Abstract
Recently we have shown that adult rats exposed to chronic stress during adolescence increase foraging performance in high-threat conditions by 43% compared to rats reared without stress. Our findings suggest that stress during adolescence can prepare rats to better function under future threat, which supports hypotheses describing an adaptive role for the long-term consequences of early stress (e.g. the thrifty phenotype and maternal mismatch hypotheses). These hypotheses often predict that early stress will impair performance in low-threat conditions later in life. However, we did not find any difference in performance under low-threat conditions between adolescent-stressed and unstressed adult animals. To understand why stress during adolescence may affect performance in high-threat but not in low-threat conditions, we discuss our findings in the framework of the Yerkes-Dodson law, a key precept of psychology that has been used for over a century to describe how stress affects performance.
Keywords: adolescence, chronic stress, chronic unpredictable stress, developmental stress, foraging, risk-taking, Yerkes-Dodson law
In Chaby et al.1 we investigated whether stress during adolescence could prepare an organism to better function under threat in the future, an idea predicted by extensions of a wealth of theory related to prenatal stress.2-5 We showed that under high-threat conditions adult rats exposed to stress during adolescence began foraging sooner, made more transitions between foraging patches, and consumed more food compared with unstressed rats, which supports the application of early hypotheses addressing prenatal stress. However, in low-threat conditions, adolescent-stressed rats took 106% longer to initiate foraging, but consumed the same amount of food as unstressed rats, which contrasts with prior hypotheses predicting decreased functionality in low-threat conditions.3,5
Here we discuss these results in the framework of the Yerkes-Dodson law, which describes a context-specific relationship between performance and arousal that we have modeled using high and low-threat conditions. The Yerkes-Dodson law is based on a series of experiments in which mice perform visual discrimination tasks under weak, moderate, and strong electrical stimulation.6 For a review see refs. 7-8. Yerkes and Dodson found a linear relationship between the acquisition of a simple discrimination task and the strength of electrical stimulation, but a curvilinear relationship between learning a task of moderate difficulty and stimulation strength. Since their seminal work over a century ago, Yerkes and Dodson's findings have been replicated in numerous taxa with modern techniques and statistical analyses 9-11 and widely applied to performance in many contexts including athletic training,12 workplace conditions,13-14 and video games.15 The Yerkes-Dodson law states that for more challenging tasks, i) moderate arousal can enhance performance16-18 in part by modulating motivation,8 but ii) high levels of arousal can decrease performance19-20 through processes such as a reduction in the amount of information that can be processed, as described in the Easterbrook hypothesis.21-22
In Chaby et al.1 we tested rats in a moderately challenging, problem-solving foraging task that required varying motor actions and object manipulations under both high-threat conditions (auditory and visual predator cues, bright light) and low-threat conditions (standard laboratory conditions, dim red light). Under high-threat conditions the control, unstressed animals decreased their performance by an average of 28% ± SE 9% (number of rewards obtained) compared to their performance in a prior low-threat test ([final-initial/initial] × 100). Remarkably, high-threat conditions did not detract from the performance of animals that had experienced adolescent-stress, on average adolescent-stressed rats showed a small increase in performance (2% ± SE 16%) compared to their performance in the prior low-threat test. The effect of adolescent-stress on the relationship between performance and threat condition could be underpinned by a shift in the curvilinear relationship between performance and arousal as described by the Yerkes-Dodson law (Fig. 1). Such a shift would allow adolescent-stressed animals to perform better at higher of arousal that exceed the optimal range of arousal for unstressed animals (the peak of the Gaussian curve). Under this framework, exposure to adolescent-stress would cause an increase in optimal arousal range, but adolescent-stressed animals would still show a decline in performance after arousal exceeds their optimal level. It would follow that adolescent-stressed rats would maintain a performance advantage over threat-naïve animals throughout the decline until their level of arousal becomes too high to permit completion of a moderately challenging task regardless of rearing environment. In this model, the effect of adolescent-stress on performance is minimal in the absence of threat (low arousal conditions). As arousal increases in the positive slope of the Gaussian curve, both unstressed and adolescent-stressed animals increase performance; no difference in performance may be detected until near the optimal arousal level of unstressed animals.
Figure 1.
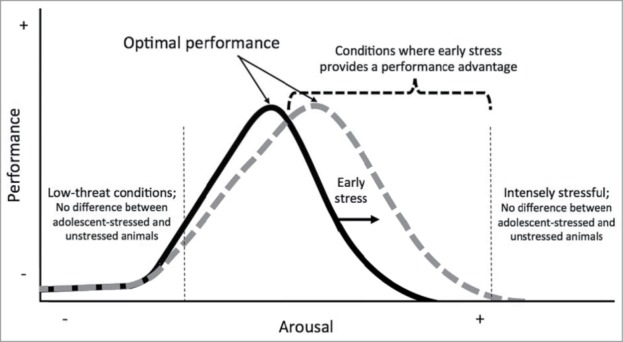
Stress during adolescence may cause a shift in the curvilinear relationship between performance and arousal, as described by the Yerkes-Dodson law for tasks of moderate difficulty. Note that under conditions that are either very low-threat or intensely stressful, animals are not predicted to differ in performance regardless of rearing conditions.
Our results emphasize the importance of context on behavior and performance, and the importance of understanding the relationship between testing environment and rearing environment. The proposed model of the capacity for stress during adolescence to cause a shift in the relationship between arousal and performance, the “arousal-shift hypothesis”, could guide future investigations of the lasting effects of early stress as animals age and threats in their environment change. In addition to understanding how performance is dependent on environmental context (threat vs. safe), we must also investigate performance in a task-dependent manner (simple vs. complex). In Chaby et al.,1 we investigated whether the lasting effects of stress during adolescence could be explained by expanding well supported hypotheses predicting the effects of prenatal stress (e.g., thrifty phenotype, maternal mismatch). The arousal-shift hypothesis presented here does not contradict crucial early hypotheses that predict that prenatal stress can prepare an individual for an adverse environment, but suggests that developmental stress may manifest though a shift in the relationship between performance and arousal. The findings discussed here inform our understanding of the role of changes in plasticity across development and the capacity for transformative change during adolescence.
Acknowledgments
We thank Erin Platz, Hannah Cooper, Brad Fetherston, Rachel Reineke, Kendall Warg, Kaitlyn Grubb, Weiyuan Tian, James Lim, David Ensminger, and Carl Hirrlinger.
Funding
We thank our funding sources, Pennsylvania State University's Huck Institutes of the Life Sciences, Eberly College of Science, and the Pennsylvania Department of Health using Tobacco CURE Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
References
- 1.Chaby LE, Sheriff MJ, Hirrlinger AM, Braithwaite VA. (2015). Does early stress prepare individuals for a stressful future? Stress during adolescence improves foraging under threat. Animal Behaviour, 105, 37-45. http://doi.org/ 10.1016/j.anbehav.2015.03.028 [DOI] [Google Scholar]
- 2.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35:595-601; PMID:1644236; http://dx.doi.org/ 10.1007/BF00400248 [DOI] [PubMed] [Google Scholar]
- 3.Breuner C. Maternal stress, glucocorticoids, and the maternal/fetal match hypothesis. Hormones and Behavior 2008; 54:485-487; PMID:18573256; http://dx.doi.org/ 10.1016/j.yhbeh.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 4.Beery AK, Francis DD. Adaptive significance of natural variations in maternal care in rats: A translational perspective. Neurosci Biobehav Rev 2011; 35:1552-1561; PMID:21458485; http://dx.doi.org/ 10.1016/j.neubiorev.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheriff MJ, Love OP. Determining the adaptive potential of maternal stress. Ecology Letters 2013; 16:271-280; PMID:23205937; http://dx.doi.org/ 10.1111/ele.12042 [DOI] [PubMed] [Google Scholar]
- 6.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol 1908; 18:459-482; http://dx.doi.org/ 10.1002/cne.920180503 [DOI] [Google Scholar]
- 7.Diamond DM. Cognitive, endocrine and mechanistic perspectives on non-linear relationships between arousal and brain function. Nonlinearity Biol Toxicol Med 2005; 3:1-7; PMID:19330153; http://dx.doi.org/ 10.2201/nonlin.003.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: A synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity 2007; e60803; http://dx.doi.org/ 10.1155/2007/60803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telegdy GA, Cohen JS. Cue utilization and drive level in albino rats. Journal of Comparative and Physiological Psychology 1971; 75:248-253; http://dx.doi.org/ 10.1037/h0030825 [DOI] [Google Scholar]
- 10.Anderson KJ. Impulsitivity, caffeine, and task difficulty: A within-subjects test of the Yerkes-Dodson law. Personality and Individual Differences 1994; 16:813-829; http://dx.doi.org/ 10.1016/0191-8869(94)90226-7 [DOI] [Google Scholar]
- 11.Dickman SJ. Dimensions of arousal: Wakefulness and vigor. Hum Factors 2002; 44:429-442; PMID:12502160; http://dx.doi.org/ 10.1518/0018720024497673 [DOI] [PubMed] [Google Scholar]
- 12.Stinson C, Bowman DA. Feasibility of training athletes for high-pressure situations using virtual reality. IEEE Trans Vis Comput Grap 2014; 20:606-615; PMID:24650988; http://dx.doi.org/ 10.1109/TVCG.2014.23 [DOI] [PubMed] [Google Scholar]
- 13.Chang LC, Mahoney JJ, Raty SR, Ortiz J, Apodaca S, De La Garza R. Neurocognitive effects following an overnight call shift on faculty anesthesiologists. Acta Anaesthesiol Scand 2013; 57:1051-1057; PMID:23593975; http://dx.doi.org/ 10.1111/aas.12120 [DOI] [PubMed] [Google Scholar]
- 14.Giddings B, Thomas J, Little L. Evaluation of the workplace environment in the UK, and the impact on users' levels of stimulation. Indoor and Built Environment 2013; 1420326X13476078; http://dx.doi.org/ 10.1177/1420326X13476078 [DOI] [Google Scholar]
- 15.Jeong EJ, Biocca FA. Are there optimal levels of arousal to memory? Effects of arousal, centrality, and familiarity on brand memory in video games. Comput Hum Behav 2012; 28:285-291; http://dx.doi.org/ 10.1016/j.chb.2011.09.011 [DOI] [Google Scholar]
- 16.Broadhurst PL. Emotionality and the Yerkes-Dodson law. J Exp Psychol 1957; 54:345; PMID:13481281; http://dx.doi.org/ 10.1037/h0049114 [DOI] [PubMed] [Google Scholar]
- 17.Ni CF. An experimental study of the influence of punishment for errors during learning upon retention. J Comparat Psychol 1934; 17:279-301; http://dx.doi.org/ 10.1037/h0075629 [DOI] [Google Scholar]
- 18.Salehi B, Cordero MI, Sandi C. Learning under stress: The inverted-U-shape function revisited. Learn. Mem 2010; 17:522-530; PMID:20884754; http://dx.doi.org/ 10.1101/lm.1914110 [DOI] [PubMed] [Google Scholar]
- 19.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 1999; 9:542-552. PMID:10560925; http://dx.doi.org/ 10.1002/(SICI)1098-1063(1999)9:5%3c542::AID-HIPO8%3e3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 2002; 3:453-462; PMID:12042880; http://dx.doi.org/ 10.1038/nrn849 [DOI] [PubMed] [Google Scholar]
- 21.Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychol Rev 1959; 66:183-201; PMID:13658305; http://dx.doi.org/ 10.1037/h0047707] [DOI] [PubMed] [Google Scholar]
- 22.Anderson KJ, Revelle W. Impulsivity, caffeine, and proofreading: A test of the Easterbrook hypothesis. J Exp Psychol: Human Percept Perform 1982; 8:614-624; PMID:6214611; http://dx.doi.org/ 10.1037/0096-1523.8.4.614 [DOI] [PubMed] [Google Scholar]


