Abstract
Atypical antipsychotics have been used to treat fear and anxiety disturbance that are highly common in schizophrenic patients. It is suggested that disruptions of N-methyl-d-aspartate (NMDA)-mediated transmission of glutamate may underlie the pathophysiology of schizophrenia. The present study was conducted to analyze the effectiveness of clozapine on the anxiety-related behavior and locomotor function of the adult brain, which had previously undergone NMDA receptor blockade during a developmental period. In order to block the NMDA receptor, male mice were administered 0.25 mg/kg of MK-801 on days 7 to 10 postnatal. In adulthood, they were administered intraperitoneally 0.5 mg/kg of clozapine and tested with open-field and elevated plus maze test, to assess their emotional behavior and locomotor activity. In the group receiving MK-801 in the early developmental period the elevated plus maze test revealed a reduction in the anxiety-related behavior (p<0.05), while the open-field test indicated a decrease in locomotor activity (p<0.01). Despite these reductions, clozapine could not reverse the NMDA receptor blockade. Also, as an atypical antipsychotic agent, clozapine could not reverse impairment in the locomotor activity and anxiety-related behavior, induced by administration of the MK-801 in neonatal period.
KEYWORDS: MK-801, clozapine, open field test, elevated plus maze test, neonatal mice
INTRODUCTION
The critical period of brain development is characterized by a number of rapid and fundamental changes. This period particularly comprises changes such as axonal growth, dendritic cell maturation, neuronal network construction, formation of novel synapses, glia-cell enlargement, and myelination. In this period, motor and emotional skills are acquired, spontaneous motor behavior reach a peak, and neurotransmitter system evolve through quantitative and qualitative changes. For instance, glutamatergic system changes, and number of NMDA receptors increases in this period. Due to the association of a number of behavioral features and cognitive functions with cholinergic and glutamatergic transmitter systems, the changes in these periods are of critical importance. Onset and duration of critical period of the brain development in mammals vary across the species. This critical period begins in the third trimester of pregnancy, and lasts for the first two years of life in humans. In rodents, such as mice and rats, lasts for the first 3 to 4 weeks of life and reaches a peak on day 10 postpartum [1, 2]. NMDA-mediated transmission of the excitatory neurotransmitter glutamate within the brain is of major importance in the process related to cognitive functions and emotional responses [3]. Some studies on the NMDA receptor antagonist during the early brain development period reported that the chronic blockade of NMDA receptor affected the emotional behaviors and cognitive functions in adults [3, 4]. It is also suggested that the disruptions of NMDA-mediated transmission of glutamate may underlie schizophrenia pathophysiology [5], and that the early postnatal stress may result in the dysfunction of the NMDA receptor [6].
Schizophrenia is a severe mental illness, quite difficult to treat by pharmacotherapy. Changes in neurochemical transmission within the dopaminergic system, especially through dopamine D2 receptors are of critical importance for both emergence of schizophrenia and its current treatment with antipsychotic drugs [7, 8]. Fear and anxiety disorders have a high rate of incidence in schizophrenic patients [9].
Atypical anti-psychotic drugs such as clozapine, olanzapine and quetiapine have been increasingly used in the treatment of anxiety-related disturbances and also in the management of schizophrenia [9]. Clozapine is an atypical antipsychotic medication which displays affinity for a number of receptors in the brain including dopamine, serotonin 5HT1A, 5HT2C, norepinephrine α1 and α2, muscarinic acetylcholine and histamine H1 receptors [8]. Clozapine was shown to play an effective role in treatment of psychotic symptoms in the majority of schizophrenic patients [10]. Previous studies have shown that clozapine possesses anxiolytic properties in certain experimental models in rodents and other species [11, 12]. In the present study, the effectivity of clozapine was investigated on the exploratory and anxiety-related behaviors in the neonatal mice exposed to MK-801, by using open-field and elevated plus maze tests.
MATERIALS AND METHODS
This study was performed following the approval obtained from Inonu University School of Medicine Ethical Board for Laboratory Animals, and the animal rights were protected as instructed by “Guidelines for the Care and Use of Experimental Animals”.
Animals
In laboratory experiments, 8- to 10-week-old and weighing approximately 31±0.2 g male Balb/c mice (n=10) and 31±0.2 g (n=30) female Balb/c mice were obtained from Inonu University Laboratory Animals Production and Research Centre. The animals were allowed free access to food and water. The animals were kept under 12-hour light/dark cycle at 21±2°C. Mice were coupled. Pregnant females were individually housed in solid plastic cages with sawdust shavings in the cage. Approximately 5-6 male mice pups were delivered by each pregnant mouse. The day of birth was accepted as postnatal day 0. The animals were not handled until the time of weaning, and the cages were cleaned once a week to minimize disruption of the mother-pups relationship. Mice pups were left with their mothers for 21 days after the birth.
Test protocol
In this study, male mice pups were randomly divided into two groups. Control group received saline, and MK-801 group received MK-801. Male mice pups were given (+)-MK-801 hydrogen maleate, whereas the control groups were given saline, from day 7 to day 10 postpartum (four days). MK-801, which is an NMDA receptor antagonist, was administered intraperitoneally twice a day (at 9:00h and 16:00h) at a dose of 0.25 mg/kg (0.1 ml/10 g body weight) for 4 days. The control groups were given intraperitoneal saline injection with the same volume as MK-801 twice a day. The mice pups were separated from their mothers 21 days after the birth, and placed into standard cages so that each cage contained 5 or 6 mice. Behavioral tests were carried out in adult (8- to 10-week-old) animals. Mice were randomly divided into four groups as follows: Saline-saline group, saline- clozapine group, MK-801-clozapine, MK-801-saline group. Thirty minutes prior to behavioral test, intraperitoneal clozapine 0.5 mg/kg was administered to some of the animals which had been exposed to MK-801 and saline in early childhood [8]. Hand and room adaptations were ensured in mice before testing the behavior. Before behavioral tests, the mice were gently handled (each mouse 1min/day, during three successive days) with both hands covered by fine latex gloves, and then they were placed into cages and transported to the testing room. Animals from each experimental group were performed open field (OF) test and elevated plus maze (EPM) test (Figure 1).
FIGURE 1.

Timeline of the experimental procedures.
Drugs
Stock solution was prepared by adjusting the volume of MK-801 (Dizocilpine hydrogen maleate, (5R, 10S)-(+)-5-Methyl-10,11-dihydro-5Hdibenzo [a,d] cyclohepten-5, 10-imine hydrogen maleate) (SIGMA-ALDRICH M107, USA) with saline up to 0.25 mg/ml, and the resulting stock solution was stored at −30°C in 1 ml Eppendorf tube. On injection days, the stock solution was reconstituted with saline prior to injection and stored at +4°C. Clozapine 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]-diazepine was supplied by SIGMA-ALDRICH C6305, USA on demand. 0.1 M HCl was added to clozapine and was dissolved in saline. Concentrated stock solution was prepared daily, and then stored at −80°C. Intraperitoneal (i.p.) clozapine (0.5 mg/kg) was administered 30 minutes before the behavioral tests.
Instruments
Open-field test
The apparatus used for the open-field test is a 60×60×24 cm open-top maze made from black plexiglass and surrounded by 1 cm thick wall. Areas of the apparatus adjacent to the walls are called peripheral squares (periphery) while the rest is called the central square. In the open-field test apparatus, peripheral squares are enclosed and these are safe locations for the animals. On the other hand, the central square is an open area where anxiety-related behavior is stimulated. Acting in their natural behavioral patterns, mice prefer the safe peripheral squares and avoid the central square where anxiety-related behavior is stimulated. Measures of anxiety-related behaviors and locomotor activity included the length of time spent in the central and periphery square, the distance traveled, and the frequency of center crossing, rearing and fecal boli [13, 14]. The behaviors of the mice were video recorded for 5 minutes followed by analysis software (Ethovision XT, Noldus, Version 4.1). Luminous intensity in the apparatus was set to 150 lx. The maze apparatus was cleaned (70% alcohol) after each trial. The scoring procedure was performed in a blinded fashion.
Elevated plus maze
The maze used in the study was made from black plexiglass in the shape of a cross and was 40 cm high above the ground. The maze had four 30-cm arms (two open and two enclosed), and the enclosed arms were surrounded by 15-cm high walls. Both the open and closed arms were combined on a platform located in the center (5×5 cm). The open arms were illuminated by 165 lx. On an elevated plus maze, the open arms are the insecure locations where anxiety-related behavior is stimulated. Acting in their natural behavioral patterns, the mice preferred the enclosed arms and avoided the open arms. Open arm activity was considered to be the indicator of anxiety-related behavior. Anxiety-related behavior was measured using the following criteria: number of entries into the open arms, number of entries into the closed arm, number of total entries to arms, time spent in open arms, time spent in closed arms, time spent in central platform, head-dipping and fecal boli [15-17]. Elevated plus maze test assesses the anxiety-related behavior in mice which is triggered by hereditary fear of heights and newly-induced. The behavior was recorded using a video camera for 5 minutes. The maze apparatus was cleaned (70% alcohol) after each trial. The parameters were measured using Ethovision Xt, Noldus, Version 4.1 software. The scoring procedure was performed in a blinded fashion.
Statistical analysis
All the data were presented as mean ± SE. Statistical analyses were performed using SPSS 11.5 software. The groups were compared using two-way analysis of variance (ANOVA), followed by Tukey’s HSD test. Non-normal distribution and homogeneous variances were tested using Kruskal-Wallis followed by post-hoc comparisons using Mann-Whitney-U test. A p value of <0.05 was considered significant.
RESULTS
Open-field test
In the terms of the distance travelled in the open-field test, ANOVA showed a significant difference in neonatal drug administration [F(1,32)=13.3 p<0.05)] and in adult drug administration [F(1,32)=19.4 p<0.05)], whereas it showed no interaction between neonatal and adult administrations. In the distance travelled, there was a significant decrease in Saline-Clozapine and MK-801-Saline groups as opposed to the Saline-Saline group (p<0.05) (Figure 2).
FIGURE 2.
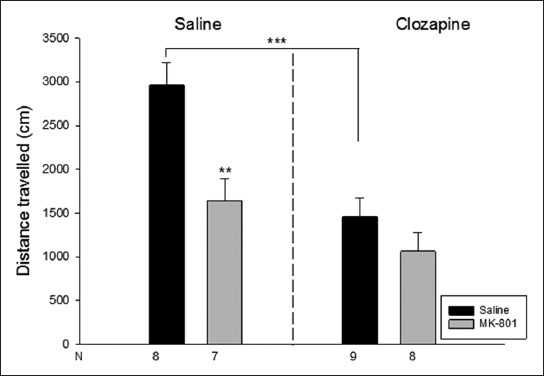
Distance travelled in the open-field test (cm). Data are expressed as mean ± SE. **p<0.01, ***p<0.001 compared to saline-saline group.
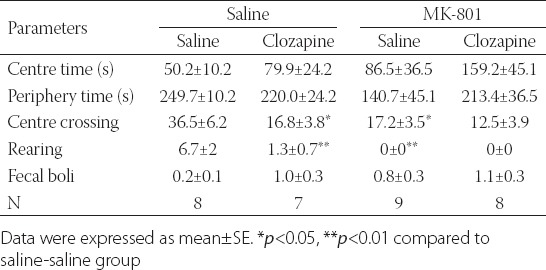
The groups showed no significant difference in terms of the length of time spent in the central and periphery square [Kruskal Wallis test, H(3, N=32)=1.8 p>0.05]. In the frequency of crossings from the periphery to the center in the open-field test, there was a significant difference in neonatal drug administration [ANOVA F(1,32)=6.6 p<0.05)] and in adult drug administration [F(1,32)=7.1 p<0.05)]; however, it no interaction was revealed between neonatal and adult drug administrations. In terms of the frequency of crossing the center, there was a significant decrease in the MK-801-Saline group as opposed to the Saline-Saline group (p<0.05) and in the Saline-Clozapine group compared to the Saline-Saline group (p<0.05). In terms of rearing frequency, the groups presented a significant difference [Kruskal Wallis test H(3, N=32)=23 p<0.05]. Also, in the frequency of rearing, significant differences were detected in the MK-801-Saline group compared to the Saline-Saline group (p<0.05) and in the MK-801-Clozapine group as opposed to the Saline-Clozapine group (p<0.05). The groups had no significant difference in fecal boli [Kruskal Wallis test H(3, N=32)=4.0 p>0.05] (Table 1).
TABLE 1.
Behaviors in the open-field test
Elevated plus maze
The groups showed significant differences both in open arm duration [Kruskal Wallis test H(3, N=30)=8.4 p<0.05] and enclosed arm duration [H(3, N=30)=7.8 p<0.05]. In open arm duration, the Saline-Clozapine and MK-801-Saline group showed a significant increase compared to the Saline-Saline group (p<0.05). In enclosed arm duration, the Saline-Clozapine group had a significant decrease compared to the Saline-Saline group (p<0.05) (Figures 3 and 4). The groups had no significant difference in central platform duration [Kruskal Wallis test H(3, N=30)=0.6 p>0.05].
FIGURE 3.
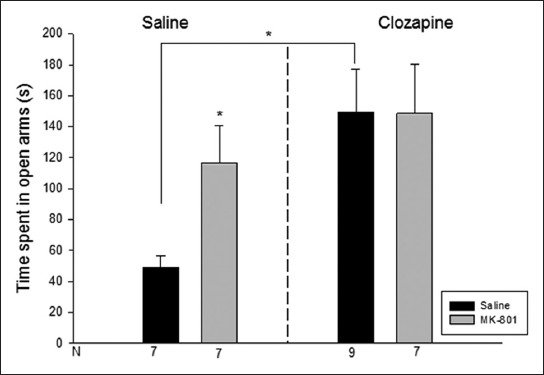
Time spent in the open arms (s) in the elevated plus maze. Data are expressed as mean ± SE. *p<0.05 compared to saline-saline group.
FIGURE 4.
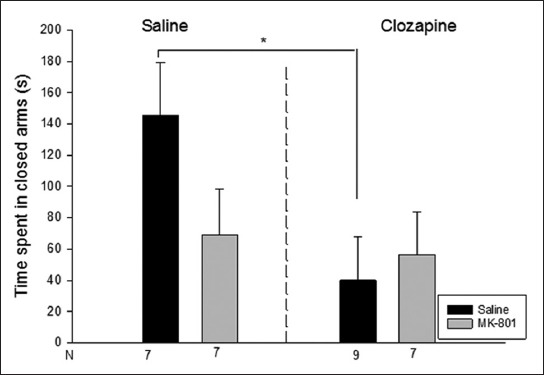
Time spent in the closed arms (s) in the elevated plus maze. Data are expressed as mean ± SE. *p<0.05 compared to saline-saline group.
In terms of the number of entries in open arm [H(3, N=30)=0.7 p>0.05], head-dipping [H(3, N=30)=3.5 p>0.05], fecal boli [H(3, N=30)=6.4 p>0.05] and number of total entries to arms [H(3, N=30)=1.1 p>0.05], the groups were not statistically different. Regarding the number of entries in closed arm the Saline-Clozapine group showed a significant decrease compared to the Saline-Saline group (p<0.05) (Table 2).
TABLE 2.
Behaviors in elevated plus maze test

DISCUSSION
MK-801 is an NMDA receptor antagonist leading to a number of cognitive disorders associated with schizophrenia. In the present study, we analyzed the effectiveness of the atypical antipsychotic clozapine on exploratory and anxiety-related behaviors in the neonatal mice administered MK-801 by using the open-field and elevated plus maze tests.
In the open-field test, a decrease was induced by the NMDA receptor blockade both in horizontal and vertical locomotor activity. It is reported that treatment with NMDA receptor antagonists during early developmental stages leads to an increase both in locomotor activity and exploratory behaviors [18-20]; however, in our study there was a decrease in the locomotor activity and exploratory behaviors. Our previous study also showed decreases in adult locomotor activity and exploratory behavior [21, 22]. Studies report that there are continuous interactions between the dopaminergic and the NMDA pathways. The increase in locomotor activity could be caused by the effects on the dopaminergic system of NMDA receptors. However, NMDA antagonists lead to alterations in other neurotransmitters including dopamine, acetylcholine and norepinephrine [23, 24]. Reduced locomotor activity may be associated with the changes in the monoaminergic (particularly in dopaminergic and serotonergic) activity following the administration of NMDA receptor antagonists during an early developmental stage. On the other hand, a recent study showed that MK-801 exposure on postnatal day 7 to 10 has no impact on locomotor activity in the open filed test in rats [25].
In the elevated plus maze test, a decrease was observed in anxiety-related behaviors caused by the NMDA receptor blockade in the early developmental period. Whether the NMDA receptor blockade during early development has an influence on anxiety-related behaviors remain a controversial issue in the literature; while some studies argue that MK-801 can increase [26] these behaviors, some others hold that MK-801 decreases [27] or even has no effect on exploratory and anxiety-related behaviors [28]. From this controversy, it can be assumed that the effectiveness of the NMDA receptor blockade during early development may be dependent on the length and timing of the NMDA receptor blockade and also on the gender and the characteristics the animals used in the experiment.
Clozapine resulted in a reduction both in vertical and horizontal locomotor activity in the open-field, and also a reduction in anxiety-related behaviors in the elevated plus maze test. The mice administered with clozapine spent more time in the open arm in the EPM. This situation may be an indicator of reduced anxiety-related behaviors. These results comply with the studies arguing that anxiety-related behaviors are reduced by the administration of clozapine [12, 29]. Consistent with some previous studies, we also found that the administration of clozapine leads to reduced locomotor activity and exploratory behaviors in the open field [7, 29].
Our findings demonstrate that neonatal NMDA receptor blockade leads to reduced exploratory and anxiety-related behaviors and these behaviors are not restored by the atypical antipsychotic clozapine. According to Scorza et al. (2010), clozapine can reverse the hyperlocomotion following the NMDA receptor blockade; however in this study only adult rodents were used for the experiment [7]. In our study, the use of MK-801 in mice led to the hypofunction of NMDA receptor 7-10 days after birth. In this period, synaptogenesis and rapid brain growth also take place, and neurotransmitter systems evolve and go through quantitative and qualitative changes. The NMDA receptor system is concerned with other neurotransmitter systems including serotonergic, dopaminergic and GABAergic [30, 31]. Dopamine is a neurotransmitter of the catecholamine and phenethylamine families that plays a number of important roles in mammalian brain, particularly in locomotor activity, cognition and emotion. In the brain, when the D2 autoreceptors are activated, the dopamine release is reduced and this leads to decreased locomotor activity; however, when the postsynaptic D2 receptors are activated, the locomotion is slightly increased [32]. The effects of clozapine on exploratory and anxiety-related behaviors in the neonatal mice administered with MK-801 may explain the potential underlying mechanisms in which dopaminergic systems probably change.
We conclude that the NMDA receptor blockade during the critical developmental period (neonatal period) leads to impairment in emotional and cognitive functions; yet, the use of clozapine could not reverse this impairment.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
ACKNOWLEDGEMENTS
This work was supported by the Scientific Research Office of Inonu University (I.U BAP) (Project no: 2011/83).
REFERENCES
- [1].Viberg H, Ponten E, Eriksson P, Gordh T, Fredriksson A. Neonatal ketamine exposure results in changes in biochemical substrates of neuronal growth and synaptogenesis, and alters adult behavior irreversibly. Toxicology. 2008;249:153–9. doi: 10.1016/j.tox.2008.04.019. http://dx.doi.org/10.1016/j.tox.2008.04.019 . [DOI] [PubMed] [Google Scholar]
- [2].Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. http://dx.doi.org/10.1016/S0149-7634(03)00005-8 . [DOI] [PubMed] [Google Scholar]
- [3].Brooks WJ, Weeks AC, Leboutillier JC, Petit TL. Altered NMDA sensitivity and learning following chronic developmental NMDA antagonism. Physiol Behav. 1997;62:955–62. doi: 10.1016/s0031-9384(97)00169-8. http://dx.doi.org/10.1016/S0031-9384(97)00169-8 . [DOI] [PubMed] [Google Scholar]
- [4].Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153:367–76. doi: 10.1016/j.bbr.2003.12.026. http://dx.doi.org/10.1016/j.bbr.2003.12.026 . [DOI] [PubMed] [Google Scholar]
- [5].Guo C, Yang Y, Su Y, Si T. Postnatal BDNF expression profiles in prefrontal cortex and hippocampus of a rat schizophrenia model induced by MK-801 administration. J Biomed Biotechnol. 2010;2010:783297. doi: 10.1155/2010/783297. http://dx.doi.org/10.1155/2010/783297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Melik E, Babar E, Kocahan S, Guven M, Akillioglu K. Enriched environment has limited capacity for the correction of hippocampal memory-dependent schizoid behaviors in rats with early postnatal NMDAR dysfunction. Int J Dev Neurosci. 2014;33:22–8. doi: 10.1016/j.ijdevneu.2013.10.004. http://dx.doi.org/10.1016/j.ijdevneu.2013.10.004 . [DOI] [PubMed] [Google Scholar]
- [7].Scorza MC, Castane A, Bortolozzi A, Artigas F. Clozapine does not require 5-HT1A receptors to block the locomotor hyperactivity induced by MK-801 Clz and MK-801 in KO1A mice. Neuropharmacology. 2010;59:112–20. doi: 10.1016/j.neuropharm.2010.04.012. http://dx.doi.org/10.1016/j.neuropharm.2010.04.012 . [DOI] [PubMed] [Google Scholar]
- [8].Mutlu O, Ulak G, Celikyurt IK, Akar FY, Erden F. Effects of olanzapine, sertindole and clozapine on learning and memory in the Morris water maze test in naive and MK-801-treated mice. Pharmacol Biochem Behav. 2011;98:398–404. doi: 10.1016/j.pbb.2011.02.009. http://dx.doi.org/10.1016/j.pbb.2011.02.009 . [DOI] [PubMed] [Google Scholar]
- [9].Mead A, Li M, Kapur S. Clozapine and olanzapine exhibit an intrinsic anxiolytic property in two conditioned fear paradigms: contrast with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav. 2008;90:551–62. doi: 10.1016/j.pbb.2008.04.014. http://dx.doi.org/10.1016/j.pbb.2008.04.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ertugrul A, Ozdemir H, Vural A, Dalkara T, Meltzer HY, Saka E. The influence of N-desmethylclozapine and clozapine on recognition memory and BDNF expression in hippocampus. Brain Res Bull. 2011;84:144–50. doi: 10.1016/j.brainresbull.2010.11.014. http://dx.doi.org/10.1016/j.brainresbull.2010.11.014 . [DOI] [PubMed] [Google Scholar]
- [11].Wiley JL, Compton AD, Porter JH. Effects of four antipsychotics on punished responding in rats. Pharmacol Biochem Behav. 1993;45:263–7. doi: 10.1016/0091-3057(93)90237-n. http://dx.doi.org/10.1016/0091-3057(93)90237-N . [DOI] [PubMed] [Google Scholar]
- [12].Millan MJ, Schreiber R, Monneyron S, Denorme B, Melon C, Queriaux S, et al. S-16924, a novel, potential antipsychotic with marked serotonin1A agonist properties IV. A drug discrimination comparison with clozapine. J Pharmacol Exp Ther. 1999;289:427–36. [PubMed] [Google Scholar]
- [13].Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European journal of pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. http://dx.doi.org/10.1016/S0014-2999(03)01272-X . [DOI] [PubMed] [Google Scholar]
- [14].Sousa N, Almeida OF, Wotjak CT. A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes, brain, and behavior. 2006;5(Suppl2):5–24. doi: 10.1111/j.1601-183X.2006.00228.x. http://dx.doi.org/10.1111/j.1601-183X.2006.00228.x . [DOI] [PubMed] [Google Scholar]
- [15].Korte SM, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. European journal of pharmacology. 2003;463:163–75. doi: 10.1016/s0014-2999(03)01279-2. http://dx.doi.org/10.1016/S0014-2999(03)01279-2 . [DOI] [PubMed] [Google Scholar]
- [16].Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of neuroscience methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. http://dx.doi.org/10.1016/0165-0270(85)90031-7 . [DOI] [PubMed] [Google Scholar]
- [17].Cole BJ, Hillmann M, Seidelmann D, Klewer M, Jones GH. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology. 1995;121:118–26. doi: 10.1007/BF02245598. http://dx.doi.org/10.1007/BF02245598 . [DOI] [PubMed] [Google Scholar]
- [18].Martinez G, Ropero C, Funes A, Flores E, Blotta C, Landa AI, et al. Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiology & behavior. 2002;76:219–24. doi: 10.1016/s0031-9384(02)00704-7. http://dx.doi.org/10.1016/S0031-9384(02)00704-7 . [DOI] [PubMed] [Google Scholar]
- [19].Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ. Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. The European journal of neuroscience. 2003;18:1706–10. doi: 10.1046/j.1460-9568.2003.02902.x. http://dx.doi.org/10.1046/j.1460-9568.2003.02902.x . [DOI] [PubMed] [Google Scholar]
- [20].Jacobs PS, Taylor BM, Bardgett ME. Maturation of locomotor and Fos responses to the NMDA antagonists, PCP and MK-801. Brain research Developmental brain research. 2000;122:91–5. doi: 10.1016/s0165-3806(00)00059-6. http://dx.doi.org/10.1016/S0165-3806(00)00059-6 . [DOI] [PubMed] [Google Scholar]
- [21].Akillioglu K, Binokay S, Kocahan S. The effect of neonatal N-methyl-D-aspartate receptor blockade on exploratory and anxiety-like behaviors in adult BALB/c and C57BL/6 mice. Behavioural brain research. 2012;233:157–61. doi: 10.1016/j.bbr.2012.04.041. http://dx.doi.org/10.1016/j.bbr.2012.04.041 . [DOI] [PubMed] [Google Scholar]
- [22].Akillioglu K, Babar Melik E, Melik E, Kocahan S. The investigation of neonatal MK-801 administration and physical environmental enrichment on emotional and cognitive functions in adult Balb/c mice. Pharmacol Biochem Behav. 2012;102:407–14. doi: 10.1016/j.pbb.2012.06.006. http://dx.doi.org/10.1016/j.pbb.2012.06.006 . [DOI] [PubMed] [Google Scholar]
- [23].Lindefors N, Barati S, O’Connor WT. Differential effects of single and repeated ketamine administration on dopamine, serotonin and GABA transmission in rat medial prefrontal cortex. Brain Res. 1997;759:205–12. doi: 10.1016/s0006-8993(97)00255-2. http://dx.doi.org/10.1016/S0006-8993(97)00255-2 . [DOI] [PubMed] [Google Scholar]
- [24].Hondo H, Yonezawa Y, Nakahara T, Nakamura K, Hirano M, Uchimura H, et al. Effect of phencyclidine on dopamine release in the rat prefrontal cortex; an in vivo microdialysis study. Brain Res. 1994;633:337–42. doi: 10.1016/0006-8993(94)91558-x. http://dx.doi.org/10.1016/0006-8993(94)91558-X . [DOI] [PubMed] [Google Scholar]
- [25].Lim AL, Taylor DA, Malone DT. A two-hit model: behavioural investigation of the effect of combined neonatal MK-801 administration and isolation rearing in the rat. J Psychopharmacol. 2012;26:1252–64. doi: 10.1177/0269881111430751. http://dx.doi.org/10.1177/0269881111430751 . [DOI] [PubMed] [Google Scholar]
- [26].Baier PC, Blume A, Koch J, Marx A, Fritzer G, Aldenhoff JB, et al. Early postnatal depletion of NMDA receptor development affects behaviour and NMDA receptor expression until later adulthood in rats--a possible model for schizophrenia. Behav Brain Res. 2009;205:96–101. doi: 10.1016/j.bbr.2009.06.018. http://dx.doi.org/10.1016/j.bbr.2009.06.018 . [DOI] [PubMed] [Google Scholar]
- [27].Amani M, Samadi H, Doosti MH, Azarfarin M, Bakhtiari A, Majidi-Zolbanin N, et al. Neonatal NMDA receptor blockade alters anxiety- and depression-related behaviors in a sex-dependent manner in mice. Neuropharmacology. 2013;73:87–97. doi: 10.1016/j.neuropharm.2013.04.056. http://dx.doi.org/10.1016/j.neuropharm.2013.04.056 . [DOI] [PubMed] [Google Scholar]
- [28].du Bois TM, Huang XF, Deng C. Perinatal administration of PCP alters adult behaviour in female Sprague-Dawley rats. Behav Brain Res. 2008;188:416–9. doi: 10.1016/j.bbr.2007.11.017. http://dx.doi.org/10.1016/j.bbr.2007.11.017 . [DOI] [PubMed] [Google Scholar]
- [29].Mc Fie S, Sterley TL, Howells FM, Russell VA. Clozapine decreases exploratory activity and increases anxiety-like behaviour in the Wistar-Kyoto rat but not the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Brain Res. 2012;1467:91–103. doi: 10.1016/j.brainres.2012.05.047. http://dx.doi.org/10.1016/j.brainres.2012.05.047 . [DOI] [PubMed] [Google Scholar]
- [30].Lim AL, Taylor DA, Malone DT. Consequences of early life MK-801 administration: long-term behavioural effects and relevance to schizophrenia research. Behav Brain Res. 2012;227:276–86. doi: 10.1016/j.bbr.2011.10.052. http://dx.doi.org/10.1016/j.bbr.2011.10.052 . [DOI] [PubMed] [Google Scholar]
- [31].du Bois TM, Deng C, Han M, Newell KA, Huang XF. Excitatory and inhibitory neurotransmission is chronically altered following perinatal NMDA receptor blockade. Eur Neuropsychopharmacol. 2009;19:256–65. doi: 10.1016/j.euroneuro.2008.12.002. http://dx.doi.org/10.1016/j.euroneuro.2008.12.002 . [DOI] [PubMed] [Google Scholar]
- [32].Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]


