Abstract
The Wnt/Wingless (Wg) signaling cascade controls a number of biological processes in animal development and adult life; aberrant Wnt/Wg signaling can cause diseases. In the 1980s genes were discovered that encode core Wnt/Wg pathway components: their mutant phenotypes were similar and an outline of a signaling cascade emerged. Over the years our knowledge of this important signaling system increased and more components were uncovered that are instrumental for Wnt/Wg secretion and transduction. Here we provide an overview of these discoveries, the technologies involved, with a particular focus on the important role Drosophila screens played in this process.
Keywords: β-catenin, development, Drosophila melanogaster, genetics, genetic screens, Wnt signaling, wingless signaling, Wnt, Wg
A Signal Essential for Development and Relevant for Disease
Wnt/Wg signaling plays important roles in animal development. In Drosophila melanogaster this signaling cascade is involved in the patterning of the embryo1-3 and in the development of adult structures from imaginal disc primordia4,5; this includes leg, wing, genitalia, antennae, and eye imaginal discs.6-8 In the 1990s Wnt/Wg signaling was first associated with human disease: the adenomatous polyposis coli (APC) tumor suppressor gene was isolated, which plays a key role in hereditary and spontaneous colorectal cancer, and a few years later APC became directly linked to Wnt/Wg signaling with the discovery that it bound to the core component β-catenin.9-13
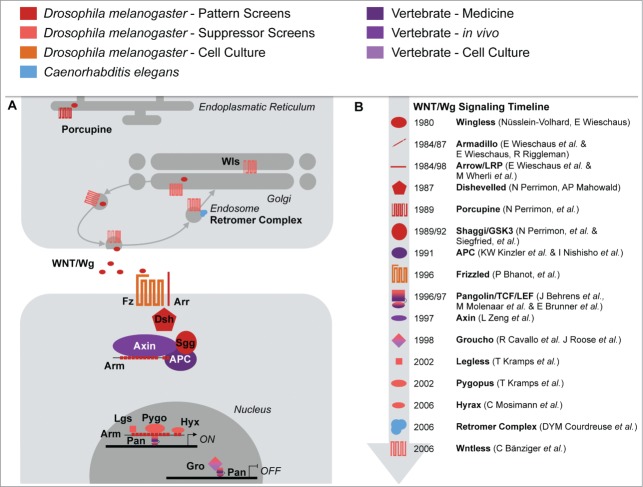
The Wnt/Wg signal acts either via calcium signaling,14 by triggering the planar cell polarity pathway15,16 or canonically by regulating the stability of Armadillo (Arm, β-catenin in vertebrates). Here we focus on the canonical signaling cascade, which principally follows 3 steps: In the sending cell Wnts first get lipid modified by the acyltransferase Porcupine (Porc) and are then secreted via the endoplasmatic reticulum and Golgi apparatus involving the transmembrane protein Wntless (Wls). In the receiving cell the Wnt ligand binds to its receptor Frizzled (Fz) and the co-receptor Arrow (Arr, LRP5/6 in mammals). When no ligand is present, a destruction complex consisting of Axin, Shaggy (Sgg, glycogen synthase kinase 3 (GSK3) in vertebrates) and APC phosphorylates Arm, and marks it for degradation by the proteasome. In presence of the Wg ligand a signal is transduced via Disheveled (Dsh) resulting in the inactivation of the destruction complex. As a consequence Arm accumulates and translocates into the nucleus, where it activates target genes together with the transcription factor Pangolin (Pan, TCF/Lef in vertebrates). 13, 17–20 A model of Wnt/Wg signaling is depicted in Figure 1A and a list of all signaling components with abbreviations can be found in Table 1.
Figure 1.
The mechanics and history of Wnt/Wg signaling. (A) The current Wnt/Wg signaling model with its core components and (B) a historic timeline overview regarding the discovery of these signaling components. The color code indicates whether the individual components were discovered in Drosophila, Caenorhabditis elegans or in vertebrates.
Table 1.
List of Wnt/Wg signaling core components. All components are shown with their full Drosophila name, abbreviation and the names of their vertebrate homologs. The information is based on Flybase (http://www.flybase.org) and the Wnt Homepage (http://wnt.stanford.edu).
| Drosophila | Vertebrate | |
|---|---|---|
| Signal-transducing components | Wingless (Wg) (6 other WNTs) | WNT1 (18 other WNTs) |
| Arrow(Arr) | LRP 5 and 6 | |
| Frizzled (Fz) and Frizzled2 (Fz2) | Fzd 1 to 9 | |
| Disheveled (Dsh) | Dvl-1 to 3 | |
| Armadillo (Arm) | β-Catenin | |
| Pangolin (Pan) | TCF1 to 4 and LEF-1 | |
| Legless (Lgs) | BCL9 | |
| Pygopus (Pygo) | PYGO 1 and 2 | |
| Porcupine (Porc) | Porc | |
| Signal-repressing components | Adenomatous polyposis coli (APC) and | APC1 and APC2 |
| APC2 | ||
| Axin | Axin 1 and 2 | |
| Groucho (Gro) | Grg/TLE 1 to 4 | |
| Shaggy (sgg) | GSK3β |
The definition of the above pathway represents more than 30 y of research. While we have a basic understanding of the mechanics and most of the core signaling components are known, there are still gaps in pathway modulation and tissue specificity, hence there are still new discoveries to be made. Here we first look back at the model systems and techniques involved in this scientific journey and how these contributed to building our comprehensive knowledge of this signaling system. We also speculate on why certain techniques and model systems were so successful and take a look into the future, to ask what technologies could contribute to an even more complete understanding of this fundamental signaling cascade.
Glazed Eyes and Absent Wings – a Chronology of Discoveries
Discovering the ligand
The discovery of the Wg signal can be attributed to Hunt Morgan and his colleagues.21 They isolated and described a dominant mutation in Drosophila, which resulted in a glazed-eye phenotype and therefore was named Glazed (Gla). 40 y later Sharma described an X-irradiation derived mutant, which frequently lacked one or both wings. This phenotype was governed by a single recessive hypomorphic mutation, which he named wg, not knowing that it was allelic to Gla.22,23 As it was found much later, neither Morgan's Gla nor Sharma's wg alleles changed the coding sequence. Gla is a gain-of function allele caused by the insertion of a roo retrotransposon,24 and Sharma's wg allele is the result of a 2.5kb deletion, downstream of the locus, which presumably contains a regulatory element involved in controlling wg expression during wing development.25,26 Other known mutations, like Sternopleural (Sp),27 spade and flag,28 were later shown to be regulatory alleles of wg as well. The first wg null allele was isolated in 1980 as a segment polarity gene influencing embryonic patterning, in the famous screen for embryonic lethal mutations in Drosophila conducted by Nüsslein-Volhard and Wieschaus,29 which laid the foundation for their Nobel Prize winning experiments. Two years later, the mouse Int-1 gene was described, as a locus activated by the integration of MMTV proviral DNA in virally induced mammary tumors (note the analogy of the retrovirus-induced Int-1 and the retrotransposon-induced Gla lesions).30 Int-1 was later found to encode a homolog of Wg,31 and the entire protein family was therefore called Wnt - a blend of the names Wg and Int.32 The discovery of wg in Drosophila showed a relevance of this pathway in fly development, whereas the characterization of the murine Int-1 gene implied a role in oncogenesis. A role in vertebrate development became evident when ectopic expression of Wnts was observed to cause axis duplications in Xenopus embryos.33 These factors, their importance in development and oncogenesis, as well as the high degree of conservation between different species, were the underpinnings of the entire Wnt/Wg signaling research field.
Sketching the pathway
Drosophila continued to play an important role early on with genetic fly screens yielding the building blocks of the pathway. In the Nüsslein-Volhard and Wieschaus screen, other genes were identified which were later shown to play a role in the Wnt/Wg pathway. Alleles of arm and arr also showed segment polarity defects, similar to those of wg null alleles, but their relationship remained obscure.34,35 In subsequent screens, where also the maternal gene function was removed, Perrimon and other researchers identified alleles of dsh, sgg (also known as zeste-white 3) and porc.36-42 This clustering of phenotypes among segment polarity genes indicated already the vague outline of a signaling cascade and therefore pushed research to the next level: The discovery that Wg stabilizes engrailed (en) expression in embryonic segmentation43 enabled researchers to add roles, functions and relationships between these genes. The Perrimon lab studied the effect of wg on en expression in sgg mutants.44 They reported that Wg inactivates the Sgg-induced repression of en and that Sgg is a homolog of GSK3 in mammals. Several epistasis experiments helped to sharpen our view of the Wnt/Wg pathway (e.g., porc, dsh, arm and sgg)44-46 and the Nusse lab could show that Porc provides a relevant function for Wg protein secretion.26
A signaling system related to cancer: the Wnt/Wg field takes off
In the early 1990s, studies in patients identified a genomic region on chromosome 5q21 and associated mutations at this locus (termed APC) with familial adenomatous polyposis.9,10 A few years later, immunoprecipitation experiments showed that the APC protein interacts with β-catenin,11 which reinforced the hypothesis that the Wnt/Wg signaling cascade is involved in cancer. Driven by this finding and its clinical relevance, the Wnt/Wg field started a quest for additional pathway components, in particular the receptor(s). Attempts to biochemically isolate Wg and its receptors failed and research focused on genetic approaches. Initially Notch was postulated to transduce the Wg signal,47 but Rulifson and Blair presented evidence that while the cross-talk between Notch and Wg functions is substantial, Notch is not the long-sought Wg receptor.48 It was established that 2 Drosophila Fz-family genes encode the Wg receptors. The Nusse and Nathan labs could identify these membrane proteins by a cell culture assays in Drosophila: Wg insensitive Schneider 2 cells, transfected with a fz2 expression construct, were able to respond to the Wg signal and stabilize Arm.49,50 Pathway specific screens in Drosophila using an ectopically active wg transgene were started in our laboratory and yielded nuclear signaling components. Brunner et al. described the in vivo role of the Drosophila pan gene in a suppressor screen.51 It encodes a homolog of vertebrate LEF-1, which can, as a transgene, substitute the Pan function.52 It was found to interact with the Wnt/Wg signaling component β-catenin in a yeast 2-hybrid assay53 and of Xenopus XTcf-3, which forms a complex with β-catenin in the nucleus. This illustrates that this family of transcription factors enables Arm and β-catenin to activate specific target genes.54
In 1997 the Costantini lab started to characterize the Fused (Fu) locus in mice.55 Fu mutations cause pleiotropic developmental effects, including axis duplications56; the gene was thus renamed to Axin. Dorsally injected Axin mRNA inhibited Xenopus axis formation and this ventralization was shown to be related to perturbed Wnt/Wg signaling. Epistasis experiments indicated that Axin acts up-stream of β-catenin. A few years later, a Drosophila screen identified Axin as a modifier of dsh over-expression in the eye.57
Soon after, the Wnt/Wg co-receptor arr was phenotypically characterized and Wehrli et al. showed that arr is essential in cells receiving Wg input, where it acts upstream of Dsh.58 The low density lipoprotein receptor-related protein (LRP) Arr was essential for proper segmentation in the Drosophila embryo and null-mutants (upon removing also the maternal contribution) were indistinguishable from wg mutants.
Events in the nucleus
Functional analyses in Drosophila, Caenorhabditis elegans and Xenopus indicated that Pan/TCF/LEF might also act as a transcriptional repressor.52,59-64 The protein Groucho (Gro) was already known as a co-repressor in segmentation, neurogenesis and sex determination in Drosophila,65-68 however there was no direct link to Wnt/Wg signaling. In the Pan/TCF/LEF yeast 2-hybrid screen that established the link of this transcription factor to β-catenin,54 a murine homolog of gro was identified and implicated in Wnt/Wg target gene repression.69 In parallel, the co-repressor function of Gro was described in Drosophila.70
After the turn of the century 3 further genes were identified, that encode products which interact with the nuclear β-catenin-Pan/TCF/LEF complex: legless (lgs), pygopus (pygo) and hyrax (hyx). Lgs recruits Pygo to the Pan/TCF/LEF complex and together with Hyx and other factors they assist β-catenin in the activation of Wg target genes.71-76 Whereas Lgs and Pygo also emerged from the ectopic Wg signaling screen in the Drosophila eye for dominant suppressors that uncovered pan,51 Hyx was identified in a complementary overexpression screen in the wing.76
Secretion of the ligand
In an improved version of the Brunner et al. suppressor screen, which can also retrieve recessive suppressors (see below), the wls gene was discovered, which encodes a factor involved in Wg secretion.77,78 At the same time, Coudreuse et al. showed that the Retromer complex, previously implicated in intracellular protein trafficking, is required for signaling in Wnt/Wg producing cells.79 The mechanism necessitating the Retromer complex was further characterized in Drosophila, where it is required for Wls protein recycling: In the absence of components of the Retromer complex, Wls is degraded and the Wg pathway is impaired.80,81Figure 1B illustrates the chronology of Wnt/Wg signaling component discoveries.
The Power of Drosophila Screens and the Contribution of Other Systems
It is striking that 13 out of 16 components were discovered first in Drosophila. This illustrates nicely the power of phenotype based screens and the associated techniques (Fig. 1A and B).
Traditional screens in Drosophila
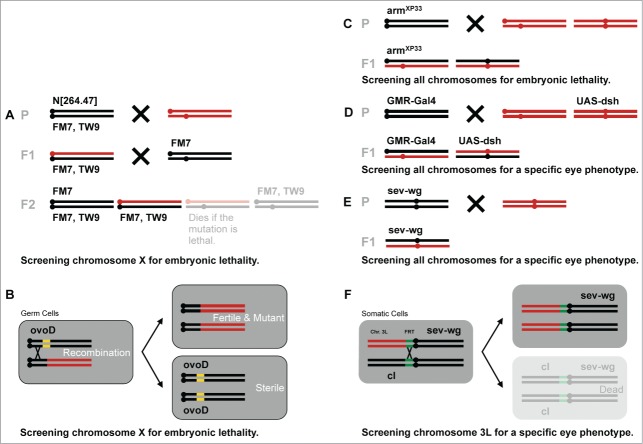
In a first phase of Wnt/Wg research, patterning screens were the key to success. Systematic searches for mutations that affect the segmental pattern, like the one from Nüsslein-Volhard and Wieschaus, identified the first components of this signaling cascade (Fig. 2A) .29,35 A number of segment polarity mutants, but not all, displayed similar phenotypes and helped to vaguely outline the scheme of the Wnt/Wg pathway. Because these experiments were conducted in embryos, where the zygotic mutant phenotype of some loci were masked by maternally provided gene product, a number of signaling components were missed. For example, maternal contribution was the reason, why mutations in the arm gene did not entirely mimic wg null alleles.35 As the embryo only gradually runs out of maternal product, the analysis of their early zygotic phenotypes is difficult. Among Wnt/Wg signaling genes, wg, arr and arm alleles show a clear segment-polarity phenotype in homozygous mutant embryos whereas other genes, such as dsh, porc and sgg were initially missed.36,38,39 Irradiation-based generation of homozygous germline clones allowed the analysis of zygotes that lacked the maternal contribution of the gene under investigation (Fig. 2B). This approach permitted Perrimon and colleagues to identify the Wnt/Wg signaling components dsh, porc and sgg, which were missed in earlier screens.
Figure 2.
Genetics of Drosophila screens for Wnt/Wg signaling components. (A) The first patterning screens were performed by Nüsslein-Volhard and Wieschaus.29,34 Flies were mutagenized and lines with interesting candidates were established. Here we show the crossing schemes for the isolation of X-linked lethal mutations. (B) Perrimon adapted these first screens for zygotic lethals and removed maternal contributions using the ovoD system, which relies on a female-sterile mutation and mitotic recombination by X irradiation. (C) The Bejsovec lab sensitized the genetic background with a hypomorphic arm allele, which was more susceptible for negative components, such as Pan/Gro and APC2 and (D) the Nusse group identified Axin in a dsh overexpression screen. (E and F) Our lab has carried out suppressor screens using wg mis-expression, induced by the activity of the sev enhancer. (E) A dominant suppressor screen for suppressors of the sev-wg phenotype yielded pan, lgs and pygo. (F) This setting was further developed for recessive suppressor screens based on Flp-induced recombination. In this screen wls was discovered. The remaining chromosome arms are screened with an improved method, where the wg transgene carries a flp-out cassette, which is removed in the eye by ey-Flp (eyeless promoter driven Flipase). The corresponding tester lines carry an FRT site as well as a cell lethal (cl) allele. Marked in red are the mutagenized chromosomes.
Suppressor screens in Drosophila
In a second phase, genetic pathways were further explored with dedicated screens in Drosophila based on sensitized phenotypes. Ectopic expression of the wg gene in the eye was used to induce a gain-of-Wg signaling phenotype, which allowed for a suppressor screening. The approach of using a wg transgene under the control of the eye-specific sevenless (sev) enhancer has proven valuable for a genome-wide screen for dominant suppressors, and led to the identification of pan, lgs and pygo (Fig. 2E).51,71 In a second version, Flp-mediated mitotic recombination was included, which allowed for a chromosome arm-specific screening for recessive suppressors (Fig. 2F). On chromosome arm 3L this approach uncovered the wls gene.77 A further improved, inducible version of this screen, taking advantage of a conditional sev-wg transgene, was recently used for all remaining chromosome arms (F. Jenny & M. Hediger Niessen et al., unpublished). A similar set-up with a sensitized background was used by the Nusse lab to carry out a screen for modifiers of a dsh mis-expression phenotype in the Drosophila eye. Flies expressing UAS-dsh driven by an eye-specific Gal4 transgene were mutagenized (Fig. 2D); this resulted in the identification of the Drosophila Axin gene.57
In a suppressor screen with an Arm-depleted sensitized background that favors the discovery of signal-repressing components, the Bejsovec lab was able to describe the negative role of Pan in Wg signaling (together with the co-repressor Gro) and to isolate one of the Drosophila APC homologues, APC2 (Fig. 2C).70,82,83
Other Approaches
While traditional and suppressor screens in Drosophila were highly successful, they also had their limitations in identifying redundant or negatively acting components.
Redundancy was the main issue in the discovery of the Wnt/Wg signaling receptors. Both fz-family genes, fz and fz2, encode functional Wg receptors and were therefore missed in genetic screens. However, the availability of well characterized Drosophila cell lines helped. It was known that Schneider 2 cells are insensitive to the Wg signal. Transfection with fz2 conferred pathway activity and thus demonstrated that fz2 encodes a Wg receptor.49 In addition, overexpression of Fz-family proteins resulted in phenotypes similar to ectopic Wg signaling.84,85 Three years later then, firm genetic data was obtained demonstrating that only when the function of both genes, fz and fz2, are abolished, Wg is no longer transduced in vivo.50,86 With the exception of Sgg, all negatively acting components were first discovered in systems other than Drosophila, perhaps because the setups in modifier screens favors the identification of suppressors (positively acting components) and not enhancers, which often unspecifically aggravate the initial phenotype and are thus difficult to incontestably score. Hence the negative component APC was first associated in clinical research with heritable colorectal cancers and later shown to interact with Arm by being part of the destruction complex.9-11 APC was missed by most Drosophila screens, perhaps because of the special nature of the 2 Drosophila APC homologs: While Wg signaling is essential for embryonic development, APC function is confined to the central nervous system.87 Only the above mentioned screen aimed at negative Wg components in the Bejsovec lab identified APC2 as a Wnt/Wg signaling component.82 Axin is another component of the destruction complex, which was first described in a Wnt-unrelated context in the mouse.56 cDNA microinjection experiments in Xenopus, an excellent vertebrate model for gain-of-function phenotypes, linked this protein then to Wnt/Wg signaling.61,88,89 In large scale screens, this system led to the discovery of Wnt-7b, Wnt-10, β-catenin90 and the Wnt-inhibitor Dickkopf.91 Since it is ideally suited to assay for gain-of-function activities, this screening system does not suffer from the redundancy issue.
Perspectives and Conclusions
At present, we can assume that most of the core components of Wnt/Wg signaling are discovered, so we may ask, whether remaining questions, such as tissue specificity and pathway modulation, can be answered by conventional Drosophila research or whether new technologies and or different model systems are needed.
RNA interference screens are already commonly used and contributed to the identification of factors influencing Wnt/Wg signaling.92,93 The method using clustered regularly inter-spaced short palindromic repeats (CRISPR) with the protein Cas9 could herald the start of a new age of knock-out screens in Drosophila. While both approaches allow systematic screens, they are limited in one aspect, compared to classical mutagenesis screens: Knock-downs by RNAi mimic hypomorphic alleles and CRISPR/Cas9 knockouts might yield most of the time null alleles (due to small deletions and frame shifts). Screens based on EMS mutagenesis, however, can lead to a wide spectrum of alleles, from hypomorphs, to dominant negatives, as well as null alleles. Missense mutations destroying functional protein domains can even identify proteins with multiple functions and help putting them into the right context. This becomes even more relevant when one of these functions is cell essential and a complete null would cause cell death. Classical mutagenesis screens have been performed since the early 1980s and millions of flies were screened ever since. So the question arises: have we reached saturation? Modifier screen setups with different sensitized backgrounds in combination with new genetic tools are still fruitfully ongoing in several labs. Hence chemical mutagenesis screens should not yet be considered experimental relics. These approaches might also be helpful, when focusing on Wnt/Wg signaling in specific tissues identifying context specific regulators.
An interesting new approach might come from population genetics: So far scientists used reductionist approaches to investigate signaling pathways. However these do not live up to the complexity of cell-to-cell communication and cross talk between pathways. The McKay lab has created a resource of 192 inbred and sequenced lines.94 They can be used as a resource for systems genetics and might reveal quantitative trait loci (QTL) influencing Wnt/Wg signaling. Even a directed evolution approach would be possible: these inbred lines could be crossed with one another, and in every generation one could select for Wnt/Wg specific features. A sensitized background (e.g., sev-wg, as in our screens) could facilitate the process of selecting for a specific trait at every generation. Isolation of QTLs, with or without prior selection, will likely also reveal components that do not have a Wnt-pathway-dedicated function.
It is foreseeable therefore, that a combination of classical and new tools in Drosophila, but also in other model organisms, will help us to shed further light on this intricate pathway.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank G Hausmann and E Brunner for their helpful comments on the manuscript. We apologize to all researchers and authors whose work was not cited in this review.
Funding
Our work is supported by the Forschungskredit of the University of Zurich and the Swiss National Science Foundation.
References
- 1. Baker NE. Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev Biol 1988; 125: 96-108; PMID:3119404; http://dx.doi.org/ 10.1016/0012-1606(88)90062-0 [DOI] [PubMed] [Google Scholar]
- 2. Bejsovec A, Martinez Arias A. Roles of wingless in patterning the larval epidermis of Drosophila. Development 1991; 113: 471-85; PMID:1782860 [DOI] [PubMed] [Google Scholar]
- 3. Bejsovec A, Wieschaus E. Segment polarity gene interactions modulate epidermal patterning in Drosophila embryos. Development 1993; 119: 501-17; PMID:8287799 [DOI] [PubMed] [Google Scholar]
- 4. Cohen SM. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature 1990; 343: 173-7; PMID:2296309; http://dx.doi.org/ 10.1038/343173a0 [DOI] [PubMed] [Google Scholar]
- 5. Cohen B, Simcox A, Cohen S. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development 1993; 117: 597-608; PMID:8330530 [DOI] [PubMed] [Google Scholar]
- 6. Morata G, Lawrence PA. The development of wingless, a homeotic mutation of Drosophila. Dev Biol 1977; 56: 227-40; PMID:849798; http://dx.doi.org/ 10.1016/0012-1606(77)90266-4 [DOI] [PubMed] [Google Scholar]
- 7. Baker N. Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development 1988; 102: 489-97; PMID:3181031 [DOI] [PubMed] [Google Scholar]
- 8. Legent K, Treisman JE. Wingless signaling in Drosophila eye development. Methods Mol Biol 2008; 469: 141-61; PMID:19109709; http://dx.doi.org/ 10.1007/978-1-60327-469-2_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger aC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science 1991; 253: 661-5; PMID:1651562; http://dx.doi.org/ 10.1126/science.1651562 [DOI] [PubMed] [Google Scholar]
- 10. Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Baba S, Hedge P, Markham A, Krush AJ, Petersen G, Hamilton SR, et al. Mutations of Chromosome 5q21 Genes in FAP and Colorectal Cancer Patients. Science 1991; 371: 665-9; http://dx.doi.org/ 10.1126/science.1651563 [DOI] [PubMed] [Google Scholar]
- 11. Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science 1993; 262: 1731-4; PMID:8259518; http://dx.doi.org/ 10.1126/science.8259518 [DOI] [PubMed] [Google Scholar]
- 12. Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol 2012; 4: a008052; PMID:22438566; http://dx.doi.org/ 10.1101/cshperspect.a008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clevers H, Nusse R. Wnt/-catenin signaling and disease. Cell 2012; 149: 1192-205; PMID:22682243; http://dx.doi.org/ 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 14. Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 2005; 38: 439-46; PMID:16099039; http://dx.doi.org/ 10.1016/j.ceca.2005.06.022 [DOI] [PubMed] [Google Scholar]
- 15. Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors 2005; 23: 111-6; PMID:16019432; http://dx.doi.org/ 10.1080/08977190500125746 [DOI] [PubMed] [Google Scholar]
- 16. Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Ann Rev Genet 2008; 42: 517-40; PMID:18710302; http://dx.doi.org/ 10.1146/annurev.genet.42.110807.091432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998; 14: 59-88; PMID:9891778; http://dx.doi.org/ 10.1146/annurev.cellbio.14.1.59 [DOI] [PubMed] [Google Scholar]
- 18. Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol 2009; 129: 1614-1627; PMID:19177135; http://dx.doi.org/ 10.1038/jid.2008.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Port F, Basler K. Wnt Trafficking: New Insights into Wnt Maturation, Secretion and Spreading. Traffic 2010; 11: 1265-71; PMID:20477987; http://dx.doi.org/ 10.1111/j.1600-0854.2010.01076.x [DOI] [PubMed] [Google Scholar]
- 20. Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J 2012; 31: 2714-36; PMID:22617422; http://dx.doi.org/ 10.1038/emboj.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgan TH, Bridges CB, Schultz J. Constitution of the germinal material in relation to heredity. Yearbook of the Carnegie Institution of Washington 1936; 35: 289-97 [Google Scholar]
- 22. Sharma R. Wingless - a new mutant in D. melanogaster. D I S 1973; 50: 134 [Google Scholar]
- 23. Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol 1976; 48: 461-65; PMID:815114; http://dx.doi.org/ 10.1016/0012-1606(76)90108-1 [DOI] [PubMed] [Google Scholar]
- 24. Brunner E, Brunner D, Fu W, Hafen E, Basler K. The dominant mutation Glazed is a gain-of-function allele of wingless that, similar to loss of APC, interferes with normal eye development. Dev Biol 1999; 206: 178-88; PMID:9986731; http://dx.doi.org/ 10.1006/dbio.1998.9136 [DOI] [PubMed] [Google Scholar]
- 25. Baker NE. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J 1987; 6: 1765-73; PMID:16453776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. EMBO J 1993; 12: 5293-302; PMID:8262072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neumann CJ, Cohen SM. Sternopleural is a regulatory mutation of wingless with both dominant and recessive effects on larval development of drosophila melanogaster. Genetics 1996; 142: 1147-55; PMID:8846894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buratovich Ma, Phillips RG, Whittle JR. Genetic relationships between the mutations spade and Sternopleural and the wingless gene in Drosophila development. Dev Biol 1997; 185: 244-60; PMID:9187086; http://dx.doi.org/ 10.1006/dbio.1997.8562 [DOI] [PubMed] [Google Scholar]
- 29. Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287: 795-801; PMID:6776413; http://dx.doi.org/ 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- 30. Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982; 31: 99-109; PMID:6297757; http://dx.doi.org/ 10.1016/0092-8674(82)90409-3 [DOI] [PubMed] [Google Scholar]
- 31. Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homology of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 1987; 50: 649-57; PMID:3111720; http://dx.doi.org/ 10.1016/0092-8674(87)90038-9 [DOI] [PubMed] [Google Scholar]
- 32. Nusse R, Brown A, Papkoff J, Scambler P, Shackerford G, McMahon A, Moon R, Varmus HA. New Nomenclature for int-1 and Related Genes: The Wnt Gene Family. Cell 1991; 64: 231-2; PMID:1846319; http://dx.doi.org/ 10.1016/0092-8674(91)90633-A [DOI] [PubMed] [Google Scholar]
- 33. McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 1989; 58: 1075-84; PMID:2673541; http://dx.doi.org/ 10.1016/0092-8674(89)90506-0 [DOI] [PubMed] [Google Scholar]
- 34. Wieschaus E, Nüsslein-Volhard C, Jürgens G. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Wilhelm Roux's Arch Dev Biol 1984; 193: 296-307; http://dx.doi.org/ 10.1007/BF00848158 [DOI] [PubMed] [Google Scholar]
- 35. Wieschaus E, Riggleman R. Autonomous requirements for the segment polarity gene armadillo during Drosophila embryogenesis. Cell 1987; 49: 177-184; PMID:3105892; http://dx.doi.org/ 10.1016/0092-8674(87)90558-7 [DOI] [PubMed] [Google Scholar]
- 36. Perrimon N, Mahowald AP. Multiple functions of segment polarity genes in Drosophila. Dev Biol 1987; 119: 587-600; PMID:3803719; http://dx.doi.org/ 10.1016/0012-1606(87)90061-3 [DOI] [PubMed] [Google Scholar]
- 37. Simpson P, Messal MEL, Prado JMDEL, Ripoll P. Stripes of positional homologies across the wing blade of Drosophila melanogaster. Development 1988; 401: 391-401. [Google Scholar]
- 38. Perrimon N, Engstromt L, Mahowald AP. Zygotic Lethals With Specific Maternal Effect Phenotypes in Drosophila melanogaster. I. Loci on the X Chromosome. Genetics 1989; 352: 333-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perrimon N, Smouse D. Multiple functions of a Drosophila homeotic gene, zeste-white 3, during segmentation and neurogenesis. Dev Biol 1989; 135: 287-305; PMID:2570722; http://dx.doi.org/ 10.1016/0012-1606(89)90180-2 [DOI] [PubMed] [Google Scholar]
- 40. Bourouis M, Heitzler P, el Messal M, Simpson P. Mutant Drosophila embryos in which all cells adopt a neural fate. Nature 1989; 341: 442-4; PMID:2797168; http://dx.doi.org/ 10.1038/341442a0 [DOI] [PubMed] [Google Scholar]
- 41. Bourouis M, Moore P, Ruel L, Grau Y, Heitzler P, Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J 1990; 9: 2877-84; PMID:2118107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siegfried E, Perkins LA, Capaci TM, Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature 1990; 345: 825-9; PMID:2113617; http://dx.doi.org/ 10.1038/345825a0 [DOI] [PubMed] [Google Scholar]
- 43. Martizez Arias A, Baker NE, Ingham PW. Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development 1988; 103: 157-70; PMID:3197626 [DOI] [PubMed] [Google Scholar]
- 44. Siegfried E, Chou T-B, Perrimon N. wingless signaling acts through zeste-white 3, the drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 1992; 71: 1167-79; PMID:1335365; http://dx.doi.org/ 10.1016/S0092-8674(05)80065-0 [DOI] [PubMed] [Google Scholar]
- 45. Noordermeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature 1994; 367: 80-3; PMID:7906389; http://dx.doi.org/ 10.1038/367080a0 [DOI] [PubMed] [Google Scholar]
- 46. Peifer M, Sweeton D, Casey M, Wieschaus E. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development 1994; 120: 369-80; PMID:8149915 [DOI] [PubMed] [Google Scholar]
- 47. Couso JP, MartinezArias A. Notch is required for wingless signaling in the epidermis of Drosophila. Cell 1994; 79: 259-72; PMID:7954794; http://dx.doi.org/ 10.1016/0092-8674(94)90195-3 [DOI] [PubMed] [Google Scholar]
- 48. Rulifson EJ, Blair SS. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 1995; 121: 2813-24; PMID:7555709 [DOI] [PubMed] [Google Scholar]
- 49. Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse RA. new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996; 382: 225-30; PMID:8717036; http://dx.doi.org/ 10.1038/382225a0 [DOI] [PubMed] [Google Scholar]
- 50. Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 1999; 126: 4175-86; PMID:10457026 [DOI] [PubMed] [Google Scholar]
- 51. Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 1997; 385: 829-33; PMID:9039917; http://dx.doi.org/ 10.1038/385829a0 [DOI] [PubMed] [Google Scholar]
- 52. Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S-C, Grosschedl R, Bienz M. LEF-1, a Nuclear Factor Coordinating Signaling Inputs from wingless and decapentaplegic. Cell 1997; 88: 777-87; PMID:9118221; http://dx.doi.org/ 10.1016/S0092-8674(00)81924-8 [DOI] [PubMed] [Google Scholar]
- 53. Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382: 638-42; PMID:8757136; http://dx.doi.org/ 10.1038/382638a0 [DOI] [PubMed] [Google Scholar]
- 54. Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3transcriptionfactormediates beta-catenin-induced axis formation in Xenopus embryos. Cell 1996; 86: 391-9; PMID:8756721; http://dx.doi.org/ 10.1016/S0092-8674(00)80112-9 [DOI] [PubMed] [Google Scholar]
- 55. Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 1997; 90: 181-92; PMID:9230313; http://dx.doi.org/ 10.1016/S0092-8674(00)80324-4 [DOI] [PubMed] [Google Scholar]
- 56. Gluecksohn-Schoenheimer S. The effects of a lethal mutation responsible for duplications and twinning in mouse embryos. J Exp Zool 1949; 110: 47-76; PMID:18113441; http://dx.doi.org/ 10.1002/jez.1401100105 [DOI] [PubMed] [Google Scholar]
- 57. Penton A, Wodarz A, Nusse R. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics 2002; 161: 747-62; PMID:12072470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 2000; 407: 527-30; PMID:11029006; http://dx.doi.org/ 10.1038/35035110 [DOI] [PubMed] [Google Scholar]
- 59. Thorpe CJ, Schlesinger A, Carter J, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to Distinguish endoderm from mesoderm. Cell 1997; 90: 695-705; PMID:9288749; http://dx.doi.org/ 10.1016/S0092-8674(00)80530-9 [DOI] [PubMed] [Google Scholar]
- 60. Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha Y-H, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 1997; 90: 707-16; PMID:9288750; http://dx.doi.org/ 10.1016/S0092-8674(00)80531-0 [DOI] [PubMed] [Google Scholar]
- 61. Lemaire P, Garrett N, Gurdon J. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 1995; 81: 85-94; PMID:7720076; http://dx.doi.org/ 10.1016/0092-8674(95)90373-9 [DOI] [PubMed] [Google Scholar]
- 62. Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 1997; 11: 2359-2370; PMID:9308964; http://dx.doi.org/ 10.1101/gad.11.18.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Han M. Gut reaction to Wnt signaling in worms. Cell 1997; 90: 581-4; PMID:9288737; http://dx.doi.org/ 10.1016/S0092-8674(00)80517-6 [DOI] [PubMed] [Google Scholar]
- 64. Bienz M. TCF: transcriptional activator or repressor? Curr Opin Cell Biol 1998; 10: 366-372; PMID:9640538; http://dx.doi.org/ 10.1016/S0955-0674(98)80013-6 [DOI] [PubMed] [Google Scholar]
- 65. Hartley DA, Preiss A, Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, Enhancer of split, shows homology to mammalian G-protein subunit. Cell 1988; 55: 785-95; PMID:3142687; http://dx.doi.org/ 10.1016/0092-8674(88)90134-1 [DOI] [PubMed] [Google Scholar]
- 66. Paroush Z. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell 1994; 79: 805-15; PMID:8001118; http://dx.doi.org/ 10.1016/0092-8674(94)90070-1 [DOI] [PubMed] [Google Scholar]
- 67. Parkhurst SM. Groucho: making its Marx as a transcriptional co-repressor. Trends Genet 1998; 14: 130-132; PMID:9594657; http://dx.doi.org/ 10.1016/S0168-9525(98)01407-3 [DOI] [PubMed] [Google Scholar]
- 68. Fisher AL, Caudy M. Groucho proteins: transcriptional corepressors for specific subsets of DNA-binding transcription factors in vertebrates andinvertebrates. Genes Dev 1998; 12: 1931-1940; PMID:9649497; http://dx.doi.org/ 10.1101/gad.12.13.1931 [DOI] [PubMed] [Google Scholar]
- 69. Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destrée O, Clevers H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 1998; 395: 608-12; PMID:9783587; http://dx.doi.org/ 10.1038/26989 [DOI] [PubMed] [Google Scholar]
- 70. Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 1998; 395: 604-8; PMID:9783586; http://dx.doi.org/ 10.1038/26982 [DOI] [PubMed] [Google Scholar]
- 71. Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Züllig S, Basler K. Wnt/Wingless signaling requires BCL9/Legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 2002; 109: 47-60; PMID:11955446; http://dx.doi.org/ 10.1016/S0092-8674(02)00679-7 [DOI] [PubMed] [Google Scholar]
- 72. Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development (Cambridge, England) 2002; 129: 2565-76; PMID:12015286 [DOI] [PubMed] [Google Scholar]
- 73. Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol 2002; 4: 367-73; PMID:11988739; http://dx.doi.org/ 10.1038/ncb786 [DOI] [PubMed] [Google Scholar]
- 74. Townsley FM, Thompson B, Bienz M. Pygopus residues required for its binding to Legless are critical for transcription and development. J Biol Chem 2004; 279: 5177-83; PMID:14612447; http://dx.doi.org/ 10.1074/jbc.M309722200 [DOI] [PubMed] [Google Scholar]
- 75. Hoffmans R, Städeli R, Basler K. Pygopus and legless provide essential transcriptional coactivator functions to armadillo/beta-catenin. Curr Biol 2005; 15: 1207-11; PMID:16005293; http://dx.doi.org/ 10.1016/j.cub.2005.05.054 [DOI] [PubMed] [Google Scholar]
- 76. Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell 2006; 125: 327- 41; PMID:16630820; http://dx.doi.org/ 10.1016/j.cell.2006.01.053 [DOI] [PubMed] [Google Scholar]
- 77. Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 2006; 125: 509-22; http://dx.doi.org/ 10.1016/j.cell.2006.02.049 [DOI] [PubMed] [Google Scholar]
- 78. Bartscherer K, Pelte N, Ingelfinger D, Boutros M. SecretionofWntligandsrequires Evi, a conserved transmembrane protein. Cell 2006; 125: 523-33; PMID:16678096; http://dx.doi.org/ 10.1016/j.cell.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 79. Coudreuse DYM, Roël G, Betist MC, Destrée O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science 2006; 312: 921-4; PMID:16645052; http://dx.doi.org/ 10.1126/science.1124856 [DOI] [PubMed] [Google Scholar]
- 80. Port F, Kuster M, Herr P, Furger E, Bänziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 2008; 10: 178-85; PMID:18193032; http://dx.doi.org/ 10.1038/ncb1687 [DOI] [PubMed] [Google Scholar]
- 81. Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent J-P. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 2008; 10: 170-7; PMID:18193037; http://dx.doi.org/ 10.1038/ncb1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McCartney BM, Dierick Ha, Kirkpatrick C, Moline MM, Baas a, Peifer M, Bejsovec A. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol 1999; 146: 1303-18; PMID:10491393; http://dx.doi.org/ 10.1083/jcb.146.6.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bejsovec A. Flying at the head of the pack: Wnt biology in Drosophila. Oncogene 2006; 25: 7442-7449; PMID:17143288; http://dx.doi.org/ 10.1038/sj.onc.1210051 [DOI] [PubMed] [Google Scholar]
- 84. Zhang J, Carthew RW. Interactions between Wingless and DFz2 during Drosophila wing development. Development 1998; 125: 3075-85; PMID:9671581 [DOI] [PubMed] [Google Scholar]
- 85. Tomlinson a, Strapps WR, Heemskerk J. Linking Frizzled and Wnt signaling in Drosophila development. Development 1997; 124: 4515-21; PMID:9409669 [DOI] [PubMed] [Google Scholar]
- 86. Chen CM, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 1999; 126: 5441-52; PMID:10556068 [DOI] [PubMed] [Google Scholar]
- 87. Hayashi S, Rubinfeld B, Souza B, Polakis P, Wieschaus E, Levine aJ. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc Natl Acad Sci U S A 1997; 94: 242-7; PMID:8990193; http://dx.doi.org/ 10.1073/pnas.94.1.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carnac G, Kodjabachian L, Gurdon J, Lemaire P. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development 1996; 122: 3055-65; PMID:8898219 [DOI] [PubMed] [Google Scholar]
- 89. Fagotto F, Guger K, Gumbiner B. Induction of the primary dorsalizing center in Xenopus by the Wnt/GSK/beta-catenin signaling pathway, but not by Vg1, Activin or Noggin. Development 1997; 124: 453-60; PMID:9053321 [DOI] [PubMed] [Google Scholar]
- 90. Grammer TC, Liu KJ, Mariani FV, Harland RM. Use of large-scale expression cloning screens in the Xenopus laevis tadpole to identify gene function. Dev Biol 2000; 228: 197-210; PMID:11112324; http://dx.doi.org/ 10.1006/dbio.2000.9945 [DOI] [PubMed] [Google Scholar]
- 91. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998; 391: 357-62; PMID:9450748; http://dx.doi.org/ 10.1038/34848 [DOI] [PubMed] [Google Scholar]
- 92. Pepperl J, Reim G, Lüthi U, Kaech A, Hausmann G, Basler K. Sphingolipid depletion impairs endocytic traffic and inhibits Wingless signaling. Mech Dev 2013; 130: 493-505; PMID:23665457; http://dx.doi.org/ 10.1016/j.mod.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 93. Reim G, Hruzova M, Goetze S, Basler K. Protection of Armadillo/β-Catenin by Armless, a novel positive regulator of Wingless signalling. PLoS Biol 2014; 12: e1001988; http://dx.doi.org/ 10.1371/journal.pbio.1001988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, et al. The drosophila melanogaster genetic reference panel. Nature 2012; 482: 173-178; PMID:22318601; http://dx.doi.org/ 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]