Abstract
Obesity, insulin resistance (IR), inflammation, and hyperandrogenism may lead to polycystic ovary syndrome (PCOS) and hypertension. Nesfatin-1 (N1) may be related to IR, obesity, and hypertension. Furthermore, a vitamin D (VD) deficiency is associated with hypertension and PCOS. We aimed to investigate N1 and VD levels in PCOS that have an effect on systolic and diastolic blood pressure (BP) and heart rate (HR). This study included 54 patients with PCOS and 48 age-body mass index (BMI)-matched healthy controls. PCOS was diagnosed according to clinical practice guidelines. Ferriman-Gallwey scores (FGS) were calculated, while N1, VD, and other hormonal and biochemical parameters were measured for all subjects. Systolic and diastolic BP was measured as well. HR was calculated using an electrocardiogram. The levels of N1 (p < 0.001), high-sensitivity C-reactive protein (hs-CRP) (p = 0.036), homeostasis model assessment as an index of insulin resistance (HOMA-IR) (p < 0.001), systolic (p < 0.001) and diastolic (p < 0.001) BP and HR (p < 0.001) in the PCOS group were significantly higher than in the control group. However, the VD levels of the PCOS group were lower than the control group (p = 0.004). N1 had a strong positive correlation with BMI, HOMA-IR, hs-CRP, luteinizing hormone, systolic and diastolic BP, and HR. VD levels were negatively correlated with HOMA-IR and luteinizing hormone. Elevated N1 and decreased VD levels may be related to the presence of high-normal BP or hypertension in PCOS subjects. N1 level may be associated with an increased BP due to its relation to inflammation and IR.
KEYWORDS: Nesfatin-1, vitamin D, polycystic ovary syndrome, insulin resistance, blood pressure, heart rate
INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common causes of female infertility. It affects nearly 5–10% of young women. It is characterized by the presence of hirsutism, acne, anovulation, hyperandrogenism, polycystic ovaries, and infertility [1]. Many mechanisms have been reported to be responsible for the pathophysiology of PCOS. The condition is thought to be determined by complex interactions between the hypothalamic-pituitary-ovarian or hypothalamic-pituitary-adrenal axis functions and metabolic disorders, such as obesity, insulin resistance (IR), and compensatory hyperinsulinemia [2]. PCOS increases the prevalence of hyperglycemia, hypertension, and dyslipidemia [3], increasing thus the risk of developing cardiovascular diseases [4].
Nesfatin-1 (N1) is derived from nucleobindin 2 (NUCB2), which is encoded by the NUCB2 gene. It is a newly identified peptide that has 82 amino acids [5]. It is released from several tissues including forebrain, hindbrain, brainstem, spinal cord, adipose tissues [6]. It has an anorexigenic effect and plays an important role in hypothalamic pathways such as regulating food intake, energy homeostasis, water intake, and body temperature [7, 8]. In addition, it exerts cardiovascular and hypertensive effects [9]. It is closely related to glucose, insulin metabolism, and IR [5, 10]. Several studies have demonstrated N1 to be associated with body mass index (BMI), IR, inflammatory stimulation in diabetes, hypertension, and PCOS [9-11]. It may also affect the control of the reproduction system, e.g. puberty onset and gonadotropin secretion [12].
Vitamin D (VD) is an important vitamin for calcium metabolism and bone structure formation. A VD deficiency is frequently observed in many countries, including Turkey [13]. The defect in calcium metabolism and the production of proinflammatory cytokines during a VD deficiency are blamed for the development of many diseases. A VD deficiency has been reported to play a role in the development of diabetes, cancer, hypertension, and atherosclerosis [14, 15]. Some studies have also reported low VD levels in POCS patients [16].
In this study, we aimed to investigate whether the N1 and VD levels, systolic and diastolic blood pressure (BP), and heart rate (HR) in PCOS patients differ from those measured in controls. Additionally, we aimed to investigate whether N1 and VD levels affect HR and systolic and diastolic BP.
MATERIALS AND METHODS
Study population
In this study, we included 54 patients with PCOS and 48 age-body mass index (BMI)-matched healthy controls. This study was performed in the period of January 2014 to March 2014. The subjects included in the patient group were diagnosed according to PCOS diagnosis criteria. They were selected from the patients referring to the Endocrinology clinic and Clinic of obstetrics and gynecology at our hospital. The subjects included in the control group were selected from the patients visiting obstetrics and gynecology clinics, presenting with non-specific complaints, having no pathology according to the physical examination and laboratory findings. Informed consent was obtained from each patient before the examination, and the study was approved by the university’s ethical committee. The gathered demographic information included the presenting complaint, age (years), age of menarche, last menstrual date, gravidity (number), parity (number), abortions (number), number of living children, and menstrual cycle regularity (number of days between cycles/number of days of menstrual bleeding/total amount of bleeding in number of pads per cycle).
Inclusion criteria
Menstrual cycle days were determined by obtaining the patient’s medical history. Transvaginal and/or transabdominal ultrasonography was performed in all subjects. Uterus size (mm), myometrial structure, endometrial thickness (mm), ovary size (mm), the number of follicles (number) as well as their diameters (mm) were determined. PCOS was diagnosed according to the criteria defined by the Rotterdam European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine-sponsored PCOS Consensus Workshop Group [17]. The revised criteria for PCOS diagnosis were as follows, with at least two of the following being required:
-
(1)
Oligo-ovulation and/or anovulation, defined by the presence of oligomenorrhea or amenorrhea, confirmed by luteal progesterone and normal serum follicle stimulating hormone (FSH) levels (1.0–10.0 IU/L).
-
(2)
Clinical hyperandrogenism, which was defined as the presence of at least one of the following three features: hirsutism, acne, and androgenic alopecia. Biochemical hyperandrogenism was defined as a serum testosterone (T) level >60 ng/dL (>2.08 nmol/L).
-
(3)
At least one ovary examined by ultrasound containing 12 or more follicles measuring 2–9 mm in diameter and/or increased ovarian volume (>10 mL).
After performing general physical and gynecological examination, the Ferriman–Gallwey scores (FGS) (points), height (cm), weight (kg), and waist circumference (WC) (cm) were measured. BMI was calculated according to the formula: BMI=body weight (kg)/square height (m2). Overweight or obesity in adults was defined by the World Health Organization (WHO) [18] as BMI >25 kg/m² for overweight and BMI>30 kg/m² for obese. The FGS system was used to assess hair growth in 11 areas of the body. The absence of terminal hair growth was scored as 0 and maximal growth as 4+. A total score of 6 or higher was defined as hirsutism.
Exclusion criteria
Exclusion criteria included the following: pregnancy, any endocrine disorder such as Cushing’s syndrome, 21-hydroxylase deficiency, congenital adrenal hyperplasia, thyroid dysfunction, hyperprolactinemia, diabetes, and a history of gestational diabetes. Subjects with chronic diseases such as cardiovascular, hepatic, hematologic, chronic renal failure, hypertension, and cancer were also excluded from the study. Women who used oral contraceptives, antiandrogenics, glucocorticoids, antihypertensives, antidiabetics and anti-obesity drugs as well as the cigarettes, alcohol, and calcium supplements were excluded from the study.
Control group
The healthy control group consisted of healthy subjects who had a hirsutism score <6, regular menstrual cycle every 21–35 days, normal androgen levels and negative history for chronic diseases. According to the ultrasound assessment, none of the women in the control group had polycystic ovaries. They were not smokers or alcohol users and did not take the calcium supplements.
Clinical, biochemical, and hormonal measurements
Morning venous blood samples were obtained between 9 and 10 am (after an 8–12h-long overnight fast), between the 3rd and 5th day of a spontaneous or progesterone-induced menstrual cycle.
The levels of fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL), and low-density lipoprotein cholesterol (LDL) were measured using the photometric assays of the Abbott Architect C16000 analyzer (Abbott Diagnostics, USA). Serum levels of fasting serum insulin (FSI), FSH, luteinizing hormone (LH), prolactin (PRL), dehydroepiandrosterone sulfate (DHEAS), total testosterone (TT), 25-hydroxyvitamin D, parathyroid hormone (PTH), and thyroid stimulating hormone (TSH) were measured using the chemiluminescent microparticle enzyme immunoassay (CMIA) method via Abbott Architect i2000 (Abbott Diagnostic, USA). Serum 17-hydroxyprogesterone (17-OHP) and free testosterone (FT) were measured by radioimmunoassay method. The concentration of hs-CRP was measured using the immunoturbidimetric method by Abbott Architect C16000 autoanalyser (Abbott Diagnostic, USA). The cut-off value for hs-CRP was < 0.5 mg/dL.
All the patients underwent a 75g oral glucose tolerance test (OGTT). If a patient was diagnosed with diabetes, she was excluded from this study. The Homeostasis Model Assessment as an index of insulin resistance (HOMA-IR) was calculated by the following formula [19]: HOMA-IR = FPG (mmol/L) × FSI (mU/mL)/22.5.
Measurement of N1
The concentration of N1 was measured using the enzyme-linked immunosorbent assay (ELISA) method. We used the commercially available human N1 ELISA kit (USCN Life Science Inc., China). The procedure for the ELISA method was performed according to the instructions provided by the manufacturer. Absorbance was measured at a wavelength of 450 ηm using the ELISA reader. The N1 levels were presented as pg/mL. The intra-assay and inter-assay coefficients of variation were <10% and <12%, respectively. The limit of detection (LOD) for the nesfatin-1 assay was 0.25 ng/mL.
BP measurement
The BP measurement using a sphygmomanometer was taken on the left upper arm after at least 15 minutes of rest. Two measurements were taken at a 5-minute interval. The mean of these two measurements was recorded.
Calculation of HR
An electrocardiogram with 12 derivations and 3 channels was taken from all PCOS patients and the controls at rest using a Nihon Kohden electrocardiogram device (with an amplitude of 1 mV/cm and a speed of 25 mm/sn). The patients were not allowed to talk during the measurement. After confirming a normal sinus rhythm, the numbers of small squares between two R waves were calculated. Then, HR was calculated according to the formula 1500/small square.
Statistical analysis
Data were analyzed using SPSS Software version 18 (IBM Inc., Chicago, IL, USA). Results were expressed as mean ± SD. The independent sample t-test for normal distribution data and Mann–Whitney U test for abnormal distribution data were used to compare the continuous variables, while the Chi-square test was used to compare categorical variables such as FGS. Pearson’s correlation test was used to calculate the associations between variables. A two-way Anova test was used for subgroup analysis. A p value of less than 0.05 was considered statistically significant.
RESULTS
Baseline clinical and laboratory characteristics
The mean age of patients in the PCOS group was 22.2±4.2 years, BMI was 30.0±7.5 kg/m2 and WC was 95.5±16.2 cm. The mean age of the subjects in the control group was 21.5±4.5 years, BMI was 29.7±5.6 kg/m2 and WC was 92.6±12.1; the difference between the two groups according to age, BMI and WC was not statistically significant. Systolic (126.0±12.4 mmHg) and diastolic (76.8±12.4 mmHg) BP, HR (80.3±8.7 beats/minute), and FGS (13.5±4.0) levels in the PCOS group were significantly higher than the systolic (112.7±9.9 mmHg, p < 0.001) and diastolic (67.9±6.6 mmHg, p < 0.001) BP, HR (74.2±8.1 beats/minute, p < 0.001), and FGS (4.2±1.0, p < 0.001) levels of the control group. The anthropometric measures, as well as the systolic and diastolic BP and HR in both groups are shown in Table 1.
TABLE 1.
The main demographic characteristics, BP, HR and biochemical parameters for the 2 groups
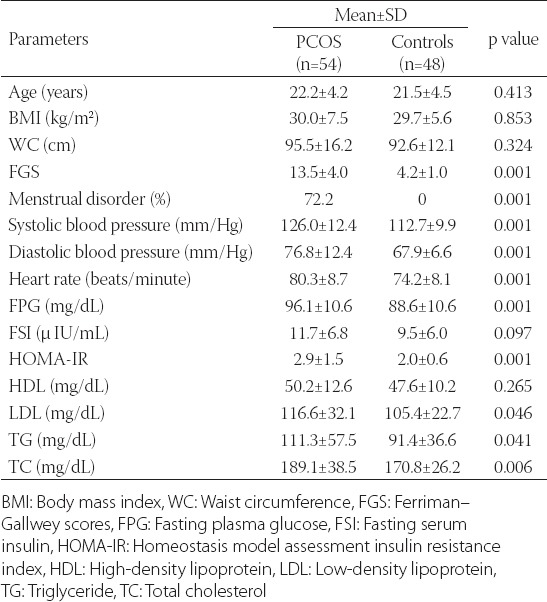
The FPG (96.1±10.6 mg/dL), HOMA-IR (2.9±1.5), N1 (10.2±5.0 ng/mL), TT (1.0±0.3 ng/mL), FT (3.4±2.0 pg/mL), LH (6.1±3.0 mIU/mL) and hs-CRP (0.5±0.9 mg/dL) levels in the PCOS group were significantly higher than the FPG (88.6±10.6 mg/dL, p < 0.001), HOMA-IR (2.0±0.6, p < 0.001), N1 (6.5±2.9 ng/mL, p < 0.001), TT (0.9±0.2 ng/mL, p = 0.028), FT (2.6±1.1 pg/mL, p = 0.017), LH (4.6±1.7 mIU/mL, p = 0.003) and hs-CRP (0.2±0.3 mg/dL, p = 0.036) levels in the control group. However, the VD levels (11.2±3.6 ng/mL) in the PCOS group were significantly lower than those in the control group (14.4±6.3 ng/mL, p = 0.004). All the biochemical and hormonal results are shown in Tables 1 and 2.
TABLE 2.
Results of the hormonal parameters and hs-CRP for the PCOS and control group

Relationship between systolic and diastolic BP and HR with clinical and laboratory parameters
HR had no positive or negative associations with age, BMI, WC, HDL, LDL, TG, TC, FT, 17OHP, DHEAS, estradiol (E2), FSH, PRL, TSH, VD, and PTH (all p values > 0.05). HR had a positive association with FSI (r = 0.209, p = 0.038), TT (r = 0.260, p = 0.009), and LH (r = 0.234, p = 0.020). HR had also a strong positive correlation with FPG, IR, N1, TT, LH, and hs-CRP (Table 3).
TABLE 3.
Pearson correlation coefficients (r) between systolic and diastolic BP, HR and measured parameters in PCOS subjects
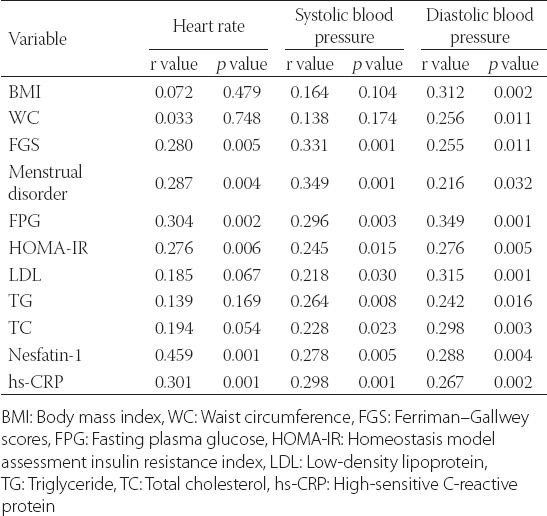
There was no association between systolic BP and age, BMI, WC, FSI, HDL, TT, FT, 17-OHP, DHEAS, E2, FSH, TSH, VD, and PTH (all p values > 0.05). There was a positive relationship between systolic BP and PRL (r = 0.207, p = 0.040). A positive association was also found between systolic BP and FPG, IR, N1, hs-CRP, LDL, and TG (Table 3). Diastolic BP had no significant relation with age, FSI, HDL, TT, FT, 17-OHP, DHEAS, E2, FSH, PRL, TSH, VD, and PTH (all p values > 0.05). There was a strong positive correlation between diastolic BP and BMI, WC, FPG, IR, LDL, TG, N1 and hs-CRP (Table 3).
Relationship between N1, VD, and Homa-IR with clinical and laboratory parameters
There was no correlation between N1 and HDL, 17OHP, DHEAS, E2, FSH, PRL, TSH, VD, and PTH (all p values > 0.05). There was a positive correlation between N1 and TT (r = 0.212, p = 0.035), LDL (r = 0.226, p = 0.024), TG (r = 0.217, p = 0.031), and TC (r = 0.119, p = 0.048). We found a strong positive association between N1 and BMI, WC, FPG, IR, TT, FT, LH, and hs-CRP (Table 4).
TABLE 4.
Pearson correlation coefficients (r) between nesfatin-1, Homa-IR, vitamin D and measured parameters in PCOS subjects
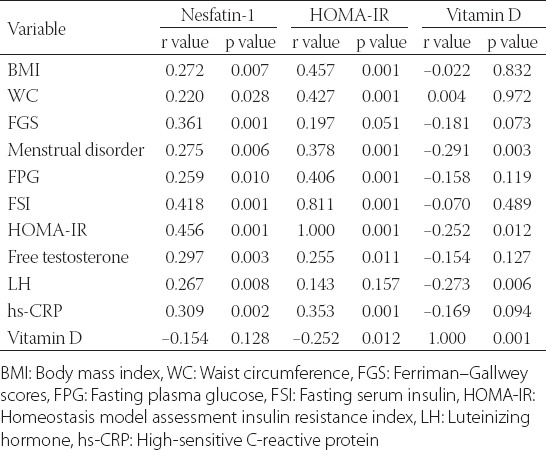
There was no significant relation between HOMA-IR and age, FGS, LDL, TG, TC, TT, 17-OHP, DHEAS, FSH, LH, PRL, and TSH (all p values > 0.05). There was a positive relationship between HOMA-IR and E2 (r = 0.234, p = 0.020). There was a negative correlation between HOMA-IR and HDL(r = -0.367, p = 0.001). We found a strong positive association between HOMA-IR and BMI, WC, menstrual disorder, FPG, FSI, FT, hs-CRP, while the association between HOMA-IR and VD was significantly negative (Table 4).
There were no positive or negative correlations between VD and age, BMI, WC, FGS, FPG, FSI, HDL, LDL, TG, TC, TT, FT, DHEAS, E2, FSH, PRL, TSH and hs-CRP (all p values > 0.05). There was a negative relationship between VD and PTH (r = -0.267, p = 0.008) and 17-OHP (r = -216, p = 0.032).
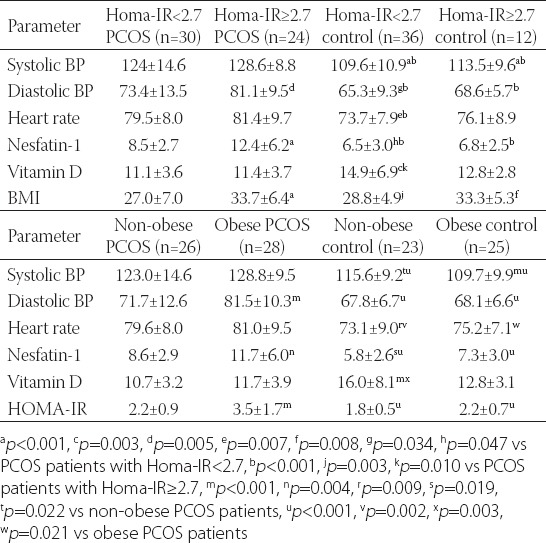
Subgroup analysis according to HOMA-IR and BMI
The cut-off level of HOMA-IR was 2.7. The subgroup analysis according to IR revealed that while the systolic and diastolic BP, HR, and N1 levels in PCOS patients with HOMA-IR <2.7 and HOMA-IR ≥2.7 were higher than in the control group, VD levels were lower than those in the control group. Systolic and diastolic BP, HR, and N1 levels of PCOS patients with HOMA-IR 2.7 or more were higher than those measured in PCOS patients with HOMA-IR lower than 2.7.
The subgroup analysis according to a BMI cut-off level of 30 kg/m2 revealed that the systolic and diastolic BP, HR, and N1 levels in both obese and non-obese PCOS patients were higher than those in the control group, while the VD levels of both obese and non-obese PCOS patients were lower than those measured in the healthy subjects. Systolic and diastolic BP, HR, and N1 levels in PCOS patients with a BMI > 30 kg/m2 were higher than those in non-obese PCOS patients. The results of the subgroup analysis are shown in Table 5.
TABLE 5.
Subgroup analysis of PCOS group and the control group defined by HOMA-IR and BMI
DISCUSSION
We found the systolic BP, diastolic BP, HR, N1, hs-CRP, FPG, HOMA-IR, TC, LH, and TT levels of PCOS patients to be higher than those measured in the age-BMI-matched healthy controls. However, the PCOS group’s VD levels were very low. Systolic and diastolic BP and HR had a strong correlation with N1, hs-CRP, and HOMA-IR. N1 had a positive correlation with BMI, WC, HOMA-IR, hs-CRP, LH as well as with the lipid panels. Decreased VD had a strong correlation with HOMA-IR and LH. The subgroup analysis showed that PCOS patients with IR had extremely higher systolic and diastolic BP, HR, and N1 levels than the other three groups. After subdivision of the groups according to obesity, the VD levels in non-obese healthy controls were obviously higher than those measured in the other three groups.
Endothelial injury that leads to the development of hypertension occurs secondary to the increased production of proinflammatory cytokines and oxidative stress in PCOS patients [20, 21]. It is known that hyperglycemia, IR, and hyperlipidemia lead to endothelial dysfunction and play a role in the development of atherosclerosis and hypertension [22]. In this study, we found that the IR, FPG, LDL and TG levels in PCOS patients are significantly higher than those in the healthy controls. The relationship between IR-induced endothelial dysfunction, hypertension and PCOS is well-known [23, 3]. In previous studies, the systolic and diastolic BP levels as well as the HR in PCOS patients have been found to be significantly higher than in healthy controls [24, 25]. In our study, the systolic and diastolic BP levels in PCOS patients, especially in those who were obese and who had high IR, were higher than in the control group.
N1 is an adipocytokine that plays a role in regulating appetite, having been reported in correlation with the low BMI levels [26, 27]. It has been shown to stimulate glycose-dependent insulin secretion [28]. Low N1 levels have been reported to be responsible for IR and metabolic syndrome [29]. However, in the organism, the majority of hormones are in equilibrium. Like other hormones, both low and high levels of N1 may cause various pathologies. In a study conducted on PCOS subjects, their N1 levels were found to be higher than in the controls, and the authors reported that N1 had a positive correlation with BMI and IR [11]. N1 levels were also found to be elevated in diabetes patients and a correlation was found between N1 and IR [30]. However, the authors underlined that the role of N1 in the pathogenesis of IR had not yet been well understood [30]. Elevated production of N1 in obese individuals and patients with IR may be a defensive mechanism of the organism against hyperglycemia and obesity. High N1 levels as well as its low levels may be dangerous. In our study, we found the BMI, FPG, HOMA-IR and lipid panel levels to have a strong positive correlation with an increased level of N1, especially in those with obesity and IR.
N1 has been reported to have an anti-inflammatory effect [31]. However, it is known that cytokines stimulate the production of N1 from adipose tissue [32]. A strong correlation has been reported between N1 and pro-inflammatory cytokines [32, 33]. There are studies reporting the association between the low N1 levels and the elevated systolic and diastolic BP [34]. Although basal N1 levels have an anti-inflammatory effect, its low-level increases inflammation, possibly leading to the development of hypertension. However, high N1 levels may also lead to hypertension by increasing inflammation and endothelial dysfunction [32, 33]. N1 has been reported to play a role in the development of hypertension, especially in obese subjects [35]. N1 has also been reported to have a hypertensive effect due to its central interaction with oxytocin receptors [9]. In our study, N1 was found to have a strong correlation with systolic and diastolic BP.
HR has been reported to be an independent predictive factor for cardiovascular mortality [24, 36]. An increase in HR of 5 beats/minute has been found to increase cardiac mortality by 17%, while the hypertensive subjects with a HR greater than 70 bpm have a threefold higher cardiovascular mortality risk [37]. In our study, the HR in PCOS patients was obviously higher than in the control group. We found a strong relationship between N1 and HR. Elevated HR in PCOS patients may be associated with IR, hyperglycemia, and hyperandrogenism, which may increase the risk of cardiovascular mortality in these patients.
It is known that VD levels lower than 20 ng/mL can cause cardiac diseases [38]. A VD deficiency leads to hypertension through many mechanisms, including an activated renin-angiotensin system, impaired glycemic control and hyperinsulinemia, and elevated inflammatory cytokines [14]. Previous studies conducted on PCOS subjects have reported the correlation between the decreased VD levels and both IR and increased LH [16]. We have found that the VD levels in PCOS patients are significantly lower than those measured in the healthy controls. Moreover, the VD levels had a negative correlation with the LH level and IR. However, the VD levels had no relationship with systolic and diastolic BP and HR. In our country, the VD levels are frequently lower than in many other countries [13, 14]; therefore, subjects in both the PCOS and control groups had in general low VD levels. Although the BP in the PCOS group was higher than in the control group, it was classified as being at a high-normal level. Low VD levels may lead both to the LH surges and to an increase in IR. In addition, it causes activation of renin-angiotensin system and greater cytokine production. Bearing in mind all these mechanisms, low VD may induce both PCOS and hypertension in PCOS patients. Additionally, we found a strong association between menstrual disorder and VD.
CONCLUSION
In conclusion, both high N1 and low VD levels may induce hyperglycemia, IR, hyperandrogenism, LH peaks as well as the chronic inflammation, playing thus a crucial role in the pathogenesis of hypertension in PCOS patients.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. doi: 10.1038/nrendo.2010.217. http://dx.doi.org/10.1038/nrendo.2010.217 . [DOI] [PubMed] [Google Scholar]
- [2].Sahin FK, Sahin SB, Balik G, Ural UM, Tekin YB, Cure MC, et al. Does low pentraxin-3 levels associate with polycystic ovary syndrome and obesity? Int J Clin Exp Med. 2014;7(10):3512–3519. [PMC free article] [PubMed] [Google Scholar]
- [3].Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038–2049. doi: 10.1210/jc.2009-2724. http://dx.doi.org/10.1210/jc.2009-2724 . [DOI] [PubMed] [Google Scholar]
- [4].Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. http://dx.doi.org/10.1210/er.2011-1034 . http://dx.doi.org/10.1210/er.2011-1034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443(7112):709–712. doi: 10.1038/nature05162. http://dx.doi.org/10.1038/nature05162 . [DOI] [PubMed] [Google Scholar]
- [6].Stengel A, Mori M, Tache Y. The role of nesfatin-1 in the regulation of food intake and body weight: recent developments and future endeavors. Obes Rev. 2013;14(11):859–870. doi: 10.1111/obr.12063. http://dx.doi.org/10.1111/obr.12063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yosten GL, Redlinger L, Samson WK. Evidence for a role of endogenous nesfatin-1 in the control of water drinking. J Neuroendocrinol. 2012;24(7):1078–1084. doi: 10.1111/j.1365-2826.2012.02304.x. http://dx.doi.org/10.1111/j.1365-2826.2012.02304.x . [DOI] [PubMed] [Google Scholar]
- [8].Könczöl K, Pinter O, Ferenczi S, Varga J, Kovacs K, Palkovits M, et al. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int J Obes (Lond) 2005;36(12):1514–1521. doi: 10.1038/ijo.2012.2. http://dx.doi.org/10.1038/ijo.2012.2 . [DOI] [PubMed] [Google Scholar]
- [9].Yosten GL, Samson WK. The anorexogenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):1642–1647. doi: 10.1152/ajpregu.00804.2009. http://dx.doi.org/10.1152/ajpregu.00804.2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: antihyperglycemia. Biochem Biophys Res Commun. 2010;391(1):1039–1042. doi: 10.1016/j.bbrc.2009.12.014. http://dx.doi.org/10.1016/j.bbrc.2009.12.014 . [DOI] [PubMed] [Google Scholar]
- [11].Ademoglu EN, Gorar S, Carlıoglu A, Yazıcı H, Dellal FD, Berberoglu Z, et al. Plasma nesfatin-1 levels are increased in patients with polycystic ovary syndrome. J Endocrinol Invest. 2014;37(8):715–719. doi: 10.1007/s40618-014-0089-2. http://dx.doi.org/10.1007/s40618-014-0089-2 . [DOI] [PubMed] [Google Scholar]
- [12].Garcia-Galiano D, Navarro VM, Roa J, Ruiz-Pino F, Sanchez-Garrido MA, Pineda R, et al. The anorexogenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30(23):7783–7792. doi: 10.1523/JNEUROSCI.5828-09.2010. http://dx.doi.org/10.1523/JNEUROSCI.5828-09.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kirbas A, Kirbas S, Anlar O, Turkyilmaz AK, Cure MC, Efe H. Investigation of the relationship between vitamin D and bone mineral density in newly diagnosed multiple sclerosis. Acta Neurol Belg. 2013;113(1):43–47. doi: 10.1007/s13760-012-0123-0. http://dx.doi.org/10.1007/s13760-012-0123-0 . [DOI] [PubMed] [Google Scholar]
- [14].Cumhur Cure M, Cure E, Yuce S, Yazici T, Karakoyun I, Efe H. Mean platelet volume and vitamin D level. Ann Lab Med. 2014;34(2):98–103. doi: 10.3343/alm.2014.34.2.98. http://dx.doi.org/10.3343/alm.2014.34.2.98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Luo C, Wong J, Brown M, Hooper M, Molyneaux L, Yue DK. Hypovitaminosis D in Chinese type 2 diabetes: lack of impact on clinical metabolic status and biomarkers of cellular inflammation. Diab Vasc Dis Res. 2009;6(3):194–199. doi: 10.1177/1479164109337974. http://dx.doi.org/10.1177/1479164109337974 . [DOI] [PubMed] [Google Scholar]
- [16].Kozakowski J, Kapuscinska R, Zgliczynski W. Associations of vitamin D concentration with metabolic and hormonal indices in women with polycystic ovary syndrome presenting abdominal and gynoidal type of obesity. Ginekol Pol. 2014;85(10):765–770. [PubMed] [Google Scholar]
- [17].ESHRE/ASRM. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome. Fertil and Steril. 2004;8:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [18].Rahman M, Berenson AB. Accuracy of current body mass index obesity classification for white, black, and Hispanic reproductive-age women. Obstet Gynecol. 2010;115(5):982–988. doi: 10.1097/AOG.0b013e3181da9423. http://dx.doi.org/10.1097/AOG.0b013e3181da9423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. http://dx.doi.org/10.1007/BF00280883 . [DOI] [PubMed] [Google Scholar]
- [20].Guleria AK, Syal SK, Kapoor A, Kumar S, Tiwari P, Dabadghao P. Cardiovascular disease risk in young Indian women with polycystic ovary syndrome. Gynecol Endocrinol. 2014;30(1):26–29. doi: 10.3109/09513590.2013.831835. http://dx.doi.org/10.3109/09513590.2013.831835 . [DOI] [PubMed] [Google Scholar]
- [21].Bajuk Studen K, Jensterle Sever M, Pfeifer M. Cardiovascular risk and subclinical cardiovascular disease in polycystic ovary syndrome. Front Horm Res. 2013;40:64–82. doi: 10.1159/000341838. http://dx.doi.org/10.1159/000341838 . http://dx.doi.org/10.1159/000341838 . [DOI] [PubMed] [Google Scholar]
- [22].Hadi HA, Carr CS, Al-Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1(3):183–198. [PMC free article] [PubMed] [Google Scholar]
- [23].Vaneckova I, Maletinska L, Behuliak M, Nagelova V, Zicha J, Kunes J. Obesity-related hypertension: possible pathophysiological mechanisms. J Endocrinol. 2014;223(3):63–78. doi: 10.1530/JOE-14-0368. http://dx.doi.org/10.1530/JOE-14-0368 . [DOI] [PubMed] [Google Scholar]
- [24].Zachurzok-Buczynska A, Szydlowski L, Gawlik A, Wilk K, Malecka-Tendera E. Blood pressure regulation and resting heart rate abnormalities in adolescent girls with polycystic ovary syndrome. Fertil Steril. 2011;96(6):1519–1525. doi: 10.1016/j.fertnstert.2011.09.043. http://dx.doi.org/10.1016/j.fertnstert.2011.09.043 . [DOI] [PubMed] [Google Scholar]
- [25].Sahin SB, Cure MC, Ugurlu Y, Ergul E, Gur EU, Alyildiz N, et al. Epicardial adipose tissue thickness and NGAL levels in women with polycystic ovary syndrome. J Ovarian Res. 2014;7:24. doi: 10.1186/1757-2215-7-24. http://dx.doi.org/10.1186/1757-2215-7-24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tsuchiya T, Shimizu H, Yamada M, Osaki A, Oh IS, Ariyama Y, et al. Fasting concentrations of nesfatin-1 are negatively correlated with body mass index in non-obese males. Clin Endocrinol (Oxf) 2010;73(4):484–490. doi: 10.1111/j.1365-2265.2010.03835.x. http://dx.doi.org/10.1111/j.1365-2265.2010.03835.x . [DOI] [PubMed] [Google Scholar]
- [27].Shimizu H, Ohsaki A, Oh IS, Okada S, Mori M. A new anorexigenic protein, nesfatin-1. Peptides. 2009;30(5):995–998. doi: 10.1016/j.peptides.2009.01.002. http://dx.doi.org/10.1016/j.peptides.2009.01.002 . [DOI] [PubMed] [Google Scholar]
- [28].Feijoo-Bandin S, Rodriguez-Penas D, Garcia-Rua V, Mosquera-Leal A, Otero MF, Pereira E, et al. Nesfatin-1 in human and murine cardiomyocytes: synthesis, secretion, and mobilization of GLUT-4. Endocrinology. 2013;154(12):4757–4767. doi: 10.1210/en.2013-1497. http://dx.doi.org/10.1210/en.2013-1497 . [DOI] [PubMed] [Google Scholar]
- [29].Aksu O, Aydın B, Doguç DK, Ilhan I, Ozturk O, Altuntas A, et al. The evaluation of Nesfatin-1 levels in patients with OSAS associated with metabolic syndrome. J Endocrinol Invest. 2015;38(4):463–469. doi: 10.1007/s40618-014-0216-0. http://dx.doi.org/10.1007/s40618-014-0216-0 . [DOI] [PubMed] [Google Scholar]
- [30].Zhang Z, Li L, Yang M, Liu H, Boden G, Yang G. Increased plasma levels of nesfatin-1 in patients with newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2012;120(2):91–95. doi: 10.1055/s-0031-1286339. http://dx.doi.org/10.1055/s-0031-1286339 . [DOI] [PubMed] [Google Scholar]
- [31].Tang CH, Fu XJ, Xu XL, Wei XJ, Pan HS. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides. 2012;36(1):39–45. doi: 10.1016/j.peptides.2012.04.014. http://dx.doi.org/10.1016/j.peptides.2012.04.014 . [DOI] [PubMed] [Google Scholar]
- [32].Leivo-Korpela S, Lehtimaki L, Hamalainen M, Vuolteenaho K, Koobi L, Jarvenpaa R, et al. Adipokines NUCB2/nesfatin-1 and visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediators Inflamm. 2014;2014:232167. doi: 10.1155/2014/232167. http://dx.doi.org/10.1155/2014/232167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Scotece M, Conde J, Abella V, Lopez V, Lago F, Pino J, et al. NUCB2/nesfatin-1: a new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties, an in vitro study. J Orthop Res. 2014;32(5):653–660. doi: 10.1002/jor.22585. http://dx.doi.org/10.1002/jor.22585 . [DOI] [PubMed] [Google Scholar]
- [34].Abaci A, Catli G, Anik A, Kume T, Bober E. The relation of serum nesfatin-1 level with metabolic and clinical parameters in obese and healthy children. Pediatr Diabetes. 2013;14(3):189–195. doi: 10.1111/pedi.12009. http://dx.doi.org/10.1111/pedi.12009 . [DOI] [PubMed] [Google Scholar]
- [35].Zhao Y, Ma X, Wang Q, Zhou Y, Zhang Y, Wu L, et al. Nesfatin-1 correlates with hypertension in overweight or obese Han Chinese population. Clin Exp Hypertens. 2015;37(1):51–56. doi: 10.3109/10641963.2014.897722. http://dx.doi.org/10.3109/10641963.2014.897722 . [DOI] [PubMed] [Google Scholar]
- [36].Cüre E, Yüce S, Ciçek Y, Cüre MC. The effect of Gilbert’ s syndrome on the dispersions of QT interval and P-wave: an observational study. Anadolu Kardiyol Derg. 2013;13(6):559–565. doi: 10.5152/akd.2013.180. http://dx.doi.org/10.5152/akd.2013.180 . [DOI] [PubMed] [Google Scholar]
- [37].Hozawa A, Ohkubo T, Kikuya M, Ugajin T, Yamaguchi J, Asayama K, et al. Prognostic value of home heart rate for cardiovascular mortality in the general population: the Ohasama study. Am J Hypertens. 2004;17(11):1005–1010. doi: 10.1016/j.amjhyper.2004.06.019. http://dx.doi.org/10.1016/S0895-7061(04)00897-0 . [DOI] [PubMed] [Google Scholar]
- [38].Lai H, Fishman EK, Gerstenblith G, Moore R, Brinker JA, Keruly JC, et al. Vitamin D deficiency is associated with development of subclinical coronary artery disease in HIV-infected African American cocaine users with low Framingham-defined cardiovascular risk. Vasc Health Risk Manag. 2013;9:729–737. doi: 10.2147/VHRM.S50537. http://dx.doi.org/10.2147/VHRM.S50537 . [DOI] [PMC free article] [PubMed] [Google Scholar]


