells with the CB1 antagonist (Figure 10). As ERKs are known to modulate Na+/K+-ATPase, we did not investigate the involvement of other MAPKs that can be triggered by cannabinoid signalling. However, we do not rule out their involvement in other kidney processes.
In conclusion our data not only confirm the presence of the ECS in renal proximal tubule cells, but also suggest, for the first time, that this system is involved in the regulation of Na+ re-absorption by direct effects on Na+/K+-ATPase mediated through different cell signalling cascades. We also hypothesize that the renal ECS interacts with other hormonal systems that control Na+ homeostasis, including the renin-angiotensin and intra-renal dopamine axis. Our results support the view that the ECS in peripheral tissues can act in a ‘functionally different’ manner from that observed in the CNS. We also showed for the first time that the peptidic cannabinoids have an important role in renal tissue. Compared with other cannabinoid agonists, such as WIN, RVD-HP and VD-HP, HP has the opposite effect in renal tissue; WIN induces its effect by signalling through PKC, whereas HP triggers the cAMP/PKA and ERK pathways. Finally, we showed that the ECS can also regulate the Na+/K+-ATPase activity by stimulating TRPV1, a mechanism shared by both WIN and HP for short-term effects. Hence, HP appeared to exhibit the same behaviour as WIN after shorter incubation times, which further illustrates the complexity of the effects of the ECS in renal proximal tubule cells.
Acknowledgments
The authors would like to thanks Mr Celso Pereira for helpful technical assistance. The work was supported by Grants from CNPq-MCT, CAPES-PROBITEC, INCT (INBEB and INNT) and FAPERJ/CONICET.
Glossary
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- CPZ
capsazepine
- DGL
diacylglycerol lipase
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- HP
haemopressin (CB1 inverse agonist)
- LLC-PK1 cells
immortalized epithelial cells derived from pig kidney proximal tubule
- MGL
monoacylglycerol lipase
- NAPE-PLD
N-acylphosphatidylethanolamine-PLD
- pERK1/2
phospho-ERK1/2
- PTX
pertussis toxin
- RVD-HP
RVD-haemopressin (haemoglobin-derived peptides; CB1 agonists)
- VD-HP
VD-haemopressin (CB1 agonist)
- WIN
WIN55,212-2
Author contributions
L. S. S. performed experiments, experimental design, discussion of data and written of the manuscript. R. T. D. S. performed experimental design and discussion of data. D. L. performed experiments. C. L. C. S. performed experiments. F. A. I. performed experiments and discussion of data. E. M. performed experiments. V. D. M. performed experimental design, discussion of data and written of the manuscript. A. V. performed discussion of data and written of the manuscript. R. A. M. R. performed experimental design, discussion of data and written of the manuscript. M. E.-L. performed experimental design, discussion of data and written of the manuscript.
Conflict of interest
Authors declare no conflict of interests.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The concise guide to pharmacology 2013/14: ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: transporters. Br J Pharmacol. 2013c;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: enzymes. Br J Pharmacol. 2013d;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya KA, David Pessoa Mahana C, González LG. Role of cannabinoid CB1 receptors and Gi/o protein activation in the modulation of synaptosomal Na+, K+-ATPase activity by WIN55,212-2 and delta(9)-THC. Eur J Pharmacol. 2007;572:32–39. doi: 10.1016/j.ejphar.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, et al. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes. 2010;59:1046–1054. doi: 10.2337/db09-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budu CE, Efendiev R, Cinelli AM, Bertorello AM, Pedemonte CH. Hormonal-dependent recruitment of Na+,K+-ATPase to the plasmalemma is mediated by PKC beta and modulated by [Na+]i. Br J Pharmacol. 2002;137:1380–1386. doi: 10.1038/sj.bjp.0704962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SX, Aizman O, Nairn AC, Greengard P, Aperia A. [Ca2+] determines the effects of protein kinases A and C on activity of rat renal Na+,K+-ATPase. J Physiol. 1999;518:37–46. doi: 10.1111/j.1469-7793.1999.0037r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- De Mello FG. The ontogeny of dopamine-dependent increase of adenosine 3′,5′-cyclic monophosphate in the chick retina. J Neurochem. 1978;31:1049–1053. doi: 10.1111/j.1471-4159.1978.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos Trans R Soc Lond B Biol Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem. 2003;278:28719–28726. doi: 10.1074/jbc.M303741200. [DOI] [PubMed] [Google Scholar]
- Feizi A, Jafari MR, Hamedivafa F, Tabrizian P, Djahanguiri B. The preventive effect of cannabinoids on reperfusion-induced ischemia of mouse kidney. Exp Toxicol Pathol. 2008;60:405–410. doi: 10.1016/j.etp.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Gildea JJ, Wang X, Shah N, Tran H, Spinosa M, Van Sciver R, et al. Dopamine and angiotensin type 2 receptors cooperatively inhibit sodium transport in human renal proximal tubule cells. Hypertension. 2012;60:396–403. doi: 10.1161/HYPERTENSIONAHA.112.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Dale CS, Casten K, Geigner MA, Gozzo FC, Ferro ES, et al. Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 2010;12:658–669. doi: 10.1208/s12248-010-9217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P, Soares-da-Silva P. Role of cAMP-PKA-PLC signaling cascade on dopamine-induced PKC-mediated inhibition of renal Na(+)-K(+)-ATPase activity. Am J Physiol Renal Physiol. 2002;282:1084–1096. doi: 10.1152/ajprenal.00318.2001. [DOI] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, de Souza LL, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–20593. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Bidaut-Russell M, Devane WA, Melvin LS, Johnson MR, Herkenham M. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13:420–423. doi: 10.1016/0166-2236(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Iannotti FA, Piscitelli F, Martella A, Mazzarella E, Allarà M, Palmieri V, et al. Analysis of the ‘endocannabinoidome’ in peripheral tissues of obese Zucker rats. Prostaglandins Leukot Essent Fatty Acids. 2013;89:127–135. doi: 10.1016/j.plefa.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Jenkin KA, McAinch AJ, Grinfeld E, Hryciw DH. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell Physiol Biochem. 2010;26:879–886. doi: 10.1159/000323997. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Takahashi I, Kobayashi E, Tamaoki T, Irie K, Koshimizu K, et al. Suppression by protein kinase C inhibitors of tumor promoter enhancement of Epstein-Barr virus-induced growth transformation. Anticancer Res. 1990;10:1051–1054. [PubMed] [Google Scholar]
- Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, et al. Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol. 2004;15:1488–1494. doi: 10.1097/01.asn.0000130561.82631.bc. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J, Wang DH. Differential mechanisms mediating depressor and diuretic effects of anandamide. J Hypertens. 2006;24:2271–2276. doi: 10.1097/01.hjh.0000249706.42230.a8. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Pan H, Rajesh M, Bátkai S, Patel V, Harvey-White J, et al. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol. 2010;160:657–668. doi: 10.1111/j.1476-5381.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci USA. 2006;103:11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruchetti DB, Pinheiro AA, Landgraf SS, Wengert M, Takiya CM, Guggino WB, et al. (Na+ + K+)-ATPase is a target for phosphoinositide 3-kinase/protein kinase B and protein kinase C pathways triggered by albumin. J Biol Chem. 2011;286:45041–45047. doi: 10.1074/jbc.M111.260737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz IL, Shlatz LJ, Kinne-Saffran E, Kinne R. Target cell polarity and membrane phosphorylation in relation to the mechanism of action of antidiuretic hormone. Proc Natl Acad Sci USA. 1974;71:2595–2599. doi: 10.1073/pnas.71.7.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima M, Di Marino S, Grimaldi M, Mastrogiacomo A, Novellino E, Bifulco M, et al. Binding of the hemopressin peptide to the cannabinoid CB1 receptor: structural insights. Biochemistry. 2010;49:10449–10457. doi: 10.1021/bi1011833. [DOI] [PubMed] [Google Scholar]
- Skorecki KL, Verkman AS, Ausiello DA. Cross talk between stimulatory and inhibitory guanosine 5′-triphosphate binding proteins: role in activation and desensitization of the adenylatecyclase response to vasopressin. Biochemistry. 1987;26:639–645. doi: 10.1021/bi00376a040. [DOI] [PubMed] [Google Scholar]
- Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


