Abstract
Maintenance and differentiation of progenitor cells is essential for proper organ development and adaptation to environmental stress and injury. In Drosophila melanogaster, the haematopietic system serves as an ideal model for interrogating the function of signaling pathways required for progenitor maintenance and cell fate determination. Here we focus on the role of the Hippo pathway effectors Yorkie and Scalloped in mediating and facilitating Notch signaling-mediated lineage specification in the lymph gland, the primary center for haematopoiesis within the developing larva. We discuss the regulatory mechanisms which promote Notch activity during normal haematopoiesis and its modulation during immune challenge conditions. We provide additional evidence establishing the hierarchy of signaling events during crystal cell formation, highlighting the relationship between Yorkie, Scalloped and Lozenge, while expanding on the role of Yorkie in promoting hemocyte survival and the developmental regulation of Notch and its ligand, Serrate, within the lymph gland. Finally, we propose additional areas of exploration that may provide mechanistic insight into the environmental and non-cell autonomous regulation of cell fate in the blood system.
Keywords: crystal cell, haematopoiesis, lymph lland, notch, serrate, scalloped, Yorkie
Abbreviations
- CZ
Cortical Zone
- LSC
Lineage Specifying Cell
- Lz
Lozenge
- MZ
Medullary Zone
- ProPO
Prophenoloxidase
- PSC
Posterior Signaling Center
- Sd
Scalloped
- Yki
Yorkie
- WT
wild-type
Introduction
The Drosophila lymph gland, the primary organ for haematopoiesis in the fruit fly, is located in the anterior region of the larva adjacent to the brain and the ring gland, where two large lobes flank the dorsal vessel.1 The two large primary lobes are the most characterized region of the lymph gland, primarily due to the presence of distinct cellular compartments. However, there are additional secondary and tertiary lobes that sequentially follow the posterior end of the primary lobes with pericardial cells interspersed between these lobes. While the secondary and tertiary lobes are smaller than the primary lobes, they also flank the dorsal vessel in a similar symmetric manner2,3 (Fig. 1A). Early observations of the primary lobes identified two distinct regions of the lymph gland based solely on morphological features. The cells that were observed in the medial region of the lobe, closer to the dorsal vessel are compact in relation to neighboring cells. However, the cells at the periphery of the organ are not as closely packed together.3 Further investigation revealed that the closely compacted region contains a population of undifferentiated haematopoietic progenitors, or prohemocytes. During the course of development, the prohemocytes along the outer edge of the lymph gland begin to differentiate forming a distinct population referred to as the Cortical Zone (CZ), while the undifferentiated prohemocytes remain in the medial region of the organ termed the Medullary Zone (MZ)3 (Fig. 1B)
Figure 1.
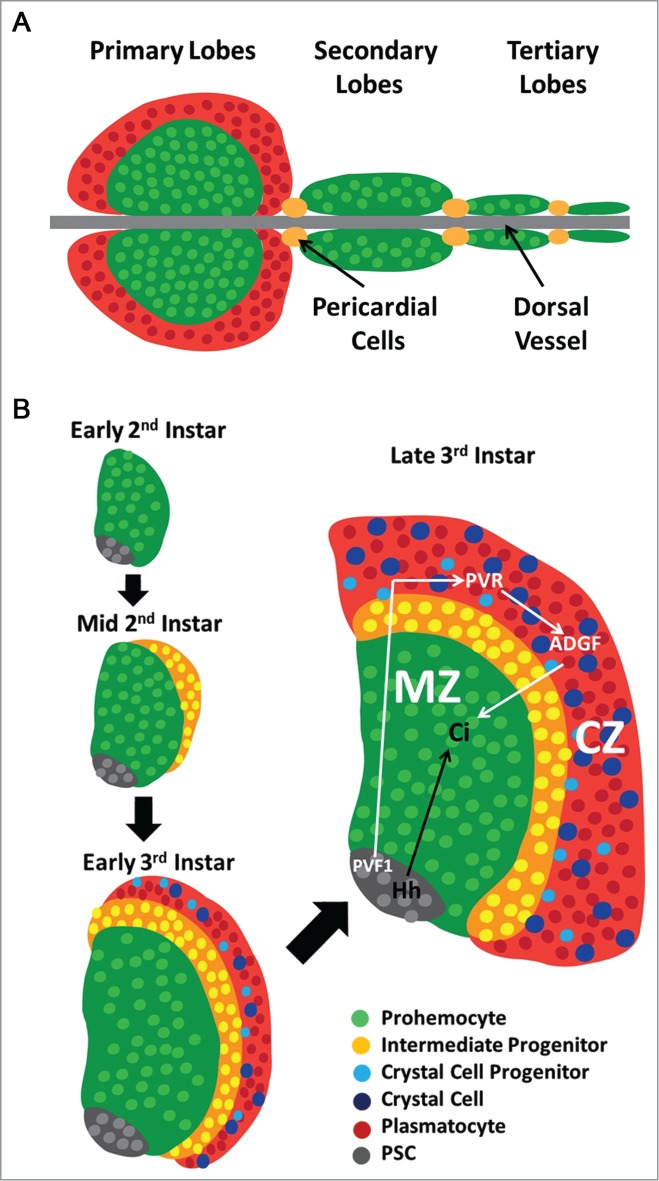
Schematic representation of lymph gland development. (A) The lymph gland is comprised of several lobes paired on either side of the dorsal vessel, separated by pericardial cells. The primary lobes are the largest and most anterior in the larva with progenitors labeled in Green and differentiated hemocytes in Red, while smaller secondary and tertiary lobes consist of mostly progenitor cells and are located posterior to the primary lobes. (B) The early lymph gland (first-second instar) is comprised of undifferentiated prohemocytes (Green) and a small number of PSC cells (Gray). The first differentiating cells are observed at the periphery of the organ at mid-second instar. By the early third instar, fully differentiated Plasmatocytes (Red) and Crystal Cells (Blue) are observed in the CZ. The PSC secretes Hedgehog (Hh, Black Arrow) to maintain Prohemocytes of the MZ (Green). Prohemocytes differentiate through an Intermediate Progenitor (Yellow) state before reaching mature hemocyte lineages found in the CZ of the lymph gland. Plasmatocytes comprise the majority of mature hemocytes, while Crystal Cell Progenitors (Light Blue) and fully mature Crystal Cells (Blue) are also present. PVF1 (White Arrow) secreted from the PSC signals through PVR expressed in differentiating cells of the CZ to maintain levels of ADGF required for the Equilibrium Signal.
These two populations of cells are defined by their unique expression of population specific proteins. The transmembrane protein Domeless, the receptor for Unpaired ligands upstream of JAK/STAT signaling, is highly expressed in the progenitor population of the MZ, while two extracellular proteins, Hemolectin and Peroxidasin, are highly expressed in differentiating hemocytes of the CZ.3 Fully differentiated plasmatocytes are phagocytic cells which express the phagocytosis receptor Nimrod (P1 Antigen)4 and comprise the majority of mature hemocytes. The other mature hemocyte lineage in the CZ are crystal cells which aid in the immune response5 and in wound healing.6 These cells are identified by the expression of the melanizing enzyme Prophenoloxidase (ProPO)7 in crystalline inclusions and the transcription factor Lozenge (Lz)8 a member of the Runx family9.
A separate population of signaling cells is located in the most posterior portion of the organ, adjacent to the Dorsal Vessel.10 This Posterior Signaling Center or PSC is maintained by the transcription factor Collier11 and is specified very early in lymph gland development by the transcription factors Antennapedia and Homothorax.12 This signaling center expresses the Notch ligand Serrate and also secretes the signaling molecules Hedgehog and PVF1 (PDGF-and VEGF-related factor 1) which are required for the maintenance of the progenitor cells in the MZ12-14. Therefore, the PSC serves as a haematopoietic niche that is required to maintain progenitors in their undifferentiated state.
Several different signaling pathways have been characterized as mediators of prohemocyte maintenance and differentiation, with Hedgehog and PVF1 having primary roles in lymph gland homeostatsis. As previously described Hedgehog and PVF1 are both secreted from the PSC (Fig. 1B), but activate signaling in distinct cellular populations. Canonical Hedgehog signaling is essential within cells of the MZ as lymph glands of Hedgehog mutant larvae are completely differentiated. Furthermore, activated Cubitus Interuptus (Ci), the downstream effector of Hedgehog signaling, is observed in the MZ.12
While PVF1 is not required in the cells of the MZ, it signals to the differentiating cells of the CZ through its receptor, PVR (PDGF-and VEGF-like receptor). PVR then activates STAT which induces expression of Adenosine Deaminase Growth Factor (ADGF). ADGF scavenges adenosine which is present in the extracellular space of the lymph gland. Excess or increased levels of adenosine leads to activation of the Adenosine Receptor in progenitor cells of the MZ, which in turn activates PKA, a cAMP dependent protein kinase, leading to the destabilization of Cubitus Interuptus and differentiation of the haematopoietic progenitors. In short, this PVF1-PVR-STAT-ADGF axis is a non-autonomous mechanism which maintains progenitors of the MZ and the balance of differentiating and non-differentiating cells in the lymph gland. This unique mechanism for progenitor cell maintenance has been termed the equilibrium signal. 14(Fig. 1B, white arrows).
In addition to these signals that are mediated by the PSC, there are also cell autonomous signals that are required for maintenance of progenitors and differentiation. The Drosophila Wnt homolog Wingless is expressed at high levels, along with its receptor Frizzled, in the undifferentiatied progenitor population and is absent in differentiating hemocytes of the CZ. Inhibition of Frizzled leads to increased differentiation, while over-expression of Wingless or the activated form of the downstream effector Armadillo leads to expansion of the progenitor population.15 In contrast to Wingless, Fibroblast Growth Factor (FGF) signaling promotes differentiation in the lymph gland. The FGF ligands Thisbe and Pyramus are both expressed in the progenitor cells of the MZ as well as their receptor Heartless. Depletion of either FGF ligand or inhibition of Heartless decreases differentiation and expands the MZ. Conversely, overexpression of the FGF ligands induces differentiation.16 These pathways function to maintain the proper balance of progenitors and differentiating cells within the lymph gland.
Another signaling pathway that has been implicated in lymph gland hemocyte differentiation is the Epidermal Growth Factor (EGF) pathway which is central to the larval cellular immune response. When presented with an immune challenge such as invading bacterium or the egg of a parasitic wasp, the Lymph Gland will undergo several changes characterized by an increase in mitoses,17,18 an observable increase in Reactive Oxygen Species (ROS) in the PSC,19 and most importantly the differentiation of a unique lineage of hemocytes called lamellocytes. These large, flat, cells function by engulfing larger invading pathogens that cannot be managed by the smaller phagocytic plasmatocytes.2 The increased levels of ROS in the PSC lead to the secretion of the EGF ligand Spitz, which is necessary and sufficient for the differentiation of lamellocytes, both within the lymph gland and in circulation.19
In this study, we clarify a number of the remaining questions regarding the mechanisms by which crystal cells differentiate in the lymph gland. We recently characterized the mechanism by which Yorkie, a transcriptional co-activator, and its partner the TEAD transcription factor Scalloped, regulate Serrate expression in a distinct population of Lineage Specifying Cells (LSCs) which induce crystal cell differentiation.20 Here we take a closer look at the temporal expression and regulation of Serrate in the lymph gland, and how precise control of this Notch ligand is critical for the differentiation of crystal cells in both normal and stress conditions. In addition, we explore the expression and function of Notch and downstream Notch signaling targets in the crystal cell differentiation program. Finally, we address the cell-autonomous requirements of Yorkie, Scalloped, and other components of the Hippo Pathway in crystal cells.
Results
Notch signaling in the developing lymph gland
The Notch signaling pathway is perhaps the best characterized signaling pathway in Drosophila haematopoiesis, as it is both necessary and sufficient for the differentiation of the crystal cell lineage of mature hemocytes. Larvae mutant for the Notch receptor are bereft of crystal cells and mutations in the downstream effector of Notch signaling, Suppressor of Hairless (Su(H)), also inhibits crystal cell differentiation.21 Furthermore, the transcription factor Lozenge is activated by Notch signaling10 and is required for crystal cell formation.8 While two Notch ligands are expressed in Drosophila, only Serrate is present in the lymph gland.10 Multiple studies have demonstrated that Serrate activity is required for initiating Notch-dependent crystal cell differentiation.10,20-22 Over-expression of an activated version of the Notch receptor or of its ligand Serrate leads to a significant increase in the number of crystal cells in the lymph gland, indicating the sensitivity of lymph gland hemocytes to Notch signaling and the specificity with which this pathway regulates crystal cell determination.10,21
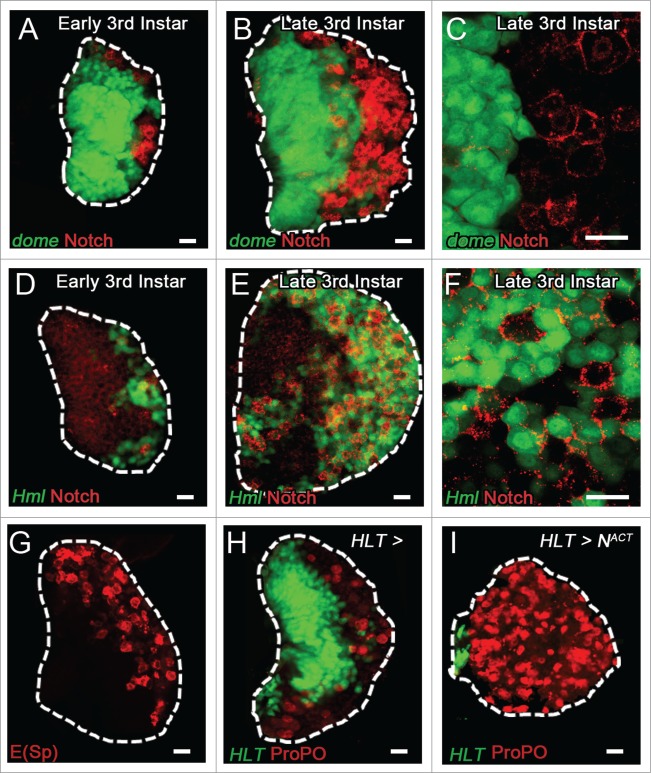
Despite its essential and sufficient requirement in crystal cell formation, the temporal and spatial pattern of Notch expression and that of its ligand Serrate remains poorly characterized. Previous reports have indicated that Notch is widely expressed throughout the late third instar lymph gland.22, 23 Similarly, Su(H) is active in low levels in most cells of the organ, while a few scattered cells show high levels of Su(H) activity which are presumed to be crystal cells.23 We took a closer look at Notch expression starting in the early 3rd instar and the late 3rd instar lymph gland and observe there is a variation in levels of Notch in the CZ compared to the MZ. In the early 3rd instar organ, the newly developing CZ expresses Notch at a high level while low levels of Notch are observed in the MZ progenitors (Fig. 2A, D). High magnification late 3rd instar lymph gland images reveal that cells from the MZ only express low levels of Notch (Fig. 2B-C) while expression in the cells of CZ is robust (Fig. 2E-F). Furthermore, we determined the specific cells that activate Notch signaling by using a separate reporter for Notch activity, Enhancer of Split, and observe that its expression is restricted to scattered cells in the CZ (Fig. 2G), likely crystal cells and crystal cell progenitors. These findings suggest a specific requirement for Notch expression in the CZ, namely, within differentiating hemocytes to impart the potential to differentiate into a crystal cell. Active Notch signaling, however, is only present in a subset of these Notch+ cells as demonstrated by restricted subset of cells within the CZ that express the Enhancer of Split reporter. These cells distinctly received the activating signal from the Notch ligand Serrate, instructing them to differentiate into crystal cells. We have previously demonstrated that activation of Notch in the lymph gland during the early second instar larval stage, when hemocyte differentiation is largely absent (Fig. 1), drives ectopic crystal cell differentiation.20 Interestingly, this effect appears to be non-autonomous, as differentiated crystal cells do not express the activated Notch receptor construct. Moreover, almost the entire organ is absent of cells actively expressing the activated Notch construct, suggesting that early Notch activation in blood progenitors affects their proliferation, quiescence, and survival (Fig. 2H-I). These findings demonstrate a specific requirement for Notch activity within the population of differentiating hemocytes, and reveal a potential non-cell autonomous effect of Notch activation on crystal cell differentiation. Notch can activate Yorkie expression non-autonomously,24 suggesting that this downstream effector of the Hippo pathway could regulate crystal cell differentiation.
Figure 2.
Notch expression in the third instar lymph gland. (A) Notch expression (Red) in the early third instar lymph gland is expressed at low levels in prohemocytes of the MZ (dome, Green). (B) A few Notch+ (Red) cells are observed in dome+ (Green) cells of the MZ, but high levels of Notch are restricted to the dome- cells of the CZ. (C) High magnification image of the MZ-CZ boundary reveals the disparity between abundant Notch expression observed in dome- cells and minimal Notch in dome+ cells. (D) Low levels of Notch (Red) expression are observed in prohemocytes of the early third instar lymph gland, while higher expression is present in hml+ hemocytes of the CZ. (E) Notch (Red) is highly expressed among hml+ (Green) cells of the CZ in the late third instar lymph gland, while hml− cells display low levels of Notch. (F) High magnification of hml+ (Green) cells in the CZ demonstrates high levels of Notch (Red) expression. (G) The Notch signaling reporter Enhancer of Split (E(Sp), Red) is active in scattered cells of the CZ. (H) Crystal cells (ProPO, Red) are traced in the CZ of a WT lymph gland with clonal GFP expression under control of HLT (Green). (I) Overexpression of Active Notch (NACT) with the HLT driver induces non-autonomous differentiation of crystal cells (ProPO, Red, not green), and inhibits expansion of HLT (Green) traced cells. Scale bar 10 μm.
Signaling downstream of Notch in crystal cells
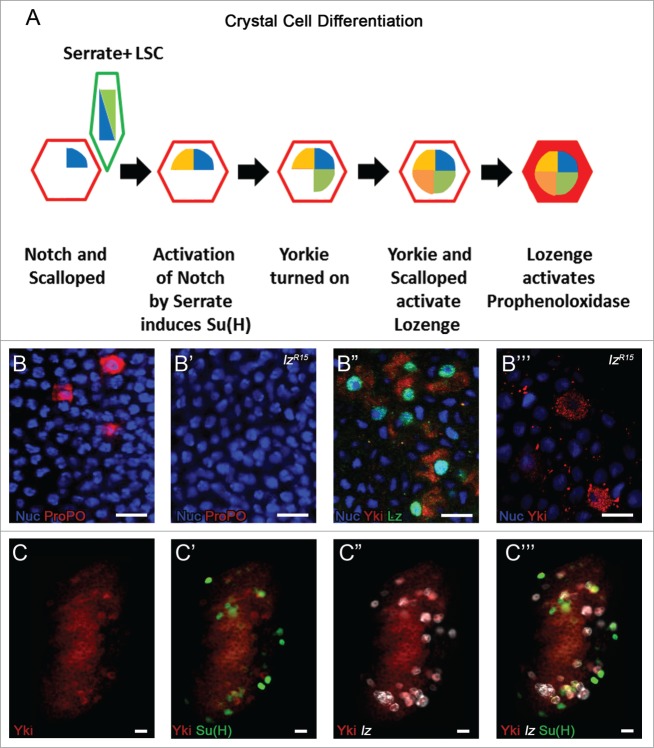
The most specific marker of crystal cell differentiation is expression of the transcription factor Lozenge.8 Inhibition of Notch signaling blocks lozenge expression in the lymph gland,10 and given that Lozenge is exclusively present in crystal cells prior to the onset of the mature crystal cell marker ProPO, we have termed Lz+ ProPO- cells crystal cell progenitors (CCPs). We recently reported20 that the transactivator protein Yorkie (Yki), an effector regulated by Hippo Pathway signaling, is also activated downstream of Notch signaling and is present with the TEAD family transcription factor Scalloped (Sd) in crystal cell progenitors and mature crystal cells (Fig. 3A). While Notch mutant lymph glands do not express Yki,20 we show here that Yki is present in a small number of cells in lozenge mutant larvae in which mature crystal cells are absent (Fig. 3B-B"’). This finding demonstrates that Yki expression is dependent on Notch but not Lozenge, consistent with a recent report demonstrating that Yki and its binding partner Sd function together to transcriptionally regulate lozenge expression in crystal cell progenitors.25 Furthermore, the expression pattern of Yki, Lz, and Su(H) in the lymph gland reveals the hierarchy of events required for crystal cell fate determination, starting with activation of Su(H) downstream of Notch, as not all Su(H)+ cells express Yki and/or Lz (Fig. 3C- C"’). This event triggers Yki expression followed by Lz which transcriptionally regulates ProPO26 (Fig. 3A). Yki is expressed at low levels throughout the lymph gland (Fig. 3C), but is upregulated specifically in crystal cell progenitors expressing Lozenge (Fig. 3C”-C"’). Taken together these findings demonstrate that Yki must first be activated by Notch signaling in crystal cell progenitors where it can than regulate the expression of downstream target genes such as Lozenge. While the role of Yorkie in this signaling cascade is clear, there are still questions regarding how Sd is regulated. Our expression analysis suggests that Sd is already present in the lymph gland prior to crystal cell formation,20 perhaps in an intermediate progenitor population awaiting Notch induction and Yki expression activation (Fig. 3A).
Figure 3.
Hierarchy of events in crystal cell differentiation. (A) A Notch+ (Red outline)/Sd+(Blue) Intermediate Progenitor is activated by a Serrate-expressing LSC (Green outline). Suppressor of Hairless (Yellow) is activated downstream of Notch. Yorkie (Green) expression is then turned on by Notch signaling and it interacts with Scalloped to transcriptionally activate Lozenge (Orange), specifying a crystal cell progenitor. The Lozenge-expressing crystal cell progenitor turns on Prophenoloxidase becoming a mature crystal cell (Filled Red). (B-B”’) Nuclear marker in Blue. (B) High magnification image of crystal cells (ProPO, Red) in the WT lymph gland. (B’) Crystal cells are absent in lzR15 mutant lymph glands. (B”) Yki (Red) and Lz (Green) in the WT lymph gland. (B”’) Yki (Red) is still present in scattered cells of the CZ in lzR15 mutant lymph glands. (C) Yki (Red) is observed at low levels throughout the lymph gland. (C’) Yki (Red) is highly co-expressed specifically with some but not all Su(H)+ (Green)cells. (C”) Yki (Red) is also co-expressed in most lozenge+ (lz, White) cells. (C”’) Expression overlap of all 3 crystal cell progenitor markers, Yki (Red), Su(H) (Green), and lozenge (White). Scale bar 10 μm.
Once the crystal cell progenitor is specified by the activation of Yki and Lz, there is some question regarding the role of Sd in these cells. Expression analysis of sd demonstrates that it is present in a subset of Notch+ cells, mature crystal cells, and crystal cell progenitors.20 Lineage tracing data using a sd-gal4 driver showed that traced mature crystal cells do not express Sd. We therefore explored if Sd is required early in crystal cell development, but is then down-regulated. Overexpression of Sd in crystal cell progenitors induces a significant increase in crystal cell numbers suggesting that down-regulation of Sd is required in crystal cells progenitors to promote the proper number of mature crystal cells present in the lymph gland. Interestingly, unlike Sd, Yki is still expressed in mature crystal cells, and both Sd and Yki are required for maintaining crystal cells in the lymph gland, as depletion of either protein in crystal cell progenitors leads to complete loss of crystal cells.20 However, the mechanism for this requirement is not clear. Given that the anti-apoptotic gene Diap1 is a well-characterized downstream target of Yki signaling,27,28 we asked if Yki and Sd are required to promote crystal cell survival through regulation of this gene. We conducted TUNEL assays on lymph glands in which yki and sd were depleted in crystal cell progenitors and observed a significant increase in apoptosis (Fig. 4A-C). Over-expression of Diap1 (Fig. 4D) or the anti-apoptotic protein p35 (Fig. 4E) rescues the loss of yki in crystal cell progenitors, demonstrating that Yki is necessary for crystal cell survival. Additionally, a Diap1-GFP reporter is active in the lymph gland with particularly strong expression in crystal cells identified by Lozenge and Prophenoloxidase (Fig. 4F). Taken together these data suggest that Yki and Sd are required for the expression of Diap1 to promote crystal cell survival.
Figure 4.
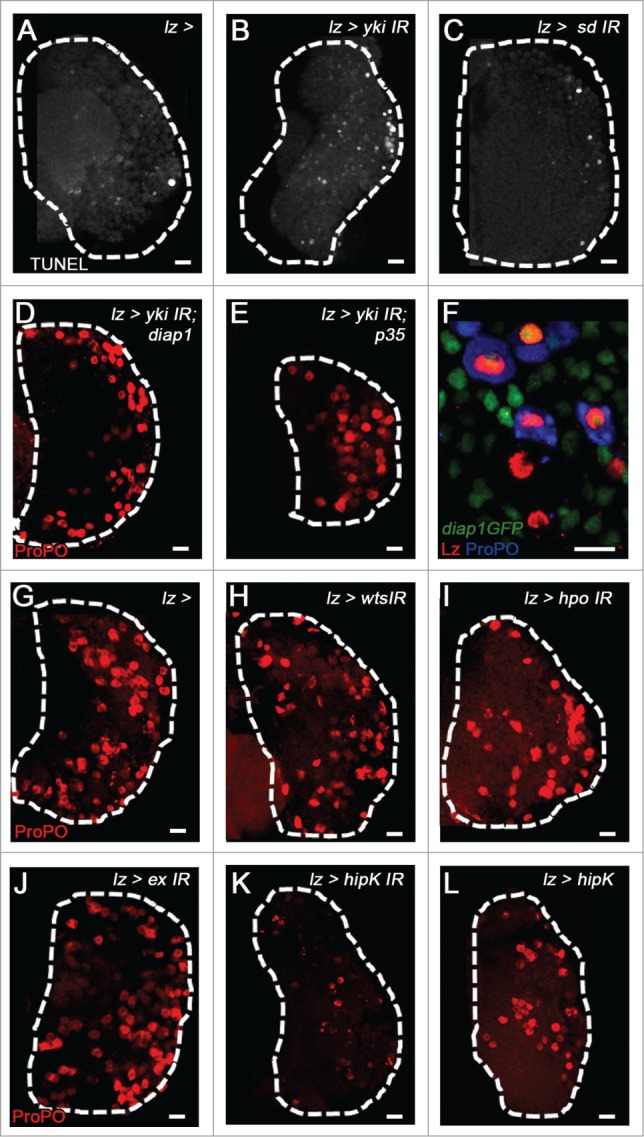
Role of Yorkie, Scalloped, and Hippo Pathway in crystal cell development. TUNEL labels apoptotic cells (White, A-C) and crystal cells are labeled with ProPO (Red, D-E, G-L). (A) WT (B) yki and (C) sd depletion in crystal cell progenitors increases apoptosis, that is rescued by over-expression of (D) Diap1 and (E) p35. (F) Diap1 (Green) is expressed in the CZ of 3rd instar lymph glands, and is specifically co-expressed with Lz (Red) and ProPO (Blue). (G) WT LG. (H) depletion of wts, (I) hippo, or (J) expanded does not have a significant effect on crystal cell numbers. (K) Overexpression of hipK does not affect crystal cell numbers, while (L) depletion of hipK leads to a significant loss of mature crystal cells. Scale bar 10 μm.
Kieran Harvey's group recently addressed the role of the Hippo pathway component Warts in regulating lymph gland haematopoiesis. Warts functions in the Hippo pathway directly upstream of Yki, as it negatively regulates Yki function via phosphorylation.28 warts mutant larvae displayed a dramatic increase in differentiation of hemocytes, resulting in a lymph gland full of plasmatocytes and crystal cells.25 We pursued a similar approach to evaluate the cell-autonomous role of the Hippo pathway in crystal cell differentiation by downregulating levels of the upstream components of the Hippo signaling pathway hippo, warts, and expanded in crystal cell progenitors. This strategy would inhibit Hippo signaling, leading to elevated levels of Yki activity in crystal cell progenitors. However, we do not observe any significant changes in mature crystal cell numbers upon Hippo pathway component depletion (Fig. 4G-J). Interestingly, we do observe a loss of crystal cells upon downregulation of homeodomain interacting protein kinase or hipK, which positively regulates Yki activity29 (Fig. 4K). Conversely, overexpression of hipK in crystal cell progenitors does not alter crystal cell number (Fig. 4L). These results are consistent with the lack of a phenotype we observed upon Yki over-expression in crystal cell progenitors.20 Taken together these findings demonstrate that, while there is a requirement for Hippo Pathway signaling in the regulation of hemocyte differentiation, Yki functions with Sd independent of canonical Hippo pathway signaling within progenitors that have already been specified to the crystal cell fate.
Serrate as a marker of a distinct population of fate determining signaling cells in the lymph gland
As previously described, Yki is strongly expressed in crystal cells and crystal cell progenitors while Sd is expressed in a more transient manner in these populations. One of our most intriguing observations from manipulating Sd and Yki in crystal cell progenitors was that while overexpression of yki had no perceivable phenotype, over-expression of sd led to a robust increase in crystal cell differentiation.20 We hypothesized that Sd levels must be carefully regulated to maintain normal crystal cell numbers in the lymph gland, but it was not immediately apparent why sd overexpression would increase crystal cell numbers. Interestingly, we observed a striking increase in the number of crystal cell progenitors upon overexpression of Yki throughout the lymph gland or in sd-expressing cells. We therefore hypothesized that Yki may function to regulate crystal cell differentiation in a non-autonomous manner, consistent with the phenotype observed in warts mutant lymph glands.25
While it had been well established that Serrate-Notch signaling is the only mechanism through which crystal cells are specified in the lymph gland, there had been little definitive evidence for how Serrate is regulated and made available to Notch+ cells in order for this process to be initiated. Serrate was first identified in the PSC of the lymph gland10 and later studies demonstrated that Serrate expression and PSC identity were dependent upon the transcription factor Collier.11 Lymph glands from col mutant larvae lack a PSC, which leads to loss of MZ progenitors and complete hemocyte differentiation.12,13 However, in these col mutants, crystal cells are still readily observed.12 In addition, although they lack a PSC, a few scattered Serrate expressing cells are still observed in the col lymph gland.11 The nature or function of these Serrate expressing cells was not addressed until we recently discovered their role as Lineage Specifying Cells (LSCs) critical for crystal cell differentiation.20 Furthermore, these cells specifically arise from sd+ cells as demonstrated by lineage tracing analysis. Removal of either yki or sd in LSCs leads to a loss of Serrate and subsequent loss of crystal cells.20 Therefore, Serrate expression in LSCs is regulated by Yki and Sd and serves as the non-autonomous mechanism by which Hippo signaling influences crystal cell fate.
While this finding demonstrated the non-autonomous function of Yki in crystal cell differentiation, it also intimated a potential requirement for Sd down-regulation in crystal cell progenitors to maintain proper numbers of crystal cells in the lymph gland. Since Yki and Sd function together to induce Serrate in LSCs, perhaps Sd must be transiently expressed in crystal cell progenitors to avoid inappropriate Serrate expression in these cells. To evaluate this possibility we ectopically over-expressed Serrate in crystal cell progenitors and observed a large increase in crystal cell numbers (Fig. 5A-B”). We further evaluated if the increased numbers of crystal cell progenitors observed upon overexpression of Sd is due to increased proliferation in early 3rd instar lymph glands. We observe that crystal cell progenitors rarely express pH3, both in WT (Fig. 5C) lymph glands and upon sd overexpression (Fig. 5D-D’), demonstrating that these cells are post-mitotic and that increased numbers of crystal cells upon sd overexpression are not due to proliferation of crystal cell progenitors. Similarly, overexpression of sd in the CZ with hml-gal4 also drives a large increase in crystal cell differentiation in a non-autonomous manner.25 indicating that ectopic Sd likely increases the number of Serrate expressing cells. Given the high levels of Notch that are present throughout the CZ (Fig. 1), Serrate expression must be tightly regulated to avoid inappropriate activation of Notch which would lead to excessive increases in crystal cell numbers. Interestingly, Serrate expression is first observed in the lymph gland at an earlier developmental stage than Notch (Fig. 5F-G), but both are clearly expressed by the early 3rd instar (Fig. 2A, D and Fig. 5H), when lymph gland differentiation is most robust. Serrate expression is restricted to a small number of cells early in development, indicating that ligand availability is a limiting factor in crystal cell differentiation and determination of the final number of crystal cells within the lymph gland.
Figure 5.
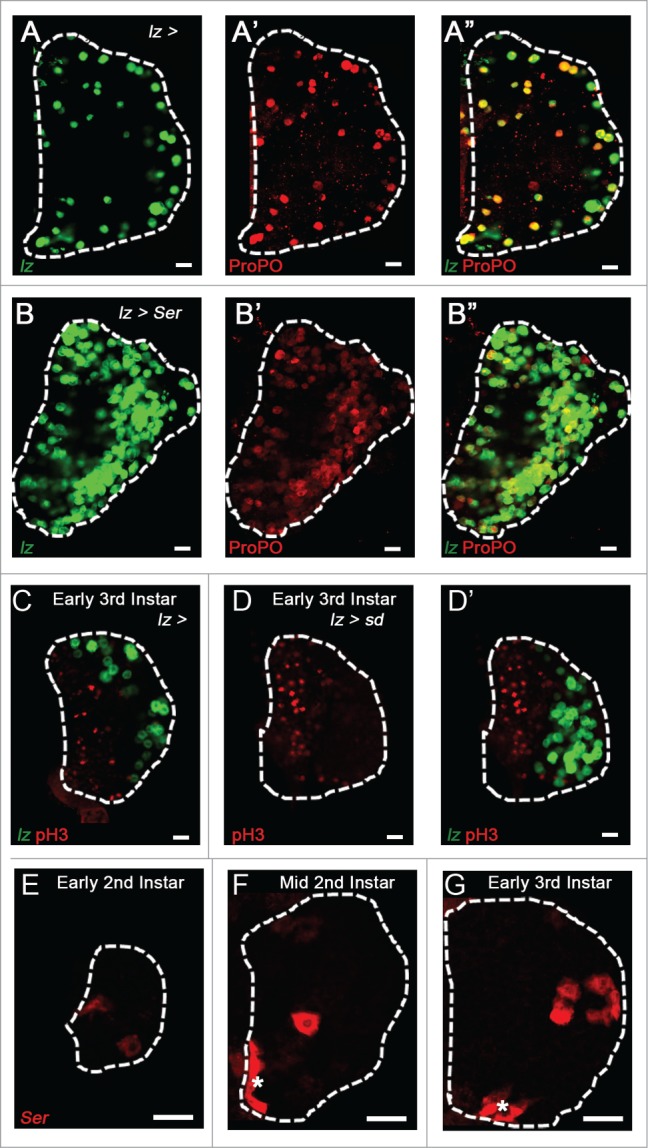
Restriction of Serrate expression in the lymph gland. (A-B”) Crystal cell progenitors labeled with lozenge (lz, Green) and mature crystal cells labeled with ProPO (Red). (A) Crystal cell progenitors and (A’) mature crystal cells in the WT lymph gland. (A”) Overlap of crystal cell progenitors and mature cells. (B) Overexpression of Serrate in crystal cell progenitors induces a large increase in the numbers of lz+ progenitors and (B’) mature crystal cells. (B”) Merge. (C) Crystal cell progenitors are labeled with lz (Green) and proliferative cells are labeled with phosphorylated Histone H3 (pH3, Red) in a WT early third instar lymph gland. (D) Proliferative cells in an early third instar lymph gland after overexpression of sd in crystal cell progenitors. (D’) Increased numbers of crystal cell progenitors are observed in early 3rd instar lymph glands upon overexpression of sd. (E-G) Serrate LacZ (Red) expression in the lymph gland. The PSC is identified with an asterisk. (E) Serrate is first observed in the early second instar lymph gland. (F) By the mid second instar, Serrate is observed in the PSC and in the first LSCs. (G) In the early third instar lymph gland, 2 distinct populations of Serrate-expressing cells are observed: the PSC and more scattered LSCs in the CZ. Scale bar 10 μm.
Immune response as a paradigm for progenitor differentiation
While levels of Serrate must be carefully controlled to limit crystal cell differentiation under normal developmental conditions, the functional significance of regulating Serrate expression is most prominent under stress conditions. Crystal cell numbers decrease significantly upon induction of the cellular immune response due to parasitization by the non-virulent wasp strain L. boulardi (Fig. 6).18,23 This has been proposed as a mechanism through which the lymph gland restricts its limited pool of hemocyte progenitors toward differentiation into lamellocytes that are required for combating large foreign invaders like parasitic wasp eggs. A recent report demonstrated that Notch signaling blocks lamelloctye differentiation in a non-autonomous manner, and that Notch signaling is decreased during the cellular immune response, correlating with the loss of crystal cell numbers in the lymph gland.23 However, the mechanism inhibiting crystal cell differentiation remained unknown until we demonstrated that Serrate expression is specifically inhibited in LSCs upon wasp parasitization.20 Loss of Serrate expression leads to the absence of crystal cells observed after parasitization, while enforced expression of Serrate in the lymph gland is able to restore normal crystal cell numbers while blocking lamellocyte differentiation. Interestingly, while overexpression of yki in lymph glands not presented with an immune challenge results in increased numbers of crystals cells, this effect is blocked in wasp parasitized larvae.20 Furthermore, sd expression is also decreased within the lymph gland of parasitized larve, and Serrate expression cannot be rescued solely by yki overexpression, suggesting that there may be a wasp-induced decrease in Yki function in parasitized larvae. Finally, forced Serrate over-expression is sufficient to maintain crystal cell differentiation during wasp parasitization, demonstrating that the decrease in crystal cell number observed in WT parasitized larvae is not due to blocking Notch signaling downstream of the receptor. Therefore, these findings demonstrate a central mechanism for the lymph gland response to an immune challenge: wasp parasitization decreases levels of Sd and/or inhibits Yki function, reducing Serrate expression and thus blocking crystal cell formation and facilitating lamellocyte differentiation.
Figure 6.
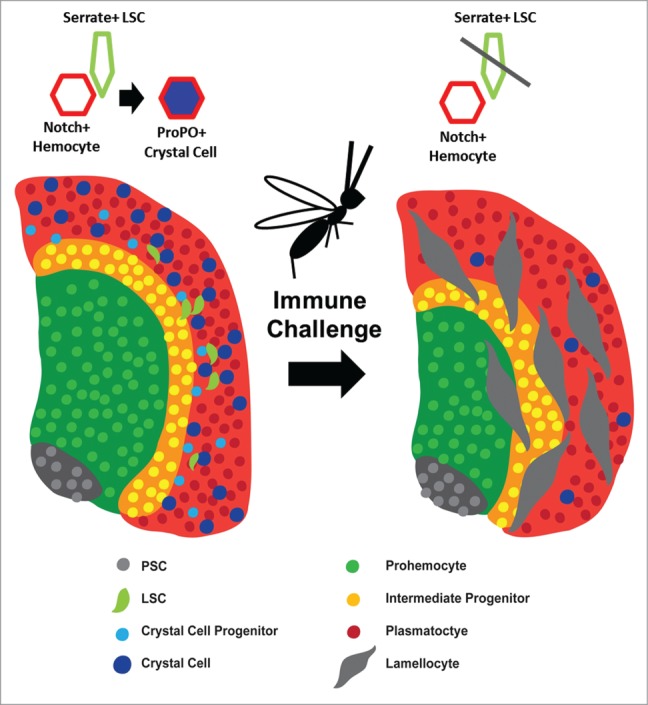
Schematic representation of the Cellular Immune Response in the lymph gland. The PSC is depicted in Gray. Prohemocytes of the MZ are shown in Green and Intermediate Progenitors are Yellow. Plasmatocytes (Red), Crystal Cell Progenitors (Light Blue) and Crystal Cells (Blue) comprise the CZ. Scattered LSCs (Light Green) are also shown in the CZ. Crystal Cell differentiation occurs when a Serrate-expressing LSC interacts with a Notch+ hemocyte. Upon immune challenge, depicted here as a parasitic wasp, the size of the MZ decreases while there is a strong up-regulation of Lamellocytes (Large Gray Cells) and loss of LSCs and Crystal Cells.
In contrast to L. boulardi, virulent strains of parasitic wasps such as L. heterotoma or G. xanthopoda, actually inhibit the larval cellular immune response by injecting virus-like particles which specifically target and destroy host lamellocytes.30 Despite their eventual destruction, the cellular immune response in the lymph gland is still present, as evidenced by the differentiation of lamellocytes,31 and the absence of lymph gland primary lobes that have disintegrated allowing hemocytes to enter circulation.32 While this mechanism of immune suppression is specific to virulent strains of parasitic wasps, perhaps the non-virulent L. boulardi wasps utilize a different approach to repress the immune response. Our findings have demonstrated that Serrate expression in the lymph gland cannot be rescued solely by yki overexpression, suggesting that there may be a wasp-induced decrease in Yki function in parasitized larvae. While the lamellocyte is primary to the larval cellular immune response, the melanizing function of crystal cells may also be required for proper destruction of encapsulated wasp eggs. It may be possible that non-virulent parasitic wasps specifically target Yki as it is paramount to both crystal cell specification and survival. We therefore asked if the loss of crystal cells upon wasp parasitization could be rescued by inhibiting apoptosis. To test this hypothesis we overexpressed p35 in crystal cell progenitors, but did not observed any change in the numbers of crystal cell progenitors after wasp parasitization (Data not shown). These findings suggest that loss of Yki dependent Serrate expression in LSCs is the primary cause for loss of crystal cells in wasp parasitized larvae.
Discussion
Our group along with others has demonstrated that larval immune challenge, either by wasp parasitzation or bacterial infection, induces meaningful cellular responses associated with changes in gene expression and progenitor cell fate. Future studies will likely focus on the mechanism and consequences of Yki inhibition during wasp parasitization. Understanding the signals that regulate Sd expression levels, particularly during wasp parasitization, would also provide insight into the critical role of Sd in cell fate determination in the lymph gland. Lineage tracing analysis of crystal cell progenitors to identify their ultimate fate and of the effectiveness of circulating larval crystal cells in the melanization response to the wasp egg would yield insight into the cellular effects of Yki inhibition during parasitization. We have preliminary evidence suggesting that wasps reduce imaginal disc overgrowth driven by Yki activation, suggesting a more wide-spread effect of infestation on other progenitor cell populations. The effects of wasp parasitization on larval tissues, including fat body,33 may be more widespread than previously scrutinized. As such, this immune challenge model could be utilized to interrogate the role of environmental stress on cell-fate responses in other progenitor/stem cell systems in the larva.
A number of questions still remain regarding the role of Notch signaling in the lymph gland. In particular, the mechanism for the non-cell autonomous effect of Notch activation on crystal cell differentiation remains unknown. Identifying the signal(s) that mediate this function would be most insightful, specifically whether they are capable of inducing Yki expression non-cell autonomously and whether this is sufficient to ectopically activate Serrate expression and thus induce crystal cell formation. The non-autonomous effects of Notch signaling extend to its negative regulation of lamellocyte formation.23 consistent with its required inhibition during wasp-induced lamellocyte formation. Given the known effect of Notch on unpaired expression in the developing eye.34 JAK-STAT pathway activation represents a candidate signal responsible for the Notch non-cell autonomous effect. Furthermore, we have previously demonstrated that AKT activation increases crystal cell number in the lymph gland.35 A study analyzing gene expression of hemocytes demonstrated that crystal cell specific genes lz and Black cells are both up-regulated when RAS is ectopically activated. Interestingly, there is also an increase in Serrate expression in hemocytes upon RAS activation.36 Taken together, these findings suggest that activation of RAS signaling and/or AKT might synergize with Yki/Sd as a common mechanism for regulating Serrate expression in the lymph gland. Overexpression of activating signals such as Notch or Ras in LSCs may provide insight into this potential interaction.
Finally, larger questions such as the extent of contribution of the lymph gland to adult circulating hemocytes and the presence of haematopoietic niches outside of the lymph gland in the developing larva or in the adult fly remain to be addressed. In contrast to crystal cell differentiation in the lymph gland, circulating crystal cells arise from a distinct haematopoietic mechanism. A small number of crystal cells are specified in the head mesoderm of the embryo.8 but this early haematopoietic event does not account for the large numbers of circulating crystal cells in the larva. Several studies have previously identified clusters of sessile hemocytes attached in iterative patterns along the larval cuticle.2,37,38 These hemocytes are maintained by a niche,39 express differentiation markers such as hml, and are capable of differentiating into mature hemocyte lineages. A recent report demonstrated that these sessile hemocytes can express the Notch ligand Serrate which is required to signal fellow sessile hemocytes into the crystal cell lineage.40 This contrasts with cells of the lymph gland where Serrate is expressed in a unique signaling population. Investigating the potential role of Yki and Sd in the differentiation of crystal cells from sessile hemocytes, particularly the pattern of Yki and Sd expression in circulating crystal cells, would establish a conserved requirement for Yki and Sd in regulating Serrate expression during haematopoiesis, regardless of the niche in which they promote crystal cell differentiation.
Material and Methods
Lymph gland dissection and Immunohistochemistry: Unless otherwise noted, lymph glands were dissected as previously described.20 from wandering third instar larvae in 1xPBS and fixed for 20 minutes in 3.7% paraformaldehyde at RT. This was followed by a 30 minute block in 10% Normal Goat Serum diluted in .4% PBT (PBS and Triton X) at RT. After blocking, lymph glands were incubated in primary antibody diluted in blocking buffer overnight at 4°C. Lymph glands were then washed 4x 15 minutes in .4% PBT followed by another 30 minute block at RT. Lymph glands were then incubated in secondary antibody diluted in blocking buffer overnight at 4°C. Lymph glands were washed 4x 15 minutes and mounted in Vectashield mounting medium. Samples were imaged using a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope.
Antibodies: Primary antibodies were diluted as follows: mouse Notch Extracellular Domain (DSHB, 1:5), mouse CD2 (for Enhancer of Split reporter)(Serotec, 1:100), rabbit Prophenoloxidase (gift from Mike Kanost, 1:200), rabbit Yorkie (gift from Ken Irvine, 1:200, preabsorbed), mouse Beta Galactosidase (for Supressor of Hairless reporter) (Millipore, 1:20), mouse Lozenge (DSHB, 1:20). Secondary antibodies were diluted as follows: anti-mouse Cy3 (Jackson Immunoresearch, 1:400), anti-rabbit Cy3 (Jackson Immunoresearch, 1:400), anti-mouse Alexa Fluor648 (Jackson Immunoresearch, 1:400), anti-rabbit FITC (Jackson Immunoresearch, 1:100).
TUNEL staining: As previously reported,20 we modified the In Situ cell death detection kit, TMR red (Roche) protocol. Larvae were dissected in PBS and fixed in 3.7% paraformaldehyde for 20 minutes at RT. Lymph glands were then washed in 0.4% PBT 4 × 15 minutes at RT. Lymph glands were then further permeabilized in 100mM NaCitrate + 0.1% PBT ( pH 6.0) on ice for 3 minutes and rinsed in PBS for 4 × 10 minutes. The TUNEL solution was made by combining Label Solution with Enzyme Solution at a 10:1 ratio. The plate containing tissues in TUNEL solution was incubated at 37°C for 1 hour and 15 minutes. Lymph glands were then rinsed in 0.1% PBT for 6 × 5 minutes, and mounted in Vectashield mounting medium.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux's Arch Dev Biol 1994; 203:266-80; http://dx.doi.org/ 10.1007/BF00360522 [DOI] [PubMed] [Google Scholar]
- 2. Lanot R, Zachary D, Holder F, Meister M. Postembryonic Hematopoiesis in Drosophila. Dev Biol 2001; 230:243-57; PMID:11161576; http://dx.doi.org/ 10.1006/dbio.2000.0123 [DOI] [PubMed] [Google Scholar]
- 3. Jung S-H, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of haematopoiesis. Dev 2005; 132:2521-33; PMID:15857916; http://dx.doi.org/ 10.1242/dev.01837 [DOI] [PubMed] [Google Scholar]
- 4. Kurucz É, Márkus R, Zsámboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, et al. Nimrod, a Putative Phagocytosis Receptor with EGF Repeats in Drosophila Plasmatocytes. Curr Biol 2007; 17:649-54; PMID:Can't14602069http://dx.doi.org/10.1016/j.cub.2007.02.041 [DOI] [PubMed] [Google Scholar]
- 5. Evans CJ, Hartenstein V, Banerjee U. Thicker Than Blood: Conserved Mechanisms in Drosophila and Vertebrate Hematopoiesis. Dev Cell 2003; 5:673-90; PMID:14602069http://dx.doi.org/ 10.1016/S1534-5807(03)00335-6 [DOI] [PubMed] [Google Scholar]
- 6. Rämet M, Lanot R, Zachary D, Manfruelli P. JNK Signaling Pathway Is Required for Efficient Wound Healing in Drosophila. Dev Biol 2002; 241:145-56; PMID:11784101; http://dx.doi.org/ 10.1006/dbio.2001.0502 [DOI] [PubMed] [Google Scholar]
- 7. Rizki TM, Rizki RM, Bellotti RA. Genetics of a Drosophila phenoloxidase. Molec Gen Genet 1985; 201:7-13; PMID:3932822; http://dx.doi.org/ 10.1007/BF00397978 [DOI] [PubMed] [Google Scholar]
- 8. Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila Hematopoietic Lineage by Conserved Transcription Factors. Science 2000; 288:146-9; PMID:10753120; http://dx.doi.org/ 10.1126/science.288.5463.146 [DOI] [PubMed] [Google Scholar]
- 9. Daga A, Karlovich CA, Dumstrei K, Banerjee U. Patterning of cells in the Drosophila eye by Lozenge, which shares homologous domains with AML1. Genes Dev 1996; 10:1194-205; PMID:8675007; http://dx.doi.org/ 10.1101/gad.10.10.1194 [DOI] [PubMed] [Google Scholar]
- 10. Lebestky T, Jung S-H, Banerjee U. A Serrate-expressing signaling center controls Drosophila haematopoiesis. Genes Dev 2003; 17:348-53; PMID:12569125; http://dx.doi.org/ 10.1101/gad.1052803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crozatier M, Ubeda J-M, Vincent A, Meister M. Cellular Immune Response to Parasitization in Drosophila Requires the EBF Orthologue Collier. LoS Biol 2004; 2:e196; PMID:15314643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 2007; 446:320-4; PMID:17361183; http://dx.doi.org/ 10.1038/nature05585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 2007; 446:325-8; PMID:17361184; http://dx.doi.org/ 10.1038/nature05650 [DOI] [PubMed] [Google Scholar]
- 14. Mondal Bama C, Mukherjee T, Mandal L, Evans Cory J, Sinenko Sergey A, Martinez-Agosto Julian A, Banerjee U. Interaction between Differentiating Cell- and Niche-Derived Signals in Hematopoietic Progenitor Maintenance. Cell 2011; 147:1589-600; PMID:22196733; http://dx.doi.org/ 10.1016/j.cell.2011.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual Role of Wingless Signaling in Stem-like Hematopoietic Precursor Maintenance in Drosophila. Dev Cell 2009; 16:756-63; PMID:19460351; http://dx.doi.org/ 10.1016/j.devcel.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dragojlovic-Munther M, Martinez-Agosto JA. Extracellular matrix-modulated Heartless signaling in Drosophila blood progenitors regulates their differentiation via a Ras/ETS/FOG pathway and target of rapamycin function. Dev Biol 2013; 384:313-30; PMID:23603494; http://dx.doi.org/ 10.1016/j.ydbio.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sorrentino RP, Carton Y, Govind S. Cellular Immune Response to Parasite Infection in the Drosophila Lymph Gland Is Developmentally Regulated. Dev Biol 2002; 243:65-80; PMID:11846478; http://dx.doi.org/ 10.1006/dbio.2001.0542 [DOI] [PubMed] [Google Scholar]
- 18. Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol 2010; 346:310-9; PMID:20707995; http://dx.doi.org/ 10.1016/j.ydbio.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 19. Sinenko SA, Shim J, Banerjee U. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO reports 2012; 13:83-9; PMID:22134547; http://dx.doi.org/25454586 10.1038/embor.2011.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferguson Gabriel B, Martinez-Agosto Julian A. Yorkie and Scalloped Signaling Regulates Notch-Dependent Lineage Specification during Drosophila Hematopoiesis. Curr Biol 2014; 24:2665-72; PMID:25454586; http://dx.doi.org/ 10.1016/j.cub.2014.09.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duvic B, Hoffmann JA, Meister M, Royet J. Notch Signaling Controls Lineage Specification during Drosophila Larval Hematopoiesis. Curr Biol 2002; 12:1923-7; PMID:12445385; http://dx.doi.org/ 10.1016/S0960-9822(02)01297-6 [DOI] [PubMed] [Google Scholar]
- 22. Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction Between Notch and Hif-α in Development and Survival of Drosophila Blood Cells. Science 2011; 332:1210-3; PMID:21636775; http://dx.doi.org/ 10.1126/science.1199643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Small C, Ramroop J, Otazo M, Huang LH, Saleque S, Govind S. An unexpected link between Notch signaling and ROS in restricting the differentiation of hematopoietic progenitors in drosophila. Genetics 2014; 197:471-83; PMID:24318532; http://dx.doi.org/ 10.1534/genetics.113.159210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graves HK, Woodfield SE, Yang C-C, Halder G, Bergmann A. Notch Signaling Activates Yorkie Non-Cell Autonomously in Drosophila. PLoS ONE 2012; 7:e37615; PMID:22679484; http://dx.doi.org/ 10.1371/journal.pone.0037615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milton Claire C, Grusche Felix A, Degoutin Joffrey L, Yu E, Dai Q, Lai Eric C, Harvey Kieran F. The Hippo Pathway Regulates Hematopoiesis in Drosophila melanogaster. Curr Biol 2014; 24:2673-80; PMID:25454587; http://dx.doi.org/ 10.1016/j.cub.2014.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gajewski KM, Sorrentino RP, Lee JH, Zhang Q, Russell M, Schulz RA. Identification of a crystal cell-specific enhancer of the black cells prophenoloxidase gene in drosophila. Genesis 2007; 45:200-7; PMID:17417793; http://dx.doi.org/ 10.1002/dvg.20285 [DOI] [PubMed] [Google Scholar]
- 27. Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF Family Protein Scalloped Mediates Transcriptional Output of the Hippo Growth-Regulatory Pathway. Dev Cell 2008; 14:388-98; PMID:18258486; http://dx.doi.org/ 10.1016/j.devcel.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 28. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005; 122:421-34; PMID:16096061; http://dx.doi.org/ 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Verheyen Esther M. Homeodomain-Interacting Protein Kinase Regulates Yorkie Activity to Promote Tissue Growth. Curr biol : CB 2012; 22:1582-6; PMID:22840522; http://dx.doi.org/ 10.1016/j.cub.2012.06.074 [DOI] [PubMed] [Google Scholar]
- 30. Rizki RM, Rizki TM. Parasitoid virus-like particles destroy Drosophila cellular immunity. Proc of the Natl Acad of Sci 1990; 87:8388-92; PMID:2122461; http://dx.doi.org/ 10.1073/pnas.87.21.8388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol 1992; 16:103-10; PMID:1499832; http://dx.doi.org/ 10.1016/0145-305X(92)90011-Z [DOI] [PubMed] [Google Scholar]
- 32. Chili H, Sorrentino R, Govind S. Suppression of the Drosophila Cellular Immune Response by Ganaspis xanthopoda. In: Beck G, Sugumaran M, Cooper E, eds. Phylogenetic Perspectives on the Vertebrate Immune System: Springer US, 2001:161-7; http://dx.doi.org/ 10.1007/978-1-4615-1291-2_14 [DOI] [PubMed] [Google Scholar]
- 33. Paddibhatla I, Lee MJ, Kalamarz ME, Ferrarese R, Govind S. Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Drosophila Larvae. PLoS Pathogens 2010; 6:e1001234; PMID:21203476; http://dx.doi.org/ 10.1371/journal.ppat.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reynolds-Kenneally J, Mlodzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Dev Biol 2005; 285:38-48; PMID:16039641; http://dx.doi.org/ 10.1016/j.ydbio.2005.05.038 [DOI] [PubMed] [Google Scholar]
- 35. Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development 2012; 139:3752-63; PMID:22951642; http://dx.doi.org/ 10.1242/dev.074203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR. Analysis of Ras-Induced Overproliferation in Drosophila Hemocytes. Genetics 2003; 163:203-15; PMID:12586708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurucz É, Váczi B, Márkus R, Laurinyecz B, Vilmos P, Zsámboki J, Csorba K, Gateff E, Hultmark D, Andó I. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biologica Hungarica 2007; 58:95-111; PMID:18297797; http://dx.doi.org/ 10.1556/ABiol.58.2007.Suppl.8 [DOI] [PubMed] [Google Scholar]
- 38. Márkus R, Laurinyecz B, Kurucz É, Honti V, Bajusz I, Sipos B, Somogyi K, Kronhamn J, Hultmark D, Andó I. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proceedings of the Natl Acad of Sci 2009; 106:4805-9; PMID:19261847; http://dx.doi.org/ 10.1073/pnas.0801766106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makhijani K, Alexander B, Tanaka T, Rulifson E, Brückner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development 2011; 138:5379-91; PMID:22071105; http://dx.doi.org/ 10.1242/dev.067322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leitão AB, Sucena É. Drosophila sessile hemocyte clusters are true hematopoietic tissues that regulate larval blood cell differentiation. eLife 2015; PMID:25650737; http://dx.doi.org/ 10.7554/eLife.06166 [DOI] [PMC free article] [PubMed] [Google Scholar]