Abstract
Burkholderia mallei is the causative agent of glanders which is a highly contagious and fatal disease of equines. Considering the nature and severity of the disease in equines, and potential of transmission to human beings, glanders is recognised as a ‘notifiable’ disease in many countries. An increasing number of glanders outbreaks throughout the Asian continents, including India, have been noticed recently. In view of the recent re-emergence of the disease, the present study was undertaken to estimate the prevalence of glanders among indigenous equines from different parts of India. Serum samples were analysed by complement fixation test (CFT) and ELISA for the detection of B mallei specific antibodies. A total of 7794 equines, which included 4720 horses, 1881 donkeys and 1193 mules were sampled from April 2011 to December 2014 from 10 states of India. Serologically, 36 equines (pony=7, mules=10, horses=19) were found to be positive for glanders by CFT and indirect-ELISA. The highest number of cases were detected in Uttar Pradesh (n=31) followed by Himachal Pradesh (n=4) and Chhattisgarh (n=1). Isolation of B mallei was attempted from nasal and abscess swabs collected from seropositive equines. Four isolates of B mallei were cultured from nasal swabs of two mules and two ponies. Identity of the isolates was confirmed by PCR and sequencing of fliP gene fragment. The study revealed circulation of B mallei in northern India and the need for continued surveillance to support the eradication.
Keywords: Equines, Glalnders, Surveillance, Zoonoses
Introduction
Burkholderia mallei, a non-motile Gram-negative bacterium, is the causative agent of contagious and fatal disease of equines known as glanders (Minett 1959, Khan and others 2013). The natural hosts for B mallei are horses, donkeys and mules. Other than equines, small ruminants (sheep and goat) may also be infected if kept in close contact with glanderous horses. Susceptibility to glanders has been proved in camels and carnivores, but cattle and pigs are resistant (Witting and others 2006). The organism has an affinity for the lymphatics and numerous foci of suppuration along lymphatic pathways may be seen. In general, three clinical forms of the disease namely nasal form, pulmonary form and cutaneous form or ‘Farcy’ are observed in B mallei infected animals (Steele 1979). Human beings may acquire the infection through direct contact with the organism and prolonged contact with diseased animals. Veterinarians, horse caretakers and laboratory workers handling this organism are considered as the professional risk groups. Although, early and aggressive treatment with combinations of systemic antibiotics can be curative (Srinivasan and others 2001), an extremely high rate of mortality can occur in untreated human beings.
Because of the economic and zoonotic impact of the infection and lack of standard therapeutics, glanders is recognised as a ‘notifiable’ disease in many countries. The disease has been eradicated from developed nations by statutory testing, elimination of infected equines and imposing import restrictions from endemic countries. However, the disease is still endemic in parts of Africa, Southern Asia, the Middle East and Central and South America (World Animal Health Information Database 2014). Lack of documented information makes it difficult to trace the early introduction of glanders in India. It is believed that glanders was first seen in the mail cart horses in 1881 (Verma 1981). Confirmed cases of B mallei infection in Indian equines were documented in 1913 (Holmes 1913). A detailed account of incidence and epidemiology of glanders in military and civilian farms in India had been reported in the early 1980s (Verma 1981, Ray and others 1984, Misra and others 1985). A sudden re-emergence of the disease was observed in 2006 and it continued to affect equids in several regions over the following five years (Malik and others 2009, 2012). As a result, glanders surveillance was intensified as part of the national policy to determine its occurrence and adopt immediate containment measures. The present study discusses the outcome of the surveillance of glanders carried out between April 2011 and December 2014 among indigenous equines.
Materials and methods
Study area and sampling
The study was conducted from April 2011 through December 2014. Based on the previous incidence of glanders outbreaks, equids were sampled from six glanders-endemic states and four non-endemic states spread across north-western and central regions of India (Fig 1 and Table 1). Geographically, the study areas covered latitude and longitude ranges from 13.70° to 36.97° North and 72.68° to 81.60° East, respectively. Surveillance was conducted by random sampling of indigenous equines. In glanders suspected cases, all of the equines in the same premises as well as in-contact equines in the working places were sampled.
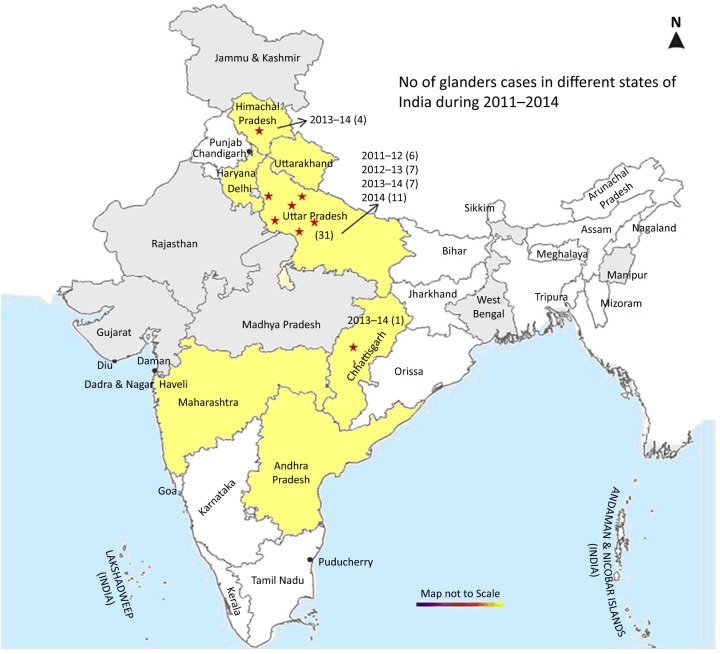
FIG 1:
Glanders-endemic and non-endemic states are indicated in yellow colour and light grey colour, respectively. Numbers in parentheses show glanders-positive cases reported from the respective states in a given year. Locations of the glanders cases are indicated by star (⋆)
TABLE 1:
Numbers of equids surveyed for evidence of glanders in different states of India during April 2011–December 2014
| S. No. | States | 2011–2012 |
2012–2013 |
2013–2014 |
2014 (April–December) |
Total |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glanders-endemic states | H | D | M | H | D | M | H | D | M | H | D | M | H | D | M | |||||
| 1 | Uttarakhand | 276 | 91 | 64 | 496 | 215 | 168 | 35 | 11 | 4 | 14 | 5 | 2 | 821 | 322 | 238 | ||||
| 2 | Haryana | 96 | 15 | 17 | 69 | 23 | 8 | 56 | 7 | 16 | 93 | 11 | 3 | 314 | 56 | 44 | ||||
| 3 | Maharashtra | 85 | 21 | 8 | 248 | 41 | 39 | 0 | 0 | 333 | 62 | 47 | ||||||||
| 4 | Uttar Pradesh | 25 | 19 | 21 | (6) | 81 | 27 | 36 | (7) | 115 | 162 | 101 | (7) | 68 | 87 | 78 | (11) | 289 | 295 | 236 |
| 5 | Andhra Pradesh | 166 | 48 | 20 | 175 | 53 | 18 | 49 | 11 | 7 | NS | 390 | 112 | 45 | ||||||
| 6 | Himachal Pradesh | NS | 86 | 21 | 19 | 115 | 17 | 23 | (4) | NS | 201 | 38 | 42 | |||||||
| 7 | Chhattisgarh* | NS | NS | 1 | (1) | NS | 1 | |||||||||||||
| Total | 648 | 194 | 130 | (6) | 1155 | 380 | 288 | (7) | 371 | 208 | 151 | (12) | 175 | 103 | 83 | (11) | 3886 (2349H, 885D, 652M) | |||
| Non-endemic states | ||||||||||||||||||||
| 1 | Jammu and Kashmir | 76 | 14 | 17 | 312 | 23 | 31 | 0 | 0 | 0 | 0 | 0 | 388 | 37 | 48 | |||||
| 2 | Rajasthan | 533 | 293 | 144 | 356 | 153 | 66 | 442 | 324 | 157 | 381 | 179 | 107 | 1712 | 949 | 474 | ||||
| 3 | Gujarat | 119 | 5 | 3 | 10 | 2 | 0 | 19 | 2 | 2 | 17 | 1 | 2 | 165 | 10 | 7 | ||||
| 4 | Madhya Pradesh | 34 | 0 | 3 | 51 | 0 | 6 | 21 | 0 | 3 | NS | 106 | 0 | 12 | ||||||
| Total | 762 | 312 | 167 | 729 | 178 | 103 | 482 | 326 | 162 | 398 | 180 | 109 | 3908 (2371H, 996D, 541M) | |||||||
| Grand total | 2213 (1410H, 506D, 297M) | 2833 (1884H, 558D, 391M) | 1700 (853H, 534D, 313M) | 1048 (573H, 283D, 192M) | 7794 (4720H, 1881D, 1193M) | |||||||||||||||
Bold faced number in parenthesis indicates glanders-positive cases
*Although the equines from Chhattisgarh were not surveyed in the present study, one positive case of glanders was detected at Raipur, Chhattisgarh in August, 2013
D, donkey; H, horse; M, mule; NS, not surveyed
Animals were examined for the presence of any clinical signs. For serological analysis, blood samples were aseptically collected from equines regardless of age or sex. Equine serum samples submitted by registered veterinarians as part of disease investigation were also included in the study. For bacteriological examination, nasal swabs and/or abscess swabs were collected from equines showing typical clinical signs of glanders as well as from apparently healthy in-contact animals. Blood samples collected from owners of suspect cases and from laboratory workers dealing with clinical samples were also included for serological assay.
Serology
Antibodies to B mallei in serum samples were detected by complement fixation test (CFT) as described by World Organization for Animal Health (OIE) (OIE 2013) with minor modification of test protocol using commercially available antigen (Bioveta, a.s., Invanovice na Hane, Czech Republic). Other reagents of CFT like sheep red blood cells (RBCs), guinea pig complement and haemolysin (rabbit anti-sheep RBCs) were obtained from or raised in respective laboratory animals with prior approval of the institute's animal ethics committee. The modification of the protocol included twofold serial dilution of serum (starting at 1:2) and incubation of serum-antigen-complement mixture at 37°C (warm incubation) for 90 minutes. The CFT titre of 1:8 and above was considered positive for glanders. In addition to the CFT, previously described in-house ELISA using recombinant B mallei TssB protein (Singha and others 2014) was used to resolve difficulties in interpretation related to the anticomplementary activities in donkey and mule serum. If available, positive reactors were resampled and retested before declaring positive.
Bacteriological culture and PCR
Nasal swabs and swabs from skin lesion were streaked on glycerol-blood agar (GBA) consisting of nutrient agar supplemented with 5 per cent defibrinated sheep blood, 5 per cent glycerol and 1 per cent dextrose. The plate was incubated at 37°C for two to four days with regular observation. Besides GBA, Burkholderia cepacia agar (Himedia Laboratories, Mumbai, India) supplemented with ticarcillin and polymyxin B was also used for isolation of organism. This plate was incubated at 30°C. A single colony simulating B mallei was streaked on fresh plate to obtain pure culture. Organism was identified by cultural and morphological characteristics, motility test as well as PCR. B mallei specific PCR assay that targets the fliP gene was performed for confirmation of the organism according to the OIE protocol (OIE 2013). PCR products were analysed in 1.2 per cent agarose gel and results were documented using Gel Documentation System (Alpha Innotech, San Leandro, California, USA). PCR amplicons were sequenced by the dideoxy method.
Data analysis
Descriptive statistics of the animals which included age, sex, species, location, season were recorded and analysed in Microsoft Excel (Microsoft, 2007). The relationship of age, sex, regional distribution and clinical presentation to serological positivity was investigated.
Results
Serological diagnosis of B mallei infection
A total of 7794 equines, which included 4720 horses, 1881 donkeys and 1193 mules distributed in glanders-endemic (n=3886) and in non-endemic (n=3908) zones were sampled for detection of B mallei specific antibodies. Year-wise and state-wise surveillance of equine species is shown in Table 1. Serologically, 36 equines (pony=7, mules=10, horses=19) were found to be positive for glanders by CFT and indirect-ELISA. The CFT titre ranged from 16 to 128 and absorbance of serum samples in ELISA were above the cut-off (data not shown). All human serum samples (n=35) were serologically negative for B mallei specific antibodies.
Clinical signs
Glanders-suspected equines exhibited common clinical signs of fever (low-grade to high-grade), drooping of the head, laboured breathing, emaciation, rough hair coat and swelling of limbs and joints (Fig 2A). In some of the animals, ‘nasal form’ of glanders was evident by yellowish-green unilateral or bilateral nasal discharge, with or without ulcerous nodules on the nasal mucosa (Fig 2B). A cutaneous manifestation of the disease, characterised by multiple papular or pustular nodules especially in the hindlimbs, was also observed in some of the equines (Fig 2C). With the progression of disease, enlargement and eruptions of nodules and coalescence of lesions were seen. Postmortem examination revealed large abscesses in the liver of one of the infected horses (Fig 2D). However, some of the seropositive equines (n=7) were apparently healthy and did not show any clinical signs suggestive of glanders. Table 2A–D shows the age and species, clinical presentation, geographical and seasonal distribution of glanders-positive equines.
FIG 2:
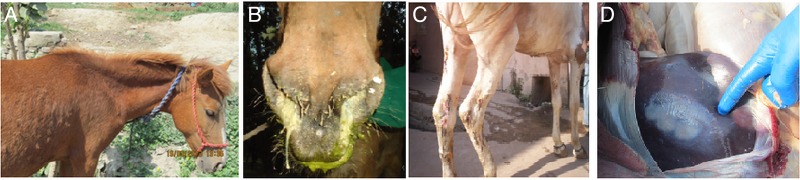
Characteristic clinical signs of glanders observed in suspected equines. A mule showed general signs of head drooping and emaciation; appearance of small papules were visible around the neck region (A). ‘Nasal form’ of glanders was demonstrated by mucopurulent bilateral nasal exudates (B). ‘Cutaneous form’ or ‘Farcy’ characterised by ulceration in the hindlimb (C). Postmortem examination revealed a large purulent abscess in the liver of one of the Burkholderia mallei infected horses (D)
TABLE 2:
Epidemiological data and clinical presentation of equines positive for Burkholderia mallei specific antibodies
| (A) Age-wise and species-wise distribution of glanders-positive equines | ||||||
| Age (years) | Below 2 | 2–4 | 5–6 | 7–8 | 9–10 | Total |
| Species | ||||||
| Horses and ponies | 3 | 5 | 7 | 6 | 5 | 26 |
| Mules | 1 | 1 | 3 | 5 | – | 10 |
| Total | 4 | 6 | 10 | 11 | 5 | 36 |
| (B) Clinical signs among equines positive for B mallei specific antibodies | |||
| Clinical signs | Fever, emaciation, laboured breathing, nasal discharge | Fever, emaciation, laboured breathing, nasal discharge+ulceration of hindlimb | Apparently healthy, no cardinal signs |
| Positive cases | 15 | 14 | 7 |
| (C) Seasonal distribution of glanders-positive cases | ||||
| Season | Spring (March–April) | Hot summer (May–June) | Rainy humid (July–August) | Fall (September–October) |
| Positive cases | 14 | 4 | 7 | 11 |
| (D) Geographical distribution of glanders-positive cases | ||
| States | Location and year | Positive case |
| Uttar Pradesh | Bulandshahr, 2011 | 6 |
| Ganjdundwara, 2012 | 1 | |
| Hardoi, 2012 | 4 | |
| Hardoi, 2013 | 4 (1) | |
| Auraiya, 2012 | 2 | |
| Badaun, 2013 | 3 | |
| Agra, 2014 | 11 (1) | |
| Himachal Pradesh | Arki, 2013 | 4 (2) |
| Chhattisgarh | Raipur, 2013 | 1 |
Number in parenthesis indicates isolates of B mallei
Bacterial isolation and PCR
Four isolates of B mallei were cultured from nasal swabs of two mules and two horses collected from Himachal Pradesh and Uttar Pradesh, respectively. However, no B mallei could be isolated from the nasal swabs obtained from the rest of the glanders-seropositive (n=32) and in-contact animals (n=35). B mallei isolates characteristically exhibited a non-haemolytic, smooth, grey, translucent colony on blood agar. However, smooth, and white to translucent colonies with changes of an agar colour to pink were observed on cepacia agar. Gram-negative coccobacilli with rounded ends were observed microscopically. The colony morphology, cultural and microscopic characteristics of the non-motile isolates were typical of B mallei. In PCR, genomic DNA of field isolates and B mallei reference strain ATCC23344 yielded specific amplification of 989 bp fliP gene fragment (Fig 3), however, no such amplification was observed with Burkholderia pseudomallei ATCC23343 DNA. Sequences of PCR products of the first two field isolates showed 100 per cent identity with B mallei fliP gene sequences available in the public database. The sequence was submitted to GenBank (Acc no. KJ814951-52).
FIG 3:
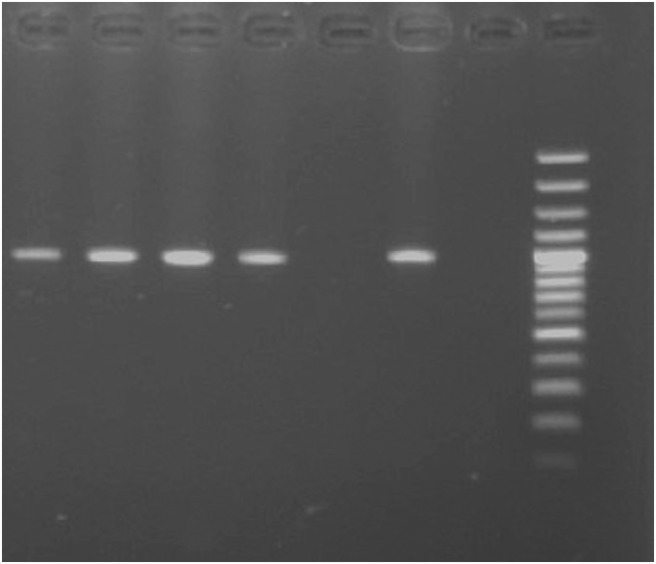
PCR amplification of 989 bp fliP gene fragment. Lane M, 100 bp DNA ladder; lanes 1–4, field isolates of Burkholderia mallei obtained from suspected cases of glanders; lane 5, negative control; lane 6, positive control B mallei ATCC23344 DNA; lane 7, Burkholderia pseudomallei DNA
Discussion
Glanders is an ancient disease that was described from the beginning of recorded history. The causative agent, B mallei, was identified by German microbiologists Loeffler and Schultz in 1882 (Loeffler 1886). Deliberate release of B mallei during World Wars I and II was associated with a sudden rise of disease in military and civilian equines across Europe, the Americas and Asia (Wheelis 1998, Kasten 2002). Heavy losses of horses and the infrequent but deadly transmission to human beings, and an empirical and challenging treatment regime forced several countries to undertake glanders control and eradication programmes. Gradually, the disease has been eradicated from many Western countries and it is now considered a rare disease in the developed world (Blancou 1994, Derbyshire 2002). In contrast, the disease is still endemic in the developing world. B mallei infection in equines, camels and zoo animals has been reported in the Middle East and Southern Asia including the Arab Emirates, Bahrain, Iran, Iraq, Kuwait, Lebanon, Mongolia, India, Myanmar, Afghanistan, Pakistan and Philippines in the recent past (Hornstra and others 2009, Wernery and others 2011, Khaki and others 2012, Malik and others 2012, World Animal Health Information Database 2014).
In the present study, surveillance and disease investigation in glanders-endemic and non-endemic states were undertaken to estimate disease prevalence and risk of spread of disease to new areas. It is pertinent to mention that samples were not equally distributed across the states which may be explained by the uneven distribution of indigenous equine populations in different states, availability of animals during the sampling period, and above all unwillingness of equine owners to participate in the survey. In Uttar Pradesh, glanders outbreaks were detected in four consecutive years involving 18 horses, 7 ponies and 6 mules. A single outbreak each involving four mules and one pony was detected in Himachal Pradesh and Chhattisgarh, respectively in 2013 (Fig 1). In Chhattisgarh, serological investigation of a closed group of horses in which a single case was detected in March 2010 did not reveal further cases over the following years. However, one pony which had reportedly been procured from Uttar Pradesh to Chhattisgarh showed B mallei specific antibodies. The present survey reveals that glanders is prevalent in six districts of Uttar Pradesh, while only focal outbreaks were observed in Himachal Pradesh and Chhattisgarh (Table 2).
Nearly 22 per cent of the Indian equine population lives in Uttar Pradesh (19 Livestock Census-2012, Ministry of Agriculture, Government of India). In rural areas equines are mainly reared by poor, landless farmers. As most of the mule breeding tracts are located in this state, contractual hiring and equine trading are more common practice in Uttar Pradesh. Unfortunately, during the last eight years the disease has invariably been reported almost every year from this state which accounts for more than 50 per cent of the reported cases of glanders. Unrestricted movement of the infected or carrier equids from this state is implicated in the spread of the disease to other areas. Previous data show that a few districts of Uttar Pradesh may be considered as hyper-endemic zones of glanders and warrants more intense surveillance and monitoring.
Season changes from winter to spring, and from summer to fall, in association with any stress, overwork, poor diet, exercise, other infections have been implicated with the flare-up of dormant equine infection such as glanders (Rutherford 1906, Huidekeoper 1907). A majority of the seropositive equines was used for draught activities mainly carrying bricks in brick kilns or construction sites, and for carrying construction materials. Because of the poor economic status of the owners, equine husbandry and welfare are often neglected. As a result the animals generally suffer from chronic malnutrition and stress. Similarly, glanders cases peaked during March–April and September–October as recorded by Malik and others (2012) and in the present study (Table 2) suggesting that the seasonal influence and stress factors may play a major role in activating the disease in latently infected equines. As the initial stage of the glanders can be difficult to differentiate from many other treatable respiratory ailments, antibiotic treatment of glanders-affected equines are routinely followed in field. Indeed, most of the clinically affected equines were repeatedly treated with multiple antibiotics. This may explain the small number of B mallei isolates (n=4) from 36 seropositive equines. Therefore, more sensitive methods such as real-time PCR should be used to detect the organism. Future work may be directed towards molecular typing of B mallei isolates using multilocus sequence typing (MLST), multiple locus variable number of tandem repeats (VNTR) and comparative whole genome sequencing to unravel the genetic relationship among isolates. In fact, MLST and VNTR typing of these isolates is currently underway and results may be published soon.
Glanders is a notifiable disease in India. At present, control measures are being followed under the guidelines of ‘The Prevention and Control of Infectious and Contagious Diseases in Animals Act, 2009’ and regulations administered by the Ministry of Agriculture, Government of India. Few positive reactors succumb to the disease and the rest of the cases are reportedly eliminated. Lack of awareness among equine owners regarding glanders and limited availability of veterinary services are key factors responsible for under-reporting of the disease. It is believed that partially treated equines with chronic or subclinical infection may be responsible for frequent and continuous outbreaks of glanders in India. Considering the prevalence of glanders in certain parts of India, rigorous disease surveillance and provision of suitable compensation for culling of infected equines should be given due priority to reduce spread and aid in eradication.
Acknowledgments
The authors sincerely acknowledge the cooperation received from staff of field veterinary hospitals during the field investigation and sample collection. Assistance of Mr Sitaram and Mr Gurudutt Sharma in sample collection from the field and processing of clinical samples in the lab is highly appreciated. The authors also thank Prof Heinrich Neubauer and Dr Mandy Elschner of OIE reference lab on glanders at FLI, Jena, Germany for providing Burkholderia mallei and Burkholderia pseudomallei reference DNA.
Footnotes
Contributors: HS, PM and SKK contributed in surveillance of glanders outbreak and testing of equine serum samples.
Funding: Indian Council of Agricultural Research.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Annual surveillance data is shared with researchers and policymakers in the form of Annual Report and News Letters. As the disease is notifiable positive incidence of glanders is also shared with the World Organization for Animal Health (OIE).
References
- Blancou J. (1994) Early methods for the surveillance and control of glanders in Europe. Revue Scientifique et Technique 13, 545–557 [DOI] [PubMed] [Google Scholar]
- Derbyshire J. B. (2002) The eradication of glanders in Canada. The Canadian Veterinary Journal 43, 722–726 [PMC free article] [PubMed] [Google Scholar]
- Holmes J. D. E. (1913) A Description of Imperial Bacteriological Laboratory, Muktesar: its Work and Products. Calcutta: Superintendent Government Printing, p 23 [Google Scholar]
- Hornstra H., Pearson T., Georgia S., Liguori A., Dale J., Price E., O'Neill M., Deshazer D., Muhammad G., Saqib M., Naureen A. & Keim P. (2009) Molecular epidemiology of glanders, Pakistan. Emerging Infectious Diseases 15, 2036–2039. doi:10.3201/eid1512.090738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huidekeoper R. S. (1907) General diseases. In Diseases of the Horse. Ed Melvin A. D. Washington DC: US Government Printing Office; pp 532–545 [Google Scholar]
- Kasten F. H. (2002) Biological weapons, war crimes, and WWI. Science 296, 1235–1237 [PubMed] [Google Scholar]
- Khaki P., Mosavari N., Khajeh N. S., Emam M., Ahouran M., Hashemi S., Taheri M. M., Jahanpeyma D. & Nikkhah S. (2012) Glanders outbreak at Tehran Zoo, Iran. Iranian Journal of Microbiology 4, 3–7 [PMC free article] [PubMed] [Google Scholar]
- Khan I., Wieler L. H., Melzer F., Elschner M. C., Muhammad G., Ali S., Sprague L. D., Neubauer H. & Saqib M. (2013) Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transboundary and Emerging Diseases 60, 204–221. doi:10.1111/j.1865-1682.2012.01342.x [DOI] [PubMed] [Google Scholar]
- Loeffler F. (1886) The etiology of glanders [in German]. Arb Kaiserl Gesundh Amt Berlin 1, 141–198 [Google Scholar]
- Malik P., Khurana S. K., Singh B. K. & Dwivedi S. K. (2009) Recent outbreak of glanders in India. The Indian Journal of Animal Sciences 79, 1015–1017 [Google Scholar]
- Malik P., Singha H., Khurana S. K., Kumar R., Kumar S., Raut A. A., Riyesh T., Vaid R. K., Virmani N., Singh B. K., Pathak S. V., Parkale D. D., Singh B., Pandey S. B., Sharma T. R., Chauhan B. C., Awasthi V., Jain S. & Singh R. K. (2012) Emergence and reemergence of glanders in India: a description of outbreaks from 2006 to 2011. Veterinaria italiana 48, 167–178 [PubMed] [Google Scholar]
- Minett F. C. (1959) Glanders and melioidosis. In Infectious Diseases of Animals: Diseases due to Bacteria, Vol 1 Eds Stableforth A. W., Galloway I. A.. London, UK: Butterworths Scientific Publications; pp 296–309 [Google Scholar]
- Misra V. C., Kaushik R. K., Dhingra P. N. & Satija K. C. (1985) Emergence of glanders epidemic in civilian equines of northern India. Journal of the Remount and Veterinary Corps 24, 110–115 [Google Scholar]
- Ray D. K. (1984) Incidence of glanders in the horses of mounted platoon of 4th AP Bn, Kahilipara, Gauhati-19: a case history. Indian Veterinary Journal 61, 264 [Google Scholar]
- Rutherford J. G. (1906) Special Report on Glanders (from the Veterinary Director-General and Livestock Commissioner). Ottawa, Canada: Canada Department of Agriculture, Health of Animals Branch [Google Scholar]
- Singha H., Malik P., Goyal S. K., Khurana S. K., Mukhopadhyay C., Eshwara V. K. & Singh R. K. (2014) Optimization and validation of indirect ELISA using truncated TssB protein for the serodiagnosis of glanders amongst equines. Scientific World Journal 2014, 469407 doi:10.1155/2014/469407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Kraus C. N., DeShazer D., Becker P. M., Dick J. D., Spacek L., Bartlett J. G., Byrne W. R. & Thomas D. L. (2001) Glanders in a military research microbiologist. New England Journal of Medicine 345, 256–258. doi:10.1056/NEJM200107263450404 [DOI] [PubMed] [Google Scholar]
- Steele J. H. (1979) Glanders. In CRC Handbook Series in Zoonoses, Section A: Bacterial, Rickettsial and Mycotic Diseases. Ed Steele J. H. Boca Raton, Florida: CRC Press; pp 339–362 [Google Scholar]
- Verma R. D. (1981) Glanders in India with special reference to incidence and epidemiology. Indian Veterinary Journal 58, 177–183 [Google Scholar]
- Wernery U., Wernery R., Joseph M., Al-Salloom F., Johnson B., Kinne J., Jose S., Tappendorf B., Hornstra H. & Scholz H. C. (2011) Natural Burkholderia mallei infection in Dromedary, Bahrain. Emerging Infectious Diseases 17, 1277–1279. doi:10.3201/eid1707.110222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. (1998) First shots fired in biological warfare. Nature 395, 213 doi:10.1038/26089 [DOI] [PubMed] [Google Scholar]
- Witting M. B., Wohlsein P., Hagene R. M., Al Dahouk S., Tomaso H., Scholz H. C., Nikolaou K., Wernery R., Wernery U., Kinne J., Elschner M. & Neubauer H. (2006) Glanders – a comprehensive review. Deutsche tierärztliche Wochenschrift 113, 323–330 [PubMed] [Google Scholar]
- World Animal Health Information Database Interface OIE-WAHID. www.oie.int. (accessed 31 Decembe 2014)
- World Organization for Animal Health (OIE) (2013) Glanders, Chapter 2. 5. 11. Paris, France: OIE Terrestrial Manual, OIE [Google Scholar]