Abstract
Background and Purpose
Memory impairment can be progressive in neurodegenerative diseases, and physiological ageing or brain injury, mitochondrial dysfunction and oxidative stress are critical components of these issues. An early clinical study has demonstrated cognitive improvement during erythropoietin treatment in patients with chronic renal failure. As erythropoietin cannot freely cross the blood–brain barrier, we tested EH-201 (2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside, also known as TSG), a low MW inducer of erythropoietin, for its therapeutic effects on memory impairment in models of neurodegenerative diseases, physiological ageing or brain injury.
Experimental Approach
The effects of EH-201 were investigated in astrocytes and PC12 neuronal-like cells. In vivo, we used sleep-deprived (SD) mice as a stress model, amyloid-β (Aβ)-injected mice as a physiological ageing model and kainic acid (KA)-injected mice as a brain damage model to assess the therapeutic effects of EH-201.
Key Results
EH-201 induced expression of erythropoietin, PPAR-γ coactivator 1α (PGC-1α) and haemoglobin in astrocytes and PC12 neuronal-like cells. In vivo, EH-201 treatment restored memory impairment, as assessed by the passive avoidance test, in SD, Aβ and KA mouse models. In the hippocampus of mice given EH-201 in their diet, levels of erythropoietin, PGC-1α and haemoglobin were increased
Conclusions and Implications
The induction of endogenous erythropoietin in neuronal cells by inducers such as EH-201 might be a therapeutic strategy for memory impairment in neurodegenerative disease, physiological ageing or traumatic brain injury.
Tables of Links
| TARGETS |
|---|
| Enzymes |
| HO-1, haem oxygenase-1 |
| LIGANDS |
|---|
| EPO, erythropoietin |
| KA, kainic acid |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Memory impairment can be progressive in neurodegenerative diseases and physiological ageing, or immediate in brain injury. Neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD), are a heterogeneous group of disorders characterized by a progressive and selective loss of memory (Huber and Paulson, 1987; Walsh and Selkoe, 2004; Weintraub et al., 2004). Memory also declines with advancing age (Craik and Rose, 2012) or after traumatic brain injury (Vakil, 2005). Regardless of its heterogeneity, mitochondrial dysfunction and oxidative stress are critical components, common to these conditions (Cui et al., 2006; Manczak et al., 2006; Schapira, 2008). Sleep has been implicated in the plastic cerebral changes that underlie learning and memory (Maquet, 2001). Sleep deprivation (SD) also results in memory loss (Chee and Chuah, 2008) and impaired mitochondrial activity (Andreazza et al., 2010). Memory loss is accompanied by the accumulation of oxidative damage to lipids, proteins and nucleic acids and by mitochondrial decay, all of which can disrupt neuronal function in ageing and disease (Harman, 1992). Increased brain oxidative stress is widely associated with cognitive impairment caused by normal ageing and neurodegenerative diseases (Silva et al., 2004). Patients with dementias, such as AD, often have nocturnally disrupted sleep (Ismail et al., 2009). Diverse evidence suggests that amyloid-β (Aβ) peptides have a causal role in the pathogenesis of these diseases (Palop and Mucke, 2010). Mitochondria are a direct site of Aβ accumulation in AD neurons, and mitochondrial dysfunction is a hallmark of AD (Manczak et al., 2006; Pagani and Eckert, 2011). Haemoglobin is the main oxygen transporter in erythrocytes. Recently, haemoglobin was unexpectedly found in many non-hematopoietic cells, and it is possible that it facilitates tissue oxygen transport or increases cellular oxygenation and therefore provides an intrinsic protective mechanism against hypoxic/ischaemic injury (Nishi et al., 2008; Biagioli et al., 2009; Tezel et al., 2010).
Recent studies implicate excitotoxicity in a variety of neuropathological conditions. Excitotoxicity is involved in a variety of neurodegenerative diseases such as AD, PD and HD (Mehta et al., 2013). Kainic acid (KA), an analogue of excitotoxic glutamate, is 30-fold more potent as a neurotoxin than glutamate, and KA-mediated excitotoxicity is used as a model for neurodegeneration. Administration of KA can induce a Ca2+ influx, production of reactive oxygen species (ROS), mitochondrial dysfunction and apoptosis in the neurons of the brain (Wang et al., 2005; Zhang and Zhu, 2011).
Erythropoietin (EPO), originally characterized as a hematopoietic cytokine produced in the kidney (Goldwasser, 1975), has been shown to have many neuroprotective effects in the CNS (van der Kooij et al., 2008). Both EPO and the EPO receptor are expressed in neurons and astrocytes, and EPO is produced primarily by astrocytes in the brain (Juul et al., 1999). Recombinant human EPO (rhEPO) is widely used to enhance erythropoiesis in patients with anaemia and has recently been found to have many non-hematopoietic beneficial effects, including cardioprotection (Parsa et al., 2003) and neuroprotection (Digicaylioglu and Lipton, 2001). An early clinical study has demonstrated cognitive improvement during EPO treatment among patients with chronic renal failure (Pickett et al., 1999). Recently, studies have shown that high-dose EPO treatments improve hippocampal plasticity and cognitive performance in patients suffering from neuropsychiatric diseases (Ehrenreich et al., 2007). However, rhEPO, given in low doses to treat anaemia or in high doses for tissue protection, increased mortality (Lund et al., 2014). In patients with ischaemic stroke, administration of only three high doses of rhEPO caused an almost twofold increase in the overall death rate (Ehrenreich et al., 2009). Therefore, the development of an inducer of this tissue-protective, endogenous EPO signalling which lacks the adverse side effects of systemic exogenous EPO is necessary.
Radix Polygoni multiflori is a traditional Chinese medicine for the treatment of anaemia, liver disease and other diseases commonly associated with ageing (Huang et al., 2007). 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside (TSG) is one of the active compounds extracted from the root of Radix Polygoni multiflori and this compound improved learning memory in both the AD model of amyloid precursor protein (APP) transgenic mice and aged rats, and was protective against cerebral ischaemic injury both in vivo and in vitro (Zhang et al., 2006; Wang et al., 2007). Recently, TSG has been shown to exhibit a protective effect against APP+-induced cell death (Sun et al., 2011). Although reports demonstrate that TSG has neuroprotective effects in neurodegenerative disorders, which are attributed to its antioxidant and free radical-scavenging properties, the pharmacological mechanism underlying these effects of TSG remains unknown. We have referred to TSG in an earlier study (Hsu et al., 2013) and here as EH-201. In the earlier study we showed EH-201 to be a potent inducer of EPO in non-haematopoietic cells. Also EH-201 showed a therapeutic effect, instead of only a preventive effect, after cisplatin-induced nephropathy or doxorubicin-induced cardiomyopathy was established in mice (Hsu et al., 2013). In the present study, we investigated the hypothesis that EPO may play a pivotal role in the treatment of the induced impairment of hippocampal learning and memory, by modulating downstream mitochondrial regulator expression as well as inducing haemoglobin, in neuronal cells. The clinical use of EPO targeting the CNS has been limited by its lack of permeation through the blood–brain barrier (BBB) (Lieutaud et al., 2008). This means that, in order to attain neuroprotective leves in the CNS, systemic high-dose rhEPO would have to be given, with the consequent unacceptable side effects (Juul et al., 2004).
As an alternative, we used EH-201 to induce EPO in neuronal cells and found EPO-mediated activation of mitochondrial function through PPAR-γ coactivator 1α (PGC-1α) and haemoglobin-β expression in these cells. We also were able to show, in vivo, recovery of memory impairment in SD, Aβ and KA mouse models after treatment with dietary EH-201. Therefore, our results suggest that the induction of EPO in neuronal cells might be a therapeutic strategy for memory impairment in neurodegenerative diseases or physiological ageing.
Methods
Animals
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of National Yang-Ming University. All efforts were made to minimize animal suffering, to reduce the number of animals used and to utilize alternatives to in vivo techniques, if available. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 110 animals were used in the experiments described here.
Eight to 12-week-old specific pathogen-free C57BL/6J mice were obtained from the National Laboratory Animal Center (Taipei, Taiwan). The animals were housed five to six per cage at a constant temperature of 22 ± 2°C, with a 12 h light–dark cycle. Rodent chow (PMI, Brentwood, MO, USA) and water were provided ad libitum.
Cell culture
Astrocyte-enriched cultures were prepared from 1-day-old C57BL/6J mice obtained from the Animal Center at the National Yang-Ming University. Briefly, cortical tissue was digested with trypsin, and the resulting dissociated cells were suspended in DMEM containing 10% FBS (Life Technologies, Grand Island, NY, USA) and incubated in a 10 cm dish for 7–8 days before use (Chou et al., 2003). Cells prepared by this method consisted of approximately 90–95% astrocytes as determined by immunochemical staining with an antibody against glial fibrillary acidic protein, a specific marker for astrocytes. Rat pheochromocytoma (PC12; PATCC, Manassas, VA, USA) cells were maintained in RPMI 1640 medium containing 5% FBS and 10% horse serum. All cultures were incubated at 37°C in a humidified atmosphere of 5% CO2.
RNA isolation and real-time quantitative PCR
Total RNA was extracted using the RNA-Bee™ reagent (Tel-Test, Friendswood, TX, USA) and reverse transcribed by AMV-RT (Promega, Madison, WI, USA). EPO, PGC-1α, haemoglobin-β, haem oxygenase-1 (HO-1) and GAPDH mRNA expression was quantified by real-time PCR with an ABI Prism 7500 Sequence Detection System using SYBR® Green Master Mix (Applied Biosystems, Foster City, CA, USA). All data were normalized to GAPDH. The relative expression levels were calculated by the 2–ΔΔCT method. All primer sequences are listed in Supporting Information Table S1.
Western blot
Cell and brain tissue lysates were prepared using a radioimmunoprecipitation assay lysis buffer. Approximately 20 μg of protein was loaded, and Western blot analysis was performed using antibodies against EPO, PGC-1α, haemoglobin-β (Santa Cruz, CA, USA) and GAPDH (Abcam, Cambridge, UK). A HRP-conjugated anti-IgG secondary antibody was used for enhanced chemiluminescence detection (Amersham, Buckinghamshire, UK).
Succinate dehydrogenase assay
Astrocytes or PC12 cells were plated at 104 cells per well in 96-well plates. Twenty-four hours later, the cells were incubated with or without EPO or EH-201-containing media (100 μL per well) for 48 h. Succinate dehydrogenase activity was determined by an MTT reduction assay. The activity was normalized to the cellular protein level with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA), and changes in absorbance were measured using a microplate reader (PerkinElmer Life Sciences Wallac Victor2; PerkinElmer, Waltham, MA, USA). Activity was expressed relative to the control condition.
Intracellular ROS generation
Astrocytes and PC12 cells were treated with EPO or EH-201 for 24 h. The culture medium was replaced with 100 μM H2O2, and cells were incubated for 6 h (astrocytes) or 30 min (PC12 cells). ROS production in cells was then measured using 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Molecular Probes, Eugene, OR, USA). Cells were incubated with 50 μM DCFH-DA at 37°C for 30 min, and fluorescence intensity was measured using a fluorescence microplate reader (PerkinElmer Life Sciences Wallac Victor2).
H2O2-induced cytotoxicity in astrocytes and neuron-like PC12 cells
Astrocytes and PC12 cells were treated with EPO or EH-201 for 24 h. Astrocyte culture medium was replaced with 500 μM H2O2, and the cells were incubated for 6 h. PC12 cell culture medium was replaced with 250 μM H2O2, and the cells were incubated for 4 h. Cell viability was determined by the exclusion of Trypan blue as assessed by light microscopy.
SD procedure
The method of SD has been previously described (Vecsey et al., 2009). Briefly, 40 C57BL/6J male mice (12 weeks of age) were housed on a 12/12 h light/dark schedule with lights on at 0600 h and were handled for 7 days. The mice were trained to complete the passive avoidance test, and the animals that did not master this task were excluded from the experiments. Because short-term SD is a quickly reversible model of memory impairment, the qualified mice (29/40) were randomly divided into five groups: control (n = 5) and SD-only groups (n = 6) were fed ad libitum with a chow diet, SD + EH-201 groups were fed ad libitum with a chow diet containing different doses of EH-201 (50, 100 or 200 mg·kg−1 per day, n = 6 for each group) for 3 days before SD; preparation of the EH-201 diet is described later. The average food intake was 4 g chow per day for each mouse (average body weight was 25 g). The SD and SD + EH-201 groups of mice were sleep-deprived in their home cages for 5 h by gentle handling beginning at 0600 h, and control mice were left undisturbed (non-SD mice). After completing all behavioural tests, the mice were killed by i.p. injection of a lethal dose of sodium pentobarbital. The brains were removed and the hippocampus was isolated rapidly for RNA isolation and Western blot analysis.
i.c.v. injection
i.c.v. injection has been described in a previous study (Park et al., 2011). Briefly, C57BL/6J female mice (12 weeks of age) were anaesthetized by i.p. injection of sodium pentobarbital (50 mg·kg−1) and placed on a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). The depth of anaesthesia was monitored by assessing the reaction to toe pinch and skin pinch tests and also by determining jaw tone. Each mouse was injected with KA or Aβ25–35 at 1 mm lateral to the midline, 0.5 mm posterior to the bregma and 3.5 mm deep using a stereotaxic injector (KD Scientific, Holliston, MA, USA) in a total volume of 3 or 5 μL at a rate of 0.5 μL·min−1. The sham-operated groups were injected with PBS.
KA injection model
Thirty-four C57BL/6J female mice (12 weeks of age) were subjected to KA injection (n = 28), and the control group was injected with PBS (n = 6). Briefly, KA (0.15 μg per 3 μl PBS; Sigma-Aldrich, St. Louis, MO, USA) was injected into the brain (i.c.v.) and induced epileptiform seizures (seizure score 3–4) (Racine, 1972). The KA-injected mice were randomly divided into four groups. Mice were fed ad libitum with a chow diet or a chow diet containing different concentrations of EH-201 (50, 100 or 200 mg·kg−1·day−1, n = 7 for each group) beginning on day 6 after KA-induced memory impairment. KA intoxication resulted in long-term damage in the brain, which means a longer recovery period is required for this model, to evaluate the effect of EH-201. Learning and memory tests were performed on day 13 and day 20 after the operation. After 2 weeks of EH-201 treatment and completing all behavioural tests, the mice were killed by i.p. injection of a lethal dose of sodium pentobarbital. The brains were removed and the hippocampus was isolated rapidly for RNA isolation and Western blot analysis.
Aβ25–35 injection model
Thirty-six C57BL/6J male mice (8 weeks of age) were subjected to Aβ25–35 injection (n = 31), and the control group was injected with PBS (n = 5). Briefly, Aβ25–35 (AnaSpec, Fremont, CA, USA) was dissolved in sterile saline at a concentration of 2 μg·μL−1 and aggregated by incubation at 37°C for 4 days before use (Maurice et al., 1996). Aβ25–35 (10 μg per 5 μL PBS) was injected into the brain (i.c.v.) to induce memory loss. After PBS or Aβ25–35 injection, the mice were trained in the passive avoidance test and 7/31 mice were excluded from the experiments. The 24 qualified Aβ25–35-injected mice were randomly divided into four groups. The Aβ25–35-injected mice were fed ad libitum with a chow diet or a chow diet containing different concentrations of EH-201 (50, 100 or 200 mg·kg−1·day−1, n = 6 for each group) beginning on day 10 after Aβ25–35-induced memory impairment. Aβ25–35 intoxication resulted in long-term damage in the brain, which means longer recovery period is required for this model to evaluate the effect of EH-201. Learning and memory tests were performed on day 17 and day 24 after the operation. After 2 weeks of EH-201 treatment and completing all behavioural tests, the mice were killed by i.p. injection of a lethal dose of sodium pentobarbital. The brains were removed and the hippocampus was isolated rapidly for RNA isolation and Western blot analysis.
Passive avoidance task
Passive avoidance experiments were conducted as previously described with minor modifications (Jarvik and Kopp, 1967). A two-way shuttle box with a guillotine door placed between the modular testing chambers was employed. One chamber was illuminated with a 40 W bulb, whereas the other chamber remained dark. In the training session, the animals were individually placed in the illuminated chamber that faced away from the guillotine door. When the animal entered the darkened chamber, the door was silently lowered and a 0.5 mA foot shock was applied for 2 s through the grid floor. In the test sessions, the animals were again placed in the illuminated chamber, but no foot shock was applied. Latency to step through was recorded in each session.
Data analysis
All results are expressed as the mean and SD. The significance of differences of means between more than two groups was determined using one-way anova followed by Tukey's post hoc test. Student's t-test was employed for the statistical comparison of paired samples. A P-value of <0.05 was considered statistically significant.
Materials
EH-201 was purified from Polygonum multiflorum Thunb., as described earlier (Hsu et al., 2013) to 99.7% purity. Chow diet containing EH201 was made in our laboratory. Briefly, we crushed Purina chow diet and then mixed it with solutions of EH-201 to provide the appropiirate content of EH-201, and reformed to the format of original Purina chow diet by lyophilization. EPO was supplied by Roche (Mannheim, Germany).
Results
EPO stimulated expression of the mitochondrial regulator PGC-1α and increased mitochondrial activity in primary astrocytes and neuron-like PC12 cells
Because EPO has been reported to activate mitochondrial biogenesis (Carraway et al., 2010) and haemoglobin expression (Tezel et al., 2010) in non-haematopoietic cells, we assessed the effects of EPO on primary astrocytes and PC12 cells. Real-time PCR analysis showed that rhEPO treatment for 24 h increased mRNA expression οf the mitochondrial regulator PGC-1α in primary astrocytes (Figure 1A) and PC12 cells (Figure 1C). rhEPO also induced mitochondrial activity in both astrocytes (Figure 1B) and PC12 cells (Figure 1D).
Figure 1.
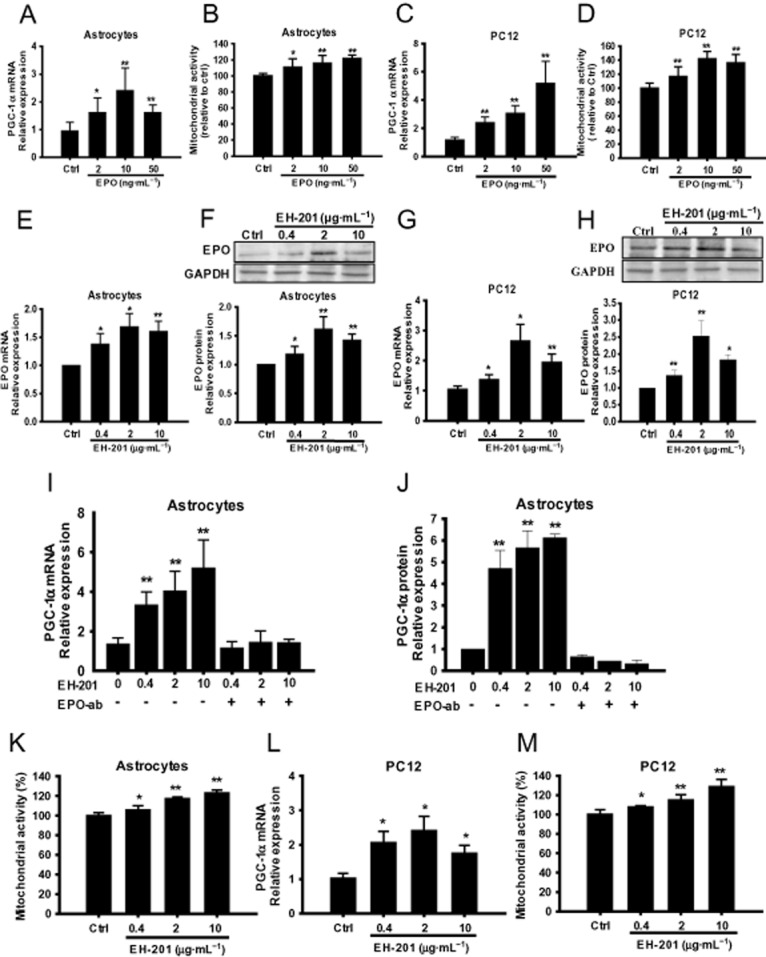
EPO and EPO inducer EH-201 stimulate mitochondrial activity in primary astrocytes and neuron-like PC12 cells. (A, C) EPO treatment for 24 h increased PGC-1α mRNA expression in primary astrocytes and PC12 cells. The expression of GAPDH was used as an internal control. The results are expressed as the relative index of untreated controls (means± SD) from at least three independent measurements. *P < 0.05, **P < 0.01; significantly different from control; one-way anova followed by Tukey's multiple comparison test. (B, D, K, M) Mitochondrial activity was determined by the succinate dehydrogenase activity of astrocytes and PC12 cells that were treated with rhEPO or EH-201 for 24 h using an MTT reduction assay and is expressed relative to the respective control conditions. The values indicate the means ± SD (n = 8), *P < 0.05, **P < 0.01 compared with untreated controls,; Student's t-test. (E, F) EH-201 treatment for 24 h increased EPO mRNA and protein expression in astrocytes and (G, H) PC12 cells. (I, J) EH-201-treated primary astrocytes with or without the neutralizing EPO antibody (nEPO-ab, 1 μg·mL−1) were analysed for PGC-1α mRNA and protein expression. (L) EH-201 treatment for 24 h increased PGC-1α mRNA expression in PC12 cells. The expression of GAPDH was used as an internal control. The results are expressed as the relative index of untreated controls means ± SD from at least three independent measurements. *P < 0.05, **P < 0.01, significantly different from control; by one-way anova followed by Tukey's multiple comparison test.
EH-201 is a neuronal EPO inducer that elevated the expression of the mitochondrial regulator PGC-1α and increased mitochondrial activity in primary astrocytes and neuron-like PC12 cells
We tested the ability of EH-201 to induce EPO expression. After treatment for 24 h, EH-201 increased EPO mRNA expression in primary astrocytes and PC12 cells (Figure 1E and G). The intracellular EPO protein expression in astrocytes and PC12 cells was up-regulated during EH-201 treatment (Figure 1F and H). Next, we examined whether EH-201 increased mitochondrial activity through EPO-induced signalling. After treatment for 24 h, EH-201 induced PGC-1α mRNA and protein expression in primary astrocytes (Figure 1I and J) and PC12 cells (Figure 1L). EH-201 also induced mitochondrial activity in both astrocytes (Figure 1K) and PC12 cells (Figure 1M). Furthermore, EPO neutralizing antibody treatment abolished the PGC-1α mRNA and protein expression induced by EH-201 (Figure 1I and J). The results showed that PGC-1α expression was induced by EH-201 via EPO-mediated signalling.
EH-201 elevated the expression of haemoglobin-β in primary astrocytes and neuron-like PC12 cells
Because PGC-1α and haemoglobin are known to be mitochondrial regulators (St-Pierre et al., 2006 Biagioli et al., 2009), we analysed which form of haemoglobin was regulated by EPO or EH-201. Real-time PCR analysis showed that rhEPO treatment for 24 h increased haemoglobin-β mRNA expression in primary astrocytes (Figure 2A) and PC12 cells (Figure 2D). After EH-201 treatment for 24 h, real-time PCR revealed that EH-201 elevated the expression of haemoglobin-β mRNA in astrocytes (Figure 2B) and PC12 cells (Figure 2E). Haemoglobin-α expression, however, was not significantly changed (data not shown). Furthermore, EPO neutralizing antibody treatment abolished the haemoglobin-β mRNA expression induced by EH-201 (Figure 2B) in astrocytes. Additionally, we observed that EH-201 increased expression of all three forms of haemoglobin protein in astrocytes (Figure 2C) and PC12 cells (Figure 2F). The results showed that haemoglobin induction by EH-201 occurred via EPO-mediated signalling.
Figure 2.
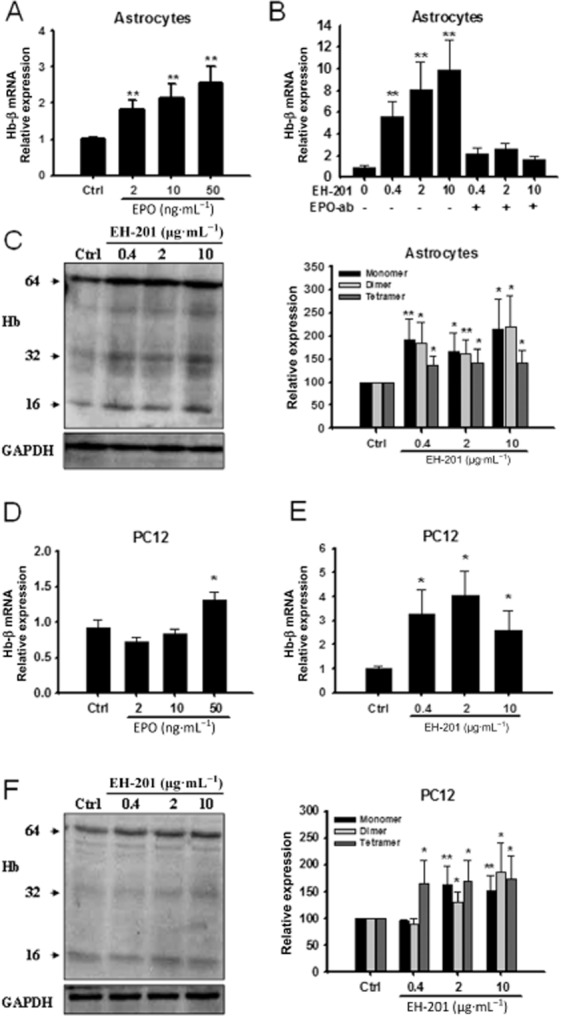
The induction of haemoglobin expression in primary astrocytes and neuron-like PC12 cells by EH-201 is mediated by EPO. (A, D) rhEPO treatment for 24 h increased haemoglobin-β mRNA expression in astrocytes and PC12 cells. (B) EH-201-treated primary astrocytes with or without the neutralizing EPO antibody (nEPO-ab, 1 μg·mL−1) were analysed for haemoglobin-β expression. (E) EH-201 treatment for 24 h increased haemoglobin-β mRNA expression in PC12 cells. The expression of GAPDH was used as an internal control. The results are expressed as the relative index of untreated controls means ± SD from at least three independent measurements. *P < 0.05, **P < 0.01; significantly different from control; one-way anova followed by Tukey's multiple comparison test. (C, F) Different forms of haemoglobin (monomer: 16 kD, dimer: 32 kD, tetramer: 64 kD) expression identified by an haemoglobin-β Ab in primary astrocytes and PC12 cells treated with EH-201. The results are expressed as the relative expression of untreated controls ± SD from at least three independent measurements. *P < 0.05, **P < 0.01, significantly different from control; Student's t-test.
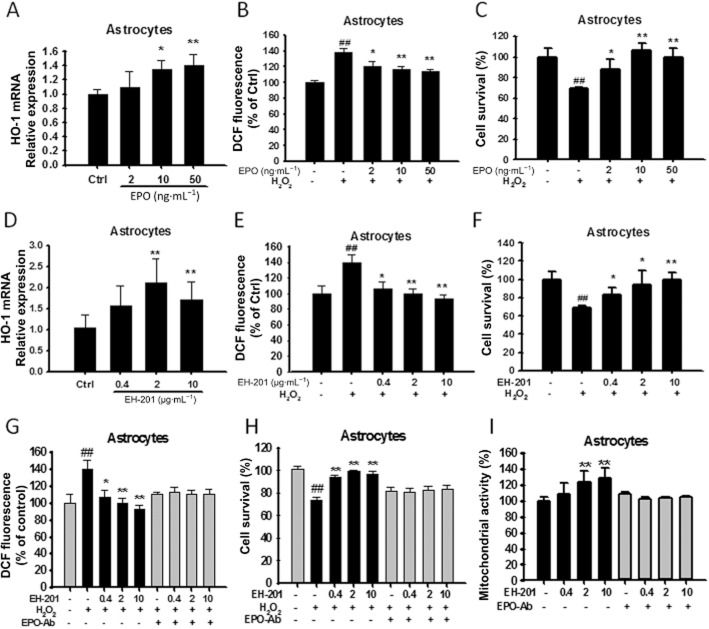
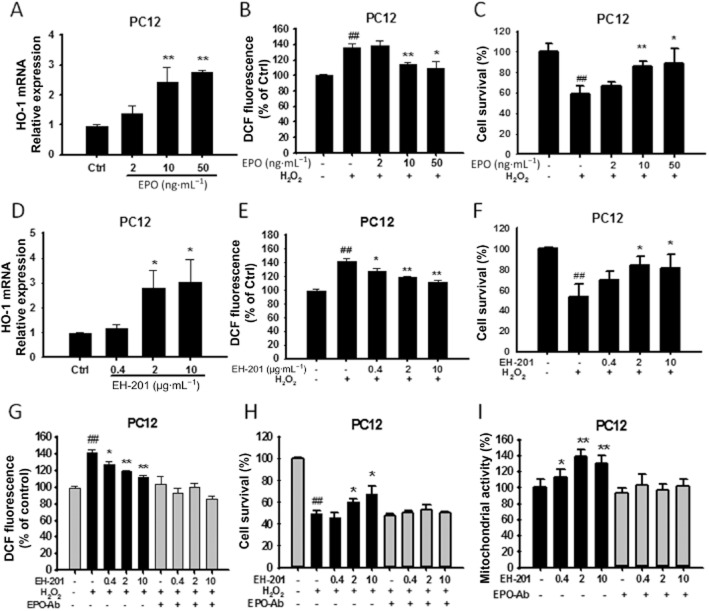
EPO is required for EH-201-mediated increased mitochondrial activity and attenuation of oxidative stress in primary astrocytes and neuron-like PC12 cells
We evaluated whether the increased mitochondrial activity and the reduction in H2O2-induced ROS generation and cytotoxicity following treatment with EH-201 in astrocytes and PC12 cells were dependent on EPO. Real-time PCR analysis showed that rhEPO or EH-201 treatment for 24 h increased mRNA expression οf the antioxidant gene HO-1 in primary astrocytes (Figure 3A and D) and PC12 cells (Figure 4A and D). Next, we estimated ROS generation in cultured cells after exposure to H2O2 using an oxidative probe, CM-H2DCFDA. rhEPO or EH-201 treatment decreased intracellular ROS in astrocytes (Figure 3B and E) and PC12 cells (Figure 4B and E). rhEPO or EH-201 also decreased cell toxicity in cells exposed to H2O2 in astrocytes (Figure 3C and F) and PC12 cells (Figure 4C and F). The reduction of ROS generation induced by H2O2 in astrocytes and PC12 cells treated with EH-201 was abolished when cells were co-incubated with an anti-EPO antibody (Figure 3G, 4G). The anti-EPO antibody also inhibited the EH-201-mediated reduction in H2O2-inducted cytotoxicity (Figure 3H, 4H). The increased mitochondrial activity observed with EH-201 treatment was blocked in the presence of an anti-EPO antibody, as measured by an MTT assay (Figure 3I, 4I). These results show that EPO is required for the increased mitochondrial activity and attenuation of oxidative stress induced by EH-201.
Figure 3.
EH-201 stimulated antioxidant gene (HO-1) expression, decreased intracellular ROS and attenuated H2O2-induced cell toxicity in primary astrocytes. (A, D) EPO or EH-201 treatment for 24 h increased HO-1 mRNA expression in primary astrocytes. The expression of GAPDH was used as an internal control. The results are expressed as the relative index of untreated controls means ± SD of at least three independent measurements. *P < 0.05, **P < 0.01; compared with untreated controls; one-way anova followed by Tukey's multiple comparison test. (B, E) Astrocytes treated with EPO or EH-201 for 24 h were exposed to 100 μM H2O2 for 6 h. Intracellular ROS formation was measured using a DCFH-DA assay. The graph shows the results in relative percentage of fluorescence units to the control. The values indicate the means ± SD (n = 8). (C, F) Astrocytes treated with EPO or EH-201 for 24 h were exposed to 500 μM H2O2 for 6 h. Cell survival was analysed with Trypan blue staining. The values indicate the means ± SD (n = 3). (G) Co-incubation of EH-201 with an anti-EPO antibody for 24 h resulted in the loss of the EH-201-mediated reduced ROS generation induced by H2O2, as assessed by a DCFH-DA assay (n = 8), and (H) reduced H2O2-mediated cytotoxicity, as assessed by Trypan blue staining (n = 3). The values indicate the means ± SD, ##P < 0.01 compared with untreated controls; *P < 0.05, **P < 0.01, compared with H2O2 controls. (I) Co-incubation of EH-201 with an anti-EPO antibody resulted in the loss of the EH-201-induced increase in succinate dehydrogenase activity, as assessed by an MTT reduction assay. The values indicate the means ± SD (n = 8), *P < 0.05, **P < 0.01 compared with controls; Student's t-test.
Figure 4.
EH-201 stimulated antioxidant gene (HO-1) expression, decreased intracellular ROS and attenuated H2O2-induced cell toxicity in neuron-like PC12 cells. (A, D) EPO or EH-201 treatment for 24 h increased HO-1 mRNA in PC12 cells. The expression of GAPDH was used as an internal control. The results are expressed as the relative index of untreated controls ± SD of at least three independent measurements. *P < 0.05, **P < 0.01, compared with untreated controls; one-way anova followed by Tukey's multiple comparison test. (B, E) PC12 cells treated with EPO or EH-201 for 24 h were exposed to 100 μM H2O2 for 6 h. Intracellular ROS formation was measured using a DCFH-DA assay. The graph shows results in relative percentage of fluorescence units to the control. The values indicate the means ± SD (n = 8). (C, F) PC12 cells treated with EPO or EH-201 for 24 h were exposed to 500 μM H2O2 for 6 h. Cell survival was analysed with Trypan blue staining. The values indicate the means ± SD (n = 3). (G) Co-incubation of EH-201 with an anti-EPO antibody for 24 h resulted in the loss of the EH-201-mediated reduction in ROS generation induced by H2O2, as assessed by a DCFH-DA assay (n = 8), and (H) reduced H2O2-mediated cytotoxicity, as assessed by Trypan blue staining (n = 3). The values indicate the means ± SD, ##P < 0.01 compared with untreated controls;*P < 0.05, **P < 0.01, compared with H2O2 controls. (I) Co-incubation of EH-201 with an anti-EPO antibody resulted in the loss of the EH-201-induced increase in succinate dehydrogenase activity, as assessed by an MTT reduction assay. The values indicate the means ± SD (n = 8), *P < 0.05, **P < 0.01, compared with control; Student's t-test.
Effects of EH-201 on memory impairment induced by SD
We next evaluated the neuroprotective effect of EH-201 on memory using the model of SD mice. The experimental procedure is outlined in Figure 5A. We analysed EPO expression in the hippocampus from each treated animal. Real-time PCR and Western blotting showed an increase in EPO expression in animals fed with a diet containing EH-201 (Figure 5B and D). Haemoglobin-β, HO-1 and PGC-1α mRNA expression in the hippocampus were also analysed by real-time PCR (Figure 5E). We further evaluated the mitochondrial succinate dehydrogenase activity using an MTT assay (Figure 5F). In the passive avoidance test, mice fed EH-201 for 3 days did not exhibit a difference in the ability to learn (Figure 5G). However, there was a significant improvement in memory performance of EH-201-fed mice after SD in the passive avoidance test (Figure 5H). Interestingly, the gene expression changes and the increased mitochondrial activity in the hippocampi from mice fed with EH-201, especially at doses of 100 and 200 mg·kg−1, correlated with the passive avoidance test.
Figure 5.
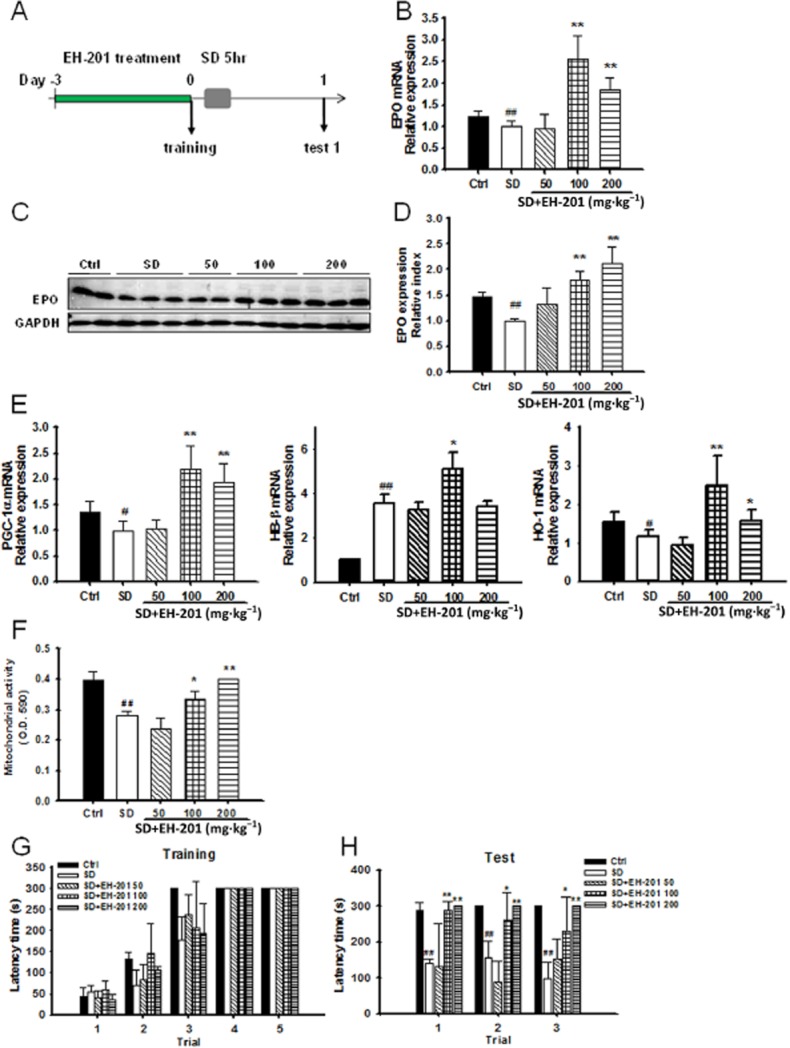
Effects of EH-201 on SD-induced memory impairment. (A) Procedure of EH-201 treatment in SD mice. (B) Real-time PCR and (C, D) Western blot analysis of EPO expression in mouse hippocampus from each group. (E) Real-time PCR analysis of PGC-1α, haemoglobin-β and HO-1 expression levels in mouse hippocampus (n = 6). (F) The MTT assay was used as a marker for mitochondrial activity. The values depict mitochondrial function after SD of untreated control mice and EH-201-treated mice. (G) Acquisition of step-through passive avoidance during five successive training trials in mice treated with or without EH-201. EH-201 treatment did not affect learning ability in mice. (H) Acquisition of step-through passive avoidance during three successive testing trials in mice treated with or without EH-201. The values indicate the means ± SD, #P < 0.05, ##P < 0.01, compared with the control: *P < 0.05, **P < 0.01, compared with the SD group; one-way anova followed by Tukey's multiple comparison test.
EH-201 improved KA-induced memory impairment in the behavioural test
Memory loss was induced in KA-injected mice. The experimental procedure is outlined in Figure 6A. Injection of KA (0.15 μg per mouse) in mice induced epileptiform seizures (scoring 3–4). There was a significant improvement in the memory performance of mice in the passive avoidance test after EH-201 diet for 1 week (day 13) and 2 weeks (day 20) (Figure 6B). EH-201 also elevated the protein level of EPO and PGC-1α expression in the hippocampus (Figure 6C and D). EH-201 improved memory performance after i.c.v. injection of KA in mice.
Figure 6.
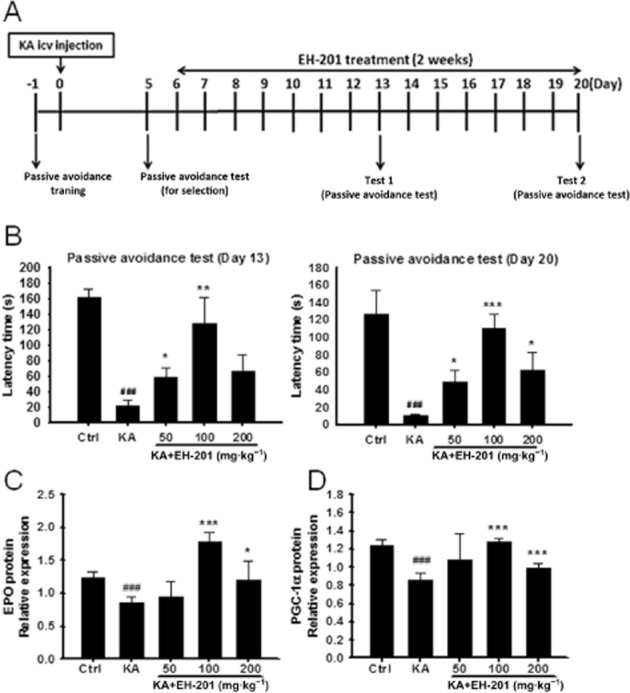
EH-201 ameliorated KA-induced memory impairment. (A) Experimental procedure of KA injection and EH-201 treatment. After KA-induced memory impairment, mice were fed a EH-201 diet on days 6–20. (B) The memory performance of KA-injected mice after EH-201 administration for 1 week (day 13) and 2 weeks (day 20) was measured using a passive avoidance task analysis. (n = 6–7). (C, D) EH-201 improved the protein expression of EPO and PGC-1α in the hippocampus (n = 5). The values indicate the means ± SEM, ###P < 0.001 compared with the control group; *P < 0.05, **P < 0.05 and ***P < 0.001 compared with the KA-injected only group; Student's t-test.
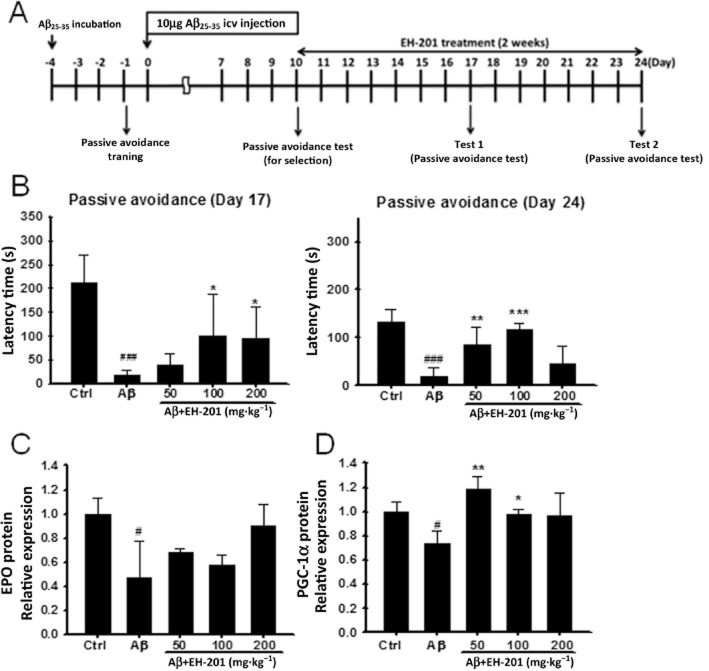
EH-201 improved Aβ25–35-induced memory impairment in the behavioural test
Memory loss was induced in mice injected with Aβ25–35. The experimental procedure is outlined in Figure 7A. There was a significant improvement in the memory performance of mice in the passive avoidance test after feeding the EH-201 diet for 1 week (day 17) and 2 weeks (day 24) (Figure 7B). EH-201 also elevated the level of EPO and PGC-1α protein expression in the hippocampus (Figure 7C and D). EH-201 improved memory performance after i.c.v. injection of Aβ25–35 in mice.
Figure 7.
EH-201 improved memory performance after i.c.v. injection of Aβ25–35 in mice. (A) Experimental procedure of Aβ25–35 injection and EH-201 treatment. After Aβ25–35-induced memory impairment, mice were fed EH-201 on days 10–24. (B) The memory performance of Aβ25–35-injected mice after EH-201 administration for 1 week (day 17) and 2 weeks (day 24) was measured using a passive avoidance task analysis (n = 5–6). (C, D) EH-201 improved the protein expression of EPO and PGC-1α in the hippocampus (n = 4–5). The values indicate the means ± SEM, #P < 0.05, compared with the control group; *P < 0.05, **P < 0.05, ***P < 0.001, compared with the Aβ25–35-injected only group; Student's t-test.
Discussion and conclusions
rhEPO has recently emerged as a highly attractive candidate for neuroprotection in both schizophrenia (Ehrenreich et al., 2007) and stroke patients (Ehrenreich et al., 2002). However, a significant challenge for future clinical use of EPO in CNS diseases is that EPO cannot easily cross the BBB (Lieutaud et al., 2008). The use of relatively high doses of exogenous rhEPO has the potential to increase thrombosis and the viscosity of the blood with potentially fatal results (Shaskey and Green, 2000). The discovery that EPO and its receptor are expressed in the brain (Juul et al., 1999) has led to the suggestion that EPO exerts a direct effect on the CNS, independent of haematopoiesis. Exogenous administration of EPO in rodents has been shown to rescue hippocampal CA1 neurons from lethal ischaemic damage and to prevent ischaemia-induced learning disabilities (Sakanaka et al., 1998) as well as providing neuroprotective effects against glutamate neurotoxicity (Morishita et al., 1997) and traumatic brain (Cherian et al., 2007) and spinal cord injury (Celik et al., 2002). EPO treatment enhances hippocampal long-term potentiation by modulating the plasticity, synaptic connectivity and activity of memory-related neuronal networks (Adamcio et al., 2008) and improves the operant conditioning stability of cognitive performance in healthy mice (El-Kordi et al., 2009). However, it has been reported that hippocampal neurons with EPO receptors respond to EPO in a limited concentration range and that high concentrations of EPO induced a rapid down-regulation of EPO receptors, resulting in a failure to transmit EPO-mediated signals to the neurons (Sakanaka et al., 1998).
EPO treatment has been shown to activate cardiac mitochondrial biogenesis through up-regulation of PGC-1α (Carraway et al., 2010), which is a transcriptional co-activator that has been identified as an inducible regulator of mitochondrial biogenesis and function (Lin et al., 2005). Haemoglobin is the main oxygen carrier in erythrocytes. Unexpectedly, haemoglobin has been found in non-haematopoietic cells, such as neurons and glial cells, which may facilitate tissue oxygen transport or increase cellular oxygenation, thus linking haemoglobin expression and mitochondrial activity (Biagioli et al., 2009). The monomer/dimer form of haemoglobin has a higher affinity for oxygen than the conventional tetrameric form (Marden et al., 1995; Richter et al., 2009). Thus, monomeric/dimeric haemoglobin expression may play an active role in facilitating oxygen extraction from capillaries as well as improving the inter- and intra-cellular oxygen transport that are associated with mitochondrial biogenesis to improve tissue oxygenation and energy production. A study also showed that hypoxia-induced, glial EPO-dependent expression of glial haemoglobin aided in free radical scavenging and NO detoxification; this intrinsic protective mechanism against hypoxic/oxidative injury may have important implications in glaucomatous neurodegeneration (Tezel et al., 2010). Haemoglobin can also protect different cell types from H2O2-induced oxidative stress and associated cell damage (Widmer et al., 2010). However, the neuroprotective doses of peripherally administered rhEPO are much higher than those needed for stimulation of haematopoiesis, and have been demonstrated to increase morbidity and mortality (Ehrenreich et al., 2009; Lund et al., 2014).
In our previous study, EH-201 acted as an EPO inducer via enhancement of EPO and EPO receptor binding to activate mitochondrial function and haemoglobin expression in non-hematopoietic cells, such as cardiomyocytes, liver cells, kidney cells and bone marrow cells (Hsu et al., 2013). In this study, we have suggested that EH-201 is a potent inducer of endogenous EPO in the brain that regulates mitochondrial biogenesis and haemoglobin expression in neuronal cells. Our results showed memory recovery in Aβ25–35-induced AD mice, KA-damaged mice and SD mice, after treatment with dietary EH-201, compared with the mice fed the control diet. There was a significant increase in the expression of EPO and its downstream genes in the EH-201-treated group in the SD model. Mitochondrial succinate dehydrogenase activity in the hippocampus was also improved in the EH-201-fed group. Also, EH-201 elevated the expression of EPO, resulting in PGC-1α expression and haemoglobin expression in the hippocampus of the Aβ25–35-induced AD mice and the KA-injected excitotoxicity model. In addition, HO-1, an anti-oxidant protein, the expression of which is induced by EPO, attenuated inflammatory or oxidative stress in the mouse brain (Chen et al., 2010; Sifringer et al., 2010). Our results showed that EH-201, an EPO inducer, provided neuroprotective effects, most likely through the up-regulation of HO-1 in primary astrocytes and neuron-like PC12 cells, and thus leads to clearance of ROS and reduced neurotoxicity in the H2O2-induced cell stress model.
According to pharmacokinetics studies performed in vivo (Lv et al., 2011; Zhao et al., 2013), EH-201 (TSG in these reports) was absorbed rapidly and distributed freely the heart, kidney, liver and lung. Samll amouints were retained in the brain, but these correlated with the effective concentrations. EH-201 was then excreted quickly after oral administration. Lv and co-workers performed a pharmacokinetic study to quantitate EH-201 (PM-SG in this report) in mouse plasma after oral administration of 100 mg·kg−1, and their results showed a the maximum concentration (Cmax) and time to reach maximum concentration (Tmax) of 29.6 μg·mL−1 and 60 min respectively. Additionally, Zhao and co-workers have also performed pharmacokinetics and tissue distribution studies of EH-201 (TSG) in rats following a single oral (100 mg·kg−1) dose, and their results showed that brain contained about 33 μg EH-201 per g tissue after 30 min of oral administration (100 mg·kg−1). Furthermore, Zhang et al. had detected the presence of EH-201 in both blood plasma and CSF in rabbits after administration by gavage, showing that that EH-201 could go through the BBB (Zhang et al., 2006). Our in vitro studies showed that EH-201 in concentrations of 0.4–2.0 μg·mL−1 induced EPO expression in neuronal cells. Such concentrations might reasonably be attained in vivo in the CNS and provide a direct effect of EH-201 in the CNS. Therefore, it is highly likely that EH-201, a low MW compound, did cross the BBB and was effective in our three mouse models of memory impairment. Furthermore, EH-201 (THSG) improved blood flow and ameliorated vascular senescence in spontaneously hypertensive rats (SHRs) (Han et al., 2012), without affecting blood pressure and body weight, after treatment with 50 mg·kg−1·day−1 for 14 weeks. Moreover, we have data (obtained by another laboratory) showing a normal haematocrit after 3 months of dosing with EH-201, at up to 2.5 g·kg−1 (data not shown). Thus, many of the toxic effects associated with exogenous rhEPO may be avoided by the use of and inducer such as EH-201.
Any new strategies to increase EPO expression in the brain will greatly broaden the clinical applications of EPO for the treatment of neurological diseases. The development of a stabilizer of the hypoxia-inducible factor (HIF) or an inhibitor of the HIF-specific prolyl hydroxylase (HIF-PH) has the potential to achieve this effect (Hsieh et al., 2007). However, HIF transcription factors regulate a wide range of biological processes, and intermittent HIF activation over prolonged periods of time may lead to profound changes in cellular metabolism, growth and differentiation. Consequently, pharmacological activation of HIF could result in serious side effects (Haase, 2010). The long-term inactivation of HIF-PH and the subsequent long-term activation of HIF has been shown to contribute to the pathogenesis of ischaemic cardiomyopathy (Moslehi et al., 2010). Moreover, in contrast to a HIF stabilizer that stimulates a high level of erythropoiesis, EH-201 treatment increased the numbers of red blood cells but these were still within the physiological range and involved a mechanism that did not produce HIF-α activation (Hsu et al., 2013).
Millions of people commonly suffer from insufficient sleep, which is a problem of considerable health, social and economical importance (Vgontzas and Kales, 1999). Clinical data have shown that SD causes deficits in several forms of learning and memory (Miyata et al., 2010) and increases in hypothalamic and thalamic oxidative stress levels were found in SD rats (D'Almeida et al., 1998). The accumulation of free radicals may further cause oxidative damage to mitochondria that leads to more ROS generation, which can disrupt neuronal function, as neurons are critically dependent on mitochondrial function to establish membrane excitability and to execute the complex processes of neurotransmission and plasticity (Kann and Kovacs, 2007). The loss of cognitive function is one of the problems that many people face as they age. Patients with AD or other types of dementia are also subject to losses in cognitive function. Current drugs for AD target cholinergic and glutamatergic neurotransmission, thus improving clinical symptoms, although their therapeutic efficacy is still debated (Mangialasche et al., 2010).
Our results showed that EPO can modulate downstream mitochondrial biogenesis and haemoglobin induction in neuronal cells. EH-201 induced EPO in neuronal cells, and its EPO-mediated activation of mitochondrial function through PGC-1α, haemoglobin-β and HO-1 expression in neuronal cells improved mitochondrial energy generation, cellular oxygenation and reduces oxidative stress, leading to the recovery of memory impairment in SD-, Aβ- and KA-induced disease mouse models. Therefore, our results suggest that the induction of endogenous EPO in neuronal cells might be a rational therapeutic strategy for memory impairment in neurodegenerative diseases, physiological ageing or traumatic brain injury.
Acknowledgments
We are grateful for a grant from the Ministry of Education, Republic of China (Taiwan), Aim for the Top University Plan and Ministry of Science and Technology, grant MOST 103-2320-B-010-016.
Glossary
- Aβ
amyloid-β
- APP
amyloid precursor protein
- EH-201 (TSG
THSG, PMSG)
- 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside: EPO
erythropoietin
- rhEPO
recombinant human EPO
- HO-1
haem oxygenase-1
- KA
kainic acid
- PGC-1α
PPAR-γ coactivator 1α
- ROS
reactive oxygen species
- SD
sleep-deprived
Author contributions
L.-Y. H. isolated the EH-201 by EPO inducer-based fractionation bioassay and also wrote carefully for this manuscript. P.-L. H. made the figures beautiful. L.-W. C. was responsible for the part of the SD animal study of this manuscript. W.-Z. T. was responsible for the part of the Aβ and KA animal study of this manuscript. K.-T. H. evaluate the memory lost and recovery in our laboratory. C.-L. W. made great contribution at the recrystallization of EH-201 at the purity of 99.67%. R.-T. W. made major modification in this manuscript.
Conflict of interest
The authors certify that they have no actual or potential conflicts of interest regarding the research reported in this paper.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.13248
Table S1. Sequences of the specific gene used for Q-PCR.
References
- Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, et al. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol. 2008;6:37. doi: 10.1186/1741-7007-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazza AC, Andersen ML, Alvarenga TA, De-Oliveira MR, Armani F, Ruiz FS, et al. Impairment of the mitochondrial electron transport chain due to sleep deprivation in mice. J Psychiatr Res. 2010;44:775–780. doi: 10.1016/j.jpsychires.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc Natl Acad Sci U S A. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway MS, Suliman HB, Jones WS, Chen CW, Babiker A, Piantadosi CA. Erythropoietin activates mitochondrial biogenesis and couples red cell mass to mitochondrial mass in the heart. Circ Res. 2010;106:1722–1730. doi: 10.1161/CIRCRESAHA.109.214353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21:417–423. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Wang YL, Lo WT, Wu CC, Hsieh CW, Huang CF, et al. Erythropoietin enhances endogenous haem oxygenase-1 and represses immune responses to ameliorate experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:210–223. doi: 10.1111/j.1365-2249.2010.04238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson C. Neuroprotection with erythropoietin administration following controlled cortical impact injury in rats. J Pharmacol Exp Ther. 2007;322:789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- Chou YC, Lin SB, Tsai LH, Tsai HI, Lin CM. Cholesterol deficiency increases the vulnerability of hippocampal glia in primary culture to glutamate-induced excitotoxicity. Neurochem Int. 2003;43:197–209. doi: 10.1016/s0197-0186(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Craik FI, Rose NS. Memory encoding and aging: a neurocognitive perspective. Neurosci Biobehav Rev. 2012;36:1729–1739. doi: 10.1016/j.neubiorev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- D'Almeida V, Lobo LL, Hipolide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9:2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, et al. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206–220. doi: 10.1038/sj.mp.4001907. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- El-Kordi A, Radyushkin K, Ehrenreich H. Erythropoietin improves operant conditioning and stability of cognitive performance in mice. BMC Biol. 2009;7:37. doi: 10.1186/1741-7007-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser E. Erythropoietin and the differentiation of red blood cells. Fed Proc. 1975;34:2285–2292. [PubMed] [Google Scholar]
- Haase VH. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299:F1–F13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Ling S, Gan W, Sun L, Duan J, Xu JW. 2,3,5,4'-tetrahydroxystilbene-2-O-beta-d-glucoside ameliorates vascular senescence and improves blood flow involving a mechanism of p53 deacetylation. Atherosclerosis. 2012;225:76–82. doi: 10.1016/j.atherosclerosis.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Harman D. Role of free radicals in aging and disease. Ann N Y Acad Sci. 1992;673:126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, et al. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PL, Horng LY, Peng KY, Wu CL, Sung HC, Wu RT. Activation of mitochondrial function and Hb expression in non-haematopoietic cells by an EPO inducer ameliorates ischaemic diseases in mice. Br J Pharmacol. 2013;169:1461–1476. doi: 10.1111/bph.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-H, Horng L-Y, Chen C-F, Wu R-T. Chinese herb Radix Polygoni multiflori as a therapeutic drug for liver cirrhosis in mice. J Ethnopharmacol. 2007;114:199–206. doi: 10.1016/j.jep.2007.07.034. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Paulson GW. Memory impairment associated with progression of Huntington's disease. Cortex. 1987;23:275–283. doi: 10.1016/s0010-9452(87)80037-0. [DOI] [PubMed] [Google Scholar]
- Ismail Z, Herrmann N, Francis PL, Rothenburg LS, Lobaugh NJ, Leibovitch FS, et al. A SPECT study of sleep disturbance and Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;27:254–259. doi: 10.1159/000203889. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Kopp R. An improved one-trial passive avoidance learning situation. Psychol Rep. 1967;21:221–224. doi: 10.2466/pr0.1967.21.1.221. [DOI] [PubMed] [Google Scholar]
- Juul SE, Yachnis AT, Rojiani AM, Christensen RD. Immunohistochemical localization of erythropoietin and its receptor in the developing human brain. Pediatr Dev Pathol. 1999;2:148–158. doi: 10.1007/s100249900103. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erythropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008;59:22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Lieutaud T, Andrews PJ, Rhodes JK, Williamson R. Characterization of the pharmacokinetics of human recombinant erythropoietin in blood and brain when administered immediately after lateral fluid percussion brain injury and its pharmacodynamic effects on IL-1beta and MIP-2 in rats. J Neurotrauma. 2008;25:1179–1185. doi: 10.1089/neu.2008.0591. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lund A, Lundby C, Olsen NV. High-dose erythropoietin for tissue protection. Eur J Clin Invest. 2014;44:1230–1238. doi: 10.1111/eci.12357. [DOI] [PubMed] [Google Scholar]
- Lv G, Lou Z, Chen S, Gu H, Shan L. Pharmacokinetics and tissue distribution of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside from traditional Chinese medicine Polygonum multiflorum following oral administration to rats. J Ethnopharmacol. 2011;137:449–456. doi: 10.1016/j.jep.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marden MC, Griffon N, Poyart C. Oxygen delivery and autoxidation of hemoglobin. Transfus Clin Biol. 1995;2:473–480. doi: 10.1016/s1246-7820(05)80074-6. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Miyata S, Noda A, Ozaki N, Hara Y, Minoshima M, Iwamoto K, et al. Insufficient sleep impairs driving performance and cognitive function. Neurosci Lett. 2010;469:229–233. doi: 10.1016/j.neulet.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- Moslehi J, Minamishima YA, Shi J, Neuberg D, Charytan DM, Padera RF, et al. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation. 2010;122:1004–1016. doi: 10.1161/CIRCULATIONAHA.109.922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Inagi R, Kato H, Tanemoto M, Kojima I, Son D, et al. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J Am Soc Nephrol. 2008;19:1500–1508. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L, Eckert A. Amyloid-beta interaction with mitochondria. Int J Alzheimer's Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Kim JH, Bae SS, Hong KW, Lee DS, Leem JY, et al. Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid beta-induced cognitive deficits associated with decreased amyloid beta accumulation. Biochem Biophys Res Commun. 2011;408:602–608. doi: 10.1016/j.bbrc.2011.04.068. [DOI] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett JL, Theberge DC, Brown WS, Schweitzer SU, Nissenson AR. Normalizing hematocrit in dialysis patients improves brain function. Am J Kidney Dis. 1999;33:1122–1130. doi: 10.1016/S0272-6386(99)70150-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr Clin Neurophysiol. 1972;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Richter F, Meurers BH, Zhu C, Medvedeva VP, Chesselet MF. Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J Comp Neurol. 2009;515:538–547. doi: 10.1002/cne.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Shaskey DJ, Green GA. Sports haematology. Sports Med. 2000;29:27–38. doi: 10.2165/00007256-200029010-00003. [DOI] [PubMed] [Google Scholar]
- Sifringer M, Brait D, Weichelt U, Zimmerman G, Endesfelder S, Brehmer F, et al. Erythropoietin attenuates hyperoxia-induced oxidative stress in the developing rat brain. Brain Behav Immun. 2010;24:792–799. doi: 10.1016/j.bbi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Sun FL, Zhang L, Zhang RY, Li L. Tetrahydroxystilbene glucoside protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Eur J Pharmacol. 2011;660:283–290. doi: 10.1016/j.ejphar.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Kain AD, Powell DW, et al. Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest Ophthalmol Vis Sci. 2010;51:907–919. doi: 10.1167/iovs.09-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Kales A. Sleep and its disorders. Annu Rev Med. 1999;50:387–400. doi: 10.1146/annurev.med.50.1.387. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- Wang R, Tang Y, Feng B, Ye C, Fang L, Zhang L, et al. Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience. 2007;149:739–746. doi: 10.1016/j.neuroscience.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17:195–200. [PubMed] [Google Scholar]
- Widmer CC, Pereira CP, Gehrig P, Vallelian F, Schoedon G, Buehler PW, et al. Hemoglobin can attenuate hydrogen peroxide-induced oxidative stress by acting as an antioxidative peroxidase. Antioxid Redox Signal. 2010;12:185–198. doi: 10.1089/ars.2009.2826. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xing Y, Ye CF, Ai HX, Wei HF, Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer's disease and intervention by using tetrahydroxystilbene glucoside. Behav Brain Res. 2006;173:246–254. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Zhu J. Kainic acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Curr Neuropharmacol. 2011;9:388–398. doi: 10.2174/157015911795596540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Zhang L, Feng YL, Chen DQ, Xi ZH, Du X, et al. Pharmacokinetics of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside in rat using ultra-performance LC-quadruple TOF-MS. J Sep Sci. 2013;36:863–871. doi: 10.1002/jssc.201200668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sequences of the specific gene used for Q-PCR.