Linked Articles
This article is a Commentary on Sandtner W, Schmid D, Schicker K, Gerstbrein K, Koenig X, Mayer FP, Boehm S, Freissmuth M and Sitte HH (2014). A quantitative model of amphetamine action on the 5-HT transporter. Br J Pharmacol 171: 1007–1018. doi: 10.1111/bph.12520. The authors reply in Schmid et al., (2015). Br J Pharmacol 172: this issue. doi: 10.1111/bph.12766
This article is a commentary upon Sendtner et al., 2014; the authors reply in Schmid et al., 2015.
A new paper has appeared in the British Journal of Pharmacology that deserves the attention of those working in the monoamine transporter field. Sandtner et al. (2014) address the important problem of how amphetamine-like drugs affect monoamine transporters (Transporter nomenclature follows Alexander et al., 2013). In particular, they studied the current that persists after p-chloroamphetamine (pCA) removal from oocytes or HEK cells that express the human serotonin transporter, hSERT. Their new work was prompted in part by a previous article we published, in which we reported a current that persists when amphetamine is removed from oocytes expressing the human dopamine transporter, hDAT (Rodriguez-Menchaca et al., 2012). Rodriguez-Menchaca et al. proposed that amphetamine enters the cell via hDAT, interacts with the inner face of hDAT, and holds the transporter open after the removal of external amphetamine. This mechanism we referred to as a molecular stent. Sandtner et al. now assert that whereas persistent currents do exist when oocytes are the expression system, they do not exist when transporters are expressed in HEK cells. Furthermore, they propose a theory for the persistent current they observed in oocytes based on the lipophilicity of compounds. Here we show that HEK cells expressing hDAT do have persistent current, and that Sandtner et al. actually saw the persistent current in HEK cells but failed to acknowledge it. We also show that the proposed lipophilicity theory that Sandtner et al. have put forward simply does not hold up on further investigation.
First, let us emphasize that Sandtner et al. found analogous results for hSERT to those we found for hDAT, both expressed in oocytes, but they have a completely different interpretation of the persistent current. Second, they expanded their study of the persistent current to include HEK cells. Their experimental approach thus compared two heterologous expression systems for hSERT, Xenopus laevis oocytes and HEK 293 cells. In both cases, cells were voltage clamped and exposed to 5-HT, pCA, or methylenedioxyamphetamine (MDMA). In hSERT-expressing oocytes, pCA-induced currents decayed, after removal of external pCA, 4× more slowly than 5HT with a half-life of 20 s and 5 s, respectively. Comparing their results with those of Rodriguez-Menchaca et al. shows that in oocytes, pCA induced a persistent current in hSERT similar to the amphetamine-induced persistent current in hDAT. Sandtner et al. go on to show that when hSERT was expressed in HEK 293 cells, the pCA-induced persistent current decayed 5× more slowly than 5HT-induced current, 2.5 s versus 0.5 s respectively. Thus, for Sandtner et al., the persistence ratio for drug to substrate was comparable in oocytes and HEK cells. However, because the ‘absolute values of the time constants differed by ∼ 10-fold’ in HEK cells as compared with oocytes, Sandtner et al. conclude on this basis alone:
Lack of ‘persistent currents’ upon removal of high concentrations of pCA in HEK293 cells.
Furthermore, in place of the originally proposed molecular stent hypothesis, Sandtner et al. assert that lipophilicity is the major player in this phenomenon when it is observed in oocytes:
(2) … there is a considerable passive pCA flux through the membrane … the cell serves as reservoir. This allows for a continuous outward leak of pCA, which results in concentrations sufficient to trigger pCA-induced currents.
Here we present data that refutes both these claims. (1) Monoamine transporters expressed in HEK cells do have a persistent current, and this is already evident in the data Sandtner et al. present. (2) The persistent current that both groups observe in oocytes has nothing to do with the lipophilicity of the compounds that are presented to the cells. Consider first only the Sandtner et al. data: it is difficult to see how they arrived at conclusion (1) while ignoring the extremely different experimental conditions that exist between data gathered in oocytes and data gathered in HEK cells. In particular, it would seem essential to normalize data when comparing results from two such disparate systems. A convenient baseline that we have used for each cell type would be how rapidly the signal recovers from the natural substrate after its removal. Data in Sandtner et al. show that the persistence of currents in both expression systems are 4–5× slower for pCA than for 5HT. Instead of using this ratio or a similar objective measure to compare these compounds, they chose to ignore the persistent current they observed and focus instead on the absolute values of persistence in two very different experimental conditions. In summary, Sandtner et al. see the same relative persistent current in oocytes and in HEK cells, contrary to their assertion expressed in (1), that no persistent current exists in HEK cells.
To emphasize the point that persistent currents do exist for monoamine transporters expressed in mammalian cells, we show new data in Figure 1 from hDAT-transfected HEK cells at 37°C. These data show a clear persistence of amphetamine-induced current, compared with dopamine-induced current, after each compound is removed. This result in HEK cells is qualitatively similar to the original persistent current we described in oocytes and quantitatively similar to that observed by Sandtner et al. in HEK cells. Comparing results obtained only from HEK cells from the two groups, both data sets show a persistent current for drug-induced currents. Considering the differences between Sandtner et al. and the present results (room temperature vs. 37°C, hSERT vs. hDAT and pCA vs. S(+)amphetamine), the persistent current in both data sets is remarkably similar.
Figure 1.
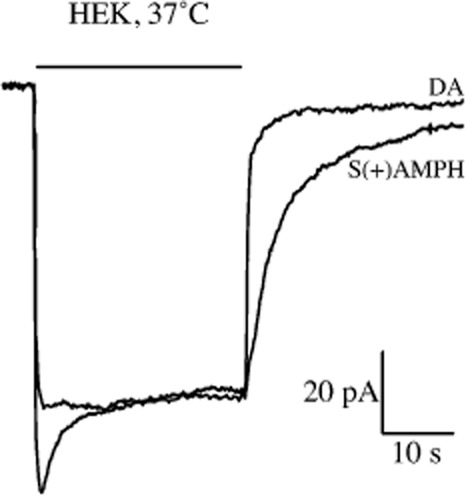
Persistent current in hDAT-transfected HEK cells. Current through hDAT transiently transfected HEK293 cells. V = −60 mV, T = 37°C. 30 s of 10 μM dopamine (DA) induces an inward current that returns to baseline when dopamine is removed. 30 s of 10 μM S(+)amphetamine (S(+)AMPH) induced a similar inward peak current; however, the return of current upon wash-out of S(+)amphetamine was slower and persists for tens of seconds and up to a minute. Dopamine and S(+)amphetamine are overlaid for better comparison of kinetics. Using a mono-exponential function, we determined the time constants for the current decays: τDA = 1.3 s, τS(+)AMPH = 5.1 s, τS(+)AMPH/τDA = 3.8 s.
Even though the relative persistent current is the same in oocytes and HEK cells, the more rapid decay of the persistent current in HEK cells compared with oocytes still remains a mystery. One possibility is that whereas sharp electrodes penetrate the oocyte, relatively large, whole-cell electrodes penetrate the HEK cell and are likely to perfuse the cell with the electrode solution. Sandtner et al. discount the importance of this difference claiming that within the time frame of their experiments no perfusion of HEK cells occurs; however, it is well known that complete perfusion of mammalian cells via whole-cell electrodes can occur within 15 seconds (Fenwick et al., 1982).
Two models of the persistent current. The persistent current has been suggested as a mechanism to enhance amphetamine-induced dopamine release (Rodriguez-Menchaca et al., 2012). It is therefore interesting to examine the current models for the persistent current in further detail.
The molecular stent model proposes that amphetamine itself or amphetamine-like drugs enter the cell primarily through the transporter (hDAT), and as the drug accumulates inside it eventually interacts with the transporter from that side. When external S(+)amphetamine is removed, internal S(+)amphetamine is still present and this presence causes the transporter to continue conducting current. The strength of this persistent current depends on initial drug concentration and drug exposure time: the more of the drug that is presented externally, the more available it becomes internally to interact with the transporter (Rodriguez-Menchaca et al., 2012).
The lipophilicity model proposes that amphetamine-like drugs enter the cell primarily through the lipid membrane. Similar to the molecular stent model, the drug accumulates inside the cell; however, when the external drug is removed the internal drug leaves the cell via the lipid membrane and despite continuous superfusion, the unstirred layer of fluid above the cell surface suffices to inhibit immediate removal of pCA … to trigger pCA-induced currents. (Sandtner et al., 2014). Sandtner et al. further report that because pCA is more lipophilic that 5HT, pCA more readily accumulates in the cells and hence produces a persistent current, whereas the natural substrate does not.
Several problems arise with the lipophilicity model. First, neither an unstirred layer nor lipophilicity could possibly explain the persistent current we observe because only the S(+)amphetamine enantiomer produces it. S(+)amphetamine and R(−)amphetamine would obviously have the same access to an unstirred layer and the same lipophilicity. Sandtner et al. use only racemates. Moreover, peak and persistent currents have unique reversal potentials confirming their different conformational states (Rodriguez-Menchaca et al., 2012). Second, in addition to this argument, which is already published, we add additional new data from experiments with hDAT expressed in oocytes that show lipophilicity is not correlated with the generation of persistent current in. Figure 2 plots the magnitude of the persistent current measured 60 s after removal for compounds with various lipophilicity, against the measure of lipophilicity used by Sandtner et al. The polar surface area (PSA) of a given molecule (expressed in Å2) quantifies its ability to partition into the lipid bilayer and the higher the PSA, the less lipophilic the molecule. The figure shows that S(+)amphetamine and R(−)amphetamine have the same PSA but a vastly different ability to generate a persistent current. Contrariwise S(+)amphetamine and S(+)methamphetamine have roughly the same persistent current but vastly different PSA values. S(−)methcathinone a synthetic cathinone, has the highest persistent current of the group we studied, but not the smallest PSA.
Figure 2.
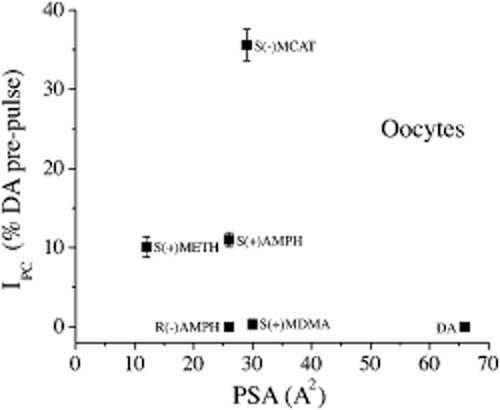
Persistent current versus polar surface area (PSA). hDAT-expressing oocytes, V = −60 mV, room temperature. All drugs are applied for 60 s and are 10 μM in concentration. The persistent current is given as a fraction of the dopamine-induced current for each oocyte to normalize for expression level from cell to cell. The PSA values were obtained from http://chemicalize.org. Mean ± SEM (n = 5–15).
Based on the hypothesis of Sandtner et al., we would expect to observe the largest persistent current with S(+)methamphetamine due to its low PSA value. We would also expect S(+)amphetamine, R(−)amphetamine, S(−)methcathinone and S(+)methylenedioxymethamphetamine to produce similar sized persistent current because their PSA values are very similar. Our data does not support these predictions and disprove the main hypothesis of the Sandtner et al. model.
We conclude that monoamine transporters do have a relative persistent current for amphetamine-like drugs, independent of the expression system, and the ability of a compound to generate a persistent current is not correlated with its ability to partition in a lipid bilayer.
Glossary
- DAT
dopamine transporter
- MCAT
methcathinone
- MDMA
methylenedioxyamphetamine
- METH
methamphetamine
- pCA
p-chloroamphetamine
- PSA
polar surface area
- SERT
serotonin transporter
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. British Journal of Pharmacology. 2013;170:1706–1796. doi: 10.1111/bph.12450. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EN, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Menchaca AA, Solis E, Jr, Cameron K, De Felice LJ. S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br J Pharmacol. 2012;165:2749–2757. doi: 10.1111/j.1476-5381.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandtner W, Schmid D, Schicker K, Gerstbrein K, Koenig X, Mayer F, et al. A quantitative model of amphetamine action on the serotonin transporter. Br J Pharmacol. 2014;171:1007–1018. doi: 10.1111/bph.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]


