Abstract
Detecting environmental change is fundamental for adaptive behavior in an uncertain world. Previous work indicates the hippocampus supports the generation of novelty signals via implementation of a match–mismatch detector that signals when an incoming sensory input violates expectations based on past experience. While existing work has emphasized the particular contribution of the hippocampus, here we ask which other brain structures also contribute to match–mismatch detection. Furthermore, we leverage the fine-grained temporal resolution of magnetoencephalography (MEG) to investigate whether mismatch computations are spectrally confined to the theta range, based on the prominence of this range of oscillations in models of hippocampal function. By recording MEG activity while human subjects perform a task that incorporates conditions of match–mismatch novelty we show that mismatch signals are confined to the theta band and are expressed in both the hippocampus and ventromedial prefrontal cortex (vmPFC). Effective connectivity analyses (dynamic causal modeling) show that the hippocampus and vmPFC work as a functional circuit during mismatch detection. Surprisingly, our results suggest that the vmPFC drives the hippocampus during the generation and processing of mismatch signals. Our findings provide new evidence that the hippocampal–vmPFC circuit is engaged during novelty processing, which has implications for emerging theories regarding the role of vmPFC in memory.
Keywords: Uncertainty, Prediction, Mismatch, Novelty, Theta entrainment, Hippocampus, Connectivity, MEG, Ventromedial prefrontal cortex
Highlights
-
•
Mismatch detection engages human hippocampus and ventromedial prefrontal cortex.
-
•
Novelty signals are spectrally confined to the theta band.
-
•
Ventromedial prefrontal cortex drives hippocampal theta induced by mismatches.
Introduction
Dealing effectively with environmental change is fundamental to adaptive behavior in an uncertain world. Failure to tolerate uncertainty has been associated with anxiety and schizophrenia (Boelen and Reijntjes, 2009; Broome et al., 2007). One mechanism for efficiently processing information is through change detection. It would be computationally inefficient to process every aspect of incoming stimuli anew. Instead, evidence suggests we learn patterns in our environment (Garrido et al., 2013) enabling us to make accurate predictions about what will happen next. We then compare these predictions with actual information and devote resources to processing mismatches. These mechanisms are often framed within predictive coding (Friston, 2005; Garrido et al., 2009; Kersten et al., 2004; Rao and Ballard, 1999; Yuille and Kersten, 2006).
Previous work has emphasized the role of the hippocampus in novelty processing (Axmacher et al., 2010; Kohler et al., 2005; Kumaran and Maguire, 2006; Ranganath and Rainer, 2003; Strange et al., 1999). A study by Kumaran and Maguire (2006) (see also Kumaran and Maguire (2009) and Strange and Dolan (2001)), showed that the hippocampus signals mismatch computations. In that paradigm, which we also employed here, each trial started with a unique sequence of four objects presented in the order ABCD. This sequence was then followed by one of three possible orders: predictable – ABCD, mismatch – ABDC, or unpredictable CADB. In that study, hippocampal activity was greatest in the mismatch condition, compared to both the predictable and unpredictable conditions – supporting the operation of match–mismatch (or comparator) computations ((Kumaran and Maguire, 2007; Lisman and Grace, 2005; Vinogradova, 2001) – as opposed to rising monotonically with the level of absolute associative novelty (greatest in the unpredictable condition), as would be predicted by the operation of a scalar familiarity-based mechanism (Brown and Aggleton, 2001; Kumaran and Maguire, 2007).
Related research has explored modulation of hippocampal theta oscillations in response to environmental novelty (Chen et al., 2013; Hasselmo and Stern, 2014; Hsieh and Ranganath, 2014). Complementary evidence in rats (Jeewajee et al., 2008) suggests theta power modulations may be more tightly linked to mismatches, rather than change per se. Direct evidence comes from an intracranial electroencephalography (iEEG) patient study showing enhanced hippocampal theta power in mismatch compared to predictable trials (Chen et al., 2013).
Two key questions remain unresolved: does the hippocampus interact with other brain structures during mismatch detection? What is the nature of these putative interactions; do hippocampal theta modulations drive other brain regions, or vice versa? One key area is the ventromedial prefrontal cortex (vmPFC) previously suggested to play an important role in making online predictions (van Kesteren et al., 2012) based on pre-existing knowledge. Moreover, theta communication in fronto-hippocampal networks is critical for information flow and memory function (Brockmann et al., 2011; Siapas et al., 2005). Work in rats suggests that theta entrainment is driven by hippocampus to prefrontal cortex (Siapas et al., 2005) but entrainment in the opposite direction has also been reported (Lesting et al., 2013). To address these questions, we used the Kumaran and Maguire (2006) paradigm and leveraged the wide spatial coverage and fine temporal resolution of MEG, in combination with dynamic causal modeling (DCM) analyses of effective connectivity between brain regions.
Methods
Experimental design
Participants
Seventeen adults with normal or corrected-to-normal vision participated in this experiment (10 female; age range: 19–48, M = 28.12, SD = 7.22 years). Each participant gave written informed consent, after full explanation of the experiment, according to procedures approved by the University College London Research Ethics Committee. Participants were monetarily compensated for their time. Data from one participant were excluded from the analysis due to excessive head movement (more than 20 mm within an experimental block). Data from two further participants were excluded from the DCM analysis due to a failure of model convergence.
Stimuli
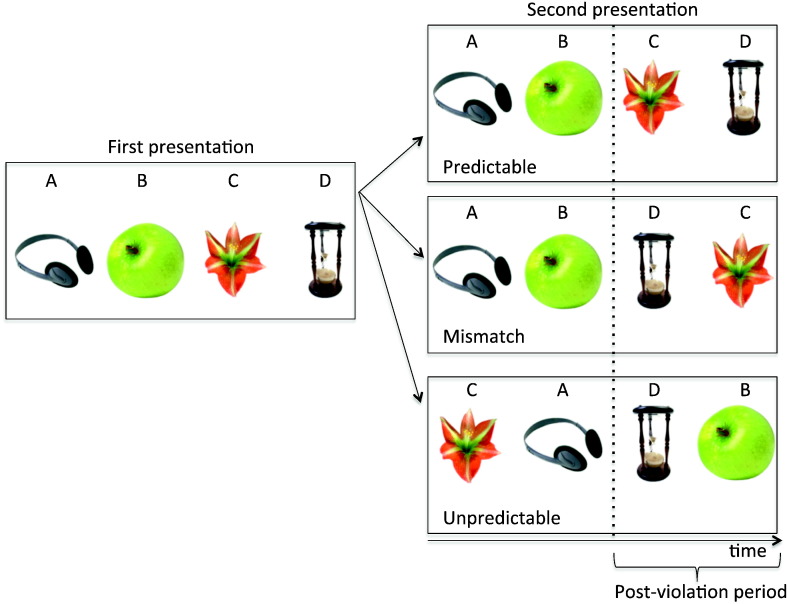
The stimuli was composed of quartets of color photos of objects that appeared one at a time on the center of the screen for a duration of 1 s for each picture and separated by a 200 ms centered fixation cross. There was a 2 s centered fixation cross between each quartet. Each trial was composed of two consecutive quartets of trial-unique objects. The first quartet consisted of a sequence of four objects (ABCD) and the second quartet presented the same four objects in one of three possible orders: (1) in the same order – predictable trials (ABCD) (2) with the last two objects presented in reversed order – mismatch trials (ABDC) or (3) completely reshuffled – unpredictable trials (CADB or BDAC – see Fig. 1). The stimuli and task parameters are described in further detail elsewhere (Kumaran and Maguire, 2006). The experiment encompassed 3 blocks each lasting 15 min with 2 short breaks in between, which yielded a total of 300 trials per participant, 100 trials per condition. In five out of seventeen participants we could only retain for analysis 2 out of 3 blocks due to excessive head movement (more than 20 mm) in one of the blocks.
Fig. 1.
Experimental design and stimuli. The stimuli was composed of quartets of objects that appeared one at a time on the center of the screen. The first presentation consisted of a sequence of four objects (ABCD) and the second presented the same four objects in one of three possible orders: (1) in the same order – predictable trials (ABCD), (2) with the last two objects presented in reversed order – mismatch trials (ABDC), or (3) in a reshuffled order – unpredictable trials (CADB or BDAC).
Task
Participants were told to look at the sequences of pictures and press a button, as fast as possible, if the same object appeared twice in a row (1-back task) within (and not between) trials. Participants were told that trials were composed by quartets of objects. Target trials – where objects appeared twice in a row – were excluded from the analysis.
Stimuli delivery and task protocol were programmed in MATLAB, using the Cogent 2000 toolbox (http://www.vislab.ucl.ac.uk/cogent.php).
MEG data acquisition and pre-processing
MEG recordings were performed with a CTF 275-channel whole-head system, with 274 functioning second-order axial gradiometers arranged in a helmet shaped array. We attached three coils to the fiducial points (nasion, and left and right preauricular), so that we could continuously monitor the head’s position of each participant with respect to the MEG sensors. Data were collected at a sampling rate of 600 Hz, were filtered with a bandpass between 0.2 and 20 Hz, and down-sampled to 200 Hz. Epochs encompassed data recorded from 400 ms before to 4600 ms after trial onset. Co-registration and the forward model were computed for each experimental block using a single-shell head model (Nolte, 2003) and data were then concatenated across blocks for each participant. All pre-processing and subsequent analysis were carried out using the SPM8 package (Litvak et al., 2011) available at http://www.fil.ion.ucl.ac.uk/spm/.
Source space estimates
The linearly constrained minimum variance (LCMV) beamformer spatial filtering (Van Veen et al., 1997) was used to make volumetric images of power change between conditions. We used the spatial filtering algorithm described in (Sekihara et al., 2004) as implemented in SPM. This was done by building a single covariance matrix for each of the bands of interest (4–8, 9–12 and 13–20 Hz) based on the data from all three conditions (predictable, mismatch, and unpredictable). The time window used for this computation was either whole temporal window in a trial [0–4.6 s] or the time window after the mismatch [2.2–4.6 s], i.e., the post-violation period. In our post-violation analysis we included both the third and fourth objects in order to provide a larger number of data points and hence increase the reliability of the beamformer estimates. For each of three bands and time windows this gave a set of beamformer weights mapping the MEG channels to a 5 mm volumetric grid. These weights were then used to produce first level contrast images of the (weight normalized) power differences between conditions. The contrasts taken to the second level consisted of the effect of (1) change detection, i.e., mismatch versus predictable (2) mismatch computations, i.e., mismatch versus unpredictable conditions.
Second-level statistical analysis
At the second level we performed F-contrasts on the single subject contrast images for those volumes constructed based on data from the whole temporal window in a trial [0–4.6 s] (Fig. 2) and then considering the data in the time window after the mismatch [2.2–4.6 s] (Fig. 3). Statistical maps are displayed at p < 0.001 uncorrected, with spatial extent threshold of zero (Figs. 2–4). Given that this exact paradigm has been employed on a previous fMRI study that yields activity in the left hippocampus and left vmPFC for mismatch computations (Kumaran and Maguire, 2006), we had good reasons to assume, a priori, that these areas would also be active here. Hence, we performed small volume corrections (SVC) for both left hippocampus and left vmPFC (Brodmann area 11, BA11) using anatomical masks defined within the SPM Anatomy toolbox v1.8 (Eickhoff et al., 2005), and report peak-level FWE-corrected statistics at p < 0.05. When using a mask including both left hippocampus and left vmPFC we use set level statistics p < 0.01.
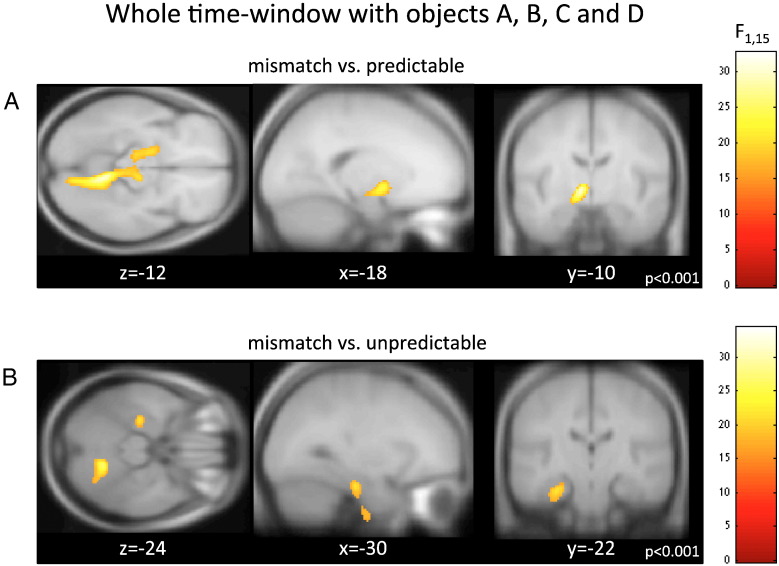
Fig. 2.
Theta activity reconstruction in the whole-time window. Hippocampal theta is induced by mismatch sequences. F-contrasts for reconstructed theta brain activity over the whole trial length [0–4.6 s] (i.e., objects A, B, C, and D) displayed at p < 0.001 uncorrected, and overlaid on a canonical T1-weighted MR image. Correction for multiple comparisons was achieved by taking into account anatomical masks for the hippocampus according to prior work (Kumaran and Maguire, 2006). A. Mismatch versus predictable contrast shows a marginally significant effect in the left hippocampus. B. Contrast between mismatch and unpredictable conditions revealed left anterior hippocampus. Note that the localization of these effects to the hippocampus is consistent with the expected decrease in MEG spatial resolution due to low sensitivity to deep sources (Hillebrand and Barnes, 2002) and typical levels of co-registration error of around ± 5 mm (Singh et al., 1997).
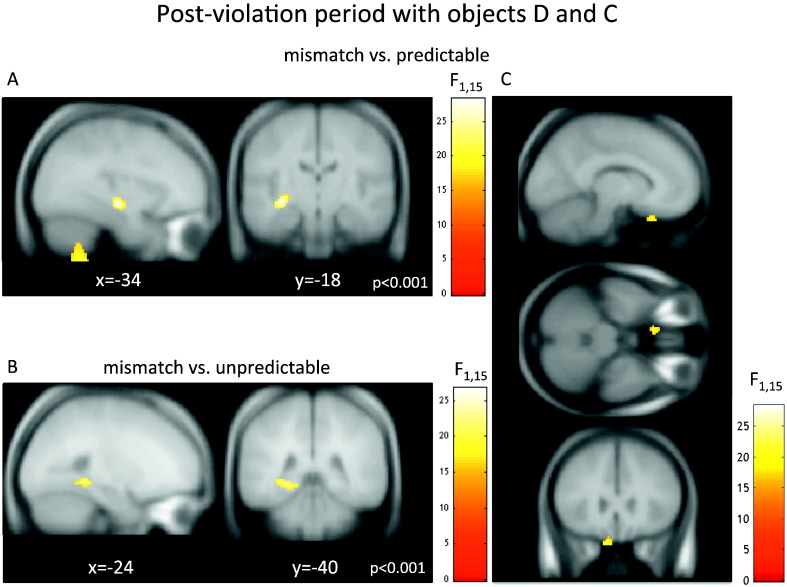
Fig. 3.
Theta activity reconstruction in the post-violation period. Hippocampal theta induced in the post-violation period. F-contrasts for reconstructed theta brain activity over the post-violation period [2.2–4.6 s] (after the third event in a trial, objects D and C) displayed at p < 0.001 uncorrected, overlaid on a canonical T1-weighted MR image. A. Mismatch versus predictable contrast revealed left anterior hippocampus B. Mismatch versus unpredictable contrast revealed left posterior hippocampus (see text for details). C. In addition to the hippocampus, mismatch versus predictable contrast revealed ventromedial prefrontal cortex (vmPFC).
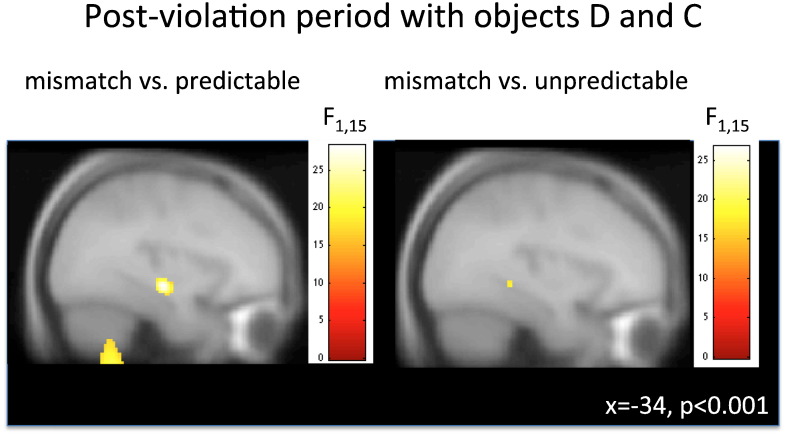
Fig. 4.
Sequence novelty and prediction violation. Anterior hippocampal theta induced in the post-violation period for mismatch versus predictable (left), and posterior hippocampal theta for the mismatch vs. unpredictable contrast (right).
Previous fMRI work shows that mismatch computations are associated with increased activity in the hippocampus (Kumaran and Maguire, 2006). However, the picture is less clear with induced theta oscillations, which may increase (Axmacher et al., 2010) or decrease (Duzel et al., 2010) with novelty. For this reason we adopted a more conservative analysis strategy using F-contrasts, instead of the more widely used T-contrasts. Nevertheless, having computed the T-maps, it is clear that these theta changes do actually correspond to increases (and not decreases) for the mismatch condition.
Bayesian model selection
The four estimated DCMs were compared statistically across participants using a random-effects Bayesian model selection (BMS) approach (Stephan et al., 2009).
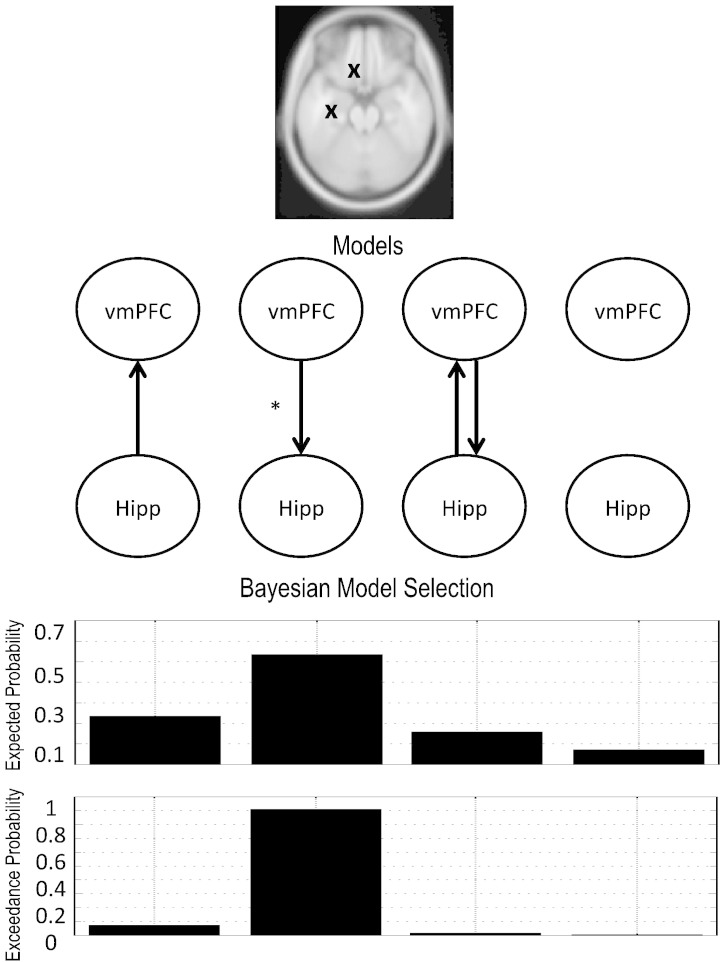
DCM for phase coupling
To study the nature of hippocampal–prefrontal interactions in the theta regime we used DCM for phase-coupled data. DCM for phase coupling (Penny et al., 2009) is an extension of the DCM framework (Chen et al., 2008; David et al., 2006; Friston et al., 2003; Moran et al., 2009) to accommodate the analysis of data coupled in phase and uses a weakly coupled oscillator model to describe the dynamics of phase changes in a network. With this model-based connectivity approach it is possible to test hypotheses of master–slave relationships or mutual entrainment between regions in a given frequency regime. Here we investigated the entrainment of hippocampus and the vmPFC in the theta band (4–8 Hz). We tested four different hypotheses with four corresponding 2-region network models: (1) hippocampus drives prefrontal cortex (Hipp → vmPFC), (2) prefrontal cortex drives hippocampus (vmPFC → Hipp), (3) hippocampus and prefrontal cortex are mutually entrained (Hipp ↔ vmPFC), and (4) hippocampus and prefrontal cortex do not interact (Fig. 5). Left hippocampus (x, y, z = − 34, − 18, − 12) and vmPFC (x, y, z = − 10, 24, − 30) nodes were defined on the basis of the SPM peaks resulting from the contrast between mismatch and predictable conditions in the post-violation period as shown in Fig. 3. We modeled data in the period of − 50 to 4600 ms and used an equivalent current dipole forward spatial model.
Fig. 5.
Dynamic causal modeling. Dynamic causal modeling for phase coupling suggests that vmPFC drives hippocampal theta activity. Models considered that: (1) hippocampus (Hipp) drives vmPFC, (2) vmPFC drives Hipp, (3) vmPFC and Hipp drive each other, and (4) vmPFC and Hipp are disconnected. Bayesian model selection (RFX) revealed that enhanced theta oscillations for mismatch vs. unpredictable are caused by vmPFC driving hippocampus.
Results
In this paper we used MEG to investigate oscillatory activity in the hippocampus while participants incidentally viewed sequences of objects that were either presented in exactly the same temporal order as previously (ABCD – predictable condition), an entirely different temporal order (e.g. CADB – unpredictable condition) or, a mismatch condition (ABDC). We used LCMV beamformer to reconstruct images of theta (4–8 Hz), alpha (9–12 Hz), and beta (13–20 Hz) oscillatory source activity for each of the three conditions and then performed planned contrasts between mismatch and predictable, and mismatch and unpredictable conditions.
Source analysis—whole time window
First, we examined whether our results replicated the fMRI results reported by Kumaran and Maguire (2006). We did this by analyzing the entire trial, that is, the time period when the object quartet was presented. Notably, whereas the original fMRI study investigated the profile of hippocampal BOLD signals across the three experimental conditions, here we contrasted beamformed images of source reconstructed theta oscillatory activity between conditions. We found marginally significant differences in the anterior hippocampal theta (peaking at x, y, z = − 18, − 10, − 12, p < 0.055 FWE-corrected) for the mismatch versus predictable (Fig. 2A) and for the mismatch versus unpredictable (Fig. 2B, peaking at x, y, z = − 30, − 22, − 24, p < 0.029 FWE-corrected) conditions. We confirmed the localization of these clusters with the Anatomy toolbox v1.8 (Eickhoff et al., 2005). The latter cluster was assigned to the left hippocampal formation (with probability of 40% to CA and 10% to subiculum). We note however that given the smoothness of the MEG images at this depth (Barnes et al., 2004; Gross et al., 2003) and other factors such as co-registration error and indeed different atlas labeling scheme (such as AAL) we cannot rule out that the source derives from an adjacent structure such as the parahippocampus. Note that by contrasting mismatch with unpredictable trials we show that the hippocampus is sensitive specifically to prediction violations, and not to sequence novelty per se, a factor present in both of these conditions (see also Fig. 4). Further, these results are consistent with the fMRI results for this task (Kumaran and Maguire, 2006), despite the expected decrease in MEG spatial resolution at this depth (Hillebrand and Barnes, 2002) and typical levels of co-registration error of around ± 5 mm (Singh et al., 1997).
Crucially, effects in the hippocampus were spectrally confined to the theta band with no significant effects identified in other frequency bands. Analysis in the remaining frequency bands revealed only an isolated cluster in the left cerebellum, relating to power in the alpha frequency range, for the mismatch versus unpredictable contrast (p < 0.001, uncorrected). Given that this effect does not survive our criteria for statistical significance (see Methods) and in the absence of an a priori hypothesis about alpha activity in the cerebellum, we do not consider this further. Likewise it is hard to infer on potential cerebellum and thalamic sources encompassed by the larger clusters shown in Fig. 2A. This contrasts with the principled use of a priori masks for the hippocampus given the prior evidence drawn from independent data reported in Kumaran and Maguire (2006).
Source analysis—post-violation period
The fine temporal resolution afforded by MEG (compared to fMRI) permitted an analysis that isolated effects of interest to the time window following the critical mismatch event: i.e. at the 3rd object. Accordingly, we performed the same contrasts considering a smaller time window that encompassed the activity elicited by the third and fourth stimuli in each trial (i.e. “post-violation” period). As in the previous analysis, we found significantly greater theta power in the mismatch condition as compared to the predictable condition in the anterior hippocampus (x, y, z = − 34, − 18, − 12, p < 0.028 FWE-corrected, see Fig. 3A). When we contrasted the mismatch and unpredictable condition in this post-violation analysis, we identified increased theta power in a region consistent with localization in the posterior hippocampus (x, y, z = − 24, − 40, − 6, p < 0.022 FWE-corrected, see Figs. 3B and 4). Again, by contrasting mismatch with unpredictable trials we were able to demonstrate that the hippocampus is sensitive specifically to prediction violations, and not to sequence novelty per se. In addition to the significant hippocampal effects, we found significant theta changes in the vmPFC when comparing the mismatch and the predictable condition (x, y, z = − 10, 24, − 30, p < 0.042 FWE-corrected, see Fig. 3C, cf. Kumaran and Maguire (2006)).
Connectivity analysis—DCM for phase coupling
Motivated by the increases in theta power in the hippocampus and vmPFC in the mismatch, as compared to predictable conditions, we next probed the existence and nature of dynamic interactions between these two brain regions. To do this, we directly compared four different connectivity models (see Fig. 5). The two-area models were built on the basis of two equivalent current dipoles placed at the peak locations of the hippocampus (Hipp, x, y, z = − 34, − 18, − 12) and the ventromedial prefrontal cortex (vmPFC, x, y, z = − 10, 24, − 30), as determined in the mismatch versus predictable contrast that resulted from source reconstruction images in the post-violation period. The first model tested the hypothesis that Hipp drives theta oscillations in vmPFC. The second model tested the opposite, that vmPFC drives Hipp. The third model tests the hypothesis that vmPFC and Hipp are mutual entrained, i.e., they drive each other through reciprocal connections. The fourth, or null, model assumed no dynamic interactions through theta oscillations between vmPFC and Hipp. We tested these models using DCM for phase coupling (see Methods) and used RFX Bayesian Model Selection to determine which of these models best explained the data. In contrast to what we had predicted – based on computational models that propose that the hippocampus performs the initial match–mismatch computations (for reviews see Kumaran and Maguire (2007); (Lisman and Grace, 2005)) – we found that the vmPFC drove hippocampal expression of theta (vmPFC → Hipp) when comparing both mismatch versus predictable conditions (expected probability = 0.5; exceedance probability = 0.81) and mismatch versus unpredictable conditions (expected probability = 0.54; exceedance probability = 0.91). We performed a further statistical analysis using a binomial test to determine the likelihood of obtaining the winning model at the group level, under the null hypothesis that each of the 4 models was equally likely. This test computes the binomial probability density function for a given number of successes, s, in a given number of independent trials, N, where the probability of success in any given trial is P. Here, s successes correspond to the number of participants for whom vmPFC → Hipp is the best model, N is the total number of participants, and P is the probability of each model, m, under the null hypothesis, i.e., P = 1/mN. We found that vmPFC drove Hipp with p-value = 0.0018 and p-value = 0.0007 for the contrasts mismatch versus predictable and mismatch versus unpredictable, respectively.
Discussion
In this paper we leveraged a wide spatial coverage and fine temporal resolution of MEG to investigate the role of hippocampal oscillations in detection of prediction violations or mismatch computations. Our data reveal that mismatch computations are spectrally confined to the theta band and spatially restricted to the hippocampus and the vmPFC, a region to which the hippocampus has direct anatomical connections (Cavada et al., 2000; Mackey and Petrides, 2010). Further, we show that sequence novelty evokes theta entrainment driven by influences from ventromedial prefrontal cortex to the hippocampus. Our findings highlight the dynamics of a neural circuit that operates during the automatic (i.e., task irrelevant) detection of mismatches in the environment, thereby providing evidence of a new function for the hippocampal–vmPFC circuit in cognition. Moreover, these findings help to constrain evolving theories concerning the complementary roles of the vmPFC and hippocampus in memory.
It may seem surprising that we use MEG to make claims about a subcortical source, such as the hippocampus. However, a growing number of empirical papers highlight source activity detected using MEG as originating from deep structures such as the hippocampus (Attal et al., 2007; Cornwell et al., 2012; Cornwell et al., 2008; Guitart-Masip et al., 2013; Poch et al., 2011; Quraan et al., 2011; Tesche and Karhu, 2000). These empirical findings are corroborated by theoretical work demonstrating, in anatomically realistic simulations, that activity in deep brain regions such as hippocampus and amygdala can be captured using MEG (Attal and Schwartz, 2013). This is rendered feasible by higher current densities generated within these deep areas compared to the neocortex, a feature that helps compensate for a greater distance to the sensors.
Previous neuroimaging data demonstrate the human hippocampus acts as an associative mismatch detector during the processing of novel arrangements of sequences of events (Kumaran and Maguire, 2006). The present demonstration that theta power in the hippocampus is significantly greater in the mismatch condition, as compared to the unpredictable and predictable conditions is consistent with these previous results. Interestingly, a recent study showed that hippocampal theta, measured by iEEG, is enhanced in a mismatch compared to a predictable condition (Chen et al., 2013). While this result complements the findings we nevertheless draw attention to two potential caveats in relation to Chen et al. (2013). First, the iEEG recordings were conducted in patients with epilepsy which makes it difficult to exclude the possibility that pathological changes in the hippocampus or medication-related effects could have contributed to the results (though see Quiroga (2012). Second, in the iEEG study, while the amplitude of hippocampal theta on unpredictable trials fell between that observed for predictable and mismatch trials, this effect did not reach statistical significance. Our results, therefore, represent the first demonstration that hippocampal novelty signals in response to mismatches are linked to increases in hippocampal theta power in healthy humans.
It is interesting to note one point of discrepancy between the current results and the previous fMRI study (Kumaran and Maguire, 2006). Here we demonstrate that a posterior region of the hippocampus exhibited a robust increase in theta power in the mismatch condition, as compared to the unpredictable condition. Interestingly, this posterior hippocampal finding was only identified in the post-violation (and not whole) time window analysis, a type of analysis for which MEG is particularly suited, given its finer temporal resolution (in contrast to fMRI). It is worth noting that no significant increase in posterior hippocampal theta power was observed when the mismatch condition was contrasted with the predictable condition in the Kumaran and Maguire (2006), or in the iEEG study using the same paradigm (Chen et al., 2013) where increases in theta power in the mismatch condition were confined to the anterior hippocampus. We suggest one possible explanation for an isolated increase in theta power in the mismatch versus unpredictable contrast and not in the mismatch versus predictable contrast that draws on ideas related to functional segregation of novelty/familiarity detection along the anterior–posterior axis of the hippocampus (e.g. (Poppenk et al., 2013; Strange et al., 2014). We think it is conceivable that posterior hippocampus might be engaged by prediction violations evoked by the mismatch condition (and not the unpredictable), but also by sequence familiarity in the predictable condition (i.e. hence accounting for the absence of a significant difference between mismatch and predictable conditions).
A key aspect of our findings is evidence that vmPFC also signals mismatches through increases in theta power, indicating that the hippocampus and vmPFC function as a circuit. This result is highly consistent with the likely existence of monosynaptic connections between the hippocampus and orbitofrontal cortex/neighboring vmPFC (Mackey and Petrides, 2010). Over recent years, multiple lines of evidence have highlighted the importance of this circuit in supporting a wide range of cognitive processes – including autobiographical memory (e.g. (Bonnici et al., 2012); Maguire (2014); (Nieuwenhuis and Takashima, 2011)), imagination (Hassabis et al., 2007), spatial navigation (Doeller et al., 2008)), goal-directed decision making (Roy et al., 2012), schema-based memory formation (van Kesteren et al., 2012) as well as constituting part of the “so-called” default network active during resting states (Fox et al., 2005). Further, in rodents phase locking – or entrainment - of hippocampal and medial PFC neurons has been shown to be an important predictor of behavioral success on a given trial, highlighting the importance of tightly coupled neural activity between these two brain structures (Hyman et al., 2011; Lesting et al., 2013; Siapas et al., 2005) (Lesting et al., 2013). It has been difficult to achieve a unifying hypothesis that can accommodate such a diverse array of functions for this hippocampal–vmPFC circuit. While a majority of previous human studies demonstrate engagement of a hippocampal–vmPFC circuit during goal-directed cognitive tasks (autobiographical memory recall, decision making), our data demonstrate that this circuit is automatically recruited during mismatch detection thereby suggesting that interactions with the vmPFC are a fundamental aspect of hippocampal processing, regardless of task/goal relevance.
A surprising aspect of our effective connectivity results is that the best fitting model involved a circuit in which signals in vmPFC drove those in the hippocampus in the theta regime. Theta entrainment of hippocampus and prefrontal cortex is thought to play an important role in memory formation and error detection (Hyman et al., 2011; Lisman and Grace, 2005). However, its mechanisms at the network level have received much less attention. Here, we used a model-based connectivity approach (DCM for phase coupling) to investigate the mechanisms and directionality of such entrainment. To our surprise we found that the vmPFC drove hippocampal theta during a mismatch. This finding is opposite to that of Siapas et al. (2005) and Brockmann et al. (2011) who found that theta oscillations in the hippocampus preceded medial prefrontal cortex in the rat. In humans, it has been suggested that mutual theta entrainment occurs, although stronger in the HIPP → PFC than in the PFC → HIPP direction (Anderson et al., 2010). However, the latter study afforded only a small sample of 3 patients with epilepsy. On the other hand, several studies have found evidence that prefrontal signals may precede hippocampal activity: Lesting et al. (2013) in the context of fear extinction, Murty and Adcock (2013) under conditions when a motivating cue precedes expectancy violations, and Brincat and Miller (2015) in a paradigm where non-human primates learnt non-spatial associations in a relatively gradual fashion (e.g. over ~ 50 trials) during simultaneous lateral PFC and hippocampus recording. In the latter study, errors induced increased theta oscillations that were driven by PFC to the hippocampus. Further, and as noted in the Introduction, the ventromedial prefrontal cortex has been previously suggested to play a role in the formation of online predictions (van Kesteren et al., 2012). Based on this hypothesis and the data cited above (e.g. Brincat and Miller (2015)), it is possible that our findings reflect the formation of online predictions by the ventromedial prefrontal cortex that are compared to incoming stimuli through mismatch computations, or detection of prediction violations, in the hippocampus. It is worth noting, however, that such has a function for the PFC has typically been proposed in the context of existing knowledge (i.e. “schema”; van Kesteren et al. (2012) or when associative learning occurs over multiple trials (Brincat and Miller, 2015) – whereas the object sequences in this study were trial-unique and therefore arbitrary (i.e. unrelated to pre-existing knowledge). While our data provide evidence for the prefrontal cortex driving hippocampus in the theta regime, we believe further work is needed to clarify this surprising observation as well as conflicting findings pertaining to the wider literature.
In conclusion, we show that mismatch signals are expressed in hippocampus and vmPFC, and that these signals are spectrally confined to the theta frequency band. Using effective connectivity analyses (DCM) we provide evidence that the hippocampus and vmPFC operate as a functional circuit during mismatch detection. Surprisingly, our data indicate that the vmPFC exerts a dominant directional influence on the hippocampus during mismatch detection. Our findings draw attention to a need to broaden current theories that focus on the contribution of the vmPFC to schema-guided memory formation, so as to inform a more unifying account of the function of the hippocampal–vmPFC circuit.
Acknowledgments
This work was funded by an Australian Research Council Discovery Early Career Researcher Award (DE130101393) and the Australian Research Council Centre of Excellence for Integrative Brain Function (ARC Centre Grant CE140100007) to MIG; a Wellcome Trust Project Grant (085316/Z/08/Z) to RJD; an MRC UK MEG Partnership Grant (MR/K005464/1) to GRB; a Wellcome Trust Fellowship to DK (WT085189MA), and a Wellcome Trust Principal Research Fellowship (101759/Z/13/Z) to EAM. We thank Heidi Bonnici and Will Penny for discussions; David Bradbury, Letitia Manyande, and Janice Glensman for help with data collection; and the volunteers for participating in this study.
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.07.016.
Appendix A. Supplementary data
Supplementary material. Glass brain and cluster information for the mismatch versus predictable F-contrast in the post-violation period.
References
- Anderson K.L., Rajagovindan R., Ghacibeh G.A., Meador K.J., Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb. Cortex. 2010;20:1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- Attal Y., Schwartz D. Assessment of subcortical source localization using deep brain activity imaging model with minimum norm operators: a MEG study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059856. e59856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal Y., Bhattacharjee M., Yelnik J., Cottereau B., Lefevre J., Okada Y., Bardinet E., Chupin M., Baillet S. Conf Proc IEEE Eng Med Biol Soc 2007. 2007. Modeling and detecting deep brain activity with MEG & EEG; pp. 4937–4940. [DOI] [PubMed] [Google Scholar]
- Axmacher N., Cohen M.X., Fell J., Haupt S., Dumpelmann M., Elger C.E., Schlaepfer T.E., Lenartz D., Sturm V., Ranganath C. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010;65:541–549. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Barnes G.R., Hillebrand A., Fawcett I.P., Singh K.D. Realistic spatial sampling for MEG beamformer images. Hum. Brain Mapp. 2004;23:120–127. doi: 10.1002/hbm.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen P.A., Reijntjes A. Intolerance of uncertainty and social anxiety. J. Anxiety Disord. 2009;23:130–135. doi: 10.1016/j.janxdis.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bonnici H.M., Chadwick M.J., Lutti A., Hassabis D., Weiskopf N., Maguire E.A. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J. Neurosci. 2012;32:16982–16991. doi: 10.1523/JNEUROSCI.2475-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat S.L., Miller E.K. Frequency-specific hippocampal–prefrontal interactions during associative learning. Nat. Neurosci. 2015;18:576–581. doi: 10.1038/nn.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann M.D., Poschel B., Cichon N., Hanganu-Opatz I.L. Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat. Neuron. 2011;71:332–347. doi: 10.1016/j.neuron.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Broome M.R., Johns L.C., Valli I., Woolley J.B., Tabraham P., Brett C., Valmaggia L., Peters E., Garety P.A., McGuire P.K. Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br. J. Psychiatry Suppl. 2007;51:s38–s42. doi: 10.1192/bjp.191.51.s38. [DOI] [PubMed] [Google Scholar]
- Brown M.W., Aggleton J.P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cavada C., Company T., Tejedor J., Cruz-Rizzolo R.J., Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Kiebel S.J., Friston K.J. Dynamic causal modelling of induced responses. NeuroImage. 2008;41:1293–1312. doi: 10.1016/j.neuroimage.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Chen J., Dastjerdi M., Foster B.L., LaRocque K.F., Rauschecker A.M., Parvizi J., Wagner A.D. Human hippocampal increases in low-frequency power during associative prediction violations. Neuropsychologia. 2013;51:2344–2351. doi: 10.1016/j.neuropsychologia.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B.R., Johnson L.L., Holroyd T., Carver F.W., Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J. Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B.R., Arkin N., Overstreet C., Carver F.W., Grillon C. Distinct contributions of human hippocampal theta to spatial cognition and anxiety. Hippocampus. 2012;22:1848–1859. doi: 10.1002/hipo.22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O., Kiebel S.J., Harrison L.M., Mattout J., Kilner J.M., Friston K.J. Dynamic causal modeling of evoked responses in EEG and MEG. NeuroImage. 2006;30:1255–1272. doi: 10.1016/j.neuroimage.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Doeller C.F., King J.A., Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E., Penny W.D., Burgess N. Brain oscillations and memory. Curr. Opin. Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Garrido M.I., Kilner J.M., Stephan K.E., Friston K.J. The mismatch negativity: a review of underlying mechanisms. Clin. Neurophysiol. 2009;120:453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido M.I., Sahani M., Dolan R.J. Outlier responses reflect sensitivity to statistical structure in the human brain. PLoS Comput. Biol. 2013;9 doi: 10.1371/journal.pcbi.1002999. e1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Timmermann L., Kujala J., Salmelin R., Schnitzler A. Properties of MEG tomographic maps obtained with spatial filtering. NeuroImage. 2003;19:1329–1336. doi: 10.1016/s1053-8119(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M., Barnes G.R., Horner A., Bauer M., Dolan R.J., Duzel E. Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. J. Neurosci. 2013;33:442–451. doi: 10.1523/JNEUROSCI.2573-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D., Kumaran D., Maguire E.A. Using imagination to understand the neural basis of episodic memory. J. Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M.E., Stern C.E. Theta rhythm and the encoding and retrieval of space and time. NeuroImage. 2014;85(Pt 2):656–666. doi: 10.1016/j.neuroimage.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. NeuroImage. 2002;16:638–650. doi: 10.1006/nimg.2002.1102. [DOI] [PubMed] [Google Scholar]
- Hsieh L.T., Ranganath C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. NeuroImage. 2014;85(Pt 2):721–729. doi: 10.1016/j.neuroimage.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman J.M., Hasselmo M.E., Seamans J.K. What is the functional relevance of prefrontal cortex entrainment to hippocampal theta rhythms? Front. Neurosci. 2011;5:24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewajee A., Lever C., Burton S., O'Keefe J., Burgess N. Environmental novelty is signaled by reduction of the hippocampal theta frequency. Hippocampus. 2008;18:340–348. doi: 10.1002/hipo.20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten D., Mamassian P., Yuille A. Object perception as Bayesian inference. Annu. Rev. Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- Kohler S., Danckert S., Gati J.S., Menon R.S. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: a comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Kumaran D., Maguire E.A. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040424. e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Maguire E.A. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007;17:735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- Kumaran D., Maguire E.A. Novelty signals: a window into hippocampal information processing. Trends Cogn. Sci. 2009;13:47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Lesting J., Daldrup T., Narayanan V., Himpe C., Seidenbecher T., Pape H.C. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PLoS One. 2013;8 doi: 10.1371/journal.pone.0077707. e77707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J.E., Grace A.A. The hippocampal–VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Litvak V., Mattout J., Kiebel S., Phillips C., Henson R., Kilner J., Barnes G., Oostenveld R., Daunizeau J., Flandin G., Penny W., Friston K. EEG and MEG data analysis in SPM8. Comput. Intell. Neurosci. 2011;2011:852961. doi: 10.1155/2011/852961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S., Petrides M. Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. Eur. J. Neurosci. 2010;32:1940–1950. doi: 10.1111/j.1460-9568.2010.07465.x. [DOI] [PubMed] [Google Scholar]
- Maguire E.A. Memory consolidation in humans: new evidence and opportunities. Exp. Physiol. 2014;99:471–486. doi: 10.1113/expphysiol.2013.072157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran R.J., Stephan K.E., Seidenbecher T., Pape H.C., Dolan R.J., Friston K.J. Dynamic causal models of steady-state responses. NeuroImage. 2009;44:796–811. doi: 10.1016/j.neuroimage.2008.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Adcock R.A. Cereb Cortex; 2013. Enriched encoding: reward motivation organizes cortical networks for hippocampal detection of unexpected events. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis I.L., Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav. Brain Res. 2011;218:325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 2003;48:3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Penny W.D., Litvak V., Fuentemilla L., Duzel E., Friston K. Dynamic causal models for phase coupling. J. Neurosci. Methods. 2009;183:19–30. doi: 10.1016/j.jneumeth.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch C., Fuentemilla L., Barnes G.R., Duzel E. Hippocampal theta-phase modulation of replay correlates with configural–relational short-term memory performance. J. Neurosci. 2011;31:7038–7042. doi: 10.1523/JNEUROSCI.6305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013;17:230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Quiroga R.Q. Concept cells: the building blocks of declarative memory functions. Nat. Rev. Neurosci. 2012;13:587–597. doi: 10.1038/nrn3251. [DOI] [PubMed] [Google Scholar]
- Quraan M.A., Moses S.N., Hung Y., Mills T., Taylor M.J. Detection and localization of hippocampal activity using beamformers with MEG: a detailed investigation using simulations and empirical data. Hum. Brain Mapp. 2011;32:812–827. doi: 10.1002/hbm.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Rainer G. Neural mechanisms for detecting and remembering novel events. Nat. Rev. Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Rao R.P., Ballard D.H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Roy M., Shohamy D., Wager T.D. Ventromedial prefrontal–subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekihara K., Nagarajan S.S., Poeppel D., Marantz A. Asymptotic SNR of scalar and vector minimum-variance beamformers for neuromagnetic source reconstruction. IEEE Trans. Biomed. Eng. 2004;51:1726–1734. doi: 10.1109/TBME.2004.827926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas A.G., Lubenov E.V., Wilson M.A. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Singh K.D., Holliday I.E., Furlong P.L., Harding G.F.A. Evaluation of MRI-MEG/EEG co-registration strategies using Monte Carlo simulation. Electroencephalogr. Clin. Neurophysiol. 1997;102:81–85. doi: 10.1016/s0921-884x(96)96570-4. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Daunizeau J., Moran R.J., Friston K.J. Bayesian model selection for group studies. NeuroImage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B.A., Dolan R.J. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Strange B.A., Fletcher P.C., Henson R.N., Friston K.J., Dolan R.J. Segregating the functions of human hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B.A., Witter M.P., Lein E.S., Moser E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Tesche C.D., Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc. Natl. Acad. Sci. U. S. A. 2000;97:919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren M.T., Ruiter D.J., Fernandez G., Henson R.N. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vinogradova O.S. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Yuille A., Kersten D. Vision as Bayesian inference: analysis by synthesis? Trends Cogn. Sci. 2006;10:301–308. doi: 10.1016/j.tics.2006.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Glass brain and cluster information for the mismatch versus predictable F-contrast in the post-violation period.