Abstract
The development of antibiotics changed the world of medicine and has saved countless human and animal lives. Bacterial resistance/tolerance to antibiotics have spread silently across the world and has emerged as a major public health concern. The recent emergence of pan-resistant bacteria can overcome virtually any antibiotic and poses a major problem for their successful control. Selection for antibiotic resistance may take place where an antibiotic is present in the skin, gut, and other tissues of humans and animals and in the environment. Borrelia burgdorferi, the etiological agents of Lyme borreliosis, evades host immunity and establishes persistent infections in its mammalian hosts. The persistent infection poses a challenge to the effective antibiotic treatment, as demonstrated in various animal models. An increasingly heterogeneous subpopulation of replicatively attenuated spirochetes arises following treatment, and these persistent antimicrobial tolerant/resistant spirochetes are non-cultivable. The non-cultivable spirochetes resurge in multiple tissues at 12 months after treatment, with B. burgdorferi-specific DNA copy levels nearly equivalent to those found in shame-treated experimental animals. These attenuated spirochetes remain viable, but divide slowly, thereby being tolerant to antibiotics. Despite the continued non-cultivable state, RNA transcription of multiple B. burgdorferi genes was detected in host tissues, spirochetes were acquired by xenodiagnostic ticks, and spirochetal forms could be visualized within ticks and mouse tissues. A number of host cytokines were up- or down-regulated in tissues of both shame- and antibiotic-treated mice in the absence of histopathology, indicating a lack of host response to the presence of antimicrobial tolerant/resistant spirochetes.
KEYWORDS: Borrelia burgdorferi, antibiotics, persistence, antimicrobial resistance, antimicrobial tolerance, ticks, small mammals
INTRODUCTION
Lyme disease, or Lyme borreliosis, was first described in 1977 as a distinctive entity in a cluster of children from Lyme, Connecticut, U.S. These children had symptoms resembling juvenile rheumatoid arthritis, a disease that does not cluster and is rare in children [1]. Based upon similarities with recognized human clinical syndromes in Europe that were of unknown etiology, but associated with the bite of Ixodes ricinus ticks, the causative agent was isolated from North American Ixodes scapularis ticks in 1982 [2], and subsequently named Borrelia burgdorferi [3, 4]. Once the connection was made between vector, agent, and the human disease, Lyme borreliosis has been diagnosed throughout the world, but its significance is greatest in North America and Europe [5-10]. Since its discovery, the importance of Lyme borreliosis has led to evolvement of all aspects of bacterial pathogenesis research.
The association of Lyme disease in humans has been demonstrated with B. burgdorferi sensu stricto (s.s.), B. afzelii, and B. garinii, and possibly with B. valaisiana. B. burgdorferi s.s., B. afzelii, and B. garinii have been associated with Lyme disease in livestock [11-13]. B. burgdorferi belongs to a guild of pathogens, including Ehrlichia, Babesia, and tick-borne encephalitis viruses that are maintained within the same vector-reservoir niches [14]. Thus, co-infection with one or more of these agents can occur and may be responsible for “para-Lyme disease” syndromes as these other agents have a wide host range.
Borreliae are unique among the pathogenic spirochetes by requiring obligate blood-feeding arthropods for their transmission and maintenance in susceptible vertebrate host populations. With one exception, ticks transmit all borreliae and nearly all species are maintained in enzootic foci with humans being only accidental victims to infection. The wide geographic distribution and the broad host range of both the vector and the bacterium provide ample opportunity for wild mammal species to be infected with B. burgdorferi. However, most of what is known about Lyme borreliosis is based upon human clinical studies, and experimental studies in laboratory rodents. The amount of well-documented information on Lyme borreliosis in wild mammals is quite limited, even though wild rodents are considered as the main reservoir host for B. burgdorferi.
ETIOLOGY
Borrelia burgdorferi was first described as a gram-negative treponema-like organism with irregular coils, 10 to 30 mm in length and 0.2 to 0.3 mm in diameter, with outer membrane and periplasmic flagella [4, 15]. For culture in vitro, this microaerophilic slow growing organism requires a complex liquid medium, and an optimal temperature of 33 to 350C [16]. Based on 16S ribosomal DNA gene sequences, the Genus Borrelia belongs to the Order Spirochaetales, family Spirochetaceae along with other genera, including Spirochaeta, Cristispira, Treponema and Brachyspira (formerly Serpulina). It was first believed that B. burgdorferi was the only bacterium that could cause Lyme disease, but differences in morphology among isolates from diverse geographic locations suggested that additional spirochetes may be involved in the etiology of Lyme disease [17].
A major effort has been undertaken to analyze the phenotypic and genotypic diversity of B. burgdorferi isolates from around the world, using PCR techniques, targeting 16S and 23S ribosomal DNA, flagellin, OspA, and bdr genes, as well as intergenic spacers. It is now apparent that B. burgdorferi is genetically diverse, and belongs to a B. burgdorferi sensu lato genospecies complex composed of several different species including: B. burgdorferi s.s., present in the USA and Europe (but not in Euroasia and Asia); B. afzelii, B. garinii, B. valaisiana, and B. lusitaniae in Euroasia; B. japonica, B. turdae, and B. tanukii in Japan; and B. bissettii and B. andersoni in the U.S. [7, 18, 19]. Evolutionary changes: mutation, genetic drift, migration, and natural selection created macro evolutionary divergence of species. The prevailing data suggest that B. burgdorferi s.l. was once a wide-ranging species in the Northern Hemisphere that rapidly separated into the species present today [19].
Lyme disease Borrelia has developed an unusual lifestyle in that they alternate between vertebrate and arthropod hosts. In addition, they belong to a group of organisms that produce no known toxins yet are capable of invading virtually any mammalian tissue and causing infection and disease manifestations for months to years. Borrelias are host-dependent, tick-transmitted, invasive, non-toxigenic, persistent pathogens that cause disease in humans and other mammals primarily through the induction of inflammatory reactions. During transmission from the infected tick, dissemination within infected host, and acquisition by a tick-vector, the bacteria undergo dramatic changes in gene expression, resulting in adaptation to the host environment [20].
EPIZOOTIOLOGY
B. burgdorferi s.l. is spread by ticks in the Ixodes ricinus species complex [21]. Ticks complete their two-year life cycle in four developmental stages: eggs, larva, nymph, and adult. Female adult ticks lay eggs in the spring. Larvae emerge from eggs in summer and seek a host, usually small rodents (mice, squirrels) and birds, which are common natural hosts of B. burgdorferi. Each larva obtains a single blood meal then drops off and molts into the nymphal stage. The nymphal stage overwinters, and seeks a host (a variety of birds, reptiles and mammals, including large animals) the following spring or early summer. After a single blood meal, the nymph drops off and molts an adult. Adult ticks are not important for maintaining B. burgdorferi in the wild, as they typically feed on incompetent reservoir hosts such as deer or livestock, but females serve a role in the transmission of the pathogen to humans (Figure 1). While B. burgdorferi is transmitted transtadially within individual ticks, the pathogen is rarely, if ever, transmitted transovarially [22].
Figure 1.
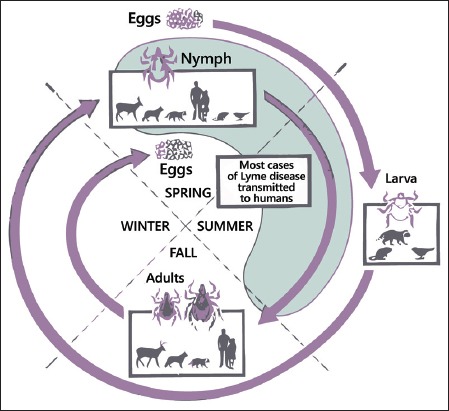
The enzootic cycle of B. burgdorferi. Ixodes spp. ticks undergo a three life cycle – larva, nymph and adult – with one blood meal per stage. Larval ticks feed on many different animals (mice, squirrel, birds), so they acquire B. burgdorferi from infected reservoir animals. There is no transovarial transmission. After molt nymphs can transmit spirochetes to a competent reservoir host (small mammals). Adult ticks are not important for maintained of B. burgdorferi in the wild, as they feed on large animals such as deer, which are incompetent hosts for B. burgdorferi. However, deer are important for maintenance of the tick population. Although all three stages can feed on humans, nymphs are responsible for the vast majority of spirochetes transmission to humans [22].
Although all three stages can feed on humans, nymphs are responsible for the vast majority of spirochete transmission to humans [23], but adult I. scapularis, I. ricinus, and I. persulcatus ticks seek and parasite large mammals, including deer or livestock, and are considered to be the most common vector for Lyme disease in large animals [24-26]. Clinical cases of Lyme borreliosis in large animals could therefore be expected in localities where these Ixodes ticks are common. B. burgdorferi and its vector ticks tend to be non-selective in their host range, as B. burgdorferi has been isolated from a wide variety of birds and mammals [27]. Many hosts harbor subclinical infections. For example, in hyper-endemic areas such as New York and Connecticut, a high percentage of dogs are seropositive (and probably infected), but only a small fraction manifest clinical signs [12].
Among a large number of hard tick species, B. burgdorferi has been primarily detected in Ixodes ricinus, which is prevalent in Europe, I. persulcatus, which is prevalent in Eastern Europe and Asia, and I. scapularis and I. pacificus in Northern America [23, 28, 29].
Lyme borreliosis shows strong spatial clustering, geographically distributed in temperate regions of the northern hemispheres. This range does not extend beyond northern Africa at its most southern extreme [27]. In the U.S. Lyme borreliosis is mainly reported from the eastern part of the country, the upper Midwest, and occasionally from the West Coast. Nevertheless, migratory birds, particularly seabirds, contribute to the opportunity for B. burgdorferi to expand its geographic distribution, with documentation of Ixodes sp. ticks, and B. burgdorferi s.l. DNA within some of them, as far south as the Falkland Islands, Crozet Islands, and off-shore islands of New Zealand [30]. Lyme borreliosis is emerging across much of the northern hemisphere, causing considerable morbidity and in some cases mortality in humans, domestic animals, and occasionally wildlife. It is the most frequently diagnosed tick-borne disease in the United States. According to the Centers for Disease Control and Prevention (CDC) there were over 240,000 reported cases of Lyme borreliosis in the United States from 1992-2006 [31]. However, the CDC estimates there are greater than 300,000 human cases of Lyme borreliosis annually (http://www.cdc.gov/lyme/stats/humanCases.html).
PATHOGENESIS
Transmission by the vector (nymphal or adult ticks) to the host is a complex three-way interaction between the tick, the host, and the pathogen. Hosts mount local inflammatory, hemostatic and immune responses to the feeding tick, while tick saliva contains substances that counter the host response, thereby facilitating a successful blood meal and transmission of the pathogens [32]. After tick attachment, B. burgdorferi spirochetes undergo striking variations in the expression of antigens, and migrate from the tick midgut to its salivary glands [20, 33-35]. After being transmitted, spirochetes stay in the skin at the attachment site for several days, then disseminate throughout host [36-38]. As a result of spirochete multiplication at the tick attachment site, the host reacts with a local rash (erythema migrans), generally weeks after tick detachment. The rash is observed in the majority of human cases [39-42].
Following local replication in the skin, spirochetes disseminate widely to multiple organs. Clinical signs of illness are generally most apparent during the early stages of disseminated infection, when spirochetes are believed to elaborate pro-inflammatory lipoproteins that appear to facilitate the process of dissemination through host tissues. Despite the highly immunogenic nature of these lipoproteins, spirochetes very effectively evade host immune clearance by yet to be understood mechanisms [43]. Whatever the mechanism, it is clear that spirochetes undergo dramatic shifts in protein expression. For example, outer surface protein A (OspA), is a highly immunogenic lipoprotein that is expressed in the midgut of flat ticks, but is rapidly down-regulated upon onset of feeding, and is minimally expressed in the host. In contrast, OspC is up regulated under similar circumstances [34, 44-46]. Up- and down-regulation have also been documented for several other lipoproteins, some of which are expressed exclusively in vivo, but their role is still unclear [20, 43, 47]. Other possible explanations of immune evasion by B. burgdorferi spirochetes have been proposed, including intracellular localization [48, 49], formation of cystic-like forms found in vivo [50], and in vitro [51, 52], existence of complement-resistant strains that regulate and control complement activation [53], and a biofilm formation [54]. Once spirochetes have disseminated, humans develop clinical sings of Lyme disease, including fever, lethargy, weight loss, swollen joints, uveitis, and sometimes encephalitis. The most common clinical feature of late-stage B. burgdorferi infection is arthritis, which usually begins months after tick bite [55]. Lyme borreliosis uncommonly affects the heart. Because of the rarity of this diagnosis and the frequent absence of other concurrent clinical manifestations of early infection, consideration of Lyme carditis demands a high level of suspicion when patients in endemic areas come to attention with cardiovascular symptoms and evidence of higher-order heart block [56].
The mechanisms by which B. burgdorferi spirochetes cause disease in infected hosts are being studied in animal models. It is suspected that the approximately 150 lipoproteins that are encoded by the B. burgdorferi genome play an important role in not only disease pathogenesis, but also host immunity. Differential expressions of pro-inflammatory lipoproteins, of which the majority are outer surface proteins, in various tissues and at different times during persistent infection appear to be critical determinants of disease [57, 58]. The genome has 12 linear and 9 circular plasmids, and loss of plasmids has been correlated with decreased infectivity and pathogenicity [59-65].
Persistence
A basic feature of Lyme borreliosis (without antibiotics) is that persistent infection is the rule, not the norm. This occurs in B. burgdorferi’s many reservoir hosts, and has been proven experimentally in Peromyscus mice [66], laboratory mice [67], rats [68], hamsters [69], gerbils [70], guinea pigs [71], dogs [72], and non-human primates [73]. Humans appear to be no different, as there are a number of documented case reports of persistent infection based on culture [74-80] and PCR [81-85].
B. burgdorferi has evolved to persist in immunologically competent hosts as a survival strategy for maintaining its natural host-vector life cycle. Natural reservoir hosts and small laboratory animals are generally rodents. In such hosts, infection is generalized and persistent, including in the skin, wherein spirochetes can most efficiently interface with the vector tick. Both in vivo animal model studies and in vitro studies have shown that B. burgdorferi spirochetes utilize an array of adhesins that engage virtually every component of the extracellular matrix to facilitate their dissemination [86], and sequester within collagen as their preferred site of persistence [87-89].
Dissemination is also facilitated by bacteremia during early infection, which is generally cleared during the immune persistent phase of infection, and intermittent thereafter. Because humans are much larger, they experience localized infections, as evidenced by erythema migrans, and sometimes disseminated, but randomly multifocal infection through bacteremia, which may result in pauciarticular arthritis, secondary erythema migrans, carditis, peripheral neuropathy, meningitis, and other objective clinical signs. It should be emphasized that Lyme disease in untreated humans (and experimental animals) is ephemeral, with “spontaneous” resolution (without antibiotic treatment) of erythema migrans, carditis, arthritis, and other signs [90, 91]. Studies in animal models have shown that resolution of arthritis and carditis is mediated by the acquired humoral immune response of the host. Under these conditions, anatomically defined inflammation resolves, but infection persists [88, 92, 93]. Indeed, even during the pre-immune phase of infection, spirochetes populate many tissues with no evidence of inflammation (thus inflammation does not necessarily correlate with spirochete presence).
CONTROL
Antibiotics such as penicillin, amoxicillin, ceftriaxone, doxycycline, and erythromycin, as the most commonly prescribed antibiotics for the treatment of human Lyme borreliosis, have shown to be effective against B. burgdorferi [55, 94]. Early treatment is desirable, and most effective when treatment is given during the early stages of infection [95]. Chronic cases require prolonged treatment, and treatment success is often less effective [39, 96-98].
There is widespread consensus among the mainstream medical community that relatively short-term courses of antibiotics can eliminate objective signs of Lyme borreliosis in patients, with the assumption that patients have been cured of infection. This has been articulated in the Infectious Diseases Society of America (IDSA) Guidelines in 2006 [99] and reaffirmed by an expert Lyme disease review panel in 2010 [100]. The IDSA Guidelines are in agreement with position statements of other medical and scientific organizations, including the European Federation of Neurological Societies, The European Union of Concerted Action on Lyme Borreliosis, the American Academy of Neurology, the Canadian Public Health Network, the German Society for Hygiene and Microbiology, several expert panels in various different countries, the American Lyme Disease Foundation, the CDC and NIH. An Ad Hoc International Lyme Disease Group has also affirmed this position [97, 101].
This consensus is based upon clinically objective criteria, in keeping with sound medical practice. However, it is well established that patients with objective criteria of Lyme borreliosis may also have widely varied and subjective manifestations that do not necessarily fit objective clinical criteria [90, 102, 103]. There is agreement that when objective clinical signs are persistent, a rare patient may have chronic Lyme disease, and when objective clinical signs return in a treated patient, a rare patient may have recurrent Lyme disease. Under both circumstances, repeated antibiotic treatment is advised. A principal area of continuing but unresolved debate involves patients who experience disabling subjective symptoms following completion of appropriate antibiotic therapy. This has been recognized by the term “post-Lyme disease syndrome” (PLDS). IDSA Guidelines state that there is “no well-accepted definition of the PLDS”, and that “there is no convincing biologic evidence for the existence of systemic chronic B. burgdorferi infection among patients after receipt of recommended treatment regimens for Lyme disease.”[99]. In the absence of objective clinical and diagnostic criteria, PLDS can never be proven to be, or not to be, associated with persistent infection with B. burgdorferi.
Nevertheless, the vagaries of PLDS have promulgated a basis of what has been euphemistically termed the “Lyme Wars” [104]: a contentious debate that can never be won simply on strongly held conviction. What is needed is research on the basic biology of B. burgdorferi, including outcome after antibiotic treatment under controlled conditions in animal models. Animals are indeed different from humans, but knowledge gained with animal models lends credence to valid hypotheses that can then be rationally approached in human trials.
ANTIMICROBIAL RESISTANCE AND ANTIMICROBIAL TOLERANCE
There is overwhelming scientific evidence of the increasingly high prevalence of antimicrobial-resistant pathogenic bacteria [105-111]. Bacterial resistance to antimicrobial agents was present even before the antimicrobial agents were introduced into human or veterinary practice. A common repeating pattern with the introduction of anti-microbial agents has existed for decades; a new drug is put into clinical use, there is an increased use of the newly discovered antimicrobial agent and sooner or later resistant bacterial strains are isolated. Resistance/tolerance to antimicrobial agents is a growing worldwide problem and it is becoming evident even for new, more potent antimicrobial agents [112]. Infections caused by antimicrobial resistant/tolerant bacteria pose major financial costs to the U.S. health care system (over $20 billion each year), associated societal costs, and costs due to premature death [113].
Selection for antimicrobial resistance/tolerance is not confined to the human body or to hospitals, clinics and farms. Resistance can occur anywhere antimicrobial agents are present, the environments most notably sewage and surface water sediments where antibiotics are likely to be coupled with high densities of various microorganisms [114-116] (Figure 2). Consequently, antibiotic resistant/tolerant pathogens and genes have been found among the bacterial flora of farm and pet animals, insects, rodents and in wild animals, including migratory birds [111, 117-122]. Methicillin-resistant S. auresus (MRSA) was isolated from wildlife in central Iowa, including cottontail rabbits and lesser yellowlegs [123]. Multidrug-resistant strains of E. coli, Salmonella spp., and Campylobacter spp. were isolated from wild rats [120]. Multidrug-resistant E. coli was isolated from wild small mammals (mice, voles, and shrews) in the proximity of swine farms and in natural environments [111]. Such population can pass to humans and animals in a number of ways, primarily through food, but also through environmental sources such as the soil, water, and plants [124]. Transmission of antimicrobial resistant/tolerant pathogens of animal origin to humans has been documented for methicillin-resistant S. aureus [125], E. coli [126], Salmonella spp. [127], and Campylobacter spp. [128]. The transmission dynamics of resistant/tolerant pathogens in farm animals and wildlife species that are exposed to antimicrobials have socio-economic and conservation importance [129]. The worldwide emergence of resistant bacteria (including methicillin-resistant S. aureus, Mycobacterium tuberculosis, Streptococcus pneumoniae, Klebsiella spp., Acinetobacter spp.), which can overcome virtually any antibiotic used, has become a major problem for their successful treatment [130]. Although limiting the spread of antibiotic resistance/tolerance strains is plausible, eradication efforts have been ineffective.
Figure 2.
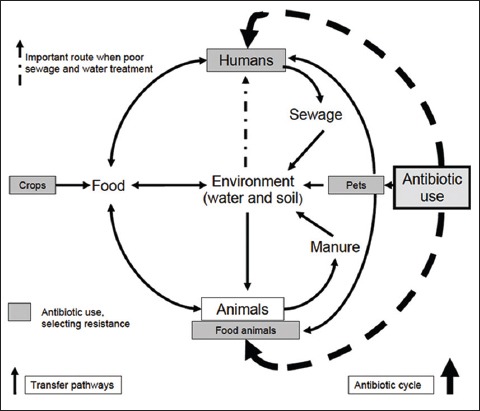
A scheme for the possible transmission of antimicrobial resistant/tolerant genes between humans, animals, food, and the environment (Source: Report by DARC and ARHAI group (2012); ESBLs: A treat to human and animal health?).
Selection for antibiotic resistance/tolerance traditionally usually occurs through exposure to higher concentrations of antibiotics; however, recent studies have documented the importance of exposure to lower levels as well [115]. Lower concentrations of antibiotics may occur in different tissues during treatment [131], and similar low concentrations may be found in sewage, soils, and many aquatic environments due to natural production and contamination from human activities [132, 133]. Recent studies have shown that resistant bacteria can be selected at concentrations several hundred-fold below the lethal concentrations for susceptible cells. Resistant mutants selected at low antibiotic concentrations are generally more fit than those selected at high concentrations but can still be highly resistant [109, 134].
The main culprit responsible for the tolerance of pathogens to antibiotics is a specialized survivor, a persister [135-137]. Persisters are not mutants; they are phenotypic variants of actively dividing cells produced stochastically in the population, and their relative abundance rises (reaching 1%) at the late-exponential phase of growth [135, 136, 138, 139]. Persisters are non-growing dormant cells [138, 140], which explains their tolerance to bactericidal antibiotics that depend on the presence of active targets for killing the cell [139]. All of the pathogens examined so far form persisters [137, 141]. Recently, significant progress was made in the study of persisters, but the importance of the persisters has not been recognized appropriately [142] and the mechanisms of their formation are largely unknown. Persisters also have an important role in the development of conventional antibiotic-resistant mutants. Persisters are killed only slowly, if at all, and resume growth when antibiotic concentrations fall [143].
Numerous studies have shown differences between antimicrobial resistance and antimicrobial persister tolerance [137, 138, 144]. These two traits are independent, and persisters represent a small subpopulation of cells that spontaneously enter a dormant, non-dividing state. Treatment with antimicrobial agents of a bacterial population results in death of regular cells, whereas persisters survive. Antimicrobial agents require active targets to be effective, which explains persister tolerance (Figure 3). In contrast, antimicrobial resistance prevents antimicrobial agents from binding to their targets [136]. An important characteristic of resistance is the ability of bacteria to continue to grow at elevated concentrations of antimicrobial agents [118, 136]. Bacterial resistance to antimicrobial agents can be defined as: (a) clinical resistance, (b) microbiological resistance, (c) inherent resistance, (d) acquired resistance, (e) cross-resistance, (f) co-resistance, and (g) multiple resistance [107, 118, 145].
Figure 3.
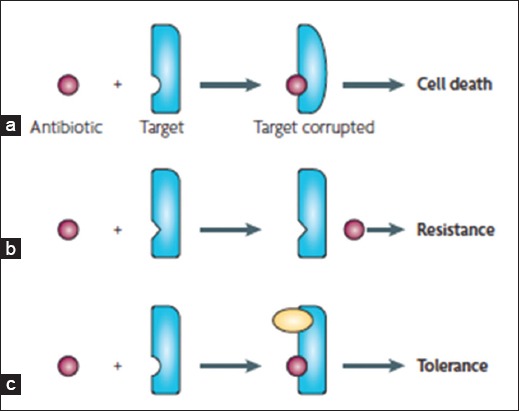
Resistance versus tolerance to antimicrobial agents. a) The antimicrobial agent (purple) binds to the target (blue) altering its function, which causes bacterial death. b) The target of the antimicrobial agents has been altered so that it fails to bind to the antimicrobial and the cell becomes resistant to treatment with the agent. c) A different molecule (yellow) inhibits the antimicrobial target. This prevents the antimicrobial agent from corrupting its functions, resulting in tolerance [136].
ANTIMICROBIAL RESISTANCE/TOLERANCE OF B. BURGDORFERI
In a recent critical review of studies involving antibiotic treatment of B. burgdorferi-infected animal models, it was stated, “in the treatment of other infections it is probably unrealistic to expect that antimicrobial therapy per se will eliminate every single microorganism from an infected host, and moreover, such an action would rarely if ever be required for a successful outcome…the role of antimicrobial therapy in vivo can be thought of in terms of “tipping the balance” in favor of the host’s own defenses against a particular pathogen” [146]. This may be true for “other infections” but when treating for B. burgdorferi, which persists in fully immunocompetent hosts as the rule of its natural behavior, “tipping the balance” in favor of the host may be a challenge.
Several reports, including those generated in our laboratory, have provided evidence of B. burgdorferi presence in collagenous tissues, following antimicrobial therapy during chronic infection in animals [63, 87, 147-151], and in humans [152-156]. One interesting finding was that uninfected ticks were able to acquire antibiotic-tolerant B. burgdorferi and transmit spirochetes to naïve hosts following the molt to the next stage. We have found no differences in the feeding efficacy and the numbers of acquired spirochetes between ticks that fed on antibiotic treated and saline treated mice [149]. Thus, seemingly adequate treatment with a variety of antimicrobial agents may not eliminate infection in some patients, which was confirmed in animal models. There is clear scientific evidence that a small heterogeneous subpopulation of surviving spirochetes shows tolerance to antimicrobial agents and can persist in a host for a prolonged period following therapy.
What is unique about all of these studies is that spirochetes can be detected by PCR for B. burgdorferi-specific DNA (BbDNA), but not by culture. In mouse studies performed in this laboratory [87, 149, 157], mice were treated with ceftriaxone, doxycycline, or tigecycline at various intervals of infection, and tissues were tested at intervals after treatment. Tissues remained BbDNA PCR-positive up to 12 months, but were consistently culture-negative. Morphologically intact spirochetes could be visualized by immunohistochemistry in tissues from treated mice; ticks could acquire morphologically intact B. burgdorferi and BbDNA from treated mice; ticks remained BbDNA-positive through molting into nymphs and adults; nymphs transmitted BbDNA to recipient immunocompromised mice; allografts from treated mice transplanted into recipient immunocompromised mice transferred BbDNA to recipient mice; and both tick- and transplant-inoculated mice had disseminated BbDNA. BbDNA-positive tissues were also positive for B. burgdorferi-specific RNA transcription. Furthermore, quantitative PCR indicated low-levels of replication during these various stages.
The IDSA Guidelines have stated, “the significance of continued PCR positivity needs to be better understood, but this phenomenon should not necessarily be construed to indicate persistence of viable B. burgdorferi” [99]. The above summarized behavior of PCR-positivity, RNA transcription, BbDNA transmission, BbDNA amplification, BbDNA dissemination, and morphologically intact spirochetes in both mouse tissues and ticks strongly indicate the presence of persistent, viable, but uncultivable spirochetes.
IDSA Guidelines also state “unless proven otherwise, culture should be regarded as the gold-standard to address viability of B. burgdorferi” [99]. Culture may indeed be a gold standard when it is positive, but it is often not. Having worked with for over 20 years, it is apparent that not all isolates or strains can be easily cultured, and this is especially apparent during long-term infection. Thus, culture cannot be relied upon as a gold standard of viability. As noted above, our studies and those of others in mice, dogs and non-human primates have all reached similar conclusions: spirochetes are persisting, but are paradoxically non-cultivable. A very intriguing observation in the recent study was the resurgence of non-cultivable spirochetes in all assessed tissues of antimicrobial treated mice after 12 months, and the overall tissue spirochete burden reached the levels detected in sham-treated mice at the same time point (Figure 4). Despite the continued non-cultivable state, RNA transcription of multiple B. burgdorferi genes was detected in host tissues, flaB DNA was acquired by xenodiagnostic ticks, and spirochetal forms could be visualized within ticks and mouse tissues by immunofluorescence and immunohistochemistry, respectively (Figure 5). A number of host cytokines were up- or down-regulated in tissues of both saline- and antibiotic-treated mice in the absence of histopathology, indicating host response to the presence of non-cultivable, despite the lack of inflammation in tissues [149].
Figure 4.
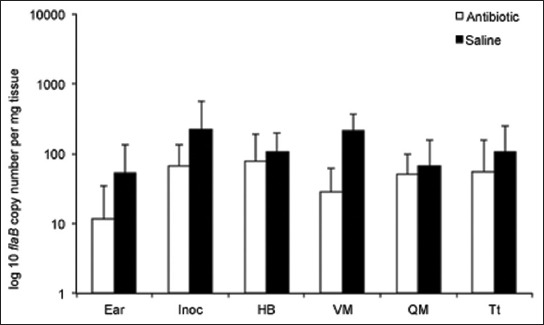
B. burgdorferi levels resurge in tissues at 12 months after antibiotic treatment. Copy numbers of B. burgdorferi flab DNA, determined by quantitative PCR, in ear, inoculation site (Inoc), heart base (HB), ventricular muscle (VM), quadriceps muscle (QM), and tibiotarsus (Tt) tissues of saline- and antibiotic-treated mice at 12 months after treatment [149].
Figure 5.
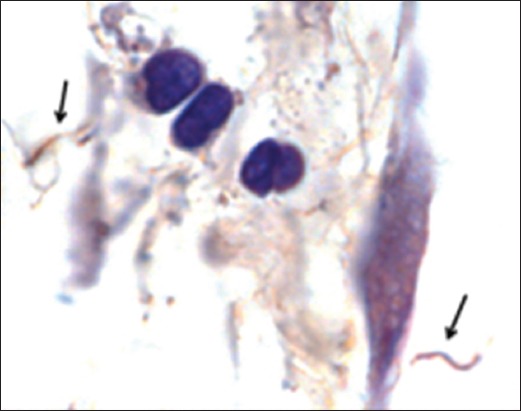
Spirochetes can be visualized within tissue of a mouse at 12 months following antibiotic treatment. Indirect immunohistochemical staining of B. burgdorferi spirochetes (arrows) in the heart base connective tissue [149].
During the course of infection, B. burgdorferi proliferates and incidentally generates an increasingly heterogeneous population of replicatively attenuated spirochetes that have lost one or more small plasmids. These “attenuated” spirochetes remain viable, but because of their plasmid loss, they divide slowly, thereby being tolerant to the effects of antibiotics, as well as being non-cultivable (unpublished data). Because persistence of non-cultivable spirochetes has been shown to occur following treatment with several different classes of antibiotics, the phenomenon is likely explained by antimicrobial tolerance (in contrast to antibiotic resistance or inadequate antibiotic treatment), in which all classes of antibiotics fail to completely eliminate non-dividing or slowly dividing subpopulations of a broad array of bacteria and fungi [136, 158]. It has been known for decades that during in vitro passage, B. burgdorferi is highly prone to plasmid loss [61, 159, 160], and therefore plasmid loss is likely to also occur during the course of infection and increase over time. This may explain why treatment success in humans [99, 103] and laboratory mice [87, 157] appears to be most effective during early infection. Treatment success is inversely correlated with spirochete populations, since spirochete burdens in mouse tissues are highest during early infection [35], when antibiotics work best. The biological (in contrast to medical) significance of attenuated spirochetes is probably insignificant, in that robustly dividing-, genetically-intact spirochetes would be selectively favored upon tick acquisition, transmission, and survival in reservoir hosts. The medical significance of attenuated persisting spirochetes is another matter, and compels further investigation.
Another interesting point is the finding of antimicrobial resistant/tolerant microorganisms, resistant genes, and integrons in wild animals [115, 123, 161-168], after being exposed to antimicrobial agents present in natural environments, most notably sewage and surface water sediments. Some such wildlife species animals are natural reservoir hosts for B. burgdorferi. These findings further support the concept that small mammals as natural hosts for B. burgdorferi could be exploited for selection of antimicrobial resistant/tolerant phenotypes. Such spirochete phenotypes could contribute to the intensity of transmission and the overall risk of Lyme borreliosis.
Based on these observations and recent evidence of persisting B. burgdorferi present in tissues of treated hosts during chronic infection, it will be plausible to speculate that there is preexisting (natural) variation in antimicrobial susceptibility among bacterial strains, which could be an alternative phenotype that offers an appreciable survival advantage within the pathogen’s original population.
CONCLUSIONS
Based on the observations stated above it can be concluded that: 1) a small population of persistent spirochetes survive antimicrobial treatment, as demonstrated in various animal models. B. burgdorferi gDNA was readily detected in tissues of mice as late as 12 months after treatment with antimicrobial agents, even after using a new class of antibiotics, tigecycline, which has a longer half-life in mice compared to ceftriaxone. 2) Morphologically intact spirochetes were visualized by immunohistochemistry in collagen rich tissues of treated mice. Transcriptional activity of antimicrobial-tolerant persistent B. burgdorferi mRNA was detected for several target genes as well as the ability to replicate at low levels. Recently published data have confirmed previous findings and showed transcriptional activity of numerous genes in spirochetes that survived for 12 months after antimicrobial treatment. These results indicate viability and metabolic activity among the persisting spirochetes. 3) It have been demonstrated that spirochetes that survived antimicrobial treatment in mice could be acquired by larval ticks, passed transtadially to the nymphs, and transmitted into naïve C3H-scid mice. Multiple tissues in the mice were PCR-positive, although inflammation was not observed. 4) A possible mechanism for the reduced infectivity of residual spirochetes might be a lack of two undetected plasmids, lp25 and lp28-1, probably due to mutation of certain genes, plasmid loss or recombination events as a result of antimicrobial treatment. The results suggest that the population of spirochetes detected after antimicrobial therapy is genetically different from the infecting population. 5) The resurgence of spirochetes in all assessed tissues of antimicrobial treated mice after 12 months was observed, and the overall tissue spirochete burden reached the levels detected in sham-treated mice at the same time point. 6) It has been shown that the antimicrobial tolerant/resistant persisters are uncultivable. These findings create an obstacle in studying the molecular mechanisms involved in persistence and properly addressing their significance in chronic Lyme borreliosis. 7) The characteristics and the role of this rare, uncultivable population are poorly understood.
DECLARATION OF INTERESTS
The author declares no competing interests.
REFERENCES
- [1].Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. Lyme arthritis: agn epidemic of oligoarticular arthritis in children and adults in three connecticut communities. Arthritis Rheum. 1977 Jan-Feb;20(1):7–17. doi: 10.1002/art.1780200102. http://dx.doi.org/10.1002/art.1780200102 . [DOI] [PubMed] [Google Scholar]
- [2].Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–9. doi: 10.1126/science.7043737. http://dx.doi.org/10.1126/science.7043737 . [DOI] [PubMed] [Google Scholar]
- [3].Johnson RC, Hyde FW, Rumpel CM. Taxonomy of the Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):529–37. [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson RC, Schmid GP, Hyde FW, Steigerwalt AG, Brenner DJ. Borrelia burgdorferi sp nov.: etiological agent of Lyme disease. Int J System Bacteriol. 1984;34(4):496–7. http://dx.doi.org/10.1099/00207713-34-4-496 . [Google Scholar]
- [5].Benach J, Garcia Monco J. The Woldwide Saga of Lyme Borreliosis. In: Samuels SD, Radolf J, editors. Borrelia, Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 21–40. [Google Scholar]
- [6].Humair P, Gern L. The wild hidden face of Lyme borreliosis in Europe. Microbes Infect. 2000 Jul;2(8):915–22. doi: 10.1016/s1286-4579(00)00393-2. http://dx.doi.org/10.1016/S1286-4579(00)00393-2 . [DOI] [PubMed] [Google Scholar]
- [7].Roberts DM, Carlyon JA, Theisen M, Marconi RT. The bdr gene families of the Lyme disease and relapsing fever spirochetes: potential influence on biology, pathogenesis, and evolution. Emerg Infect Dis. 2000 Mar-Apr;6(2):110–22. doi: 10.3201/eid0602.000203. http://dx.doi.org/10.3201/eid0602.000203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sigal LH. Summary of the Fifth International Congress on Lyme Borreliosis. Arthritis Rheum. 1994 Jan;37(1):10–4. doi: 10.1002/art.1780370103. http://dx.doi.org/10.1002/art.1780370103 . [DOI] [PubMed] [Google Scholar]
- [9].Smith R, O’Connell S, Palmer S. Lyme disease surveillance in England and Wales, 1986-1998. Emerg Infect Dis. 2000 Jul-Aug;6(4):404–7. doi: 10.3201/eid0604.000416. http://dx.doi.org/10.3201/eid0604.000416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro surveillance: bulletin Europeen sur les maladies transmissibles =European communicable disease bulletin. 2011;16(27) [PubMed] [Google Scholar]
- [11].Lischer CJ, Leutenegger CM, Braun U, Lutz H. Diagnosis of Lyme disease in two cows by the detection of Borrelia burgdorferi DNA. Vet Rec. 2000 Apr 22;146(17):497–9. doi: 10.1136/vr.146.17.497. http://dx.doi.org/10.1136/vr.146.17.497 . [DOI] [PubMed] [Google Scholar]
- [12].Magnarelli LA, Ijdo JW, Van Andel AE, Wu C, Padula SJ, Fikrig E. Serologic confirmation of Ehrlichia equi and Borrelia burgdorferi infections in horses from the northeastern United States. J Am Vet Med Assoc. 2000;217(7):1045–50. doi: 10.2460/javma.2000.217.1045. http://dx.doi.org/10.2460/javma.2000.217.1045 . [DOI] [PubMed] [Google Scholar]
- [13].Tuomi J, Rantamaki LK, Tanskanen R. Experimental infection of laboratory mice and rabbits with several isolates of Borrelia burgdorferi sensu lato;comparison of antigens from different genospecies in serological measurement of immune responses. Comp Immunol Microbiol Infect Dis. 2002;25(2):109–25. doi: 10.1016/s0147-9571(01)00027-3. http://dx.doi.org/10.1016/S0147-9571(01)00027-3 . [DOI] [PubMed] [Google Scholar]
- [14].Telford SR, 3rd, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997 Apr-Jun;3(2):165–70. doi: 10.3201/eid0302.970209. http://dx.doi.org/10.3201/eid0302.970209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–5. [PMC free article] [PubMed] [Google Scholar]
- [17].Hayes SF, Burgdorfer W. II Characteristics of Borrelia burgdorferi 3 Ultrastructure of Borrelia burgdorferi. In: Weber K, Burgdorfer W, Schierz G, editors. Aspects of Lyme Borreliosis. Berlin, Heidelberg, New York, London, Paris, Tokyo, Hong Kong, Barcelona: Budapest Springer-Verlag; 1993. pp. 29–43. http://dx.doi.org/10.1007/978-3-642-77614-4_3 . [Google Scholar]
- [18].Casjens SR, Eggers CH, Schwartz I. Borrelia Genomics: Chromosome, Plasmids, Bacteriphages and Genetic Variation. In: Samuels SD, Radolf J, editors. Borrelia; Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 21–47. [Google Scholar]
- [19].Dykhuizen D, Brisson D. Evolutionary Genetics of Borrelia burgdorferi sensu lato. In: Samuels SD, Radolf J, editors. Borrelia; Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 215–43. [Google Scholar]
- [20].Norris DE, Coburn J, Leong JM, Hu LT, Hook M. Pathobiology of Lyme Disease Borrelia. In: Samuels DS, Radolf J, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 295–325. [Google Scholar]
- [21].Daix V, Schroeder H, Praet N, Georgin JP, Chiappino I, Gillet L, et al. Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol Biol. 2007 Apr;16(2):155–66. doi: 10.1111/j.1365-2583.2006.00710.x. http://dx.doi.org/10.1111/j.1365-2583.2006.00710.x . [DOI] [PubMed] [Google Scholar]
- [22].Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012 Feb;10(2):87–99. doi: 10.1038/nrmicro2714. http://dx.doi.org/10.1038/nrmicro2714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Piesman J, Schwan T. Ecology of Borreliae and their Arthropod Vectors. In: Samuels SD, Radolf J, editors. Borrelia; Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 245–72. [Google Scholar]
- [24].Madigan JE. Lyme disease (Lyme borreliosis) in horses. Vet Clin North Am Equine Pract. 1993 Aug;9(2):429–34. doi: 10.1016/s0749-0739(17)30409-1. [DOI] [PubMed] [Google Scholar]
- [25].Parker JL, White KK. Lyme borreliosis in cattle and horses: a review of the literature. Cornell Vet. 1992 Jul;82(3):253–74. [PubMed] [Google Scholar]
- [26].Schmidtmann ET, Schlater JL, Maupin GO, Mertins JW. Vegetational association of host-seeking adult blacklegged ticks, Ixodes scapularis Say (Acari: Ixodidae), on dairy farms in northwestern Wisconsin. J Dairy Sci. 1998 Mar;81(3):718–21. doi: 10.3168/jds.s0022-0302(98)75627-9. http://dx.doi.org/10.3168/jds.S0022-0302(98)75627-9 . [DOI] [PubMed] [Google Scholar]
- [27].Anderson JF, Magnarelli LA. Epizootiology of Lyme disease-causing borreliae. Clin Dermatol. 1993 Jul-Sep;11(3):339–51. doi: 10.1016/0738-081x(93)90088-t. http://dx.doi.org/10.1016/0738-081X(93)90088-T . [DOI] [PubMed] [Google Scholar]
- [28].Spielman A, Levine JF, Wilson ML. Vectorial capacity of North American Ixodes ticks. Yale J Biol Med. 1984 Jul-Aug;57(4):507–13. [PMC free article] [PubMed] [Google Scholar]
- [29].Peavey CA, Lane RS. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J Parasitol. 1995;81(2):175–8. http://dx.doi.org/10.2307/3283916 . [PubMed] [Google Scholar]
- [30].Olsen B, Duffy DC, Jaenson TG, Gylfe A, Bonnedahl J, Bergstrom S. Transhemispheric exchange of Lyme disease spirochetes by seabirds. J Clin Microbiol. 1995 Dec;33(12):3270–4. doi: 10.1128/jcm.33.12.3270-3274.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States 1992-2006. MMWR Surveill Summ. 2008 Oct 3;57(10):1–9. [PubMed] [Google Scholar]
- [32].Nuttall PA. Displaced tick-parasite interactions at the host interface. Parasitology. 1998;116(Suppl):S65–72. doi: 10.1017/s003118200008495x. http://dx.doi.org/10.1017/S003118200008495X . [DOI] [PubMed] [Google Scholar]
- [33].De Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53(4):397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- [34].Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92(7):2909–13. doi: 10.1073/pnas.92.7.2909. http://dx.doi.org/10.1073/pnas.92.7.2909 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun. 2003 Sep;71(9):5042–55. doi: 10.1128/IAI.71.9.5042-5055.2003. http://dx.doi.org/10.1128/IAI.71.9.5042-5055.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hodzic E, Borjesson DL, Feng S, Barthold SW. Acquisition dynamics of Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis at the host-vector interface. Vector Borne Zoonotic Dis. 2001 Summer;1(2):149–58. doi: 10.1089/153036601316977750. http://dx.doi.org/10.1089/153036601316977750 . [DOI] [PubMed] [Google Scholar]
- [37].Hodzic E, Feng S, Fish D, Leutenegger CM, Freet KJ, Barthold SW. Infection of mice with the agent of human granulocytic ehrlichiosis after different routes of inoculation. J Infect Dis. 2001 Jun 15;183(12):1781–6. doi: 10.1086/320735. http://dx.doi.org/10.1086/320735 . [DOI] [PubMed] [Google Scholar]
- [38].Shih CM, Pollack RJ, Telford SR, 3rd, Spielman A. Delayed dissemination of Lyme disease spirochetes from the site of deposition in the skin of mice. J Infect Dis. 1992;166(4):827–31. doi: 10.1093/infdis/166.4.827. http://dx.doi.org/10.1093/infdis/166.4.827 . [DOI] [PubMed] [Google Scholar]
- [39].Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. 2008 Jun;22(2):341–60. doi: 10.1016/j.idc.2007.12.011. vii-viii. http://dx.doi.org/10.1016/j.idc.2007.12.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steere AC, Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003 Jun 12;348(24):2472–4. doi: 10.1056/NEJM200306123482423. http://dx.doi.org/10.1056/NEJM200306123482423 . [DOI] [PubMed] [Google Scholar]
- [41].Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004 Apr;113(8):1093–101. doi: 10.1172/JCI21681. http://dx.doi.org/10.1172/JCI21681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nadelman RB. Erythema Migrans. Infect Dis Clin North Am. 2015 Jun;29(2):211–39. doi: 10.1016/j.idc.2015.02.001. http://dx.doi.org/10.1016/j.idc.2015.02.001 . [DOI] [PubMed] [Google Scholar]
- [43].Barthold SW. Lyme borreliosis. In: Nataro JP, Blaser MJ, Cunningham-Rundles S, editors. Persistent bacterial infection. Washington, DC: ASM Press; 2000. pp. 281–304. http://dx.doi.org/10.1128/9781555818104.ch14 . [Google Scholar]
- [44].Barthold SW, Fikrig E, Bockenstedt LK, Persing DH. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63(6):2255–61. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Montgomery RR, Malawista SE, Feen KJ, Bockenstedt LK. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183(1):261–9. doi: 10.1084/jem.183.1.261. http://dx.doi.org/10.1084/jem.183.1.261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Srivastava SY, de Silva AM. Reciprocal expression of ospA and ospC in single cells of Borrelia burgdorferi. J Bacteriol. 2008 May;190(10):3429–33. doi: 10.1128/JB.00085-08. http://dx.doi.org/10.1128/JB.00085-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pulzova L, Bhide M. Outer surface proteins of Borrelia: peerless immune evasion tools. Curr Protein Pept Sci. 2014 Feb;15(1):75–88. doi: 10.2174/1389203715666140221124213. http://dx.doi.org/10.2174/1389203715666140221124213 . [DOI] [PubMed] [Google Scholar]
- [48].Montgomery RR, Nathanson MH, Malawista SE. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery. J Immunol. 1993;150(3):909–15. [PubMed] [Google Scholar]
- [49].Pachner AR, Basta J, Delaney E, Hulinska D. Localization of Borrelia burgdorferi in murine Lyme borreliosis by electron microscopy. Am J Trop Med Hyg. 1995;52(2):128–33. doi: 10.4269/ajtmh.1995.52.128. [DOI] [PubMed] [Google Scholar]
- [50].Hulinska D, Bartak P, Hercogova J, Hancil J, Basta J, Schramlova J. Electron microscopy of Langerhans cells and Borrelia burgdorferi in Lyme disease patients. Zentralbl Bakteriol. 1994 Jan;280(3):348–59. doi: 10.1016/s0934-8840(11)80597-9. http://dx.doi.org/10.1016/S0934-8840(11)80597-9 . [DOI] [PubMed] [Google Scholar]
- [51].Brorson O, Brorson SH. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection. 1998;26(3):144–50. doi: 10.1007/BF02771839. http://dx.doi.org/10.1007/BF02771839 . [DOI] [PubMed] [Google Scholar]
- [52].Alban PS, Johnson PW, Nelson DR. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology. 2000 Jan;146(Pt 1):119–27. doi: 10.1099/00221287-146-1-119. [DOI] [PubMed] [Google Scholar]
- [53].Kraiczy P, Hanssen-Hubner C, Kitiratschky V, Brenner C, Besier S, Brade V, et al. Mutational analyses of the BbCRASP-1 protein of Borrelia burgdorferi identify residues relevant for the architecture and binding of host complement regulators FHL-1 and factor H. Int J Med Microbiol. 2009 Apr;299(4):255–68. doi: 10.1016/j.ijmm.2008.09.002. http://dx.doi.org/10.1016/j.ijmm.2008.09.002 . [DOI] [PubMed] [Google Scholar]
- [54].Sapi E, Bastian SL, Mpoy CM, Scott S, Rattelle A, Pabbati N, et al. Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS One. 2012;7(10):e48277. doi: 10.1371/journal.pone.0048277. http://dx.doi.org/10.1371/journal.pone.0048277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Arvikar SL, Steere AC. Diagnosis and Treatment of Lyme Arthritis. Infect Dis Clin North Am. 2015 Jun;29(2):269–80. doi: 10.1016/j.idc.2015.02.004. http://dx.doi.org/10.1016/j.idc.2015.02.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Robinson ML, Kobayashi T, Higgins Y, Calkins H, Melia MT. Lyme Carditis. Infect Dis Clin North Am. 2015 Jun;29(2):255–68. doi: 10.1016/j.idc.2015.02.003. http://dx.doi.org/10.1016/j.idc.2015.02.003 . [DOI] [PubMed] [Google Scholar]
- [57].Porcella SF, Schwan TG. Borrelia burgdorferi and Treponema pallidum: a comparison of functional genomics, environmental adaptations, and pathogenic mechanisms. J Clin Invest. 2001;107(6):651–6. doi: 10.1172/JCI12484. http://dx.doi.org/10.1172/JCI12484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liang FT, Nelson FK, Fikrig E. DNA Microarray Assessment of Putative Borrelia burgdorferi Lipoprotein Genes. Infect Immun. 2002;70(6):3300–3. doi: 10.1128/IAI.70.6.3300-3303.2002. http://dx.doi.org/10.1128/IAI.70.6.3300-3303.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Labandeira-Rey M, Seshu J, Skare JT. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun. 2003 Aug;71(8):4608–13. doi: 10.1128/IAI.71.8.4608-4613.2003. http://dx.doi.org/10.1128/IAI.71.8.4608-4613.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun. 2001;69(1):446–55. doi: 10.1128/IAI.69.1.446-455.2001. http://dx.doi.org/10.1128/IAI.69.1.446-455.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97(25):13865–70. doi: 10.1073/pnas.97.25.13865. http://dx.doi.org/10.1073/pnas.97.25.13865 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thomas V, Anguita J, Samanta S, Rosa PA, Stewart P, Barthold SW, et al. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect Immun. 2001;69(5):3507–9. doi: 10.1128/IAI.69.5.3507-3509.2001. http://dx.doi.org/10.1128/IAI.69.5.3507-3509.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bockenstedt LK, Mao J, Hodzic E, Barthold SW, Fish D. Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J Infect Dis. 2002 Nov 15;186(10):1430–7. doi: 10.1086/345284. http://dx.doi.org/10.1086/345284 . [DOI] [PubMed] [Google Scholar]
- [64].Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390(6660):580–6. doi: 10.1038/37551. http://dx.doi.org/10.1038/37551 . [DOI] [PubMed] [Google Scholar]
- [65].Dulebohn DP, Bestor A, Rego RO, Stewart PE, Rosa PA. Borrelia burgdorferi linear plasmid 38 is dispensable for completion of the mouse-tick infectious cycle. Infect Immun. 2011 Sep;79(9):3510–7. doi: 10.1128/IAI.05014-11. http://dx.doi.org/10.1128/IAI.05014-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus) J Clin Microbiol. 1988;26(5):893–5. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143(3):959–71. [PMC free article] [PubMed] [Google Scholar]
- [68].Moody KD, Barthold SW, Terwilliger GA, Beck DS, Hansen GM, Jacoby RO. Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg. 1990;42(2):165–74. doi: 10.4269/ajtmh.1990.42.165. [DOI] [PubMed] [Google Scholar]
- [69].Goodman JL, Jurkovich P, Kodner C, Johnson RC. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J Clin Microbiol. 1991 May;29(5):894–6. doi: 10.1128/jcm.29.5.894-896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Preac Mursic V, Patsouris E, Wilske B, Reinhardt S, Gross B, Mehraein P. Persistence of Borrelia burgdorferi and histopathological alterations in experimentally infected animals. A comparison with histopathological findings in human Lyme disease. Infection. 1990 Nov-Dec;18(6):332–41. doi: 10.1007/BF01646399. http://dx.doi.org/10.1007/BF01646399 . [DOI] [PubMed] [Google Scholar]
- [71].Sonnesyn SW, Manivel JC, Johnson RC, Goodman JL. A guinea pig model for Lyme disease. Infect Immun. 1993 Nov;61(11):4777–84. doi: 10.1128/iai.61.11.4777-4784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Straubinger RK, Summers BA, Chang YF, Appel MJ. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol. 1997 Jan;35(1):111–6. doi: 10.1128/jcm.35.1.111-116.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Roberts ED, Bohm RP, Jr, Cogswell FB, Lanners HN, Lowrie RC, Jr, Povinelli L, et al. Chronic lyme disease in the rhesus monkey. Lab Invest. 1995 Feb;72(2):146–60. [PubMed] [Google Scholar]
- [74].Asbrink E, Hovmark A. Successful cultivation of spirochetes from skin lesions of patients with erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):161–3. doi: 10.1111/j.1699-0463.1985.tb02870.x. http://dx.doi.org/10.1111/j.1699-0463.1985.tb02870.x . [DOI] [PubMed] [Google Scholar]
- [75].Kuiper H, van Dam AP, Spanjaard L, de Jongh BM, Widjojokusumo A, Ramselaar TC, et al. Isolation of Borrelia burgdorferi from biopsy specimens taken from healthy-looking skin of patients with Lyme borreliosis. J Clin Microbiol. 1994 Mar;32(3):715–20. doi: 10.1128/jcm.32.3.715-720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Maraspin V, Ogrinc K, Ruzic-Sabljic E, Lotric-Furlan S, Strle F. Isolation of Borrelia burgdorferi sensu lato from blood of adult patients with borrelial lymphocytoma, Lyme neuroborreliosis, Lyme arthritis and acrodermatitis chronica atrophicans. Infection. 2011 Feb;39(1):35–40. doi: 10.1007/s15010-010-0062-8. http://dx.doi.org/10.1007/s15010-010-0062-8 . [DOI] [PubMed] [Google Scholar]
- [77].Miklossy J, Khalili K, Gern L, Ericson RL, Darekar P, Bolle L, et al. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J Alzheimers Dis. 2004 Dec;6(6):639–49. doi: 10.3233/jad-2004-6608. discussion 73-81. [DOI] [PubMed] [Google Scholar]
- [78].Snydman DR, Schenkein DP, Berardi VP, Lastavica CC, Pariser KM. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann Intern Med. 1986 Jun;104(6):798–800. doi: 10.7326/0003-4819-104-6-798. http://dx.doi.org/10.7326/0003-4819-104-6-798 . [DOI] [PubMed] [Google Scholar]
- [79].Stanek G, Klein J, Bittner R, Glogar D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med. 1990 Jan 25;322(4):249–52. doi: 10.1056/NEJM199001253220407. http://dx.doi.org/10.1056/NEJM199001253220407 . [DOI] [PubMed] [Google Scholar]
- [80].Strle F, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Nelson JA, et al. Persistence of Borrelia burgdorferi sensu lato in resolved erythema migrans lesions. Clin Infect Dis. 1995 Aug;21(2):380–9. doi: 10.1093/clinids/21.2.380. http://dx.doi.org/10.1093/clinids/21.2.380 . [DOI] [PubMed] [Google Scholar]
- [81].Bradley JF, Johnson RC, Goodman JL. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann Intern Med. 1994 Mar 15;120(6):487–9. doi: 10.7326/0003-4819-120-6-199403150-00007. http://dx.doi.org/10.7326/0003-4819-120-6-199403150-00007 . [DOI] [PubMed] [Google Scholar]
- [82].Fraser DD, Kong LI, Miller FW. Molecular detection of persistent Borrelia burgdorferi in a man with dermatomyositis. Clin Exp Rheumatol. 1992 Jul-Aug;10(4):387–90. [PubMed] [Google Scholar]
- [83].Moter SE, Hofmann H, Wallich R, Simon MM, Kramer MD. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by ospA-specific PCR. J Clin Microbiol. 1994 Dec;32(12):2980–8. doi: 10.1128/jcm.32.12.2980-2988.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994 Jan 27;330(4):229–34. doi: 10.1056/NEJM199401273300401. http://dx.doi.org/10.1056/NEJM199401273300401 . [DOI] [PubMed] [Google Scholar]
- [85].von Stedingk LV, Olsson I, Hanson HS, Asbrink E, Hovmark A. Polymerase chain reaction for detection of Borrelia burgdorferi DNA in skin lesions of early and late Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1995 Jan;14(1):1–5. doi: 10.1007/BF02112610. http://dx.doi.org/10.1007/BF02112610 . [DOI] [PubMed] [Google Scholar]
- [86].Cabello FC, Godfrey HP, Newman SA. Hidden in plain sight: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 2007 Aug;15(8):350–4. doi: 10.1016/j.tim.2007.06.003. http://dx.doi.org/10.1016/j.tim.2007.06.003 . [DOI] [PubMed] [Google Scholar]
- [87].Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother. 2008 May;52(5):1728–36. doi: 10.1128/AAC.01050-07. http://dx.doi.org/10.1128/AAC.01050-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Barthold SW, Hodzic E, Tunev S, Feng S. Antibody-mediated disease remission in the mouse model of lyme borreliosis. Infect Immun. 2006 Aug;74(8):4817–25. doi: 10.1128/IAI.00469-06. http://dx.doi.org/10.1128/IAI.00469-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004 Sep;165(3):977–85. doi: 10.1016/S0002-9440(10)63359-7. http://dx.doi.org/10.1016/S0002-9440(10)63359-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Steere AC. Lyme disease. N Engl J Med. 2001 Jul 12;345(2):115–25. doi: 10.1056/NEJM200107123450207. http://dx.doi.org/10.1056/NEJM200107123450207 . [DOI] [PubMed] [Google Scholar]
- [91].Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987 Nov;107(5):725–31. doi: 10.7326/0003-4819-107-5-725. http://dx.doi.org/10.7326/0003-4819-107-5-725 . [DOI] [PubMed] [Google Scholar]
- [92].Barthold SW, deSouza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Invest. 1996;74(1):57–67. [PubMed] [Google Scholar]
- [93].Barthold SW, Feng S, Bockenstedt LK, Fikrig E, Feen K. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25(Suppl 1):S9–17. doi: 10.1086/516166. http://dx.doi.org/10.1086/516166 . [DOI] [PubMed] [Google Scholar]
- [94].Wormser GP, Nadelman RB, Dattwyler RJ, Dennis DT, Shapiro ED, Steere AC, et al. Practice guidelines for the treatment of Lyme disease. The Infectious Diseases Society of America. Clin Infect Dis. 2000 Jul;31(Suppl 1):1–14. doi: 10.1086/314053. http://dx.doi.org/10.1086/314053 . [DOI] [PubMed] [Google Scholar]
- [95].Wormser GP, Ramanathan R, Nowakowski J, McKenna D, Holmgren D, Visintainer P, et al. Duration of antibiotic therapy for early Lyme disease A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003 May 6;138(9):697–704. doi: 10.7326/0003-4819-138-9-200305060-00005. http://dx.doi.org/10.7326/0003-4819-138-9-200305060-00005 . [DOI] [PubMed] [Google Scholar]
- [96].Bockenstedt LK, Radolf JD. Xenodiagnosis for posttreatment Lyme disease syndrome: resolving the conundrum or adding to it? Clin Infect Dis. 2014 Apr;58(7):946–8. doi: 10.1093/cid/cit942. http://dx.doi.org/10.1093/cid/cit942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Feder HM, Jr, Johnson BJ, O’Connell S, Shapiro ED, Steere AC, Wormser GP, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007 Oct 4;357(14):1422–30. doi: 10.1056/NEJMra072023. http://dx.doi.org/10.1056/NEJMra072023 . [DOI] [PubMed] [Google Scholar]
- [98].Gaubitz M, Dressler F, Huppertz HI, Krause A, Kommission Pharmakotherapie der D. Diagnosis and treatment of Lyme arthritis. Recommendations of the Pharmacotherapy Commission of the Deutsche Gesellschaft fur Rheumatologie (German Society for Rheumatology) Z Rheumatol. 2014 Jun;73(5):469–74. doi: 10.1007/s00393-014-1370-7. http://dx.doi.org/10.1007/s00393-014-1370-7 . [DOI] [PubMed] [Google Scholar]
- [99].Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006 Nov 1;43(9):1089–134. doi: 10.1086/508667. http://dx.doi.org/10.1086/508667 . [DOI] [PubMed] [Google Scholar]
- [100].Lantos PM, Charini WA, Medoff G, Moro MH, Mushatt DM, Parsonnet J, et al. Final report of the Lyme disease review panel of the Infectious Diseases Society of America. Clin Infect Dis. 2010 Jul 1;51(1):1–5. doi: 10.1086/654809. http://dx.doi.org/10.1086/654809 . [DOI] [PubMed] [Google Scholar]
- [101].Baker PJ. Chronic Lyme disease: in defense of the scientific enterprise. FASEB J. 2010 Nov;24(11):4175–7. doi: 10.1096/fj.10-167247. http://dx.doi.org/10.1096/fj.10-167247 . [DOI] [PubMed] [Google Scholar]
- [102].Nadelman RB, Nowakowski J, Forseter G, Goldberg NS, Bittker S, Cooper D, et al. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med. 1996 May;100(5):502–8. doi: 10.1016/s0002-9343(95)99915-9. http://dx.doi.org/10.1016/S0002-9343(95)99915-9 . [DOI] [PubMed] [Google Scholar]
- [103].Stanek G, Strle F. Lyme borreliosis. Lancet. 2003 Nov 15;362(9396):1639–47. doi: 10.1016/S0140-6736(03)14798-8. http://dx.doi.org/10.1016/S0140-6736(03)14798-8 . [DOI] [PubMed] [Google Scholar]
- [104].Tonks A. Lyme wars. BMJ. 2007 Nov 3;335(7626):910–2. doi: 10.1136/bmj.39363.530961.AD. http://dx.doi.org/10.1136/bmj.39363.530961.AD . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011 Apr 1;145(1):39–53. doi: 10.1016/j.cell.2011.02.022. http://dx.doi.org/10.1016/j.cell.2011.02.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011 May 12;473(7346):216–20. doi: 10.1038/nature10069. http://dx.doi.org/10.1038/nature10069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Aminov RI, Mackie RI. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol Lett. 2007 Jun;271(2):147–61. doi: 10.1111/j.1574-6968.2007.00757.x. http://dx.doi.org/10.1111/j.1574-6968.2007.00757.x . [DOI] [PubMed] [Google Scholar]
- [108].Boumart Z, Roche SM, Lalande F, Virlogeux-Payant I, Hennequet-Antier C, Menanteau P, et al. Heterogeneity of persistence of Salmonella enterica serotype Senftenberg strains could explain the emergence of this serotype in poultry flocks. PLoS One. 2012;7(4):e35782. doi: 10.1371/journal.pone.0035782. http://dx.doi.org/10.1371/journal.pone.0035782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gullberg E, Cao S, Berg OG, Ilback C, Sandegren L, Hughes D, et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011 Jul;7(7):e1002158. doi: 10.1371/journal.ppat.1002158. http://dx.doi.org/10.1371/journal.ppat.1002158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hamad MA, Austin CR, Stewart AL, Higgins M, Vazquez-Torres A, Voskuil MI. Adaptation and antibiotic tolerance of anaerobic Burkholderia pseudomallei. Antimicrob Agents Chemother. 2011 Jul;55(7):3313–23. doi: 10.1128/AAC.00953-10. http://dx.doi.org/10.1128/AAC.00953-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kozak GK, Boerlin P, Janecko N, Reid-Smith RJ, Jardine C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl Environ Microbiol. 2009 Feb;75(3):559–66. doi: 10.1128/AEM.01821-08. http://dx.doi.org/10.1128/AEM.01821-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother. 2012 Apr;67(4):810–8. doi: 10.1093/jac/dkr578. http://dx.doi.org/10.1093/jac/dkr578 . [DOI] [PubMed] [Google Scholar]
- [113].Roberts RR, Hota B, Ahmad I, Scott RD, 2nd, Foster SD, Abbasi F, et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009 Oct 15;49(8):1175–84. doi: 10.1086/605630. http://dx.doi.org/10.1086/605630 . [DOI] [PubMed] [Google Scholar]
- [114].Boxall AB. The environmental side effects of medication. EMBO Rep. 2004 Dec;5(12):1110–6. doi: 10.1038/sj.embor.7400307. http://dx.doi.org/10.1038/sj.embor.7400307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sandegren L. Selection of antibiotic resistance at very low antibiotic concentrations. Upsala journal of medical sciences. 2014 May;119(2):103–7. doi: 10.3109/03009734.2014.904457. http://dx.doi.org/10.3109/03009734.2014.904457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils - a review. Plant Nutr Soil Sci 2003. 2003 Feb 11;155:145–67. [Google Scholar]
- [117].Bonnedahl J, Jarhult JD. Antibiotic resistance in wild birds. Upsala journal of medical sciences. 2014 May;119(2):113–6. doi: 10.3109/03009734.2014.905663. http://dx.doi.org/10.3109/03009734.2014.905663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004 Dec;10(12 Suppl):S122–9. doi: 10.1038/nm1145. http://dx.doi.org/10.1038/nm1145 . [DOI] [PubMed] [Google Scholar]
- [119].Lloyd DH. Reservoirs of antimicrobial resistance in pet animals. Clin Infect Dis. 2007 Sep 1;45(Suppl 2):S148–52. doi: 10.1086/519254. http://dx.doi.org/10.1086/519254 . [DOI] [PubMed] [Google Scholar]
- [120].Nkogwe C, Raletobana J, Stewart-Johnson A, Suepaul S, Adesiyun A. Frequency of Detection of Escherichia coli, Salmonella spp and Campylobacter spp. in the Faeces of Wild Rats (Rattus spp.) in Trinidad and Tobago. Veterinary medicine international 2011. 2011:686923. doi: 10.4061/2011/686923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Pichon B, Egan D, Rogers M, Gray J. Detection and identification of pathogens and host DNA in unfed host-seeking Ixodes ricinus L. (Acari: Ixodidae) J Med Entomol. 2003 Sep;40(5):723–31. doi: 10.1603/0022-2585-40.5.723. http://dx.doi.org/10.1603/0022-2585-40.5.723 . [DOI] [PubMed] [Google Scholar]
- [122].Zurek L, Ghosh A. Insects represent a link between food animal farms and the urban environment for antibiotic resistance traits. Appl Environ Microbiol. 2014 Jun;80(12):3562–7. doi: 10.1128/AEM.00600-14. http://dx.doi.org/10.1128/AEM.00600-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wardyn SE, Kauffman LK, Smith TC. Methicillin-resistant Staphylococcus aureus in central Iowa wildlife. J Wildl Dis. 2012 Oct;48(4):1069–73. doi: 10.7589/2011-10-295. http://dx.doi.org/10.7589/2011-10-295 . [DOI] [PubMed] [Google Scholar]
- [124].Moodley A, Guardabassi L. Transmission of IncN plasmids carrying blaCTX-M-1 between commensal Escherichia coli in pigs and farm workers. Antimicrob Agents Chemother. 2009 Apr;53(4):1709–11. doi: 10.1128/AAC.01014-08. http://dx.doi.org/10.1128/AAC.01014-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, et al. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007 Dec;13(12):1834–9. doi: 10.3201/eid1312.070384. http://dx.doi.org/10.3201/eid1312.070384 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vieira AR, Collignon P, Aarestrup FM, McEwen SA, Hendriksen RS, Hald T, et al. Association between antimicrobial resistance in Escherichia coli isolates from food animals and blood stream isolates from humans in Europe: an ecological study. Foodborne Pathog Dis. 2011 Dec;8(12):1295–301. doi: 10.1089/fpd.2011.0950. http://dx.doi.org/10.1089/fpd.2011.0950 . [DOI] [PubMed] [Google Scholar]
- [127].Haeusler GM, Curtis N. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol. 2013;764:13–26. doi: 10.1007/978-1-4614-4726-9_2. http://dx.doi.org/10.1007/978-1-4614-4726-9_2 . [DOI] [PubMed] [Google Scholar]
- [128].Endtz HP, Ruijs GJ, van Klingeren B, Jansen WH, van der Reyden T, Mouton RP. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother. 1991 Feb;27(2):199–208. doi: 10.1093/jac/27.2.199. http://dx.doi.org/10.1093/jac/27.2.199 . [DOI] [PubMed] [Google Scholar]
- [129].MacGregor LH, Cumming GS, Hockey PAR. Understanding pathogen transmission dynamics in waterbird communities: At what scale should interactions be studied? South African Journal of Science. 2011;107(9/10):1–10. http://dx.doi.org/10.4102/sajs.v107i9/10.283 . [Google Scholar]
- [130].Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008 Jan 15;46(2):155–64. doi: 10.1086/524891. http://dx.doi.org/10.1086/524891 . [DOI] [PubMed] [Google Scholar]
- [131].Baquero F, Negri MC, Morosini MI, Blazquez J. Antibiotic-selective environments. Clin Infect Dis. 1998 Aug;27(Suppl 1):S5–11. doi: 10.1086/514916. http://dx.doi.org/10.1086/514916 . [DOI] [PubMed] [Google Scholar]
- [132].Chander Y, Kumar K, Goyal SM, Gupta SC. Antibacterial activity of soil-bound antibiotics. J Environ Qual. 2005 Nov-Dec;34(6):1952–7. doi: 10.2134/jeq2005.0017. http://dx.doi.org/10.2134/jeq2005.0017 . [DOI] [PubMed] [Google Scholar]
- [133].Kummerer K. Antibiotics in the aquatic environment--a review--part I. Chemosphere. 2009 Apr;75(4):417–34. doi: 10.1016/j.chemosphere.2008.11.086. http://dx.doi.org/10.1016/j.chemosphere.2008.11.086 . [DOI] [PubMed] [Google Scholar]
- [134].Liu A, Fong A, Becket E, Yuan J, Tamae C, Medrano L, et al. Selective advantage of resistant strains at trace levels of antibiotics: a simple and ultrasensitive color test for detection of antibiotics and genotoxic agents. Antimicrob Agents Chemother. 2011 Mar;55(3):1204–10. doi: 10.1128/AAC.01182-10. http://dx.doi.org/10.1128/AAC.01182-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gefen O, Balaban NQ. The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress. FEMS Microbiol Rev. 2009 Jul;33(4):704–17. doi: 10.1111/j.1574-6976.2008.00156.x. http://dx.doi.org/10.1111/j.1574-6976.2008.00156.x . [DOI] [PubMed] [Google Scholar]
- [136].Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007 Jan;5(1):48–56. doi: 10.1038/nrmicro1557. http://dx.doi.org/10.1038/nrmicro1557 . [DOI] [PubMed] [Google Scholar]
- [137].Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–72. doi: 10.1146/annurev.micro.112408.134306. http://dx.doi.org/10.1146/annurev.micro.112408.134306 . [DOI] [PubMed] [Google Scholar]
- [138].Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004 Sep 10;305(5690):1622–5. doi: 10.1126/science.1099390. http://dx.doi.org/10.1126/science.1099390 . [DOI] [PubMed] [Google Scholar]
- [139].Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004 Jan 15;230(1):13–8. doi: 10.1016/S0378-1097(03)00856-5. http://dx.doi.org/10.1016/S0378-1097(03)00856-5 . [DOI] [PubMed] [Google Scholar]
- [140].Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K. Persisters: a distinct physiological state of E. coli. BMC Microbiol. 2006;6:53. doi: 10.1186/1471-2180-6-53. http://dx.doi.org/10.1186/1471-2180-6-53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Grenfell BT, Pybus OG, Gog JR, Wood JL, Daly JM, Mumford JA, et al. Unifying the epidemiological and evolutionary dynamics of pathogens. Science. 2004 Jan 16;303(5656):327–32. doi: 10.1126/science.1090727. http://dx.doi.org/10.1126/science.1090727 . [DOI] [PubMed] [Google Scholar]
- [142].Kint CI, Verstraeten N, Fauvart M, Michiels J. New-found fundamentals of bacterial persistence. Trends Microbiol. 2012 Dec;20(12):577–85. doi: 10.1016/j.tim.2012.08.009. http://dx.doi.org/10.1016/j.tim.2012.08.009 . [DOI] [PubMed] [Google Scholar]
- [143].Kiani D, Quinn EL, Burch KH, Madhavan T, Saravolatz LD, Neblett TR. The increasing importance of polymicrobial bacteremia. Jama. 1979 Sep 7;242(10):1044–7. http://dx.doi.org/10.1001/jama.1979.03300100022015 . [PubMed] [Google Scholar]
- [144].Fauvart M, De Groote VN, Michiels J. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J Med Microbiol. 2011 Jun;60(Pt 6):699–709. doi: 10.1099/jmm.0.030932-0. http://dx.doi.org/10.1099/jmm.0.030932-0 . [DOI] [PubMed] [Google Scholar]
- [145].Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, et al. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother. 2003 Aug;52(2):145–8. doi: 10.1093/jac/dkg312. http://dx.doi.org/10.1093/jac/dkg312 . [DOI] [PubMed] [Google Scholar]
- [146].Wormser GP, Schwartz I. Antibiotic treatment of animals infected with Borrelia burgdorferi. Clin Microbiol Rev. 2009 Jul;22(3):387–95. doi: 10.1128/CMR.00004-09. http://dx.doi.org/10.1128/CMR.00004-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Barthold SW, Cadavid D, Philipp MT. Animal Models of Borreliosis. In: Samuels DS, Radolf J, editors. Borrelia, Molecular Biology, Host Interaction and Pathogenesis. Norfolk, UK: Caister Academic Press; 2010. pp. 353–405. [Google Scholar]
- [148].Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One. 2012;7(1):e29914. doi: 10.1371/journal.pone.0029914. http://dx.doi.org/10.1371/journal.pone.0029914 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One. 2014;9(1):e86907. doi: 10.1371/journal.pone.0086907. http://dx.doi.org/10.1371/journal.pone.0086907 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Straubinger RK, Straubinger AF, Harter L, Jacobson RH, Chang YF, Summers BA, et al. Borrelia burgdorferi migrates into joint capsules and causes an up-regulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infect Immun. 1997 Apr;65(4):1273–85. doi: 10.1128/iai.65.4.1273-1285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yrjanainen H, Hytonen J, Hartiala P, Oksi J, Viljanen MK. Persistence of borrelial DNA in the joints of Borrelia burgdorferi-infected mice after ceftriaxone treatment. Apmis. 2010 Sep 1;118(9):665–73. doi: 10.1111/j.1600-0463.2010.02615.x. http://dx.doi.org/10.1111/j.1600-0463.2010.02615.x . [DOI] [PubMed] [Google Scholar]
- [152].Breier F, Khanakah G, Stanek G, Kunz G, Aberer E, Schmidt B, et al. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br J Dermatol. 2001 Feb;144(2):387–92. doi: 10.1046/j.1365-2133.2001.04034.x. http://dx.doi.org/10.1046/j.1365-2133.2001.04034.x . [DOI] [PubMed] [Google Scholar]
- [153].Honegr K, Hulinska D, Beran J, Dostal V, Havlasova J, Cermakova Z. Long term and repeated electron microscopy and PCR detection of Borrelia burgdorferi sensu lato after an antibiotic treatment. Cent Eur J Public Health. 2004 Mar;12(1):6–11. [PubMed] [Google Scholar]
- [154].Hunfeld KP, Ruzic-Sabljic E, Norris DE, Kraiczy P, Strle F. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrob Agents Chemother. 2005 Apr;49(4):1294–301. doi: 10.1128/AAC.49.4.1294-1301.2005. http://dx.doi.org/10.1128/AAC.49.4.1294-1301.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Oksi J, Marjamaki M, Nikoskelainen J, Viljanen MK. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann Med. 1999 Jun;31(3):225–32. doi: 10.3109/07853899909115982. http://dx.doi.org/10.3109/07853899909115982 . [DOI] [PubMed] [Google Scholar]
- [156].Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis. 1998;57(2):118–21. doi: 10.1136/ard.57.2.118. http://dx.doi.org/10.1136/ard.57.2.118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother. 2010 Feb;54(2):643–51. doi: 10.1128/AAC.00788-09. http://dx.doi.org/10.1128/AAC.00788-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31. doi: 10.1007/978-3-540-75418-3_6. http://dx.doi.org/10.1007/978-3-540-75418-3_6 . [DOI] [PubMed] [Google Scholar]
- [159].Biskup UG, Strle F, Ruzic-Sabljic E. Loss of plasmids of Borrelia burgdorferi sensu lato during prolonged in vitro cultivation. Plasmid. 2011 Oct;66(1):1–6. doi: 10.1016/j.plasmid.2011.02.006. http://dx.doi.org/10.1016/j.plasmid.2011.02.006 . [DOI] [PubMed] [Google Scholar]
- [160].Schwan TG, Burgdorfer W, Garon CF. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56(8):1831–6. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010 Apr;8(4):251–9. doi: 10.1038/nrmicro2312. http://dx.doi.org/10.1038/nrmicro2312 . [DOI] [PubMed] [Google Scholar]
- [162].Dolejska M, Cizek A, Literak I. High prevalence of antimicrobial-resistant genes and integrons in Escherichia coli isolates from Black-headed Gulls in the Czech Republic. J Appl Microbiol. 2007 Jul;103(1):11–9. doi: 10.1111/j.1365-2672.2006.03241.x. http://dx.doi.org/10.1111/j.1365-2672.2006.03241.x . [DOI] [PubMed] [Google Scholar]
- [163].Gakuya FM, Kyule MN, Gathura PB, Kariuki S. Antimicrobial susceptibility and plasmids from Escherichia coli isolated from rats. East Afr Med J. 2001 Oct;78(10):518–22. doi: 10.4314/eamj.v78i10.8960. http://dx.doi.org/10.4314/eamj.v78i10.8960 . [DOI] [PubMed] [Google Scholar]
- [164].Gaukler SM, Linz GM, Sherwood JS, Dyer NW, Bleier WJ, Wannemuehler YM, et al. Escherichia coli, Salmonella, and Mycobacterium avium subsp. paratuberculosis in wild European starlings at a Kansas cattle feedlot. Avian diseases. 2009 Dec;53(4):544–51. doi: 10.1637/8920-050809-Reg.1. http://dx.doi.org/10.1637/8920-050809-Reg.1 . [DOI] [PubMed] [Google Scholar]
- [165].Power ML, Emery S, Gillings MR. Into the wild: dissemination of antibiotic resistance determinants via a species recovery program. PLoS One. 2013;8(5):e63017. doi: 10.1371/journal.pone.0063017. http://dx.doi.org/10.1371/journal.pone.0063017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Radhouani H, Silva N, Poeta P, Torres C, Correia S, Igrejas G. Potential impact of antimicrobial resistance in wildlife, environment and human health. Front Microbiol. 2014;5:23. doi: 10.3389/fmicb.2014.00023. http://dx.doi.org/10.3389/fmicb.2014.00023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Sherley M, Gordon DM, Collignon PJ. Variations in antibiotic resistance profile in Enterobacteriaceae isolated from wild Australian mammals. Environmental microbiology. 2000 Dec;2(6):620–31. doi: 10.1046/j.1462-2920.2000.00145.x. http://dx.doi.org/10.1046/j.1462-2920.2000.00145.x . [DOI] [PubMed] [Google Scholar]
- [168].Souza V, Rocha M, Valera A, Eguiarte LE. Genetic structure of natural populations of Escherichia coli in wild hosts on different continents. Appl Environ Microbiol. 1999 Aug;65(8):3373–85. doi: 10.1128/aem.65.8.3373-3385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


